Abstract
Multiple strains of Acinetobacter baumannii have developed multidrug resistance (MDR), leaving colistin as the only effective treatment. The cecropin-α–melittin hybrid BP100 (KKLFKKILKYL-NH2) and its analogs have previously shown activity against a wide array of plant and human pathogens. In this study, we investigated the in vitro antibacterial activities of 18 BP100 analogs (four known and 14 new) against the MDR A. baumannii strain ATCC BAA-1605, as well as against a number of other clinically relevant human pathogens. Selected peptides were further evaluated against strains of A. baumannii that acquired resistance to colistin due to mutations of the lpxC, lpxD, pmrA, and pmrB genes. The novel analogue BP214 showed antimicrobial activity at 1 to 2 μM and a hemolytic 50% effective concentration (EC50) of >150 μM. The lower activity of its enantiomer suggests a dual, specific and nonspecific mode of action. Interestingly, colistin behaved antagonistically to BP214 when pmrAB and lpxC mutants were challenged.
INTRODUCTION
Multidrug-resistant (MDR) Acinetobacter baumannii infections often occur in intensive care units, where patients are typically immunosuppressed or have been subjected to invasive procedures (1). The therapy outcome is further threatened by the common coexistence of multiple heteroresistant subpopulations (2, 3).
Due to the growing prevalence of carbapenem resistance (4, 5), colistin as a last-resort treatment is increasingly critical. Unfortunately, colistin-resistant clinical isolates of A. baumannii have been reported several times (6–8).
Polymyxins are well known for binding to the LPS of Gram-negative bacteria, with concomitant displacement of Ca2+ and Mg2+ ions (9). From a chemical perspective, this interaction is very specific, and studies on polymyxin nonapeptides (i.e., lacking the lipidated N-terminal amino acid) have revealed that the enantiomers are inactive (10). This specificity provides the basis for both the high activity and selectivity of polymyxins against Gram-negative bacteria. Unfortunately, it also provides pathogens with a clear escape route: known mechanisms (11) behind colistin resistance in A. baumannii consist of (i) the addition of ethanolamine to the lipid A moiety of the LPS mediated by the pmrA, pmrB, pmrAB, and pmrC genes (12) and (ii) loss of LPS due to mutations in the lpxA, lpxC, and lpxD genes (13).
Its high specificity ultimately makes the self-promoted uptake process very effective but also very delicate, and colistin appears to work in an “all-or-nothing” fashion: susceptible strains are typically inhibited at concentrations of <0.5 μM, whereas resistant strains appear unaffected at concentrations of <128 μM.
Many studies in recent years have focused on modifying polymyxins to address their shortcomings (9, 14), but only a few have dealt with identifying a novel lead for their potential replacement. We envisaged that a less specific antimicrobial peptide could offer the advantage of better robustness and reliability in the critical clinical scenario whereby a last-resort drug is employed. This was based on the assumption that the activity of a peptide able to rapidly kill bacteria of different genera, both Gram positive and Gram negative, could not depend on a single molecular target. This characteristic was expected to lower the survival probability of heteroresistant populations, as well as to overcome resistance mechanisms based on target modification.
Given the previous reports (15, 16) of cecropin-α–melittin hybrids showing activity against colistin-resistant strains of A. baumannii, we developed an interest in the BP peptide family (17–19). In the present study, we investigated the in vitro antibacterial activity of 18 BP100 (KKLFKKILKYL-NH2) analogs, four known and 14 new, against MDR A. baumannii ATCC BAA-1605, as well as against a number of other clinically relevant human pathogens. Selected peptides were then evaluated against four colistin-susceptible and -resistant clinical isolates of A. baumannii. We report that BP214, a novel analogue, showed only slightly reduced activity compared to colistin and a hemolytic EC50 of >150 μM. The peptide displayed rapid bactericidal properties, and its high activity was also maintained against colistin-resistant strains featuring mutated lpxC, pmrA, and pmrB genes.
MATERIALS AND METHODS
Abbreviations.
