Abstract
Dried blood spot (DBS) antibiotic assays can facilitate pharmacokinetic/pharmacodynamic (PK/PD) studies in situations where venous blood sampling is logistically and/or ethically problematic. In this study, we aimed to develop, validate, and apply a DBS ceftriaxone assay. A liquid chromatography-tandem mass spectroscopy (LC-MS/MS) DBS ceftriaxone assay was assessed for matrix effects, process efficiency, recovery, variability, and limits of quantification (LOQ) and detection (LOD). The effects of hematocrit, protein binding, red cell partitioning, and chad positioning were evaluated, and thermal stability was assessed. Plasma, DBS, and cell pellet ceftriaxone concentrations in 10 healthy adults were compared, and plasma concentration-time profiles of DBS and plasma ceftriaxone were incorporated into population PK models. The LOQ and LOD for ceftriaxone in DBS were 0.14 mg/liter and 0.05 mg/liter, respectively. Adjusting for hematocrit, red cell partitioning, and relative recovery, DBS-predicted plasma concentrations were comparable to measured plasma concentrations (r > 0.95, P < 0.0001), and Bland-Altman plots showed no significant bias. The final population PK estimates of clearance, volume of distribution, and time above threshold MICs for measured and DBS-predicted plasma concentrations were similar. At 35°C, 21°C, 4°C, −20°C, and −80°C, ceftriaxone retained >95% initial concentrations in DBS for 14 h, 35 h, 30 days, 21 weeks, and >11 months, respectively. The present DBS ceftriaxone assay is robust and can be used as a surrogate for plasma concentrations to provide valid PK and PK/PD data in a variety of clinical situations, including in studies of young children and of those in remote or resource-poor settings.
INTRODUCTION
Antimicrobial resistance in pathogenic microorganisms represents a major threat to global health. One way to prevent/retard the emergence of antimicrobial resistance and to mitigate its consequences is to optimize dosing of antibiotics through pharmacokinetic/pharmacodynamic (PK/PD) studies. This approach explores the triangular relationship between antibiotic concentrations, the antibiotic susceptibility of the infecting organism (taken as the MIC), and predefined clinical outcomes (1). This allows estimation of microbiological susceptibility breakpoints, facilitates design of optimal dosing regimens (2), and provides data relating to the emergence of drug-resistant organisms (3, 4).
A lack of PK/PD data for older drugs such as ceftriaxone (CFX) has been identified as a research priority (4). The few human studies with well-defined clinical outcomes have been performed mainly as part of the development of newer antibiotics (5, 6). In contrast, there are few comparable PK/PD data that relate to older drugs that are often used as first-line treatments for severe infections (7). This has particular relevance for neonatal and pediatric patients because studies performed in adults may not be applicable due to differences in drug disposition (8, 9), the spectrum of clinical disease, and bacterial pathogens (10). Additionally, the need for “rich” blood sampling in young children and neonates may be unethical because multiple, large-volume venesections could result in adverse hemodynamic effects (11).
The measurement of drug concentrations in dried blood spots (DBS) represents a novel way to overcome these limitations, particularly when laboratory facilities that process, store, and transport blood or plasma samples are not accessible. DBS have been used in the diagnosis of in-born errors of metabolism (e.g., the Guthrie test) (12) and in the detection and quantification of HIV, hepatitis B virus, and malaria parasites in research settings. More recent studies have demonstrated that accurate measurement of drug concentrations in DBS is feasible in animal models and human studies of antibiotics (9, 13, 14) and other drugs (15–17). Very low blood volumes (10 to 20 μl) from finger or heel prick samples can be taken serially and impregnated onto filter paper which is stored with desiccant, without the need for processing of plasma/cell pellets or for frozen storage. A small-diameter disc, or chad, can be subsequently punched out from the filter paper, and the drug can be eluted into a liquid matrix prior to liquid chromatography-mass spectrometry (LC-MS) assay (13, 15, 17). However, few studies have performed the detailed validation experiments in humans that enable DBS drug concentrations to be used in PK/PD studies as a reliable surrogate for plasma concentrations.
Given this background, we have developed and validated a DBS assay for CFX in samples taken from healthy adult volunteers. Our DBS assay was comprehensively validated to ensure that it could be applied broadly, including in challenging situations such as pediatric PK/PD studies in remote clinical settings.
MATERIALS AND METHODS
Approvals, patients, and sample collection.
The present study was approved by the Fremantle Hospital Human Research Ethics Committee (13/22). Ten healthy adult employees at Fremantle Hospital (Western Australia, Australia) were recruited as the study participants. All patients had no history of anaphylaxis, allergy, or other adverse reaction to CFX or other beta-lactam antibiotics, had not been diagnosed with diabetes or psychiatric, cardiac, renal, or hepatic disease, and were not pregnant or breast feeding. All patients gave witnessed informed consent to study procedures. Each volunteer was examined, and his/her height and weight were measured. An intravenous (i.v.) cannula was inserted, and a baseline heparinized blood sample was taken for drug assay as well as routine biochemical and hematologic tests.
CFX (Ceftriaxone Sandoz; Sandoz Australia, Pyrmont, NSW, Australia) at 1 g in 10 ml of water for injection was administered i.v. over 3 min (at 0 h) in accordance with the manufacturer's recommendations and local prescribing guidelines. The i.v. line was then flushed with 10 ml of normal saline. Additional 5-ml heparinized venous blood samples were drawn at 0.25, 1, 4, and 8 h, with prior removal of 3 ml of dead space and flushing with normal saline after sampling on each occasion, and then the cannula was removed. A final 24-h venous blood sample was collected by venipuncture.
A venous hematocrit was measured on all blood samples, which were then centrifuged promptly. Plasma and cell pellets were separated and placed on dry ice before freezer storage at −80°C. At each sampling time point, duplicate DBS were collected onto filter paper cards (Whatman 903 Protein Saver Cards; GE Healthcare Australia Pty. Ltd., Parramatta, NSW, Australia) from the venous blood sample, and mixed capillary blood after a finger prick, taken at the same time as the venous blood sample, was (i) collected into a heparinized capillary tube and (ii) spotted onto filter paper directly from the finger. In both of these situations, the mixed capillary blood was allowed to be drawn by capillary action into the tube and to distribute evenly over the filter paper. The DBS cards were air dried at room temperature for 1 h, placed in an airtight foil envelope with a single desiccant sachet, and transported on dry ice before being stored at −80°C.
Analytical materials and reagents.
