Abstract
Lopinavir-ritonavir is frequently prescribed to HIV-1-infected women during pregnancy. Decreased lopinavir exposure has been reported during pregnancy, but the clinical significance of this reduction is uncertain. This analysis aimed to evaluate the need for lopinavir dose adjustment during pregnancy. We conducted a population pharmacokinetic analysis of lopinavir and ritonavir concentrations collected from 84 pregnant and 595 nonpregnant treatment-naive and -experienced HIV-1-infected subjects enrolled in six clinical studies. Lopinavir-ritonavir doses in the studies ranged between 400/100 and 600/150 mg twice daily. In addition, linear mixed-effect analysis was used to compare the area under the concentration-time curve from 0 to 12 h (AUC0–12) and concentration prior to dosing (Cpredose) in pregnant women and nonpregnant subjects. The relationship between lopinavir exposure and virologic suppression in pregnant women and nonpregnant subjects was evaluated. Population pharmacokinetic analysis estimated 17% higher lopinavir clearance in pregnant women than in nonpregnant subjects. Lopinavir clearance values postpartum were 26.4% and 37.1% lower than in nonpregnant subjects and pregnant women, respectively. As the tablet formulation was estimated to be 20% more bioavailable than the capsule formulation, no statistically significant differences between lopinavir exposure in pregnant women receiving the tablet formulation and nonpregnant subjects receiving the capsule formulation were identified. In the range of lopinavir AUC0–12 or Cpredose values observed in the third trimester, there was no correlation between lopinavir exposure and viral load or proportion of subjects with virologic suppression. Similar efficacy was observed between pregnant women and nonpregnant subjects receiving lopinavir-ritonavir at 400/100 mg twice daily. The pharmacokinetic and pharmacodynamic results support the use of a lopinavir-ritonavir 400/100-mg twice-daily dose during pregnancy.
INTRODUCTION
The use of combination antiretroviral therapy (cART) is recommended in all pregnant women with HIV infection for prevention of perinatal transmission as well as for maternal health. Such use has resulted in a significant reduction of perinatal transmission from 25% to 0–3.6% (1, 2). Treatment guidelines include the use of protease inhibitors in combination with two nucleoside reverse transcriptase inhibitors (NRTIs) (3–7).
Lopinavir (LPV) is a peptidomimetic HIV type 1 (HIV-1) protease inhibitor. When LPV is coadministered with low-dose ritonavir (RTV), which acts as a pharmacokinetic enhancer by blocking the cytochrome P450 3A (CYP3A)-mediated metabolism of LPV, serum levels of LPV are significantly increased, and half-life is prolonged. The high LPV exposures achieved with coformulated lopinavir-ritonavir (LPV/r) have the advantage of providing a pharmacologic barrier to the emergence of HIV-1 viral resistance in patients with wild-type virus, as well as enhanced activity against some forms of drug-resistant HIV-1 (8).
Because of the potency of LPV/r, lack of CD4 count-dependent toxicity, and favorable tolerability profile in general, most treatment guidelines of national, regional, and global organizations and agencies (e.g., Department of Health and Human Services perinatal guidelines, WHO, British HIV Association, and the European AIDS Clinical Society) recommend LPV/r as a protease inhibitor during pregnancy (3–7). Several clinical studies have demonstrated LPV/r efficacy in achieving virologic suppression in mothers during pregnancy and preventing HIV transmission to their children (9–16). Reports of higher LPV clearance during pregnancy have prompted some investigators to propose the use of a higher dose during the third trimester, while others advocated no adjustment to the standard 400/100-mg twice-daily (BID) regimen (17–20). As an important component of cART given during pregnancy, further assessment of LPV/r dosing during pregnancy would support its appropriate use in this population. This analysis employed a model-based approach to analyze LPV pharmacokinetics and pharmacodynamics in pregnant and nonpregnant HIV-infected subjects to evaluate dosing of LPV/r in pregnant women utilizing data collected in six studies.
MATERIALS AND METHODS
Clinical studies and patient population.
Six clinical studies of HIV-infected adults were included in the analyses, three of which were conducted with pregnant women and three of which were with nonpregnant subjects. For each study, the study protocol was approved by the Institutional Review Board of the individual study site, and written informed consent was obtained from each subject prior to enrollment. All subjects were ≥18 years of age and received cART regimens comprising two NRTIs plus LPV/r (Table 1). All studies used validated high-performance liquid chromatography with tandem mass spectrometric detection (HPLC-MS/MS) or UV detection (HPLC-UV) to quantitate LPV and RTV concentrations.
TABLE 1.
Overview of the clinical studies included in the analysis
| Study no. (reference) | No. of subjects | Population | LPV/r dosinga | Coadministered NRTIsb | PK sampling regimen type and timesc |
|---|---|---|---|---|---|
| 1 (20) | 53 | HIV-infected pregnant women | 400/100-mg or 600/150-mg tablet BIDa | Zidovudine-lamivudine | Intensive (predose and 1, 2, 3, 4, 5, 6, 8, 10, and 12 h postdosing) |
| 2 (17) | 19 | HIV-infected pregnant women | 400/100-mg tablet BID or 400/100-mg SGC BID | Zidovudine-lamivudine, abacavir-lamivudine, tenofovir-emtricitabine, abacavir-zidovudine-lamivudine, or zidovudine-tenofovir | Intensive (predose and 1, 2, 4, 6, 9, and 12 h postdosing) |
| 3 (21) | 12 | HIV-infected pregnant women | 400/100-mg or 500/125-mg tablet BID | Zidovudine- lamivudine | Intensive (30 min predose and 2, 4, 6, 8, 10, and 12 h postdose) |
| 4 (22) | 316 | Antiretroviral-naive HIV-1-infected subjects (nonpregnant) | 400/100-mg tablet BID or 400/100-mg SGC BID | Tenofovir disoproxil fumarate-emtricitabine or tenofovir disoproxil fumarate-lamivudine | Sparse (weeks 1, 2, 4, 8, 10, 12, 16, 24, 32, 40, and 48 and every 12 weeks thereafter)c |
| 5 (23) | 261 | Antiretroviral-experienced, HIV-1-infected subjects (nonpregnant) | 400/100-mg tablet BID | At least two investigator-selected NRTIs | Sparse (weeks 4, 8, 16, 24, 32, 40, and 48) |
| 6 (24) | 18 | Antiretroviral-naive HIV-1-infected subjects (nonpregnant) | 400/100-mg SGC BID | Stavudine-lamivudine | Intensive (0, 2, 4, 6, 8, 10, and 12 h at steady state) |
BID, twice daily; LPV/r, lopinavir-ritonavir.
NRTIs, nucleoside analog reverse transcriptase inhibitors.
Intensive samplings at 0, 2, 4, 6, 8, and 12 h postdose were performed for a cohort of participating subjects.
