Abstract
We assessed the prevalence of six biocide resistance genes among 82 methicillin-resistant Staphylococcus aureus (MRSA) and 219 methicillin-susceptible S. aureus (MSSA) isolates from three African countries; the prevalence was very high for sepA (95.3%), mepA (89.4%), and norA (86.4%), intermediate for lmrS (60.8%) and qacAB (40.5%), and low for smr (3.7%). A significant association between biocide resistance genes and antibiotic resistance was observed, and a new cutoff MIC of ≥1 mg/liter for chlorhexidine nonsusceptibility was defined.
TEXT
Biocides, as quaternary ammonium compounds (QACs) or biguanides, are widely used in infection control programs, including hand washing and skin decolonization prior to invasive procedures. However, its overuse led to the emergence of Staphylococcus aureus with decreased antiseptic susceptibility, which became a problem in hospitals in different regions of the world (1–3). Although biocide resistance might be multifactorial, multidrug resistance efflux pumps are the predominant mechanisms mediating cross-resistance to both antibiotics and biocides (4). Numerous genes encoding multidrug efflux pumps, such as qacA, qacB, smr, norA, lmrS, mepA, and sepA, have been described in clinical S. aureus isolates from humans, animals, and environmental samples (2, 5–10). Moreover, reduced susceptibility to chlorhexidine, one of the most frequently used biocides, is usually associated with both the qacAB and smr gene families (11, 12). Consequently, daily chlorhexidine baths in combination with mupirocin nasal ointment, a usual practice in preventive decolonization programs, and the usage of chlorhexidine hand soap as an infection control measure might be compromised (13). Although qacAB prevalence in methicillin-resistant S. aureus (MRSA) has been reported as highly varied, from <1% in a few Asiatic countries to 80% in Brazil (1, 2, 14–20), the geographic distribution of additional biocide resistance genes has been poorly studied and, to date, there are no data from the African continent, where the prevalence of MRSA is considerably high (up to 60%) (21).
In the present study, we aimed to fulfil this gap by determining the prevalence of six biocide resistance genes among representative S. aureus isolates from previous nasal carriage surveillance studies in Angola, São Tomé and Príncipe, and Cape Verde (21, 22). Antimicrobial resistance profiles, molecular characterizations, and information on the presence of virulence determinants were available for all isolates (21, 22). In order to obtain the highest degree of clonal variability, convenience samples of 82 MRSA and 219 methicillin-susceptible S. aureus (MSSA) isolates were selected, including one isolate from each pulsed-field gel electrophoresis (PFGE) subtype described in each country and during each screening period: (i) Angola, 57 MRSA and 62 MSSA isolates; (ii) Cape Verde, 6 MRSA and 99 MSSA isolates; and (iii) São Tomé and Príncipe, 19 MRSA and 58 MSSA isolates.
Internal fragments of six efflux pump genes (qacAB, smr, norA, lmrS, mepA, and sepA) were amplified by PCR in all isolates. The primer sequences, modifications of published protocols, and specific controls are listed in Table S1 in the supplemental material.
The chlorhexidine MIC and minimum bactericidal concentration (MBC) were determined for all isolates using a Clinical and Laboratory Standards Institute broth microdilution method (23). S. aureus strains ATCC 29213 and ATCC 12228 were used as quality controls.
Categorical variables were compared using Fisher's exact test, and prevalence increasing trends were verified by chi-square test using the GraphPad software version 6.0. In all cases, P values of <0.05 were considered statistically significant.
The global prevalence of six biocide resistance genes in the present S. aureus collection was very high for sepA (95.3%), mepA (89.4%), and norA (86.4%), intermediate for lmrS (60.8%) and qacAB (40.5%), and low for smr (3.7%). A similar distribution was observed in a comparison of the MRSA and MSSA populations (see Table S2 in the supplemental material), except for sepA and qacAB, which were more prevalent among MRSA isolates (P = 0.0137 and <0.0001, respectively). Although qacAB has commonly been reported among MRSA isolates (1, 3, 24, 25), no direct relation was described between methicillin resistance and qacAB prevalence (26).
Except for sepA, which was prevalent in all countries (P = 0.1249), the distributions of other genes were significantly different (see Table S2 in the supplemental material); namely, a higher percentage of qacAB was detected in Angola (56.3%, P < 0.0001). The smr gene, sporadically reported in Europe and Asia (1, 2), was detected only among MSSA isolates from Angola (5.9%) and Cape Verde (3.8%).