AMP, antimicrobial peptide; DCM, dichloromethane; DIC, N,N′-diisopropylcarbodiimide; DIEA, diisopropylethylamine; DMF, N,N′-dimethylformamide; EC50, 50% effective concentration; Et2O, diethyl ether; EtOH, ethanol; Fmoc, fluoren-9-ylmethoxycarbonyl; HATU, O-(7-azabenzotriazol-1-yl)-1,1,3,3,-tetramethylaminium hexafluorophosphate; HOAt, 1-hydroxy-7-aza-benzotriazole; HPLC, high-performance liquid chromatography; LPS, lipopolysaccharide; MALDI-TOF MS, matrix-assisted laser desorption ionization–time of flight mass spectrometry; MeCN, acetonitrile; MDR, multidrug-resistant; MHB, Mueller-Hinton broth; MRSA, methicillin-resistant Staphylococcus aureus; OD600, optical density at 600 nm; PBS, phosphate-buffered saline; PTFE, polytetrafluoroethylene; RBC, red blood cells; TFA, trifluoroacetic acid; TIS, triisopropylsilane; VRE, vancomycin-resistant Enterococcus faecium.
Solid-phase peptide synthesis.
Disposable 5-ml polypropylene reactors fitted with a PTFE filter were acquired from Thermo Scientific. Hypogel RAM 200 resin and Fmoc-protected amino acids were purchased from Iris Biotech GmbH. The resin was allowed to swell overnight in DMF and was then washed with DMF (five times). The Fmoc group was removed by treatment with a 20% (vol/vol) piperidine solution in DMF (three times for 4 min each); then, the resin was washed with DMF (three times), DCM (three times), and DMF again (five times). Chain elongation was achieved with single couplings using 3.5 equivalents each of amino acid, HOAt, and HATU and 7 equivalents of DIEA (based on declared resin loading). Fmoc-protected amino acids were dissolved in DMF together with HOAt (both at a concentration of 0.4 M); they were then activated by the sequential addition of the HATU solution (0.4 M in DMF) and of DIEA (pure), and the mixture was immediately transferred into the reactor. After 2 h, the resin was washed with DMF (three times), DCM (three times), and DMF again (five times); then, Fmoc removal with piperidine was performed as described above. Cycles of amino acid coupling and Fmoc removal were alternated until the chain elongation process was completed. After the last deprotection cycle for the N-terminal amino acid, the resin was additionally washed with EtOH (five times) and dried in vacuo. The release from the solid support and the cleavage of the side chain protecting groups were performed by treatment with a TFA-H2O-TIS (95:2.5:2.5) solution for 2 h. The cleavage solution was collected and concentrated to ∼300 μl with a gentle stream of N2, and then the peptide was precipitated (and washed) with Et2O (three times). After spontaneous evaporation of the residual Et2O, the residue was dissolved in H2O-MeCN (8.5:1.5) and freeze-dried.
Peptoid synthesis.
Peptide-peptoid hybrids were synthesized via the submonomer approach as previously described (20). Briefly, bromoacetic acid (20 equivalents, 0.6 M in DMF) was coupled (twice for 20 min each time) to the free N terminus of the growing resin-bound peptide after preactivation (3 min) with diisopropyl carbodiimide (DIC, 19 equivalents). After washing with DMF (10×), a solution of the appropriate amine (20 equivalents; 1 M in DMF) was added, and the reactor was placed on a shaker for 2 h.
Peptide purity and identity.
The verification was performed using analytical HPLC and MALDI-TOF MS. The α-cyano-4-hydroxycinammic acid matrix was used for MALDI-TOF MS experiments. Peptides were purified via preparative HPLC; purity was ≥95% for all peptides tested. Results are presented in the supplemental material.
Hemolytic activity.
Peptide-induced hemolysis was determined in triplicate by mixing 75 μl of peptide solution in PBS with 75 μl of a 0.5% RBC (blood type O+) suspension in PBS, incubating the mixture at 37°C for 1 h, and then measuring hemoglobin release with a spectrophotometer (λ = 414 nm). Results were normalized to a positive (melittin) and negative (PBS) control.
Determination of antimicrobial activity.
MICs were determined in triplicate using the tube microdilution method according to CLSI guidelines. Bacterial inocula were prepared by diluting an overnight culture 1:100 with preheated MHB-II. The suspension was allowed to reach an OD600 of 0.2 to 0.4 and then diluted to 1 × 106 CFU/ml.
Tests against colistin-resistant strains were performed using two alternative procedures. When colistin was maintained through all stages, including the final test tubes, the overnight culture and all following dilutions were made using a colistin-enriched (10 μg/ml colistin sulfate) MHB-II medium. Alternatively, colistin was present only in overnight culture medium, and all following dilutions were performed with standard MHB-II medium.
Peptide solutions were mixed with an equal volume of bacterial suspension in a polypropylene microtiter plate and incubated at 37°C for 16 h. Inhibition of bacterial growth was assessed visually.