Table 1 summarizes the clinical studies used in this analysis and their dosing and pharmacokinetic sampling schemes. Study 1 was a randomized, open-label prospective study that enrolled 53 HIV-infected pregnant women between 14 and 30 weeks of gestation (20). Subjects were randomized in a 1:1 ratio to receive LPV/r tablets either at 400/100 mg BID or 600/150 mg BID during pregnancy; all participants then received LPV/r at 400/100 mg BID for at least 6 weeks postpartum. Pharmacokinetic evaluations were performed at least 2 weeks after treatment initiation at the following time points: second trimester (between 20 and 28 weeks of gestation), third trimester (between 30 and 36 weeks of gestation), at delivery, and postpartum (4 to 6 weeks after delivery), depending on the gestational age at study enrollment.
Study 2 was a single-center, open-label study that compared the LPV pharmacokinetics of tablet and soft gelatin capsule (SGC) formulations in 19 HIV-infected pregnant women (17). Throughout the study, subjects received LPV/r at 400/100 mg BID either as an SGC (cohort 1, n = 8) or as tablets (cohort 2, n = 11). Pharmacokinetic evaluations were performed in the second and third trimesters as well as 4 to 6 weeks after delivery.
Study 3 was a two-center, open-label study that compared the pharmacokinetics of the LPV/r tablet at 400/100 mg BID and 500/125 mg BID during the third trimester of pregnancy (21). The 500/125-mg dose was achieved by adding a half-strength tablet of LPV/r of 100/25-mg to two 200/50-mg tablets. In this study, 12 HIV-infected pregnant women receiving LPV/r at 400/100 mg BID underwent intensive LPV pharmacokinetic analyses in the second trimester and at 30 weeks of gestation in the third trimester. The LPV/r dose was increased in all women to 500/125 mg BID after the week 30 pharmacokinetic visit, with subsequent pharmacokinetic sampling at 32 weeks of gestation. Two weeks after delivery, the LPV/r dose was decreased to 400/100 mg BID, and pharmacokinetics were reassessed 8 weeks after delivery.
Study 4 was an open-label, randomized phase 3 study comparing the pharmacokinetics and pharmacodynamics of LPV/r at 800/200 mg once daily (QD) and 400/100 mg BID in 664 treatment-naive HIV-infected male and nonpregnant female subjects receiving the LPV/r tablet and SGC formulations (22) Data from 316 subjects receiving the 400/100-mg BID regimen were included in this analysis.
Study 5 was a randomized, open-label phase 3 study comparing the pharmacokinetics and pharmacodynamics of LPV/r tablets at 800/200 mg QD and 400/100 mg BID in 599 treatment-experienced HIV-infected male and nonpregnant female subjects (23). Data from 261 subjects receiving the 400/100-mg BID regimen were included in this analysis.
Study 6 was a randomized, double-blind, multicenter phase 1/2 study of LPV/r soft gelatin capsules BID in 100 HIV-infected male and nonpregnant female subjects without prior antiretroviral therapy (24). Data from 18 subjects receiving 400/100-mg BID doses were included in this analysis.
Population pharmacokinetic analysis.
Concentration-time data were pooled across all studies and analyzed using a nonlinear mixed-effects population analysis approach with NONMEM (version 7.3.0) (25, 26). The first-order conditional estimation (FOCE) method with eta-epsilon (η-ε) interaction was employed throughout the model development. The graphic processing of the NONMEM output was performed with SAS (version 9.4).
Population pharmacokinetic models were built for LPV using total plasma concentrations. After dose proportionality was established, both one- and two-compartment models with first-order absorption and elimination (ADVAN 2 and ADVAN 3 subroutines in NONMEM) were fitted to the data. Both a proportional plus additive-error model and proportional residual-error model were assessed. Individual pharmacokinetic parameters were assumed to be log-normally distributed, and the interindividual variability in pharmacokinetic parameters was modeled using an exponential error model.
Two approaches were attempted to describe the RTV inhibition of LPV clearance. First, RTV inhibition of LPV clearance was modeled using a competitive inhibition model according to the following equation (27): CL = TVCL × RTVConc/[1 + (RTVConc/Ki)], where CL is clearance, TVCL is the typical value for LPV clearance in the absence of RTV, RTVConc is the observed RTV concentration, and Ki is the inhibition constant.
Second, RTV inhibition of LPV clearance was modeled using a maximum-inhibition model according to the following equation (28): CL = TVCL × [1 − RTVConc/(IC50 + RTVConc)], where IC50 represents the RTV concentration at which a half-maximal inhibition effect on LPV clearance is obtained.
Covariate modeling was performed using the forward-inclusion (P < 0.05), backward-elimination (P < 0.001) approach and was guided by evaluation of the empirical Bayesian pharmacokinetic parameter estimates versus covariate plots as well as changes in the estimates of pharmacokinetic parameter variability and residual variability. Nested models were compared using a likelihood ratio test, while nonnested models were compared using the Akaike information criterion.
Precision of the final model parameter estimates was assessed using the asymptotic standard errors obtained by the covariance routine in NONMEM as well as by the bootstrap confidence intervals. In bootstrapping, subjects were randomly sampled with replacement from the data set that was used in model development to obtain 1,000 data sets that have the same number of subjects as the original data set. The final model was then fitted to each of these data sets, and the parameter estimates were compared to the estimates from the original data set. The final model was qualified by visual predictive check where the final parameter estimates were used to simulate 1,000 replicates of the observed data set. The median and 5th and 95th percentile concentrations of the simulated data sets were then plotted against the original observations.
Statistical analysis.
To establish the pharmacokinetic comparability of LPV between nonpregnant subjects and pregnant women, the linear mixed-model methodology using PROC MIXED in SAS system software, version 9.4, was used to compare the area under the concentration-time curve from 0 to 12 h (AUC0–12) and concentration prior to dosing (Cpredose) in pregnant women receiving the tablet formulation in their second and third trimesters and nonpregnant subjects receiving the SGC formulation. To assess statistical differences between the dose groups across studies, the differences in means were estimated with the corresponding least-squares means obtained from the mixed model on the logarithm of AUC0–12 and Cpredose. Corresponding P values for the comparisons were estimated.
Exposure-virologic response relationship.
LPV exposure-response relationships in pregnant women were explored using both scatter plots and quartile plots. In scatter plots, LPV AUC0–12 and Cpredose values in pregnant women were plotted against HIV-1 viral load collected at the same visit. Furthermore, the percentage of subjects with viral load less than 50 copies per ml (percent responders) was compared across the four quartiles of LPV AUC0–12 and Cpredose values in both pregnant women and nonpregnant subjects. Bayesian post hoc estimates obtained from the population pharmacokinetic model were used to obtain AUC0–12 and Cpredose values for subjects with sparse pharmacokinetic sampling. For all other subjects, observed AUC0–12 and Cpredose values were used in the analysis.
RESULTS
Population pharmacokinetic analysis.
The analysis included 3,079 total LPV plasma concentrations and 3,077 total RTV plasma concentrations from 84 pregnant women and 595 nonpregnant subjects.