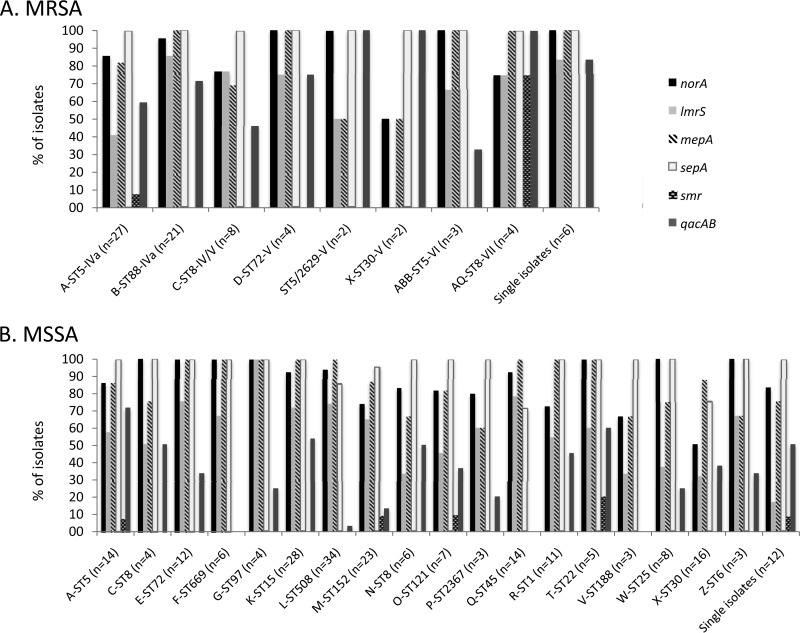
Although the presence of some biocide resistance genes has been associated with major S. aureus clonal lineages (9, 13, 18, 19, 27, 28), this was not the case in our collection, in which all genes but one (smr) were present among the 14 MRSA clonal types (Fig. 1A). Concerning the major clones currently circulating in the three African countries (A-ST5-IVa, B-ST88-IVa, and C-ST8-IV/V) (21), mepA, norA, and qacAB were predominant not only in the B-ST88-IVa lineage (100%, 95%, and 77%, respectively) but in the majority of A-ST5-IVa isolates (81%, 85%, and 59%, respectively) as well. Moreover, lmrS was widespread between the B-ST88-IVa (86%) and C-ST8-IV/V (77%) lineages and also found in the A-ST5-IVa lineage (41%). Therefore, biocide resistance genes are highly prevalent among major MRSA clonal lineages in these African countries.
FIG 1.
Distribution of the six biocide resistance genes by major MRSA (A) and MSSA (B) clonal lineages circulating in Angola, São Tomé and Príncipe, and Cape Verde.
The MSSA population showed higher clonal lineage variability (30 clones) than that of the MRSA population (21), with a parallel variability in the distribution of biocide resistance genes (Fig. 1B). Although a direct correlation between the presence of a biocide resistance gene and a specific clone was not traced, qacAB was poorly represented in two of the most predominant MSSA lineages (Q-ST45 and M-ST152) and absent in V-ST188 (Fig. 1B).
In this study, biocide resistance genes were significantly associated with antibiotic resistance (see Table S3 in the supplemental material). qacAB was associated with resistance to β-lactams (cefoxitin), aminoglycosides, rifampin, trimethoprim-sulfamethoxazole, and chloramphenicol, which has also been reported in S. aureus clinical isolates in China (3). qacAB and smr resistance genes are located on mobile genetic elements, namely, plasmids that frequently carry other antibiotic resistance genes, which results in the coselection of antibiotic-resistant bacteria (4, 8, 29). Rifampin resistance, which was high in these African countries (21), was associated with the presence of four out of the six antiseptic resistance genes (see Table S3). Rifampin resistance is driven by mutations in the rpoB gene, and several reports have suggested that the fitness burden associated with rifampin resistance might influence the prevalence of specific resistance genotypes in clinical strains of S. aureus (30). Although these suggestions might justify the maintenance of antiseptic genes as an advantage for bacterial persistence in this environment, this association should not be excluded as a confounding parameter.