Peptides were tested as TFA salts against Escherichia coli ATCC 25922 (reference strain), Staphylococcus aureus ATCC 33591 (MRSA), Enterococcus faecium ATCC 700221 (VRE), Pseudomonas aeruginosa ATCC 27853 (reference strain), Klebsiella pneumoniae ATCC 700603 (MDR), and Acinetobacter baumannii ATCC BAA-1605 (MDR). ATCC strains were obtained commercially. Selected peptides were tested against additional strains of A. baumannii, namely, ATCC 19606, Ab-167 (MDR, colistin-susceptible clinical isolate) (21), Ab-176 (MDR, colistin-susceptible clinical isolate) (21), CS01 (colistin-susceptible clinical isolate) (8), Ab-167R (strain Ab-167 containing an ISAba1 insertion at nucleotide 321 of lpxC) (22), Ab-176R (strain Ab-176 with a G739T substitution at nucleotide 739 of the lpxD gene, producing a premature stop codon) (22), RC64 (derivative of ATCC 19606 containing R134C and A227V mutations in pmrB) (23), and CR17 (colistin-resistant derivative of CS01 containing an M12K mutations in pmrA) (8).
Time course experiments.
Time-kill curves were measured by growing single colonies of ATCC 19606 or RC64 in MHB-II. In the case of RC64, the growth medium was supplemented with colistin sulfate (10 μg/ml) to prevent reversal of resistance. For stationary-phase experiments, overnight cultures were used directly. For exponential-phase experiments, the overnight cultures were diluted 1:100 in 50 ml of preheated (37°C) MHB-II (with the addition of colistin sulfate in the case of RC64) and transferred into Erlenmeyer flasks placed in a water bath under shaking. When the cultures reached an OD600 of 0.5, they were divided into fresh flasks and treated with the test compound. Spot plating was performed in triplicate at 0, 1, 3, and 5 h by transferring 10 μl of a 10-fold-diluted suspension on a plate containing MHB-II medium. In persister assays, the procedure was the same, but the cultures were divided at two time points, i.e., before and after ciprofloxacin treatment. Spot plating was performed as described above.
RESULTS
The antimicrobial activities of all peptides investigated in this study are presented in Table 1, along with the hemolytic activities observed at 150 μM. For consistency with previous literature, the “BP” designation has been maintained for the novel sequences presented in this study, with a new numeration starting from 201. BP100 and RW-BP100 were synthesized and tested to ensure comparability with previously reported data.
TABLE 1.
Antimicrobial and hemolytic activities of all peptides investigated in this studya
| Compound | Sequenceb | % HAc | MIC (μg/ml) (MIC [μM]) |
|||||
|---|---|---|---|---|---|---|---|---|
| S. aureus | E. faecium | E. coli | P. aeruginosa | K. pneumoniae | A. baumanniid | |||
| BP100 | KKLFKKILKYL-NH2 | 33 | 134.5 (64) | 67 (32) | 17 (8) | 33.5 (16) | 17 (8) | 8.5 (4) |
| RW-BP100 | RRLFRRILRWL-NH2 | 100 | 18.5 (8) | 18.5 (8) | 4.5 (2) | 18.5 (8) | 18.5 (8) | 18.5 (8) |
| BP143 | KKLfKKILKYL-NH2 | <8 | 134.5 (64) | 67 (32) | 8.5 (4) | 33.5 (16) | 17 (8) | 8.5 (4) |
| BP157 | KKLFKkilkyl-NH2 | 8 | 269 (128) | 134.5 (64) | 33.5 (16) | 134.5 (64) | 67 (32) | 33.5 (16) |
| BP201 | RKLFKRILKYL-NH2 | 45 | 137.5 (64) | 275 (128) | 275 (128) | 137.5 (64) | 137.5 (64) | 69 (32) |
| BP202 | KRLFRKILKYL-NH2 | 44 | 8.5 (4) | 8.5 (4) | 17 (8) | 17 (8) | 8.5 (4) | 4.5 (2) |
| BP203 | KKLFKKILRYL-NH2 | 31 | 33.5 (16) | 8.5 (4) | 8.5 (4) | 17 (8) | 8.5 (4) | 4 (2) |
| BP204 | KRLFRKILRYL-NH2 | 69 | 70.5 (32) | 35 (16) | 70.5 (32) | 70.5 (32) | 35 (16) | 17.5 (8) |
| BP205 | KKLFRRILKYL-NH2 | 63 | 8.5 (4) | 8.5 (4) | 34.5 (16) | 17 (8) | 8.5 (4) | 4.5 (2) |
| BP206 | RRLFKKILKYL-NH2 | 68 | 34.5 (16) | 17 (8) | 34.5 (16) | 17 (8) | 17 (8) | 4.5 (2) |
| BP207 | KKLFKKiLKYL-NH2 | ND | >269 (>128) | 269 (128) | 269 (128) | 269 (128) | 269 (128) | 134.5 (64) |