Ceftriaxone disodium (molecular weight [MW], 661.6; Sigma-Aldrich Chemicals, St. Louis, MO, USA) was used as the reference standard, and cefazolin sodium salt (CFZ; MW, 476.49; Merck Chemicals, Darmstadt, Germany) was the internal standard. Formic acid (Sigma-Aldrich, Ltd., Gillingham, United Kingdom), acetonitrile (Fisher Scientific, Fair Lawn, NJ, USA), and all other chemicals were of analytical or high-performance liquid chromatography (HPLC) grade. Stock solutions of CFX and CFZ standards were prepared separately (20 mg/ml) in water and stored at −80°C. Quality control (QC) samples were prepared in blank plasma or whole blood, using a positive-displacement pipette, at spiked concentrations of 5, 20, 50, and 200 mg/liter and stored at −80°C prior to use. The working standards were prepared by serial dilution from the primary stock. To prepare the standards and the QC samples in the blood and plasma, 10 μl of different concentrate levels was spiked to 1 ml of each sample in order to maintain consistency.
Plasma and red cell pellet sample preparation.
Plasma was extracted using a protein precipitation method. Plasma (20 μl) was precipitated by adding 300 μl of acetonitrile containing 5 mg/liter CFZ, and this mixture was vortexed for 1 min and centrifuged at 1,500 × g for 10 min. The supernatant (200 μl) was separated and then diluted with an equal amount of deionized water. Subsequent sample preparations were adapted in a deep-well plate. For this, 20 μl of plasma was added to the deep-well plate, followed by an addition of 300 μl of acetonitrile with CFZ. The plate was shaken on a Thermomixer C (Eppendorf, Hamburg, Germany) at 1,000 rpm for 30 min, centrifuged at 2,000 × g for 10 min, and then processed in the same manner as the plasma. For the red cell pellet, CFX extraction required sonication of the sample for 30 min in order to optimize recovery.
Dried blood spot preparation.
Optimization of DBS extraction was performed in different water-acetonitrile and water-methanol ratios; 30% water and 70% (vol/vol) acetonitrile provided the highest extraction of CFX. The DBS chad (6 mm) was placed into a borosilicate glass tube, with 300 μl of extraction solution (water-acetonitrile at 30:70 containing 5 mg/liter of CFZ). The sample was sonicated for 30 min and centrifuged at 1,500 × g for 10 min. The supernatant (200 μl) was diluted with 200 μl of deionized water. Method validation was later adapted to a deep-well plate, whereby chads were placed into a well, and 300 μl of extraction solution was added. The plate was then shaken at 1,000 rpm for 1 h. The sample was centrifuged at 2,000 × g, and 200 μl of supernatant was diluted with 200 μl of water.
Instrumentation and chromatographic conditions.
A triple quadrupole mass spectrometer (LCMS-8030; Shimadzu, Kyoto, Japan) was used for the compound analysis. The instrument comprised a Nexera UHPLC pump (LC-30A), degasser (DGU-20A5), autosampler (SIL-30A), and column oven (CTO-30A). Authentic CFX and CFZ standards were scanned for parent and product ions. The identified precursor and product ions of CFX and CFZ were further allowed to auto-optimize in the instrument. This gave a more specific precursor-product ion pair and collision energy for each compound of interest. Quantitation was performed by multiple reaction monitoring (MRM) in electrospray ionization-positive (ESI+) mode. The precursor-product ion pair for CFX was mz 554.95 → 396.05, and for CFZ it was m/z 454.9 → 323.0. The optimized mass spectra were acquired with an interface voltage of 4.5 kV, a detector voltage of 1.0 kV, a heat block temperature of 400°C, and a desolvation temperature of 220°C. Nitrogen was used as the nebulizer gas at a flow rate of 2.5 liters/min and as a drying gas at a flow of 8 liters/min. Argon was used as the collision gas at 230 kPa. The dwell time for both CFX and CFZ was 100 msec, and their collision energies were −15 V and −13 V, respectively.
Chromatographic separation was performed on an ultraperformance liquid chromatography (UPLC) C18 column (Waters Acquity T3; 2.1 by 50 mm, 1.7-μm particle size) connected to a C18 precolumn (VanGuard Acquity UPLC, 2.1 by 5 mm, 1.7-μm particle size; Waters Corp., Wexford, Ireland) while the column oven was set to 40°C. The mobile phase comprised solvent A (0.1% formic acid in water) and solvent B (0.1% formic acid in acetonitrile). It was run in a gradient mode of 0.5 to 2.0 min with solvent B at 10 to 90% and of 2.1 to 5 min with solvent B at 10%, for a total run time of 5 min. The flow rate was 0.4 ml/min, and the injection volume was 5 μl. The retention times for CFX and CFZ were 1.35 min and 1.39 min, respectively.
Method validation.
For both plasma and DBS, a linear calibration curve (1 to 200 mg/liter) was constructed (r2 ≥ 0.998). There was no observable difference between the plasma linear calibration curves prepared with EDTA or lithium heparin anticoagulant. Samples above the upper calibration point were diluted further and rerun. Chromatographic data (peak area ratio of CFX/CFZ) was processed using LAB Solution (version 5.56; Shimadzu, Japan). Matrix effects (ion suppression/enhancement), absolute recovery, and process efficiency were determined from the analysis of three concentrations (5, 50, and 200 mg/liter) (18). Three sets of matrix were prepared: set 1 comprised blank plasma or DBS spiked first and then extracted; set 2 comprised blank plasma or blank DBS extracted first and then spiked postextraction; and set 3 comprised pure solutions of analyte in acetonitrile and water. The matrix effect (percent) was determined as follows: (set 2 response × 100)/(set 3 response). The process efficiency (percent) was determined as follows: (set 1 response × 100)/(set 3 response). The absolute recovery (percent) was determined as follows: (set 1 response × 100)/(set 2 response). Intraday (n = 5) and interday (n = 15) precision and accuracy (n = 20) were assessed using relative standard deviations (RSD) for both plasma and DBS assays using standard solutions.
Effect of different hematocrit values on matrix, process efficiency, and recovery.
A range of hematocrit values (0.31 to 0.67) were artificially prepared by adding plasma or red cells to whole blood from the same healthy volunteer. Blood was spiked with CFX to concentrations of 10, 50, and 200 mg/liter and placed onto Whatman cards. The whole spot was cut out, extracted, and assayed as described above. Experiments were performed in triplicate to evaluate the matrix effect, process efficiency, and absolute recovery.
Red cell partitioning (blood-to-plasma ratio).
Fresh control blood samples were collected in EDTA tubes from a healthy volunteer, and the hematocrit was measured. The blood-to-plasma partition ratio was assessed at CFX concentrations of 10, 50, and 200 mg/liter using the following equation (19): partition ratio = {[CFXBlood]/[CFXPlasma] − (1 − hematocrit)}/hematocrit.
The blood sample was separated into two parts: (i) blank blood which was centrifuged, after which the separated plasma was spiked with the appropriate concentration of drug; (ii) blank blood which was spiked with drug and processed as whole blood. The samples were incubated at 37°C in a water bath for 1 h to optimize the equilibration of CFX with plasma and blood. For the volunteer samples, CFX was measured in red cell pellets using the same extraction methods described above.