A one-compartment disposition model with first-order absorption and elimination best described the LPV plasma concentration-time data. A lag time describing a potential absorption delay was not observed in the majority of subjects and hence was not added to the model. Both the competitive inhibition and the maximum effect (Emax)-type inhibition models for evaluating the effect of RTV on LPV clearance were supported by the data and led to similar results. It was decided to move forward with the Emax-type inhibition model on clearance as the interpretation of the IC50 term allowed comparison of the estimates to results of other studies that used the same approach.
The data supported including interindividual variability terms for apparent clearance and apparent central volume of distribution, which were estimated with high precision. The correlation between CL/F and Vc/F (Vc is the apparent central volume of distribution) was estimated to be 0.23 in the base model. Inclusion of interindividual variability terms for the absorption rate constant and IC50 was attempted. These terms were associated with high shrinkage of greater than 50% and hence were not added to the model. The residual unexplained variability was best characterized using a combined-additive and proportional-error model because both additive and proportional residual-error models provided inferior fits.
The final model parameter estimates and the precision associated with their estimations are shown in Table 2. Both fixed and random effects were precisely estimated with percent relative standard error (RSE) of 16% or less. Accounting for the difference in bioavailability levels between the tablet and SGC formulations was found to significantly improve the fit; the bioavailability of the tablet formulation was estimated to be 20% higher than that of the SGC formulation in the final model. Pregnancy status was also found to be a significant predictor of LPV clearance. Both the second and third trimesters had similar increases of 17% in clearance; postpartum women had a decrease of 26.4% in clearance compared with that of nonpregnant subjects.
TABLE 2.
Population pharmacokinetic parameter estimates obtained after fitting the final model to the original data set and to 1,000 bootstrap samplesa
| Parameter | Model result |
Bootstrap result |
|||
|---|---|---|---|---|---|
| Population estimate (SEE)b | % RSEc | Mean | Median | 95% confidence interval | |
| Volume of distribution (liters) | 63.70 (3.2) | 5.0 | 63.44 | 63.3 | 57.50–69.90 |
| Clearance (liters/h) | 6.62 (0.26) | 3.8 | 6.70 | 6.64 | 5.980–7.770 |
| Absorption rate constant (1/h) | 0.712 (0.03) | 4.3 | 0.73 | 0.721 | 0.551–0.940 |
| Pregnancy on CL | 1.17 (0.03) | 2.6 | 1.19 | 1.18 | 1.030–1.390 |
| Postpartum on CL | 0.736 (0.02) | 2.7 | 0.74 | 0.735 | 0.646–0.837 |
| IC50 (μg/ml) | 0.239 (0.01) | 4.3 | 0.24 | 0.235 | 0.131–0.373 |
| Tablet on bioavailability | 1.200 (0.05) | 4.0 | 1.19 | 1.190 | 1.110–1.290 |
| Interindividual variance of volume of distribution | 0.183 (0.03) | 16.0 | 0.19 | 0.189 | 0.112–0.280 |
| Interindividual variance of clearance | 0.130 (0.01) | 6.6 | 0.13 | 0.131 | 0.101–0.166 |
Based on 964/1,000 successful runs.
SEE, standard error of the estimate.
RSE, relative standard error.
Finally, body weight was found to be a significant covariate on apparent volume of distribution but not on LPV clearance. The need for estimating the exponent of the allometric model for body weight on volume of distribution was tested to ensure model parsimony. Fixing the exponents to 1 resulted in a nonsignificant increase in the objective function value (OFV) (P value = 0.156), and hence estimating the exponent was deemed unnecessary and was kept fixed at 1 in the model, which indicates that an increase in body weight is predicted to be associated with a proportional increase in volume of distribution.
Lopinavir apparent clearance population estimates were 6.62, 7.75, and 4.87 liters/h in nonpregnant adults, pregnant women, and postpartum women, respectively. The lopinavir apparent volume of distribution population estimate was 63.7 liters, with no differences between nonpregnant adults and pregnant and postpartum women.
The final equations and typical values of LPV parameters were as follows. Bioavailability was 1.2 for the tablet and 1 for the SGC. Clearance was equal to 6.62 × θTrimester × (1 − RTVConc)/(0.239 + RTVConc), where θ is 1.17 for the second or third trimester of pregnancy, 0.736 for the postpartum subjects, and 1 for nonpregnant subjects. The volume of distribution was calculated as 63.7 × (WTKG/71), where WTKG is weight in kilograms.
In order to confirm the stability of the model precision of estimated pharmacokinetic parameters, a nonparametric bootstrap analysis was performed, and 96.4% of the bootstrap replicates converged successfully. In accordance with the estimated standard errors of estimates for pharmacokinetic parameters in the LPV pharmacokinetic model, the bootstrap showed narrow confidence intervals for all parameters. The medians and 5th and 95th percentiles of the parameter estimates from the fit of the final model to the bootstrap samples are shown in Table 2. The asymptotic estimates obtained from the original data set showed close agreement with the median and were all included within the 2.5th to the 97.5th percentiles of the bootstrapping values, indicating model stability. None of the 95% confidence intervals for the parameters from the bootstrap data sets included zero, confirming the robustness of parameters.
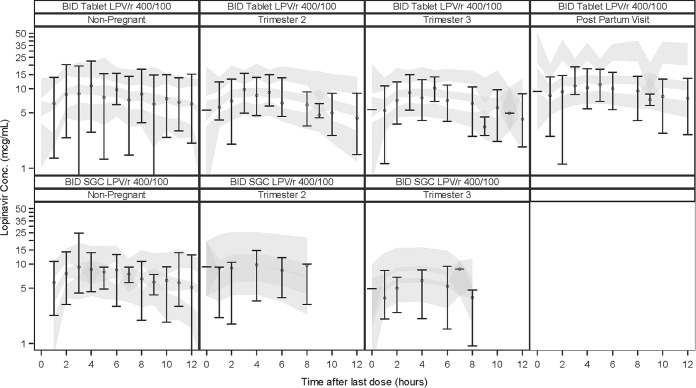
For the visual predictive checks, observed plasma concentration-time data, and 5th, 50th, and 95th percentiles of observed data and confidence intervals of the 5th, 50th, and 95th percentiles of simulated data are shown in Fig. 1, indicating sufficient predictive ability of the model to describe LPV concentrations.
FIG 1.
Visual predictive check for the final model. Circles represent the medians, and error bars represent the 5th and 95th percentiles of observations. Shaded areas represent 95% confidence intervals of the 5th, 50th, and 95th percentiles of simulated data.
Statistical analysis.