| BP208 | KKLFKKILKYL-NH2 | ND | >269 (>128) | 269 (128) | 134 (64) | 134 (64) | 269 (128) | 134 (64) |
| BP209 | KKLFKKLLKFL-NH2 | ND | >269 (>128) | 269 (128) | 269 (128) | 269 (128) | >269 (>128) | >269 (>128) |
| BP210 | RRL(2-Nal)RRILRYL-NH2 | 100 | 37 (16) | 18.5 (8) | 73.5 (32) | 147 (64) | 37 (16) | 37 (16) |
| BP211 | KKLfKKILRYL-NH2 | 43 | 17 (8) | 17 (8) | 4.5 (2) | 8.5 (4) | 8.5 (4) | 4 (2) |
| BP212 | KKL(D-2-Nal)KKILKYL-NH2 | 85 | 17 (8) | 17 (8) | 4.5 (2) | 8.5 (4) | 8.5 (4) | 4.5 (2) |
| BP213 | KKLFKkilryl-NH2 | <8 | 134.5 (64) | 67 (32) | 33.5 (16) | 67 (32) | 33.5 (16) | 17 (8) |
| BP214 | kklfkkilryl-NH2 | 42 | 33.5 (16) | 8.5 (4) | 8.5 (4) | 33.5 (16) | 8.5 (4) | 4 (2) |
All experiments were performed in triplicate.
Lowercase letters indicate d-amino acids. Underlining identifies peptoid residues (e.g., F indicates NPhe).
HA, hemolytic activity at 150 μM. ND, not determined.
A. baumannii ATCC BAA-1605.
In this discussion, the term “persister” is used to describe metabolically inactive bacteria that survive antibiotic treatment (24). The term “heteroresistant” describes metabolically active subpopulations that display a lower susceptibility to antibiotics due to phenotypic variations (25).
Design of optimized analogs.
The BP peptide family comprises numerous analogs with various degrees of antimicrobial and hemolytic activity. Our design approach was based on combining elements from different analogs, as well as introducing novel modifications.
BP201 to BP206 are BP100 analogs featuring single and double Lys-to-Arg substitutions. A second set of analogs (BP207 to BP209) was designed by introducing stereochemical modifications that included unprecedented d/l-amino acid combinations and peptoids. BP210 was based on the structure of RW-BP100, but the bulky aromatic group (2-Nal for Trp) was moved from position 10 to position 4. We included the previously reported BP143 (KKLfKKILKYL-NH2) and BP157 (KKLFKkilkyl-NH2; lowercase indicates d-amino acids) in our initial screening against clinically relevant human pathogens. These peptides were selected on the basis of their low toxicity and good activity previously published against phytopathogenic strains of the Pseudomonas genus (18).
Among the novel sequences, BP203 stood out as a net improvement: by introducing a single arginine at position 9, we were able to match and surpass the high activity of RW-BP100 without any detectable increment in toxicity to RBC. BP207 to BP209 did not produce any encouraging results and were not further investigated. BP143 showed an activity profile similar or identical to that of the more hemolytic BP100, while BP157 proved considerably less active.
Analogs BP211 to BP213 were designed by combining elements of BP203 with elements from the less hemolytic BP143 and BP157. Partial d-amino acid substitution did not result in any reduction of hemolysis, which was instead slightly increased. Finally, BP214 was designed as the all-d BP203 enantiomer and behaved similarly.
Activity against colistin-resistant A. baumannii strains.
The peptides BP202, BP203, BP211, BP213, and BP214 showed good activity against MDR A. baumannii ATCC BAA-1605 and different degrees of selectivity between Gram-positive and -negative bacteria and caused <50% hemolysis at 150 μM. These peptides, along with BP100 and RW-BP100, were tested against a wider array of colistin-susceptible and -resistant strains of A. baumannii (Table 2). Overall, the resistant mutants proved 4- to 16-fold less susceptible to BP peptides than their parent strains and practically insusceptible to colistin. However, the all-d BP214 showed even higher activity than BP203 and the overall highest of the series. Together with RW-BP100, it proved the least affected by the lack or modification of the LPS.