Protein binding.
Plasma (200 μl) was spiked with four concentrations of CFX (10, 50, 200, and 500 mg/liter) and incubated at 37°C in a water bath for 30 min. Five replicates were performed for each concentration. The samples were transferred into microcentrifuge filters (Vivaspin 500-μl; Sartorius Stedim Biotech, Gottingen, Germany) and centrifuged at 15,000 × g for 15 min to separate protein-free supernatant. Both protein-free supernatant and plasma (20 μl) were extracted and analyzed to determine protein binding of CFX.
The effect of the site of chad sampling from the dried blood spot.
Since blood diffusion can be influenced by its viscosity, chads were taken from different areas of the DBS in order to assess the effect of unequal diffusion. Five chad locations were chosen (Fig. 1) and tested using standard concentrations (10, 50, and 200 mg/liter, respectively).
FIG 1.
Testing the effect on ceftriaxone concentrations of chad positioning in relation to the blood spot.
Ceftriaxone thermal stability in dried blood spots.
Whole blood (freshly collected) was spiked with CFX at concentrations of 10, 50, and 200 mg/liter. Aliquots (50 μl) were placed in microcentrifuge tubes or spotted onto blood collection cards and air dried at room temperature. The microcentrifuge tubes and dried blood spot cards were stored at 35°C (incubator; similar to ambient tropical temperatures), 21°C (room temperature, monitored), 4°C (laboratory refrigerator), −20°C, and −80°C until analyzed. The extraction and processing of samples were as described above, using triplicate DBS at each time point. Samples were analyzed at predetermined times, based on a pilot study, over a 9-week period at 35°C and 21°C and over at least 6 months at 4°C, −20°C, and −80°C.
Preliminary determination of the degradation reaction order was determined graphically (20). At 4°C, −20°C, and −80°C, a first-order reaction was apparent, and a single exponential equation was fitted to the concentration-time data to determine the degradation rate constant (kD). Degradation of CFX at 35°C and 21°C was not consistent with zero-, first-, or second-order kinetic profiles. Single exponential, bi-exponential, and sigmoid equations were fitted to the concentration-time data at room temperature (21°C) and at 35°C to determine the most likely reaction model. The goodness of fit was determined according to the Akaike information criterion. A bi-exponential equation provided the most plausible model and a goodness of fit superior to that of all other relevant equations, suggesting a consecutive first-order degradation reaction (21). Using these curves, the time required to reach 95% of the starting concentration (t95) was calculated.
Statistical methods.
Correlations between plasma and DBS concentrations are provided and were assessed using Spearman's rank correlation coefficient (rs). Bland-Altman plots were constructed using plasma CFX concentrations as a reference standard (GraphPad Prism, version 6.05; GraphPad Software, Inc., La Jolla, CA, USA). Predicted plasma CFX concentrations were calculated by using the DBS concentrations from mixed capillary blood collected directly onto the filter paper and adjusting for hematocrit, relative absolute recoveries between plasma and DBS, and red cell partitioning. Thermal stability data analysis and representation were performed with SigmaPlot, version 13.0 (Systat Software, Inc., San Jose, CA).
Pharmacokinetic modeling.
Loge plasma concentration-time data sets for CFX in plasma and the derived CFX plasma concentration from DBS samples were analyzed separately by nonlinear mixed effects modeling using NONMEM (version 7.2.0; Icon Development Solutions, Ellicott City, MD, USA) with an Intel Visual Fortran 10.0 compiler. The first-order conditional estimation (FOCE) with interaction estimation method was used. Allometric scaling was employed a priori, with volume terms multiplied by (wt/70)1.0 and clearance terms by (wt/70)0.75, where wt is body weight (22). Two structures for residual variability (RV), equivalent to proportional and combined RV structures on the normal scale, were tested for the log-transformed data. Secondary pharmacokinetic parameters including area under the concentration-time curve from 0 h to infinity (AUC0–∞) and elimination half-lives (t1/2) for the participants were obtained from post hoc Bayesian prediction in NONMEM using the final model parameters. Base models were parameterized using ka (absorption rate constant), VC/F (central volume of distribution, where F is bioavailability), CL/F (clearance, relative to bioavailability), and VP/F and Q/F [peripheral volumes of distribution(s) and their respective intercompartmental clearance(s)]. The minimum value of the objective function (OFV), goodness-of-fit plots including conditional weighted residuals (CWRES), and condition number (<1,000) were used to choose suitable models during the model-building process. A significance level, P value, of <0.01 was set for comparison of OFVs for nested models using a chi-squared distribution.
One-, two-, and three-compartment models (ADVAN models 1, 3, and 11) with bolus dosing were tested. Once the structure of the models was established, interindividual variability (IIV; also termed ETA) and correlations between IIV terms were estimated, where supported by the data. Finally, relationships between model parameters with age, albumin, hematocrit, and creatinine clearance were assessed through inspection of ETA-versus-covariate scatter plots and box plots, and subsequently evaluated within NONMEM. A stepwise forward inclusion and backward elimination method was used with a P value of <0.05 required for inclusion of a covariate relationship and a P value of <0.01 to retain a covariate relationship.
Model evaluation.
A bootstrap using Perl-speaks-NONMEM (PsN) with 1,000 samples was performed, and the parameters derived from this analysis were summarized as median and 2.5th and 97.5th percentiles (95% empirical confidence interval [CI]) to facilitate evaluation of final model parameter estimates. In addition, prediction-corrected visual predictive checks (pcVPCs) were performed with 1,000 data sets simulated from the final models using PsN. The observed 10th, 50th, and 90th percentiles were plotted with their respective simulated 95% CIs. Numerical predictive checks (NPCs) were performed to complement the pcVPCs in assessing the predictive performance of the model.
To determine if the differences between the two models were significant, nested models with a difference term for each model parameter were established. Given that both models had the same structure, it was possible to quantify these differences statistically using a chi-squared distribution with 2 degrees of freedom to compare the difference between the resultant OFVs.
RESULTS
Subject characteristics.
Six female and four male volunteers were recruited. The median (range) age, body mass index, and hematocrit and serum creatinine were 42 (19 to 62) years, 24.6 (23.3 to 32.4) kg/m2, and 0.42 (0.36 to 0.42) and 69.5 (59 to 87) μmol/liter, respectively.
Assay validation.