The MIC for chlorhexidine ranged between 0.125 and 8 mg/liter in the entire collection, although the overwhelming majority of the isolates (90%) showed an MIC of 0.5 to 1 mg/liter. Higher MICs of 4 and 8 mg/liter were observed in three Angolan isolates only. Similar chlorhexidine MICs (0.5 to 1 mg/liter) were found in MRSA and MSSA populations (85.0% [70/82 isolates] of MRSA versus 91.8% [201/219 isolates] of MSSA; P = 0.1285), as previously reported for MRSA isolates from human and animal origins (10, 31). To date, there is no breakpoint consensus to define biocide-reduced susceptibility, and independent reports have proposed different epidemiological cutoff (ECOFF) values based on the normal distribution of MICs in different S. aureus populations (27, 32, 33). In our study, an MIC cutoff of ≥1 mg/liter was defined, since <1% of the isolates showed an MIC of ≥2 mg/liter. In Cape Verde, 40% of the S. aureus isolates showed a chlorhexidine MIC of ≥1 mg/liter, which is significantly higher than the values observed in São Tomé and Príncipe (34%) and Angola (23%) (P = 0.0186). Chlorhexidine nonsusceptibility still seems to be low in these African countries, despite the high percentage of biocide resistance genes detected in S. aureus isolates there. The presence of qacAB and norA genes was not associated with increased chlorhexidine nonsusceptibility in our collection (P = 0.7069 and 0.7196, respectively), although these associations have commonly been described in staphylococci (11).
One of the limitations of our study was the lack of availability of quantitative data concerning biocide usage. Although the prevalence of biocide-resistant isolates in the African population might be underestimated due to the use of convenience samples, these data show that the use of antiseptics might be selecting for antibiotic-resistant strains and assisting their survival in the health care environment, which is of major concern for future infection control programs.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by project PTDC/SAU-SAP/118813/2010 and grant PEst-OE/EQB/LA0004/2011 from the Fundação para a Ciência e a Tecnologia (FCT). T.C. and C.C. were supported by grants SFRH/BPD/72422/2010 and 036/BI-BI/2012, respectively, from FCT, Portugal.
We declare no conflicts of interest.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.02140-15.
REFERENCES
- 1.Mayer S, Boos M, Beyer A, Fluit AC, Schmitz FJ. 2001. Distribution of the antiseptic resistance genes qacA, qacB and qacC in 497 methicillin-resistant and -susceptible European isolates of Staphylococcus aureus. J Antimicrob Chemother 47:896–897. doi: 10.1093/jac/47.6.896. [DOI] [PubMed] [Google Scholar]
- 2.Noguchi N, Suwa J, Narui K, Sasatsu M, Ito T, Hiramatsu K, Song JH. 2005. Susceptibilities to antiseptic agents and distribution of antiseptic-resistance genes qacA/B and smr of methicillin-resistant Staphylococcus aureus isolated in Asia during 1998 and 1999. J Med Microbiol 54:557–565. doi: 10.1099/jmm.0.45902-0. [DOI] [PubMed] [Google Scholar]
- 3.Zhang M, O'Donoghue MM, Ito T, Hiramatsu K, Boost MV. 2011. Prevalence of antiseptic-resistance genes in Staphylococcus aureus and coagulase-negative staphylococci colonising nurses and the general population in Hong Kong. J Hosp Infect 78:113–117. doi: 10.1016/j.jhin.2011.02.018. [DOI] [PubMed] [Google Scholar]
- 4.Costa SS, Viveiros M, Amaral L, Couto I. 2013. Multidrug efflux pumps in Staphylococcus aureus: an update. Open Microbiol J 7:59–71. doi: 10.2174/1874285801307010059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bjorland J, Sunde M, Waage S. 2001. Plasmid-borne smr gene causes resistance to quaternary ammonium compounds in bovine Staphylococcus aureus. J Clin Microbiol 39:3999–4004. doi: 10.1128/JCM.39.11.3999-4004.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Floyd JL, Smith KP, Kumar SH, Floyd JT, Varela MF. 2010. LmrS is a multidrug efflux pump of the major facilitator superfamily from Staphylococcus aureus. Antimicrob Agents Chemother 54:5406–5412. doi: 10.1128/AAC.00580-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sierra JM, Ruiz J, Jimenez De Anta MT, Vila J. 2000. Prevalence of two different genes encoding NorA in 23 clinical strains of Staphylococcus aureus. J Antimicrob Chemother 46:145–146. doi: 10.1093/jac/46.1.145. [DOI] [PubMed] [Google Scholar]