TABLE 2.
Antimicrobial activity of BP100, RW-BP100, BP202, BP203, BP211, BP213, and BP214 against selected colistin-susceptible and -resistant strains of A. baumanniia
| Compound | MIC (μg/ml) for Acinetobacter baumannii |
|||||||
|---|---|---|---|---|---|---|---|---|
| Colistin susceptible |
Colistin resistant |
|||||||
| Ab-167 | Ab-176 | ATCC 19606 | CS01 | Ab-167R | Ab-176R | RC64 | CR17 | |
| BP100 | 8.5 | 17 | 8.5 | 8.5 | 17 (33.5) | 67 (67) | 8.5 (67) | 33.5 (67) |
| RW-BP100 | 8.5 | 4.5 | 8.5 | 8.5 | 8.5 (4.5) | 17 (17) | 8.5 (8.5) | 4.5 (8.5) |
| BP202 | 4.5 | 8.5 | 4.5 | 4.5 | 33.5 (17) | 33.5 (33.5) | 17 (33.5) | 17 (33.5) |
| BP203 | 4.5 | 17 | 8.5 | 8.5 | 67 (33.5) | 67 (67) | 8.5 (33.5) | 33.5 (67) |
| BP211 | 4.5 | 17 | 8.5 | 17 | 33.5 (33.5) | 67 (67) | 4.5 (33.5) | 17 (67) |
| BP213 | 8.5 | 17 | 17 | 17 | 33.5 (17) | 67 (67) | 17 (134.5) | 67 (269) |
| BP214 | 2 | 4.5 | 4.5 | 2 | 8.5 (17) | 33.5 (33.5) | 4.5 (17) | 8.5 (33.5) |
| Colistin | 0.5 | 0.5 | 0.25 | 0.25 | >128 | >128 | 128 | >128 |
Colistin sulfate is included as a reference. Values in parentheses were obtained with a colistin-enriched medium (10 μg/ml colistin sulfate). All tests were performed in triplicate.
Susceptibility tests against colistin-resistant strains were carried out both in standard MHB-II medium and in a modified version containing 10 μg/ml of colistin sulfate. With the exception of RW-BP100, the presence of colistin resulted in a marked antagonistic effect on BP peptides in a challenge of pmr mutants.
Time course experiments.
The most interesting analog, BP214, was selected for further investigation and compared to colistin. Time course experiments were carried out with stationary and exponentially growing cultures of A. baumannii ATCC 19606 and RC64 (Fig. 1 and 2). For practical reasons, tests on exponentially growing cultures were carried out from an initial bacterial concentration of approximately 5 × 108 CFU/ml (OD600 ≈ 0.5); tested peptide concentrations were, however, based on multiples of the MICs determined for a standard inoculum of 5 × 105 CFU/ml (Table 2).
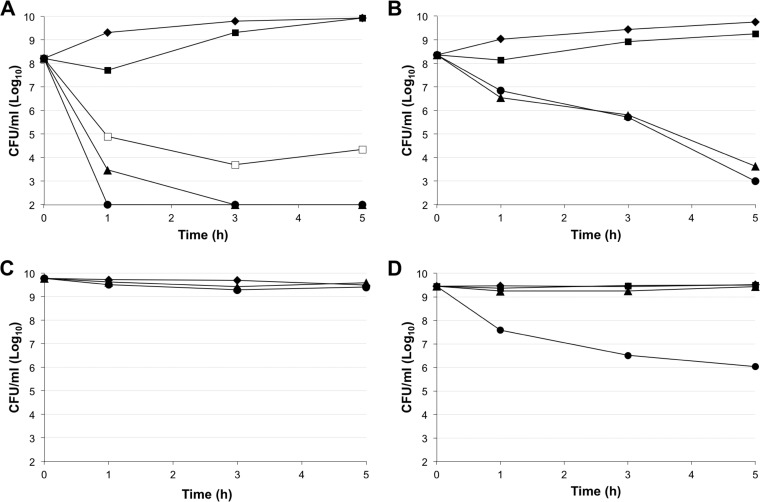
FIG 1.
Time-kill curves for colistin and BP214 against A. baumannii ATCC 19606 in exponential phase (A and B) and stationary phase (C and D). Each data point is the average of readings from at least three independent experiments. (A and C) BP214. ◆, control; ■, 1× MIC; □, 2× MIC; ▲, 4× MIC; ●, 8× MIC. (B and D) Colistin. ◆, control; ■, 16× MIC; ▲, 32× MIC; ●, 64× MIC.