The calibration curve (1 to 200 mg/liter) for the plasma CFX assay was accurate (r2 = 0.998). The limits of quantification (LOQ) and detection (LOD) were 0.06 mg/liter and 0.02 mg/liter, respectively, for plasma ceftriaxone and 0.14 mg/liter and 0.05 mg/liter, respectively, for the DBS assay. The matrix effects, process efficiency, absolute recovery, intraday variation, interday variation, and accuracy for CFX extraction from plasma and DBS are shown in Table 1. Ceftriaxone was highly protein bound (90 to 95%) except at very high concentrations (Table 1). Red cell partitioning was low (0.55 to 0.61) (Table 1). Chad positioning had limited effect on DBS CFX concentrations (RSD <20%) (Table 1). The hematocrit did not have significant impact on the matrix effect, process efficiency, or recovery of CFX from DBS (Table 2).
TABLE 1.
Ceftriaxone liquid chromatography-mass spectroscopy assay characteristics at different concentrations when measured from plasma and dried blood spots
| Parameter and/or sample sourcea | Value for the parameter with: |
|||||
|---|---|---|---|---|---|---|
| Cefazolin (1.5 mg/liter)b | Ceftriaxone at the indicated concn (mg/liter) |
|||||
| 5 | 10 | 20 | 50 | 200 | ||
| Plasma | ||||||
| Matrix effect (% ± SD, n = 5) | 88.5 (±8.2) | 104.1 (±4.6) | 101.6 (±14.5) | 90.4 (±8.2) | ||
| Process efficiency (% ± SD, n = 5) | 96.3 (±11.9) | 103.1 (±5.7) | 107.6 (±6.9) | 96.8 (±5.1) | ||
| Absolute recovery (% ± SD, n = 5) | 109.0 (±13.9) | 97.3 (±8.1) | 108.9 (±13.2) | 106.3 (±11.5) | ||
| Interday variation (RSD%, n = 5) | 6.5 | 5.9 | 4.8 | 3.9 | ||
| Intraday variation (RSD%, n = 15) | 7.8 | 6.3 | 5.4 | 4.9 | ||
| Accuracy (% ± SD, n = 20) | 102 (±9.4) | 96 (±8.7) | 105 (±7.5) | 98.2 (±8.6) | ||
| Protein binding (% ± SD, n = 5)c | 94.5 (±0.2) | 95.3 (±0.1) | 90.1 (±0.2) | |||
| Red cell partitioning ratio (±SD, n = 5) | 0.55 (±.09) | 0.61 (±.06) | 0.57 (±.08) | |||
| Dried blood spots | ||||||
| Matrix effect (% ± SD, n = 5) | 82.6 (±3.5) | 103.5 (±6.1) | 112.6 (±7.7) | 109.2(±3.1) | ||
| Process efficiency (% ± SD, n = 5) | 80.1 (±2.6) | 86.4 (±4.6) | 93.9 (±6.9) | 92.6 (±2.2) | ||
| Absolute recovery (% ± SD, n = 5) | 97.9 (±1.9) | 83.5 (±2.0) | 83.3 (±2.7) | 84.8 (±2.1) | ||
| Interday variation (RSD%, n = 5) | 5.9 | 6.8 | 4.7 | 2.8 | ||
| Intraday variation (RSD%, n = 15) | 6.9 | 7.4 | 5.8 | 3.9 | ||
| Accuracy (% ± SD, n = 20) | 104.7 (±9) | 92.5 (±9.1) | 98.3 (±7.9) | 97.3 (±4.0) | ||
| Chad positioning (RSD%, n = 5; hematocrit = 0.41) | 12.7 | 14.6 | 18.4 | |||
SD, standard deviation; RSD, residual standard deviation.
Internal standard.
Protein binding at a CFX concentration of 500 mg/liter was 63.5% (±0.5%).
TABLE 2.
Matrix effect, process efficiency, and absolute recovery of ceftriaxone from dried blood spots
| Parameter and CFX concn (mg/liter) | Value (%) at the indicated hematocrita |
||||
|---|---|---|---|---|---|
| 0.31 | 0.40 | 0.44 | 0.51 | 0.67 | |
| Matrix effect | |||||
| 10 | 95.6 | 98.3 | 108.7 | 107.2 | 107.7 |
| 50 | 109.7 | 116.0 | 105.8 | 124.6 | 107.3 |
| 200 | 112.6 | 110.4 | 109.2 | 104.6 | 108.9 |
| Process efficiency | |||||
| 10 | 79.0 | 85.1 | 88.5 | 90.6 | 88.9 |
| 50 | 91.6 | 95.0 | 92.4 | 104.9 | 85.9 |
| 200 | 89.4 | 91.2 | 92.6 | 87.4 | 88.1 |
| Absolute recovery | |||||
| 10 | 82.6 | 86.5 | 81.4 | 84.5 | 82.6 |
| 50 | 83.4 | 81.9 | 87.3 | 84.2 | 80.1 |
| 200 | 79.4 | 82.6 | 84.8 | 83.5 | 80.9 |
Mean values ± standard deviations were as follows: matrix effect, 108% ± 7%; process efficiency, 90% ± 6%; absolute recovery, 83% ± 2%.
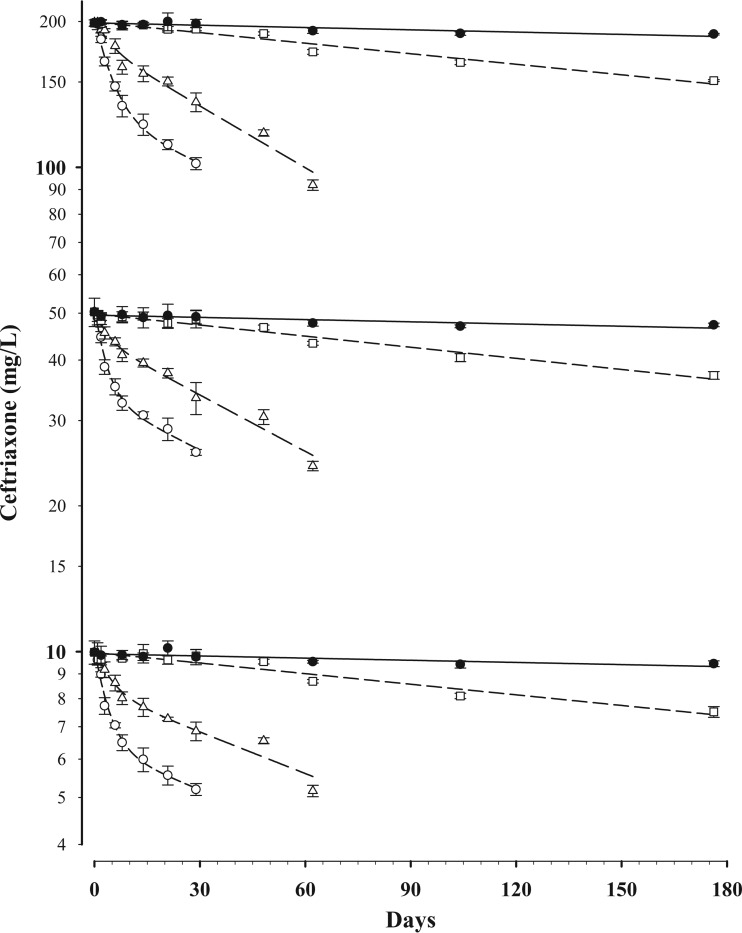
Concentration-time data for CFX stability in DBS are shown (Fig. 2). At 35°C the mean ± standard error of the mean (SEM) t95 was 14.0 ± 1.5 h in DBS and 18.7 ± 4.2 h in whole blood. At room temperature (21°C) the t95 was 35.4 ± 3.1 h in DBS and 32.5 ± 2.7 h in whole blood.