- 8.Costa SS, Mourato C, Viveiros M, Melo-Cristino J, Amaral L, Couto I. 2013. Description of plasmid pSM52, harbouring the gene for the Smr efflux pump, and its involvement in resistance to biocides in a meticillin-resistant Staphylococcus aureus strain. Int J Antimicrob Agents 41:490–492. doi: 10.1016/j.ijantimicag.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 9.Kosmidis C, Schindler BD, Jacinto PL, Patel D, Bains K, Seo SM, Kaatz GW. 2012. Expression of multidrug resistance efflux pump genes in clinical and environmental isolates of Staphylococcus aureus. Int J Antimicrob Agents 40:204–209. doi: 10.1016/j.ijantimicag.2012.04.014. [DOI] [PubMed] [Google Scholar]
- 10.Couto N, Belas A, Kadlec K, Schwarz S, Pomba C. 2015. Clonal diversity, virulence patterns and antimicrobial and biocide susceptibility among human, animal and environmental MRSA in Portugal. J Antimicrob Chemother 70:2483–2487. doi: 10.1093/jac/dkv141. [DOI] [PubMed] [Google Scholar]
- 11.Horner C, Mawer D, Wilcox M. 2012. Reduced susceptibility to chlorhexidine in staphylococci: is it increasing and does it matter? J Antimicrob Chemother 67:2547–2559. doi: 10.1093/jac/dks284. [DOI] [PubMed] [Google Scholar]
- 12.Noguchi N, Hase M, Kitta M, Sasatsu M, Deguchi K, Kono M. 1999. Antiseptic susceptibility and distribution of antiseptic-resistance genes in methicillin-resistant Staphylococcus aureus. FEMS Microbiol Lett 172:247–253. doi: 10.1111/j.1574-6968.1999.tb13475.x. [DOI] [PubMed] [Google Scholar]
- 13.Batra R, Cooper BS, Whiteley C, Patel AK, Wyncoll D, Edgeworth JD. 2010. Efficacy and limitation of a chlorhexidine-based decolonization strategy in preventing transmission of methicillin-resistant Staphylococcus aureus in an intensive care unit. Clin Infect Dis 50:210–217. doi: 10.1086/648717. [DOI] [PubMed] [Google Scholar]
- 14.Fritz SA, Hogan PG, Camins BC, Ainsworth AJ, Patrick C, Martin MS, Krauss MJ, Rodriguez M, Burnham CA. 2013. Mupirocin and chlorhexidine resistance in Staphylococcus aureus in patients with community-onset skin and soft tissue infections. Antimicrob Agents Chemother 57:559–568. doi: 10.1128/AAC.01633-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith K, Gemmell CG, Hunter IS. 2008. The association between biocide tolerance and the presence or absence of qac genes among hospital-acquired and community-acquired MRSA isolates. J Antimicrob Chemother 61:78–84. [DOI] [PubMed] [Google Scholar]
- 16.Longtin J, Seah C, Siebert K, McGeer A, Simor A, Longtin Y, Low DE, Melano RG. 2011. Distribution of antiseptic resistance genes qacA, qacB, and smr in methicillin-resistant Staphylococcus aureus isolated in Toronto, Canada, from 2005 to 2009 Antimicrob Agents Chemother 55:2999–3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang JT, Sheng WH, Wang JL, Chen D, Chen ML, Chen YC, Chang SC. 2008. Longitudinal analysis of chlorhexidine susceptibilities of nosocomial methicillin-resistant Staphylococcus aureus isolates at a teaching hospital in Taiwan. J Antimicrob Chemother 62:514–517. doi: 10.1093/jac/dkn208. [DOI] [PubMed] [Google Scholar]
- 18.Wang C, Cai P, Zhan Q, Mi Z, Huang Z, Chen G. 2008. Distribution of antiseptic-resistance genes qacA/B in clinical isolates of meticillin-resistant Staphylococcus aureus in China. J Hosp Infect 69:393–394. doi: 10.1016/j.jhin.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 19.Ho J, Branley J. 2012. Prevalence of antiseptic resistance genes qacA/B and specific sequence types of methicillin-resistant Staphylococcus aureus in the era of hand hygiene. J Antimicrob Chemother 67:1549–1550. doi: 10.1093/jac/dks035. [DOI] [PubMed] [Google Scholar]
- 20.Miyazaki NH, Abreu AO, Marin VA, Rezende CA, Moraes MT, Villas Bôas MH. 2007. The presence of qacA/B gene in Brazilian methicillin-resistant Staphylococcus aureus. Mem Inst Oswaldo Cruz 102:539–540. doi: 10.1590/S0074-02762007000400018. [DOI] [PubMed] [Google Scholar]