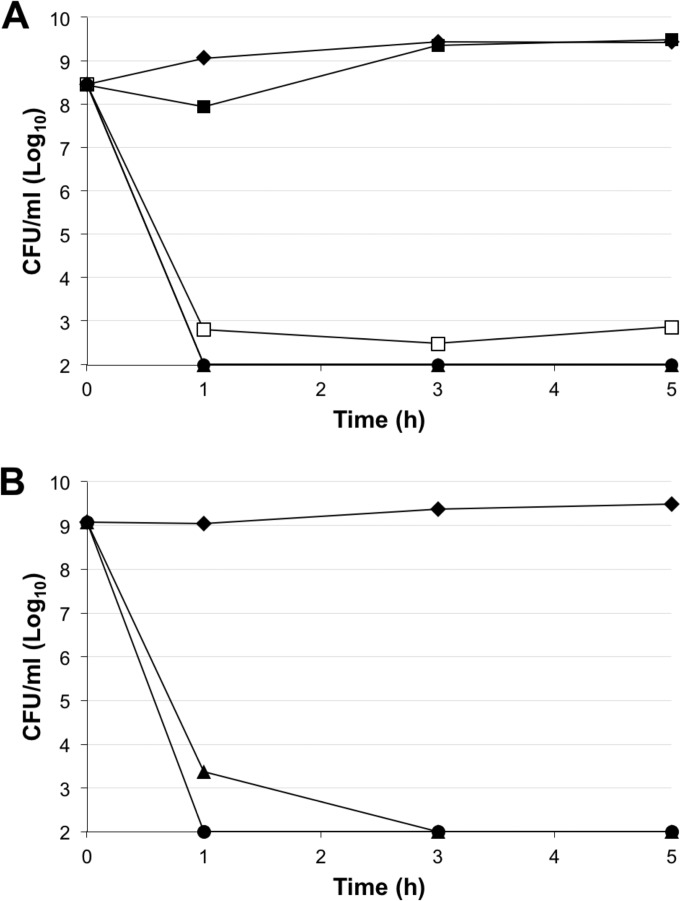
FIG 2.
Time-kill curves for BP214 against A. baumannii RC64 in exponential phase (A) and stationary phase (B). Each data point is the average of readings from at least three independent experiments. ◆, control; ■, 1× MIC; □, 2× MIC; ▲, 4× MIC; ●, 8× MIC.
Both colistin and BP214 appeared to be affected by the higher bacterial inoculum, although to different extents. At 1× MIC, the bactericidal effect of BP214 was moderate and, after less than 3 h, the bacterial population showed full recovery (Fig. 1A); this behavior is compatible with heteroresistance phenomena (3) or with peptide sequestration by membrane debris. At 4× MIC and above, BP214 was able to reduce the number of CFU of the colistin-susceptible strain ATCC 19606 to below our detection level (Fig. 1A). The >3-log reduction in CFU per milliliter clearly indicates a bactericidal action. Colistin proved visibly affected by the high bacterial concentrations and/or heteroresistance phenomena (Fig. 1B), being unable to eradicate a growing culture even after 5 h at 64× MIC. None of the tested concentrations of BP214 had any effect on stationary-phase ATCC 19606 cells (Fig. 1C). Even at high concentrations, colistin only had a modest effect on stationary-phase cells (Fig. 1D).
A different picture emerged when BP214 challenged the colistin-resistant pmrB mutant RC64. Concentrations of BP214 corresponding to 4× MIC and above could reduce the number of CFU/ml in the culture to below our detection level for both exponential-phase (Fig. 2A) and stationary-phase (Fig. 2B) cultures.