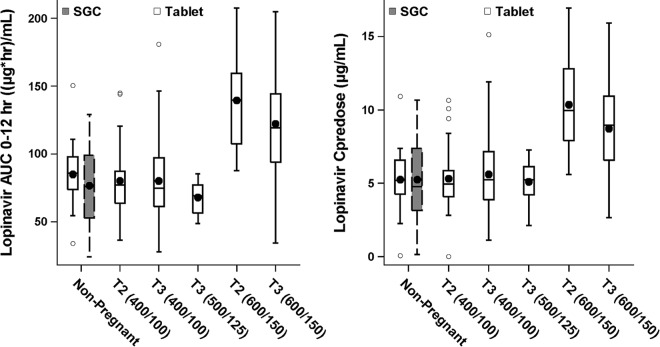
There were no statistically significant differences in mean LPV AUC0–12 and Cpredose values between pregnant women in the second trimester receiving the LPV/r tablet and nonpregnant subjects receiving the SGC formulation at the 400/100-mg BID dose (P values of 0.66 and 0.21, respectively). Furthermore, there were no statistically significant differences in LPV AUC0–12 or Cpredose values between women in the third trimester receiving 400/100-mg tablets BID and nonpregnant HIV-infected subjects receiving the LPV/r SGC formulation at the 400/100-mg BID dose (P values of 0.83 and 0.25, respectively). A comparison of the LPV AUC0–12 and Cpredose values in pregnant women during second and third trimesters receiving the tablet formulation at different doses and nonpregnant subjects receiving the SGC formulation at the 400/100-mg BID dose is shown in Fig. 2.
FIG 2.
Box plots of LPV AUC0–12 and Cpredose values in nonpregnant subjects and pregnant women in the second (T2) and third (T3) trimesters. Values for the 25th to the 75th percentile of exposure are boxed, the horizontal line represents the median, and the symbol in the box represents the mean. Whiskers represent the 5th to 95th percentiles of exposure, and additional symbols outside the box represent the outlying data.
Exposure-virologic response relationship.
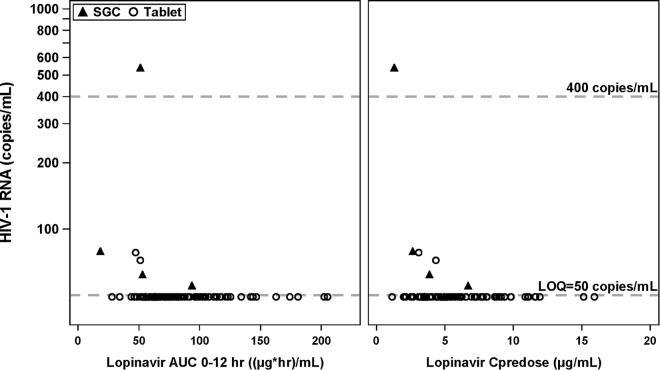
Figure 3 shows the relationship between individual LPV AUC0–12 and Cpredose values achieved in the third trimester and HIV-1 virologic response measured as plasma HIV-1 RNA levels (copies/ml). Substantial reductions in HIV-1 RNA were achieved by the third trimester for all doses of LPV/r administered. In the range of LPV AUC0–12 or Cpredose values observed in the third trimester, there does not appear to be a relationship between observed exposure and plasma HIV-1 RNA levels.
FIG 3.
HIV-1 RNA response versus LPV AUC0–12 and Cpredose values in the third trimester of pregnancy. LOQ, limit of quantitation.
Of the 84 pregnant women included in this analysis, 21/22 (95.5%) treatment-experienced and 57/62 (91.9%) treatment-naive subjects had plasma HIV-1 RNA levels of less than 50 copies/ml. Only one treatment-experienced pregnant woman receiving 400/100-mg BID SGC had an observed HIV-1 RNA level greater than 400 copies/ml during the third trimester. Five treatment-naive pregnant women had an observed plasma HIV-1 RNA level between 50 and 400 copies/ml, with three receiving LPV/r SGC at 400/100 mg BID and two receiving an LPV/r tablet at 400/100 mg BID.
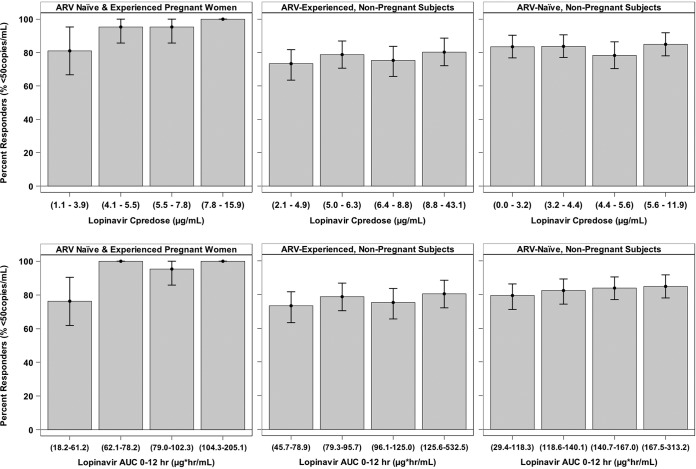
Quartile plots of the percentage of subjects with viral loads of less than 50 copies/ml stratified by Cpredose and AUC0–12 values are shown in Fig. 4. While a lower response rate was observed in pregnant women in the lowest quartile, the response rates were similar between this quartile and those of nonpregnant adults across all quartiles.
FIG 4.
Quartile plots of the percentages of HIV-1 RNA responders stratified by LPV exposure in pregnant women (third trimester) and nonpregnant subjects. The exposure range in each quartile is shown in parentheses on the x axis. Each error bar represents the 90% confidence interval of the percentage of responders based on binomial distribution. ARV, antiretroviral therapy.
DISCUSSION
We evaluated the pharmacokinetic differences between pregnant women and nonpregnant subjects using nonlinear mixed-effect analysis of LPV and RTV plasma concentrations pooled from studies of both populations. The final model estimates for LPV clearance and volume of distribution in nonpregnant subjects were similar to those of previously published pharmacokinetic studies (27, 28). RTV concentrations increased LPV concentrations, with an IC50 of 0.239 μg/ml in this model, which reflects the potency of the RTV inhibitory effect on LPV clearance and is consistent with prior estimates (29, 30). The model was qualified using both bootstrapping and visual predictive checks.
The final population pharmacokinetic model estimated LPV clearance to be 17% higher in pregnant women during their second and third trimesters than in nonpregnant subjects. This increase in clearance is consistent with the induction of hepatic CYP3A and P-glycoprotein (P-gp) activity during pregnancy (31, 32). The model also estimated the tablet formulation to be 20% more bioavailable than the SGC formulation in both pregnant women and nonpregnant subjects. The increase in bioavailability is consistent with that observed in a bioequivalence study of healthy volunteers, where the LPV/r tablet formulation demonstrated 18 to 24% higher bioavailability than the SGC formulation under fed conditions (33). This indicates that despite the increase in clearance during pregnancy, the bioavailability of the tablet formulation may compensate for the increased clearance, and thus pregnant women receiving the tablet formulation still maintain LPV exposure that is similar to the efficacious exposure in nonpregnant subjects receiving the SGC formulation. Statistical analysis confirmed the similarity in LPV AUC0–12 and Cpredose values between the pregnant and nonpregnant populations.