- 21.Conceição T, Coelho C, Santos Silva I, de Lencastre H, Aires-de-Sousa M. 2015. Staphylococcus aureus in former Portuguese colonies from Africa and the Far East: missing data to help fill the world map. Clin Microbiol Infect 21:842.e1–842.e10. doi: 10.1016/j.cmi.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 22.Aires-de-Sousa M, Santos Sanches I, Ferro ML, De Lencastre H. 2000. Epidemiological study of staphylococcal colonization and cross-infection in two West African Hospitals. Microb Drug Resist 6:133–141. doi: 10.1089/107662900419447. [DOI] [PubMed] [Google Scholar]
- 23.Clinical and Laboratory Standards Institute. 2012. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard, 9th ed. CLSI document M07-A9, Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 24.Opacic D, Lepsanovic Z, Sbutega-Milosevic G. 2010. Distribution of disinfectant resistance genes qacA/B in clinical isolates of meticillin-resistant and -susceptible Staphylococcus aureus in one Belgrade hospital. J Hosp Infect 76:266–267. doi: 10.1016/j.jhin.2010.04.019. [DOI] [PubMed] [Google Scholar]
- 25.Alam MM, Kobayashi N, Uehara N, Watanabe N. 2003. Analysis on distribution and genomic diversity of high-level antiseptic resistance genes qacA and qacB in human clinical isolates of Staphylococcus aureus. Microb Drug Resist 9:109–121. doi: 10.1089/107662903765826697. [DOI] [PubMed] [Google Scholar]
- 26.Lambert RJ. 2004. Comparative analysis of antibiotic and antimicrobial biocide susceptibility data in clinical isolates of methicillin-sensitive Staphylococcus aureus, methicillin-resistant Staphylococcus aureus and Pseudomonas aeruginosa between 1989 and 2000. J Appl Microbiol 97:699–711. doi: 10.1111/j.1365-2672.2004.02345.x. [DOI] [PubMed] [Google Scholar]
- 27.Otter JA, Patel A, Cliff PR, Halligan EP, Tosas O, Edgeworth JD. 2013. Selection for qacA carriage in CC22, but not CC30, methicillin-resistant Staphylococcus aureus bloodstream infection isolates during a successful institutional infection control programme. J Antimicrob Chemother 68:992–999. doi: 10.1093/jac/dks500. [DOI] [PubMed] [Google Scholar]
- 28.Sheng WH, Wang JT, Lauderdale TL, Weng CM, Chen D, Chang SC. 2009. Epidemiology and susceptibilities of methicillin-resistant Staphylococcus aureus in Taiwan: emphasis on chlorhexidine susceptibility. Diagn Microbiol Infect Dis 63:309–313. doi: 10.1016/j.diagmicrobio.2008.11.014. [DOI] [PubMed] [Google Scholar]
- 29.Sidhu MS, Heir E, Leegaard T, Wiger K, Holck A. 2002. Frequency of disinfectant resistance genes and genetic linkage with beta-lactamase transposon Tn552 among clinical staphylococci. Antimicrob Agents Chemother 46:2797–2803. doi: 10.1128/AAC.46.9.2797-2803.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O'Neill AJ, Huovinen T, Fishwick CW, Chopra I. 2006. Molecular genetic and structural modeling studies of Staphylococcus aureus RNA polymerase and the fitness of rifampin resistance genotypes in relation to clinical prevalence. Antimicrob Agents Chemother 50:298–309. doi: 10.1128/AAC.50.1.298-309.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clark SM, Loeffler A, Bond R. 2015. Susceptibility in vitro of canine methicillin-resistant and -susceptible staphylococcal isolates to fusidic acid, chlorhexidine and miconazole: opportunities for topical therapy of canine superficial pyoderma. J Antimicrob Chemother 70:2048–2052. doi: 10.1093/jac/dkv056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morrissey I, Oggioni MR, Knight D, Curiao T, Coque T, Kalkanci A, Martinez JL, BIOHYPO Consortium. 2014. Evaluation of epidemiological cut-off values indicates that biocide resistant subpopulations are uncommon in natural isolates of clinically-relevant microorganisms. PLoS One 9:e86669. doi: 10.1371/journal.pone.0086669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Coelho JR, Carriço JA, Knight D, Martinez JL, Morrissey I, Oggioni MR, Freitas AT. 2013. The use of machine learning methodologies to analyse antibiotic and biocide susceptibility in Staphylococcus aureus. PLoS One 8:e55582. doi: 10.1371/journal.pone.0055582. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.