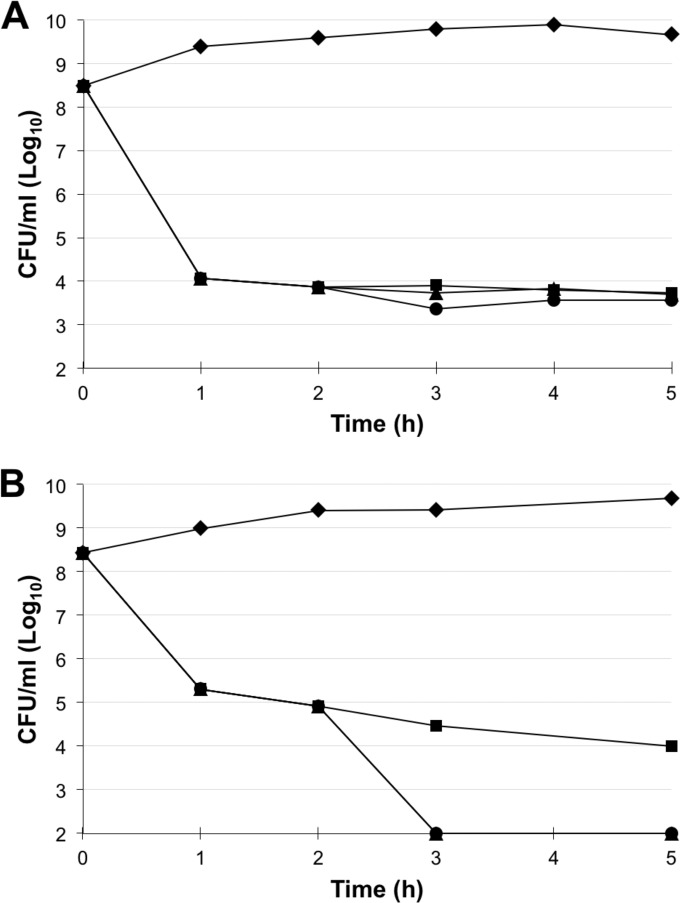
We found that ciprofloxacin induced persister formation in both ATCC 19606 and RC64 cultures (Fig. 3). After BP214 was added (2 h), no bactericidal effect was observed for ATCC 19606, whereas the number of CFU of RC64 per milliliter was reduced to below our detection limit. Overall, the susceptibility of persisters to BP214 was thus quite similar to that of stationary-phase cultures.
FIG 3.
Determination of the time efficiency of BP214 in clearing ATCC 19606 (A) and RC64 (B) persisters remaining after 2 h of ciprofloxacin treatment. Each data point is the average of readings from at least three independent experiments. ◆, control; ■, ciprofloxacin, 2× MIC; ▲, ciprofloxacin, 2× MIC + BP214, 4× MIC (added at 2 h); ●, ciprofloxacin, 2× MIC + BP214, 8× MIC (added at 2 h).
DISCUSSION
As previous studies have highlighted the worrying ease with which colistin-resistant mutants of A. baumannii can be isolated (22), more robust alternatives are needed.
From a drug design perspective, colistin's case indicates that an LPS-dependent mechanism of action might be a disadvantageous approach for achieving selectivity against Gram-negative bacteria, and this appears to apply particularly well to A. baumannii. On the other hand, the LPS is a constitutive element of the Gram-negative cell wall and thus an attractive (and obligate) target. Furthermore, the partial or complete loss of LPS has been connected to increased susceptibility to many antibiotics and decreased virulence (11, 26); therefore, pmr mutants of A. baumannii have been suggested to be of greater clinical importance (11). From this perspective, it is clear that colistin-resistant strains are not intrinsically more threatening or virulent, but they become clinically important due to the increasing prevalence of carbapenem resistance and the role of colistin as a last-resort treatment (4, 27).
We envisaged that a highly attractive alternative to colistin for the treatment of A. baumannii infections would be able to strongly interact with wild-type and modified LPS while also being able to exert a bactericidal effect via alternative mechanisms. Ideally, such a peptide should also be short, nontoxic, and resistant to proteolysis.
Due to their small size and good antimicrobial activity, the BP series of cecropin-α-melittin hybrids constituted a good starting point. Our synthetic approach to improved BP100 analogs is described in Results, and MICs for all compounds are presented in Tables 1 and 2.
As hypothesized, the broad-spectrum BP peptides were less affected overall than colistin by modifications or loss of the LPS. This proved true in particular for RW-BP100, as the full arginine substitution grants it an advantage in electrostatic interactions, due to the higher basicity of the guanidino group (pKa = 12.5) versus primary amino groups (pKa = 10.5). The superior hydrogen bonding capability, as well as the increased size and lipophilicity of Trp versus Tyr, can also be expected to play a role in stabilizing peptide-membrane interactions. Taken together, these characteristics make RW-BP100 a potent and nonspecific membrane-active agent, as further evidenced by its high hemolytic activity. At the same time, however, RW-BP100 did not provide any significant advantage over its less hemolytic analogues against colistin-susceptible strains.