The results of our population pharmacokinetic analysis estimated LPV clearance postpartum to be 26.4% and 37.1% lower than the clearance in nonpregnant subjects and pregnant women, respectively. A recent study estimated a similar magnitude of higher CYP3A4 activity in pregnant women (35%) than in postpartum subjects as measured by the urinary ratio of 6-β-hydroxycortisol to cortisol as a marker (34). Several other studies investigating the pharmacokinetics of LPV/r tablets in pregnant women similarly reported LPV exposure during the third trimester with the 400/100-mg BID tablet dose to be 35% to 45% lower than the postpartum exposure (18, 35–40). Other clinical studies evaluated LPV pharmacokinetics for the SGC formulation in pregnant women (19, 41, 42). Similar to the results with the LPV/r tablet formulation, following administration of LPV/r as 400/100-mg SGC BID, LPV exposure in the third trimester was approximately 30% lower than the exposure observed 2 weeks after delivery.
It is important to note that the LPV Cpredose was above 1 μg/ml in most of the pregnant women receiving 400/100 mg BID of either the LPV/r tablet or SGC formulation. This plasma LPV trough target is approximately 15 times the protein binding-adjusted plasma concentration required to obtain 50% of the maximum effect in vivo (EC50) for the wild-type HIV-1 (43–46).
We assessed virologic suppression in pregnant women receiving LPV/r during pregnancy. Virologic suppression was maintained in the vast majority of the pregnant women included in this analysis. As most of the subjects in these studies initiated treatment during their pregnancy, it is not unexpected that some subjects would not be fully suppressed with a treatment duration of less than 26 weeks, even when adequate plasma LPV levels are achieved. Overall, quartile plots of the percentage of subjects with virologic suppression versus LPV AUC0–12 and Cpredose values in pregnant women showed response rates comparable to, if not higher than, those of nonpregnant subjects with similar or longer treatment durations.
We also explored the relationship between LPV exposure and the proportion of subjects with virologic suppression. There was no correlation between the HIV-1 RNA virologic response and LPV exposures achieved in pregnant women or nonpregnant subjects, with similar response rates achieved in all four quartiles of AUC0–12 and Cpredose values. The high response rate leading to a lack of exposure response correlation limited the ability to model the relationship between lopinavir concentrations and the viral load. In addition, a similar HIV-1 RNA virologic response rate was achieved in both ART-naive and ART-experienced women in their third trimesters of pregnancy. There are insufficient data to recommend dosing in pregnant women with any documented lopinavir-associated resistance substitutions.
LPV is highly protein bound to plasma proteins with higher affinity to alpha-1 acid glycoprotein (AAG) than to albumin. Conflicting reports on the fraction of LPV unbound during pregnancy have been reported. One study demonstrated no change in the fraction of LPV unbound, and four studies reported an increase in the fraction of LPV unbound (16, 20, 28, 47, 48). An increase in the fraction unbound may potentially offset at least part of the increase of the LPV clearance during pregnancy. Aweeka et al. reported an 18% higher LPV unbound fraction in the plasma during the third trimester than at postpartum (n = 28) (49). This higher LPV unbound fraction was associated with 52% and 15% reductions in AAG and albumin concentrations, respectively. Kiser et al. reported 40% and 50% higher LPV unbound fractions in the plasma during the second and third trimesters, respectively, than at postpartum (n = 9) (47). More recently, Else et al. reported that the LPV unbound fraction was 27% and 17% higher in the second and third trimesters, respectively, than at postpartum (n = 11) (17). Lastly, Fayet-Mello et al. reported an increase of 7% to 29% in the LPV unbound fraction and a decrease in AAG and albumin during pregnancy (38). The increase in the unbound fraction may be explained by displacement of LPV from binding sites by steroid and placental hormones and dilutional decreases in albumin and AAG concentrations (39, 48, 50, 51).
A systematic review has recently assessed the effects of LPV/r on maternal and infant clinical and safety outcomes in HIV-infected pregnant women (52). Nine studies were identified, comprising 2,675 LPV/r-treated women. The results from this systematic review suggested no unique safety or efficacy concerns with use of the standard dose LPV/r as part of cART in pregnant women. Furthermore, in 1,333 and 2,371 women exposed to LPV/r in the first and second/third trimesters, respectively, in the French perinatal cohort, no association was found between birth defects and LPV or RTV (53). As of January 2013, the Antiretroviral Pregnancy Registry has cumulatively received prospective reports of 3,335 exposures to LPV/r-containing regimens. Birth defects occurred in 24 of the 1,049 (2.3%) live births with first trimester exposure and in 67 of the 2,286 (2.9%) live births with second/third trimester exposure (54). The prevalence of congenital birth defects among the offspring of women with first- and second/third-trimester LPV/r exposures was not significantly different from the prevalence of congenital birth defects in offspring of pregnant women in the United States reference population, whose background rate of birth defects is 2.7%. No pattern of birth defect signal was detected for LPV/r (54).
In conclusion, dosing recommendations for antiretroviral agents in HIV-infected, pregnant women should be based on the assessment of the pharmacokinetic exposures and virologic response during pregnancy, rather than from exposure comparison with nonpregnant subjects or postpartum measurements (i.e., the initial weeks after delivery). For LPV, nonlinear mixed-effects modeling as utilized in our analysis is the most appropriate approach to make this evaluation since it can account for inter- and intrasubject variability between pregnant women and nonpregnant subjects. Although the LPV exposure in the third trimester of pregnancy was lower than that of nonpregnant subjects receiving the same formulation or in the postpartum period, the increase in LPV clearance during pregnancy is compensated by the higher bioavailability of the LPV/r tablet formulation than that of the SGC formulation along with potentially a decrease in protein binding. Over 90% of the pregnant women in this analysis were virologically suppressed. Analysis of virologic response in pregnant women receiving 400/100 mg of LPV/r BID demonstrated no association with LPV exposure and no difference compared with responses of nonpregnant subjects, which is consistent with demonstration of LPV plasma trough concentrations above 1 μg/ml. The virologic response is also consistent with the demonstrated efficacy in both treatment-naive and treatment-experienced HIV subjects of the original SGC formulation of LPV/r (21, 55), which has lower LPV exposure than the tablet formulation. Pregnant women achieved virologic suppression with the 400/100-mg twice-daily regimen of LPV/r tablets, and there has been no signal of increased rate of congenital birth defects seen in their offspring. Based on our pharmacokinetic and pharmacodynamic evaluation, LPV/r tablets in doses of 400/100 mg twice daily during pregnancy is appropriate, and no dosage adjustment for LPV/r is recommended.
ACKNOWLEDGMENTS
AbbVie participated in the design, study conduct, analysis, and interpretation of data as well as the writing, review, and approval of the publication. A.H.S., A.K.J., A.M.N., and C.E.K. are employed by AbbVie and may own stock.
Funding Statement
M.S.-O. received a grant from the Brazilian National AIDS Program, Ministry of Health, and clinical drug supply and internal standards for the analytical assay from Abbott/AbbVie for the conduct of one of the studies included in this analysis. K.B.P. and G.P.T. received financial support from Abbott/AbbVie for the conduct of two of the studies included in this analysis. AbbVie provided financial support for the study.