FIG 2.
Concentration-time data for ceftriaxone stability in DBS at initial concentrations of 10, 50, and 200 mg/liter and stored at 35°C (○), 21°C (△), 4°C (□), and −20°C (●). Data are means ± standard deviations. A monoexponential (first-order) equation was fitted to the data at 4°C and −20°C, and a bi-exponential equation was fitted to the data at 35°C and 21°C.
In contrast, at 4°C the t95 was substantially longer, with 30.1 ± 0.8 days in DBS and 28.5 ± 1.6 days in whole blood. At −20°C the t95 was 146 ± 4 days in DBS and 101 ± 3 days in whole blood (P < 0.001). Although our stability data currently extend to only 6 months, the estimated, extrapolated t95 for CFX in DBS at −80°C is >11 months.
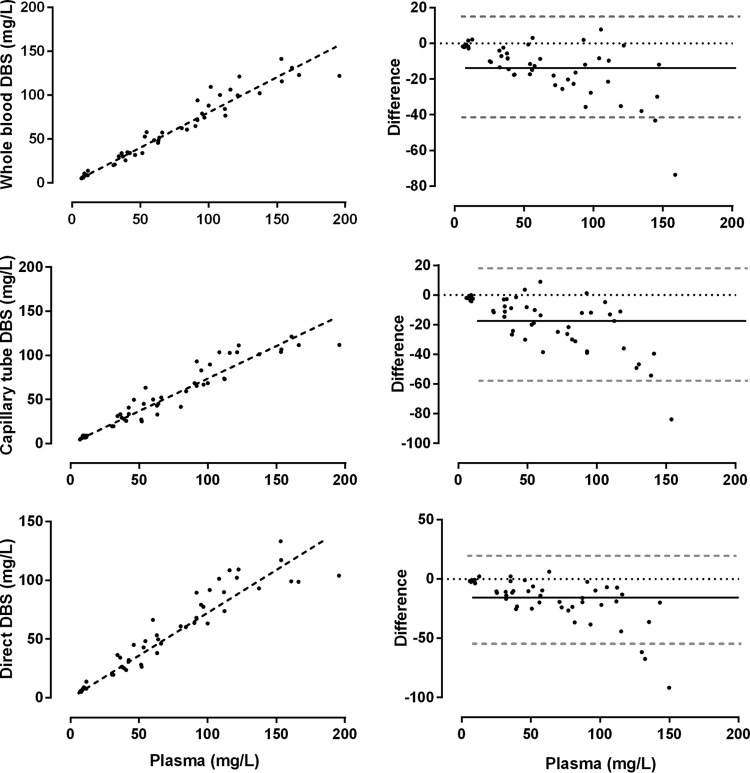
The relationship between plasma concentrations and unadjusted whole blood DBS, capillary tube DBS, and direct capillary DBS with their associated Bland-Altman plots is shown in Fig. 3. Ceftriaxone plasma concentrations correlated well with DBS concentrations taken from whole blood and from mixed capillary blood collected in a heparinized tube and directly from the finger (r > 0.95) (Table 3). DBS concentrations from mixed capillary blood collected in a heparinized capillary tube and directly from the finger onto the filter paper were tightly correlated (r = 0.97) (Table 3). Cell pellet concentrations had lower correlations with the other methods (0.66 ≤ r ≤ 0.71). The mean red cell pellet/plasma concentration was 10.5%. The slope of the linear regression line for unadjusted DBS concentrations collected directly from the finger was 0.74 (range, 0.70 to 0.78).
FIG 3.
Relationship between raw dried blood spot (DBS) measurements from whole blood, capillary blood from a heparinized tube, and capillary blood directly and plasma ceftriaxone concentrations from healthy volunteers. Linear regression lines (dashed black lines) are provided (left graphs). Bland-Altman plots show the mean difference (solid black line) and 95% confidence intervals (dashed gray lines) (right graphs).
TABLE 3.
Correlation matrix of different methods compared with plasma ceftriaxone concentrationsa
| Sample source | Pearson r or P valuea |
||||
|---|---|---|---|---|---|
| Plasma | Whole-blood DBS | Capillary tube DBS | Direct DBS | Cell pellet | |
| Plasma | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |
| Whole blood | 0.97 | <0.0001 | <0.0001 | <0.0001 | |
| Capillary tube DBS | 0.95 | 0.98 | <0.0001 | <0.0001 | |
| Direct DBS | 0.95 | 0.98 | 0.97 | <0.0001 | |
| Cell pellet | 0.69 | 0.71 | 0.66 | 0.69 | |
Pearson r values are shown in the lower left corner, and P values are shown in the upper right corner.
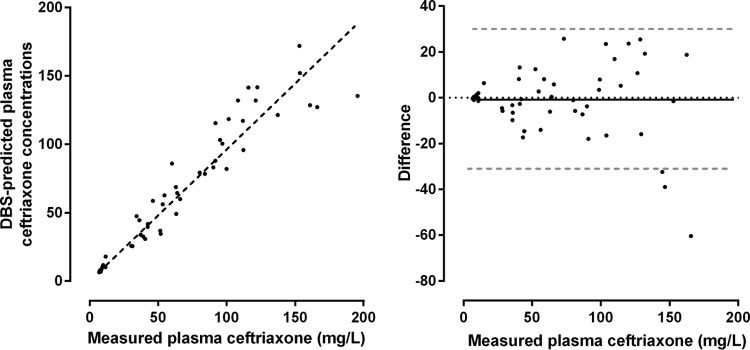
The relative recovery between plasma (104.2%) and DBS (83.9%) was calculated at 0.80. Using this value and after adjustments for hematocrit and red cell partitioning (10.5% average), mean predicted plasma concentrations from DBS concentrations were compared with measured plasma CFX concentrations. Predicted plasma concentrations were tightly correlated with measured plasma concentrations (r = 0.95 [CI95, 0.90 to 0.98], P < 0.0001) (Fig. 4). The slope of the linear regression line was 0.96 (range, 0.91 to 1.01). Visual inspection of the Bland-Altman plot suggested a larger absolute difference at higher measured plasma concentrations. The slope of the linear regression line improved slightly (1.03 [range, 0.98 to 1.08]) when data points with measured plasma CFX concentrations of >150 mg/liter were removed.