REFERENCES
- 1.Garcia PM, Kalish LA, Pitt J, Minkoff H, Quinn TC, Burchett SK, Kornegay J, Jackson B, Moye J, Hanson C. 1999. Maternal levels of plasma human immunodeficiency virus type 1 RNA and the risk of perinatal transmission. N Engl J Med 341:394–402. doi: 10.1056/NEJM199908053410602. [DOI] [PubMed] [Google Scholar]
- 2.Cooper ER, Charurat M, Mofenson L, Hanson IC, Pitt J, Diaz C, Hayani K, Handelsman E, Smeriglio V, Hoff R. 2002. Combination antiretroviral strategies for the treatment of pregnant HIV-1-infected women and prevention of perinatal HIV-1 transmission. J Acquir Immune Defic Syndr 29:484–494. doi: 10.1097/00042560-200204150-00009. [DOI] [PubMed] [Google Scholar]
- 3.Günthard HF, Aberg JA, Eron JJ, Hoy JF, Telenti A, Benson CA, Burger DM, Cahn P, Gallant JE, Glesby MJ. 2014. Antiretroviral treatment of adult HIV infection: 2014 recommendations of the International Antiviral Society-USA Panel. JAMA 312:410–425. doi: 10.1001/jama.2014.8722. [DOI] [PubMed] [Google Scholar]
- 4.Panel on Treatment of HIV-Infected Pregnant Women and Prevention of Perinatal Transmission. 2015. Recommendations for use of antiretroviral drugs in pregnant HIV-1-infected women for maternal health and interventions to reduce perinatal HIV transmission in the United States. Public Health Service Task Force, NIH, Rockville, MD: https://aidsinfo.nih.gov/contentfiles/lvguidelines/PerinatalGL.pdf. [Google Scholar]
- 5.European AIDS Clinical Society. 2014. Guidelines, version 7.1, November 2014. European AIDS Clinical Society, Brussels, Belgium: http://www.eacsociety.org/files/guidelines_english_71_141204.pdf. [Google Scholar]
- 6.Taylor G, Clayden P, Dhar J, Gandhi K, Gilleece Y, Harding K, Hay P, Kennedy J, Low-Beer N, Lyall H. 2012. British HIV Association guidelines for the management of HIV infection in pregnant women 2012. HIV Med 13:87–157. doi: 10.1111/j.1468-1293.2012.01030.x. [DOI] [PubMed] [Google Scholar]
- 7.World Health Organization. 2010. Antiretroviral drugs for treating pregnant women and preventing HIV infection in infants: recommendations for a public health approach, 2010 version. World Health Organization, Geneva, Switzerland. [PubMed] [Google Scholar]
- 8.Chandwani A, Shuter J. 2008. Lopinavir/ritonavir in the treatment of HIV-1 infection: a review. Ther Clin Risk Manag 4:1023–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Vincenzi I. 2011. Triple antiretroviral compared with zidovudine and single-dose nevirapine prophylaxis during pregnancy and breastfeeding for prevention of mother-to-child transmission of HIV-1 (Kesho Bora study): a randomised controlled trial. Lancet Infect Dis 11:171–180. doi: 10.1016/S1473-3099(10)70288-7. [DOI] [PubMed] [Google Scholar]
- 10.Shapiro R, Hughes M, Ogwu A, Kitch D, Lockman S, Moffat C, Makhema J, Moyo S, Thior I, McIntosh K. 2010. Antiretroviral regimens in pregnancy and breast-feeding in Botswana. N Engl J Med 362:2282–2294. doi: 10.1056/NEJMoa0907736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Floridia M, Ravizza M, Masuelli G, Giacomet V, Martinelli P, Degli Antoni A, Spinillo A, Fiscon M, Francisci D, Liuzzi G. 2014. Atazanavir and lopinavir profile in pregnant women with HIV: tolerability, activity and pregnancy outcomes in an observational national study. J Antimicrob Chemother 69:1377–1384. doi: 10.1093/jac/dkt497. [DOI] [PubMed] [Google Scholar]
- 12.Cohan D, Natureeba P, Koss CA, Plenty A, Luwedde F, Mwesigwa J, Ades V, Charlebois ED, Gandhi M, Clark TD, Nzarubara B, Achan J, Ruel T, Kamya MR, Havlir DV. 2015. Efficacy and safety of lopinavir/ritonavir versus efavirenz-based antiretroviral therapy in HIV-infected pregnant Ugandan women. AIDS 29:183–191. doi: 10.1097/QAD.0000000000000531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roberts SS, Martinez M, Covington DL, Rode RA, Pasley MV, Woodward WC. 2009. Lopinavir/ritonavir in pregnancy. J Acquir Immune Defic Syndr 51:456–461. doi: 10.1097/QAI.0b013e3181a2813f. [DOI] [PubMed] [Google Scholar]
- 14.Peixoto MF, Pilotto JH, Stoszek SK, Kreitchmann R, Mussi-Pinhata MM, Melo VH, João EC, Ceriotto M, Souza RDSD, Read J. 2011. Lopinavir/ritonavir dosing during pregnancy in Brazil and maternal/infant laboratory abnormalities. Braz J Infect Dis 15:253–261. doi: 10.1016/S1413-8670(11)70185-4. [DOI] [PubMed] [Google Scholar]
- 15.Azria E, Moutafoff C, Schmitz T, Le Meaux JP, Krivine A, Pannier E, Firtion G, Compagnucci A, Finkielsztejn L, Taulera O. 2009. Pregnancy outcomes in women with HIV type-1 receiving a lopinavir/ritonavir-containing regimen. Antivir Ther 14:423–432. [PubMed] [Google Scholar]
- 16.Senise J, Cruz R, Palacios R, Bonafé S, Vaz MJR, Lacerda AP. 2008. Low-birth weight and pre-term delivery in relation to lopinavir/ritonavir use in pregnancy. Am J Infect Dis 4:209–214. doi: 10.3844/ajidsp.2008.209.214. [DOI] [Google Scholar]
- 17.Else L, Douglas M, Dickinson L, Back D, Khoo S, Taylor G. 2012. Improved oral bioavailability of lopinavir in melt-extruded tablet formulation reduces impact of third trimester on lopinavir plasma concentrations. Antimicrob Agents Chemother 56:816–824. doi: 10.1128/AAC.05186-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Best BM, Stek AM, Mirochnick M, Hu C, Li H, Burchett SK, Rossi SS, Smith E, Read JS, Capparelli EV. 2010. Lopinavir tablet pharmacokinetics with an increased dose during pregnancy. J Acquir Immune Defic Syndr 54:381–388. doi: 10.1097/QAI.0b013e3181d6c9ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mirochnick M, Best BM, Stek AM, Capparelli E, Hu C, Burchett SK, Holland DT, Smith E, Gaddipati S, Read JS. 2008. Lopinavir exposure with an increased dose during pregnancy. J Acquir Immune Defic Syndr 49:485–491. doi: 10.1097/QAI.0b013e318186edd0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Santini-Oliveira M, Estrela RDCE, Veloso