FIG 4.
Relationship between measured and dried blood spot-predicted plasma ceftriaxone concentrations. Linear regression lines (dashed black lines) are provided (left). The Bland-Altman plot (right) shows the mean difference (solid black line) and 95% confidence intervals (dashed gray lines).
Pharmacokinetic modeling.
There were 50 individual CFX plasma and DBS-derived plasma concentrations available for analysis. None of the concentrations were below the lower limit of quantification. For both models, a two-compartment model improved the fit of the model, with a significant reduction in the OFV (P < 0.001) and resolution of the bias seen in the CWRES plots with a one-compartment model. The addition of an additional third compartment did not improve the fit further. The structural model parameters for both models were therefore VC/F, VP/F, CL/F, and Q/F. Of these, the IIV of VC/F and CL/F were able to be estimated along with the correlation between these two variability parameters. No significant covariate relationships were identified for either model.
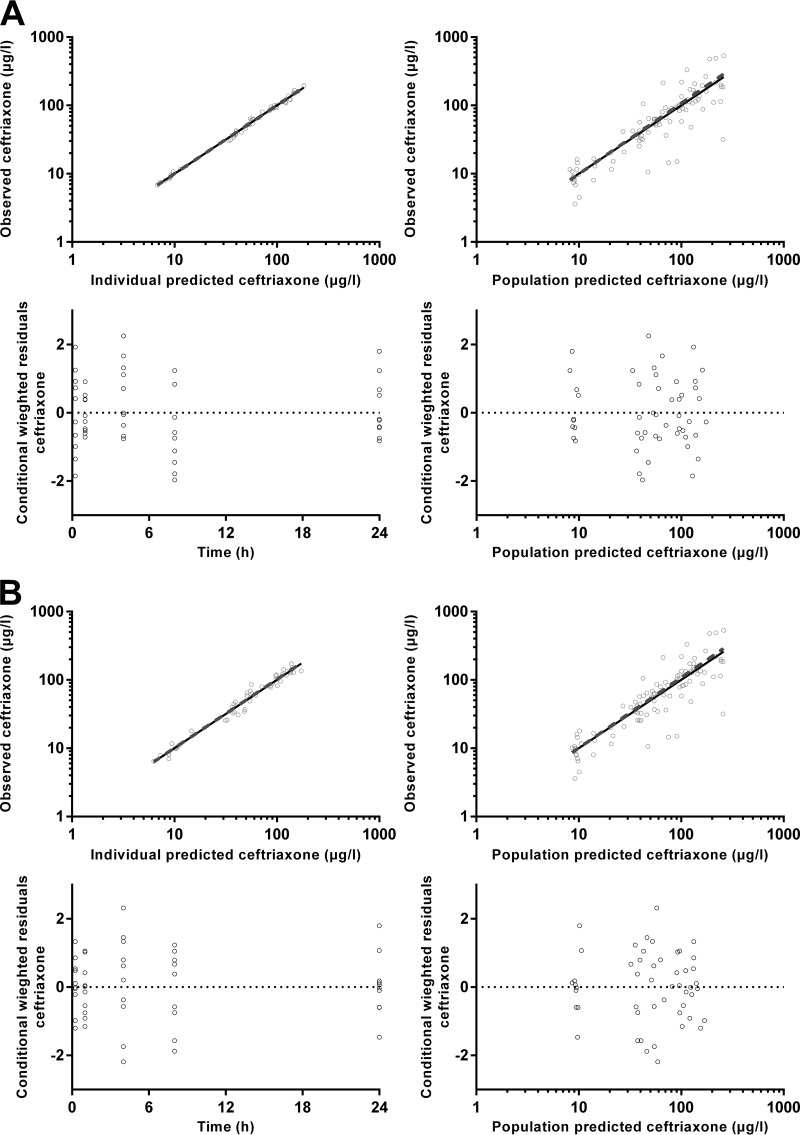
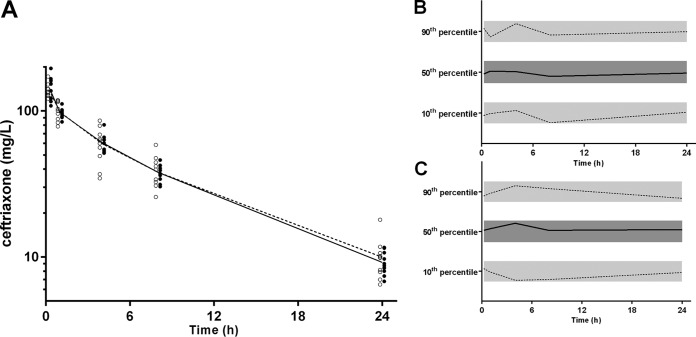
The final model parameter estimates and the bootstrap results for both CFX models are summarized in Table 4. Bias was less than 10% for all structural and random model parameters for both models. P values for differences between each model parameter for the two models are also presented in Table 4. There were no significant differences for any of the structural model parameters. The RV for the model using derived plasma concentrations from DBS samples was significantly higher (17 versus 8%, P < 0.001); however, there was no difference between the estimates of IIV terms. Shrinkage for the IIV terms was higher in the DBS model (6 and 13% versus 1 and 4%); however, despite the small number of subjects, it was still relatively low in the DBS model. Figure 5 shows goodness-of-fit plots, and Fig. 6 presents pcVPCs for both of the models.
TABLE 4.