VG, Cattani VB, Yanavich C, Velasque L, Torres TS, Marins LM, Pilotto JH, João EC, Goncalves JC, Grinsztejn B. 2014. Randomized clinical trial comparing the pharmacokinetics of standard-and increased-dosage lopinavir-ritonavir coformulation tablets in HIV-positive pregnant women. Antimicrob Agents Chemother 58:2884–2893. doi: 10.1128/AAC.02599-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patterson KB, Dumond JB, Prince HA, Jenkins AJ, Scarsi KK, Wang R, Malone S, Hudgens MG, Kashuba AD. 2013. Protein binding of lopinavir and ritonavir during four phases of pregnancy: implications for treatment guidelines. J Acquir Immune Defic Syndr 63:51–58. doi: 10.1097/QAI.0b013e31827fd47e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.González-García J, Cohen D, Johnson M, Sloan L, Fredrick L, Naylor C, da Silva B, Bernstein B. 2010. Short communication: comparable safety and efficacy with once-daily versus twice-daily dosing of lopinavir/ritonavir tablets with emtricitabine + tenofovir DF in antiretroviral-naive, HIV type 1-infected subjects: 96 week final results of the randomized trial M05-730. AIDS Res Hum Retroviruses 26:841–845. doi: 10.1089/aid.2009.0307. [DOI] [PubMed] [Google Scholar]
- 23.Zajdenverg R, Podsadecki TJ, Badal-Faesen S, Andrade-Villanueva J, Gathe J, Mingrone H, Fredrick LM, Gaultier IA, Woodward WC, Bernstein BM. 2010. Similar safety and efficacy of once-and twice-daily lopinavir/ritonavir tablets in treatment-experienced HIV-1-infected subjects at 48 weeks. J Acquir Immune Defic Syndr 54:143–151. doi: 10.1097/QAI.0b013e3181cbd21e. [DOI] [PubMed] [Google Scholar]
- 24.Murphy RL, Brun S, Hicks C, Eron JJ, Gulick R, King M, White AC Jr, Benson C, Thompson M, Kessler HA. 2001. ABT-378/ritonavir plus stavudine and lamivudine for the treatment of antiretroviral-naive adults with HIV-1 infection: 48-week results. AIDS 15:F1–F9. doi: 10.1097/00002030-200101050-00002. [DOI] [PubMed] [Google Scholar]
- 25.Lindbom L, Pihlgren P, Jonsson N. 2005. PsN-Toolkit—a collection of computer intensive statistical methods for non-linear mixed effect modeling using NONMEM. Comput Methods Programs Biomed 79:241–257. doi: 10.1016/j.cmpb.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 26.Beal S, Sheiner L, Boeckmann A, Bauer R. 2009. NONMEM user's guides (1989–2009). Icon Development Solutions, Ellicott City, MD. [Google Scholar]
- 27.Wang K, D'Argenio DZ, Acosta EP, Sheth AN, Delille C, Lennox JL, Kerstner-Wood C, Ofotokun I. 2014. Integrated population pharmacokinetic/viral dynamic modelling of lopinavir/ritonavir in HIV-1 treatment-naïve patients. Clin Pharmacokinet 53:361–371. doi: 10.1007/s40262-013-0122-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vezina HE, Park J-G, Wallis CL, Bartlett JA, Kumarasamy N, Stevens WS, Klingman KL, Ribaudo HJ, Katzenstein DA. 2013. Lopinavir population pharmacokinetics in HIV-infected patients from resource-limited settings receiving second-line treatment with lopinavir/ritonavir monotherapy in AIDS Clinical Trials Group (ACTG) Study 5230. J Pharmacokinet Pharmacodyn 40:S31–S32. doi: 10.1007/s10928-013-9308-2. [DOI] [Google Scholar]
- 29.Moltó J, Barbanoj MJ, Miranda C, Blanco A, Santos JR, Negredo E, Costa J, Domingo P, Clotet B, Valle M. 2008. Simultaneous population pharmacokinetic model for lopinavir and ritonavir in HIV-infected adults. Clin Pharmacokinet 47:681–692. doi: 10.2165/00003088-200847100-00005. [DOI] [PubMed] [Google Scholar]
- 30.Crommentuyn K, Kappelhoff B, Mulder J, Mairuhu A, Van Gorp E, Meenhorst P, Huitema A, Beijnen J. 2005. Population pharmacokinetics of lopinavir in combination with ritonavir in HIV-1-infected patients. Br J Clin Pharmacol 60:378–389. doi: 10.1111/j.1365-2125.2005.02455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hebert M, Easterling T, Kirby B, Carr D, Buchanan M, Rutherford T, Thummel K, Fishbein D, Unadkat J. 2008. Effects of pregnancy on CYP3A and P-glycoprotein activities as measured by disposition of midazolam and digoxin: a University of Washington specialized center of research study. Clin Pharmacol Ther 84:248–253. doi: 10.1038/clpt.2008.1. [DOI] [PubMed] [Google Scholar]
- 32.Tracy TS, Venkataramanan R, Glover DD, Caritis SN. 2005. Temporal changes in drug metabolism (CYP1A2, CYP2D6 and CYP3A activity) during pregnancy. Am J Obstet Gynecol 192:633–639. doi: 10.1016/j.ajog.2004.08.030. [DOI] [PubMed] [Google Scholar]
- 33.Klein CE, Chiu Y-L, Awni W, Zhu T, Heuser RS, Doan T, Breitenbach J, Morris JB, Brun SC, Hanna GJ. 2007. The tablet formulation of lopinavir/ritonavir provides similar bioavailability to the soft-gelatin capsule formulation with less pharmacokinetic variability and diminished food effect. J Acquir Immune Defic Syndr 44:401–410. doi: 10.1097/QAI.0b013e31803133c5. [DOI] [PubMed] [Google Scholar]
- 34.Aweeka F, Hu C, Huang L, Best B, Stek A, Lizak P, Burchett S, Read J, Watts H, Mirochnick M. 2015. Alteration in cytochrome P450 3A4 activity as measured by a urine cortisol assay in HIV-1-infected pregnant women and relationship to antiretroviral pharmacokinetics. HIV Med 16:176–183. doi: 10.1111/hiv.12195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baroncelli S, Villani P, Floridia M, Pirillo MF, Galluzzo CM, Cusato M, Amici R, Pinnetti C, Sabbatini F, Molinari A. 2008. Trough concentrations of lopinavir, nelfinavir, and nevirapine with standard dosing in human immunodeficiency virus-infected pregnant women receiving 3-drug combination regimens. Ther Drug Monit 30:604–610. doi: 10.1097/FTD.0b013e3181867a6e. [DOI] [PubMed] [Google Scholar]