Final population pharmacokinetic estimates and bootstrap results for ceftriaxone plasma concentrations and dried blood spot-derived plasma concentration for 10 healthy volunteers
| Parameter and/or modela | Value in plasma |
Value in DBS-derived plasma |
P value | ||||
|---|---|---|---|---|---|---|---|
| Mean | RSE (%)b | Bootstrap median (95% CI) | Mean | RSE (%) | Bootstrap median (95% CI) | ||
| Objective function value | −170.155 | −176.526 (−201.801 to −162.576) | −102.657 | −110.603 (−137.205 to −94.312) | |||
| Structural model parameters | |||||||
| CL/F (liters/h/70 kg) | 0.976 | 4 | 0.976 (0.902 to 1.06) | 0.974 | 7 | 0.966 (0.844 to 1.11) | 0.442 |
| VC/F (liters/70 kg) | 5.49 | 8 | 5.48 (4.65 to 6.55) | 5.93 | 9 | 5.88 (5.04 to 7.15) | 0.331 |
| Q/F (liters/h/70 kg) | 3.07 | 18 | 3.10 (1.63 to 4.03) | 2.36 | 44 | 2.40 (0.87 to 4.67) | 0.488 |
| VP/F (liters/70 kg) | 4.18 | 8 | 4.18 (3.38 to 4.77) | 4.23 | 16 | 4.26 (3.14 to 6.11) | 0.274 |
| Variable model parameters (shrinkage %) | |||||||
| IIV in CL/F (%) | 12 (1) | 19 | 11 (6 to 16) | 16 (6) | 24 | 15 (7 to 24) | 0.176 |
| IIV in VC/F (%) | 14 (4) | 13 | 14 (9 to 18) | 16 (13) | 28 | 17 (3 to 24) | 0.499 |
| r (CL/F, VC/F) | 0.874 | 31 | 0.901 (0.612 to 1.00) | 0.865 | 0.944 (−0.999 to 1.00) | ||
| RV for ceftriaxone (%) | 8 (16) | 14 | 8 (6 to 9) | 18 | 11 | 17 (12 to 21) | <0.001 |
CL/F, clearance relative to bioavailability; VC/F, central volume of distribution relative to bioavailability; Q/F, intercompartmental clearance; VP/F, peripheral volume of distribution relative to bioavailability; IIV, interindividual variability; RV, residual variability. IOV and IIV are presented as 100% × variability estimate.
RSE, relative standard error.
FIG 5.
Goodness-of-fit plots for plasma ceftriaxone (A) and derived plasma ceftriaxone from dried blood spots (B).
FIG 6.
(A) Time-concentration profile of ceftriaxone (mg/liter on a log10 scale) for dried blood spot-derived (DBS; open circles) and measured plasma concentrations (closed circles; DBS and plasma data points have been artificially separated to aid comparison). Graphs on the left are the normalized VPC values for dried blood spot-derived (B) and measured plasma (C) concentrations, showing the 10th, 50th, and 90th percentiles with the actual data (solid and dashed lines) within their respective 95% prediction intervals (gray shaded areas).
Based on individual patient time-concentration curves, the time above threshold concentrations of 0.5 mg/liter and 2 mg/liter was also calculated. The median (interquartile range [IQR]) time that concentrations exceeded 0.5 mg/liter was 17.5 (range, 16.2 to 18.3) h and 18.0 (range, 16.5 to 18.4) h for measured and DBS-predicted plasma concentrations, respectively (P = 0.75). Concentrations were above 2 mg/liter for 2.6 h for both measured (range, 2.2 to 3.2 h) and DBS-predicted (range, 2.1 to 2.9 h) plasma concentrations (P = 0.78).
DISCUSSION
The present data show that CFX can be accurately quantified in DBS, and this approach can therefore be used as an alternative to measuring plasma CFX concentrations in PK/PD studies. The potential effects of sample hematocrit, red cell partitioning, CFX protein binding, assay sensitivity, chad positioning, and temperature stability were assessed, and either they were noninfluential or they could be incorporated as variables in the calculation of DBS-predicted plasma concentrations. DBS-predicted plasma CFX concentration-time profiles and PK parameters derived from population PK analyses in our healthy volunteers were also consistent with published data relating to the CFX disposition and elimination. For example, the elimination t1/2 of the second compartment (8.0 h) calculated in our study accords well with data published elsewhere (6 to 9 h) (23, 24).
The PK properties of CFX were not significantly different when the plasma and DBS-predicted plasma concentration models were compared. There was no difference in estimates of structural model parameters (CL/F, VC/F Q/F, and VP/F) or in the estimates of population variability (IIV for CL/F and VC/F). Any difference identified between the two models does not represent any bias from using DBS sampling, namely, higher residual variability and IIV shrinkage.
When measures of overall antibiotic exposure such as the area under the curve (AUC)/MIC ratio or the time above the MIC are used to predict clinical outcome, assay sensitivity is less of a concern than in formal PK and toxicology studies. In this case, the LOQ and LOD were 0.14 mg/liter and 0.05 mg/liter, respectively. Both of these values are well below the median free-CFX concentrations at 24 h (0.3 mg/liter) and published susceptibility breakpoints for Streptococcus pneumoniae (25). These considerations suggest that the LOQ and LOD for our DBS assay are acceptable for CFX PK/PD studies.
Studies incorporating DBS sampling have many advantages over conventional PK protocols. Repeated small-volume samples can be collected with minimal processing, thus overcoming ethical concerns associated with the volume of blood taken from young children and allowing recruitment of subjects where laboratory facilities such as centrifugation and freezer storage are not available. Furthermore, our thermal stability data indicate that DBS CFX samples do not have to be transported and assayed promptly. For the purposes of our PK studies, we considered t95 as a stability threshold. In the present study, CFX was stable for 14 h at typical ambient tropical temperatures (35°C), for 30 days under refrigeration at 4°C, and for 21 weeks in a commercial freezer (−20°C). This provides reassurance that CFX DBS can be air dried at room temperature for 1 to 2 h, refrigerated in desiccant-containing sealed plastic bags, and transported cold to a central laboratory for assay within a month or stored at −80°C for much longer periods before assay.
The detailed approach to laboratory-based and clinical validation in healthy adults that we adopted could serve as a template for validation of DBS assays of other antimicrobial agents for PK studies and for therapeutic drug monitoring in a variety of health care settings. Staff training is simple and straightforward, disposables (Whatman cards, desiccant, and plastic bags) are relatively inexpensive, and the equipment needed at the field site comprises only a refrigerator and hematocrit centrifuge.
ACKNOWLEDGMENTS
We thank the Fremantle Hospital employees who participated in this study. We are also grateful to Elliot Nunn for his logistical assistance with the collection of clinical samples.
Funding Statement
T.M.E.D. is supported by an NHMRC Practitioner Fellowship. B.R.M. is supported by an NHMRC Early Career Fellowship (1036951). The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
REFERENCES
- 1.Ambrose PG, Bhavnani SM, Rubino CM, Louie A, Gumbo T, Forrest A, Drusano GL. 2007. Pharmacokinetics-pharmacodynamics of antimicrobial therapy: it's not just for mice anymore. Clin Infect Dis 44:79–86. doi: 10.1086/510079. [DOI] [PubMed] [Google Scholar]
- 2.Drusano GL, Ambrose PG, Bhavnani SM, Bertino JS, Nafziger AN, Louie A. 2007. Back to the future: using aminoglycosides again and how to dose them optimally. Clin Infect Dis 45:753–760. doi: 10.1086/520991. [DOI] [PubMed] [Google Scholar]
- 3.Andes D. 2001. Pharmacokinetic and pharmacodynamic properties of antimicrobials in the therapy of respiratory tract infections. Curr Opin Infect Dis 14:165–172. doi: 10.1097/00001432-200104000-00010. [DOI] [PubMed] [Google Scholar]
- 4.Mouton JW, Ambrose PG, Canton R, Drusano GL, Harbarth S, MacGowan A, Theuretzbacher U, Turnidge J. 2011. Conserving antibiotics for the future: new ways to use old and new drugs from a pharmacokinetic and pharmacodynamic perspective. Drug Resist Updat 14:107–117. doi: 10.1016/j.drup.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 5.Bhavnani SM, Passarell JA, Owen JS, Loutit JS, Porter SB, Ambrose PG. 2006. Pharmacokinetic-pharmacodynamic relationships describing the efficacy of oritavancin in patients with Staphylococcus aureus bacteremia. Antimicrob Agents Chemother 50:994–1000. doi: 10.1128/AAC.50.3.994-1000.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rayner CR, Forrest A, Meagher AK, Birmingham MC, Schentag JJ. 2003. Clinical pharmacodynamics of linezolid in seriously ill patients treated in a compassionate use programme. Clin Pharmacokinet 42:1411–1423. doi: 10.2165/00003088-200342150-00007. [DOI] [PubMed] [Google Scholar]
- 7.Ohno A, Ishii Y, Kobayashi I, Yamaguchi K. 2007. Antibacterial activity and PK/PD of ceftriaxone against penicillin-resistant Streptococcus pneumoniae and beta-lactamase-negative ampicillin-resistant Haemophilus influenzae isolates from patients with community-acquired pneumonia. J Infect Chemother 13:296–301. doi: 10.1007/s10156-007-0539-2. [DOI] [PubMed] [Google Scholar]
- 8.Ayalew K, Nambiar S, Yasinskaya Y, Jantausch BA. 2003. Carbapenems in pediatrics. Ther Drug Monit 25:593–599. doi: 10.1097/00007691-200310000-00009. [DOI] [PubMed] [Google Scholar]
- 9.Suyagh M, Collier PS, Millership JS, Iheagwaram G, Millar M, Halliday HL, McElnay JC. 2011. Metronidazole population pharmacokinetics in preterm neonates using dried blood-spot sampling. Pediatrics 127:e367–e374. doi: 10.1542/peds.2010-0807. [DOI] [PubMed] [Google Scholar]
- 10.Elliott SR, Beeson JG. 2008. Estimating the burden of global mortality in children aged <5 years by pathogen-specific causes. Clin Infect Dis 46:1794–1795. doi: 10.1086/588049. [DOI] [PubMed] [Google Scholar]
- 11.Howie SR. 2011. Blood sample volumes in child health research: review of safe limits. Bull World Health Organ 89:46–53. doi: 10.2471/BLT.10.080010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kissinger PT. 2011. Thinking about dried blood spots for pharmacokinetic assays and therapeutic drug monitoring. Bioanalysis 3:2263–2266. doi: 10.4155/bio.11.235. [DOI] [PubMed] [Google Scholar]
- 13.la Marca G, Malvagia S, Filippi L, Innocenti M, Rosati A, Falchi M, Pellacani S, Moneti G, Guerrini R. 2011. Rapid assay of rufinamide in dried blood spots by a new liquid chromatography-tandem mass spectrometric method. J Pharm Biomed Anal 54:192–197. doi: 10.1016/j.jpba.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 14.Vu DH, Koster RA, Alffenaar JW, Brouwers JR, Uges DR. 2011. Determination of moxifloxacin in dried blood spots using LC-MS/MS and the impact of the hematocrit and blood volume. J Chromatogr B Analyt Technol Biomed Life Sci 879:1063–1070. doi: 10.1016/j.jchromb.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 15.la Marca G, Giocaliere E, Villanelli F, Malvagia S, Funghini S, Ombrone D, Filippi L, De Gaudio M, De Martino M, Galli L. 2012. Development of an UPLC-MS/MS method for determination of antibiotic ertapenem on dried blood spots. J Pharm Biomed Anal 61:108–113. doi: 10.1016/j.jpba.2011.12.018. [DOI] [PubMed] [Google Scholar]
- 16.la Marca G, Malvagia S, Filippi L, Fiorini P, Innocenti M, Luceri F, Pieraccini G, Moneti G, Francese S, Dani FR, Guerrini R. 2008. Rapid assay of topiramate in dried blood spots by a new liquid chromatography-tandem mass spectrometric method. J Pharm Biomed Anal 48:1392–1396. doi: 10.1016/j.jpba.2008.09.025. [DOI] [PubMed] [Google Scholar]
- 17.ter Heine R, Rosing H, van Gorp EC, Mulder JW, van der Steeg WA, Beijnen JH, Huitema AD. 2008. Quantification of protease inhibitors and non-nucleoside reverse transcriptase inhibitors in dried blood spots by liquid chromatography-triple quadrupole mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 867:205–212. doi: 10.1016/j.jchromb.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 18.Matuszewski BK, Constanzer ML, Chavez-Eng CM. 2003. Strategies for the assessment of matrix effect in quantitative bioanalytical methods based on HPLC-MS/MS. Anal Chem 75:3019–3030. doi: 10.1021/ac020361s. [DOI] [PubMed] [Google Scholar]
- 19.Hung TY, Davis TM, Ilett KF. 2003. Measurement of piperaquine in plasma by liquid chromatography with ultraviolet absorbance detection. J Chromatogr B Analyt Technol Biomed Life Sci 791:93–101. doi: 10.1016/S1570-0232(03)00209-5. [DOI] [PubMed] [Google Scholar]
- 20.Pugh J. 2002. Kinetics and product stability, p 101–112. In Aulton M. (ed), Pharmaceutics: the science of dosage form design, 2nd ed Churchill Livingstone, Edinburgh, United Kingdom. [Google Scholar]
- 21.Sinko P. 2011. Martin's physical pharmacy and pharmaceutical sciences, 6th ed Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 22.Anderson BJ, Holford NH. 2009. Mechanistic basis of using body size and maturation to predict clearance in humans. Drug Metab Pharmacokinet 24:25–36. doi: 10.2133/dmpk.24.25. [DOI] [PubMed] [Google Scholar]
- 23.Zhou HH, Chan YP, Arnold K, Sun M. 1985. Single-dose pharmacokinetics of ceftriaxone in healthy Chinese adults. Antimicrob Agents Chemother 27:192–196. doi: 10.1128/AAC.27.2.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patel IH, Weinfeld RE, Konikoff J, Parsonnet M. 1982. Pharmacokinetics and tolerance of ceftriaxone in humans after single-dose intramuscular administration in water and lidocaine diluents. Antimicrob Agents Chemother 21:957–962. doi: 10.1128/AAC.21.6.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clinical and Laboratory Standards Institute. 2014. Performance standards for antimicrobial susceptibility testing; twenty-fourth informational supplement. Document M100-S24. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]