- 36.Cressey TR, Jourdain G, Rawangban B, Varadisai S, Kongpanichkul R, Sabsanong P, Yuthavisuthi P, Chirayus S, Ngo-Giang-Huong N, Voramongkol N. 2010. Pharmacokinetics and virologic response of zidovudine/lopinavir/ritonavir initiated during the third trimester of pregnancy. AIDS 24:2193–2200. doi: 10.1097/QAD.0b013e32833ce57d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lambert J, Else L, Jackson V, Breiden J, Gibbons S, Dickinson L, Back D, Brennan M, Connor E, Boyle N. 2011. Therapeutic drug monitoring of lopinavir/ritonavir in pregnancy. HIV Med 12:166–173. doi: 10.1111/j.1468-1293.2010.00865.x. [DOI] [PubMed] [Google Scholar]
- 38.Fayet-Mello A, Buclin T, Guignard N, Cruchon S, Cavassini M, Grawe C, Gremlich E, Popp KA, Schmid F, Eap CB. 2013. Free and total plasma levels of lopinavir during pregnancy, at delivery and postpartum: implications for dosage adjustments in pregnant women. Antivir Ther 18:171–182. doi: 10.3851/IMP2328. [DOI] [PubMed] [Google Scholar]
- 39.Calza L, Manfredi R, Trapani F, Salvadori C, Colangeli V, Borderi M, Grossi G, Motta R, Viale P. 2012. Lopinavir/ritonavir trough concentrations with the tablet formulation in HIV-1-infected women during the third trimester of pregnancy. Scand J Infect Dis 44:381–387. doi: 10.3109/00365548.2011.642306. [DOI] [PubMed] [Google Scholar]
- 40.Shapiro RL, Rossi S, Ogwu A, Moss M, Leidner J, Moffat C, Lockman S, Moyo S, Makhema J, Essex M. 2013. Therapeutic levels of lopinavir in late pregnancy and abacavir passage into breast milk in the Mma Bana Study, Botswana. Antivir Ther 18:585–590. doi: 10.3851/IMP2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stek AM, Mirochnick M, Capparelli E, Best BM, Hu C, Burchett SK, Elgie C, Holland DT, Smith E, Tuomala R. 2006. Reduced lopinavir exposure during pregnancy. AIDS 20:1931–1939. doi: 10.1097/01.aids.0000247114.43714.90. [DOI] [PubMed] [Google Scholar]
- 42.Cressey TR, Van Dyke R, Jourdain G, Puthanakit T, Roongpisuthipong A, Achalapong J, Yuthavisuthi P, Prommas S, Chotivanich N, Maupin R. 2009. Early postpartum pharmacokinetics of lopinavir initiated intrapartum in Thai women. Antimicrob Agents Chemother 53:2189–2191. doi: 10.1128/AAC.01091-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Molla A, Vasavanonda S, Kumar G, Sham HL, Johnson M, Grabowski B, Denissen JF, Kohlbrenner W, Plattner JJ, Leonard JM. 1998. Human serum attenuates the activity of protease inhibitors toward wild-type and mutant human immunodeficiency virus. Virology 250:255–262. doi: 10.1006/viro.1998.9383. [DOI] [PubMed] [Google Scholar]
- 44.Ananworanich J, Kosalaraksa P, Hill A, Siangphoe U, Bergshoeff A, Pancharoen C, Engchanil C, Ruxrungtham K, Burger D, HIV-NAT 017 Study Team. 2005. Pharmacokinetics and 24-week efficacy/safety of dual boosted saquinavir/lopinavir/ritonavir in nucleoside-pretreated children. Pediatr Infect Dis J 24:874–879. doi: 10.1097/01.inf.0000180578.38584.da. [DOI] [PubMed] [Google Scholar]
- 45.Kappelhoff BS, Crommentuyn KM, de Maat MM, Mulder JW, Huitema AD, Beijnen JH. 2004. Practical guidelines to interpret plasma concentrations of antiretroviral drugs. Clin Pharmacokinet 43:845–853. doi: 10.2165/00003088-200443130-00002. [DOI] [PubMed] [Google Scholar]
- 46.La Porte C, Back D, Blaschke T, Boucher C, Fletcher C, Flexner C, Gerber J, Kashuba A, Schapiro J, Burger D. 2006. Updated guideline to perform therapeutic drug monitoring for antiretroviral agents. Rev Antivir Ther 2006:4–14. [Google Scholar]
- 47.Kiser JJ, Mawhinney S, Kinzie K, Barr E, Simons A, Paul S, Hoody D, Fletcher C, Allshouse A, Weinberg AL. 2009. Total and unbound lopinavir/ritonavir (LPV/RTV) pharmacokinetics (PK) in a concentration-guided study of HIV infected women throughout pregnancy and post-partum (PP), abstr 946. Abstr 16th Conf Retrovir Opportunistic Infect, Montreal, Canada, 8 to 11 February 2009. [Google Scholar]
- 48.Perucca E, Crema A. 1982. Plasma protein binding of drugs in pregnancy. Clin Pharmacokinet 7:336–352. doi: 10.2165/00003088-198207040-00004. [DOI] [PubMed] [Google Scholar]
- 49.Aweeka F, Stek A, Best B, Hu C, Holland D, Hermes A, Burchett S, Read J, Mirochnick M, Capparelli E. 2010. Lopinavir protein binding in HIV-1-infected pregnant women. HIV Med 11:232–238. doi: 10.1111/j.1468-1293.2009.00767.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Anderson GD. 2005. Pregnancy-induced changes in pharmacokinetics. Clin Pharmacokinet 44:989–1008. doi: 10.2165/00003088-200544100-00001. [DOI] [PubMed] [Google Scholar]
- 51.Aweeka FT, Rosenkranz SL, Segal Y, Coombs RW, Bardeguez A, Thevanayagam L, Lizak P, Aberg J, Watts DH, NIAID . AIDS Clinical Trials Group. 2006. The impact of sex and contraceptive therapy on the plasma and intracellular pharmacokinetics of zidovudine. AIDS 20:1833–1841. doi: 10.1097/01.aids.0000244202.18629.36. [DOI] [PubMed] [Google Scholar]
- 52.Pasley MV, Martinez M, Hermes A, d'Amico R, Nilius A. 2013. Safety and efficacy of lopinavir/ritonavir during pregnancy: a systematic review. AIDS Rev 15:38–48. [PubMed] [Google Scholar]
- 53.Sibiude J, Mandelbrot L, Blanche S, Le Chenadec J, Boullag-Bonnet N, Faye A, Dollfus C, Tubiana R, Bonnet D, Lelong N. 2014. Association between prenatal exposure to antiretroviral therapy and birth defects: an analysis of the French perinatal cohort study (ANRS CO1/CO11). PLoS Med 11:e1001635. doi: 10.1371/journal.pmed.1001635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Antiretroviral Pregnancy Registry Steering Committee. 2013. Antiretroviral Pregnancy Registry international interim report for 1 January 1989 through 31 January 2013. Antiretroviral Pregnancy Registry, Wilmington, NC. [Google Scholar]
- 55.Benson CA, Deeks SG, Brun SC, Gulick RM, Eron JJ, Kessler HA, Murphy RL, Hicks C, King M, Wheeler D. 2002. Safety and antiviral activity at 48 weeks of lopinavir/ritonavir plus nevirapine and 2 nucleoside reverse-transcriptase inhibitors in human immunodeficiency virus type 1-infected protease inhibitor-experienced patients. J Infect Dis 185:599–607. doi: 10.1086/339014. [DOI] [PubMed] [Google Scholar]