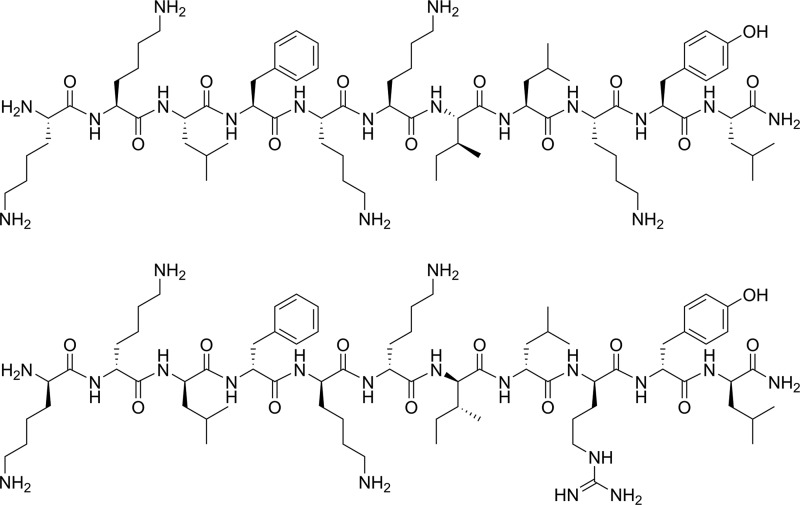
Our efforts in designing an AMP that would ideally be equally active against colistin-susceptible and -resistant strains of A. baumannii have resulted in the identification of BP214 (Fig. 4). This all-d undecapeptide displayed robust activity (MIC ≈ 4 μg/ml as TFA-salt; ≈2 μM peptide concentration) against several strains, including clinically important pmr mutants, and a modest hemolytic EC50, >150 μM. The evaluation of this peptide in microbiological assays led to several interesting observations.
FIG 4.
Structures of the lead compound BP100 (top) and the novel analog BP214 (bottom).
In time course experiments, remarkable differences were observed between exponential- and stationary-phase cultures of the colistin-susceptible strain ATCC 19606 and its pmrB mutant RC64. Specifically, stationary-phase cultures of the susceptible strain proved immune to BP214 and only moderately susceptible to high concentrations of colistin, while RC64 proved instead susceptible to BP214. Interestingly, persisters remaining after ciprofloxacin treatment behaved identically. Previous studies showed differences in cell shape and membrane appearance between exponential- and stationary-phase cultures of colistin-susceptible and -resistant strains of A. baumannii (28). Upon entering stationary phase, A. baumannii considerably changes its transcriptome and upregulates maintenance and protective processes, several of which can play a role in determining the susceptibility to membrane-active agents (29–31). From this perspective, it is plausible that the fitness cost involved in colistin resistance would prevent RC64 from dedicating sufficient resources to these protective mechanisms (11). Another possibility, as shown for lpx mutants, is related to the zeta potential of the bacterial outer membrane. Colistin-susceptible strains have shown a less negative potential in stationary than in exponential phase, whereas resistant mutants behaved oppositely (32).
In terms of in vitro MIC, the enantiomeric pair BP203 and BP214 behaved very similarly against most species (Table 1). This is expected for membrane-active peptides that do not bind specifically to any target, e.g., cecropin, melittin, and their hybrids (33). However, moderate but consistent differences in MIC were observed with several A. baumannii strains (Table 2), suggesting the presence of binding targets with strict chiral requirements, as is the case for, e.g., colistin and drosocin (10, 34). Ultimately, all BP peptides are able to kill bacteria via nonspecific, amphipathicity-driven membrane damage; additionally, as far as A. baumannii is concerned, BP214 appeared able to interact with certain structural elements also in a more specific fashion. For wild-type strains and pmr mutants, the high number of stereocenters in the saccharide portion of the LPS may very well account for the observed enantiomeric discrimination. Moreover, being a prominent feature of the cell wall, the LPS can be expected to play a major role in determining the susceptibility of Gram-negative bacteria to membrane-active agents in general. Accordingly, LPS-deficient lpx mutants proved consistently less susceptible than LPS-modified pmr mutants to the investigated BP peptides—again, with the exception of the RW analog.
However, the lpxC mutant Ab-167R unexpectedly proved very susceptible to BP214. Interestingly, the same strain had been previously reported to be 100-fold less susceptible to LL-37 than its parent strain, whereas other mutants proved as susceptible (22). BP203 also was 16-fold less active than its enantiomer. While the advantages of RW-BP100 can be rationalized as described above, for LL-37, BP203, and BP214, the same task is more arduous without assuming the presence of specific binding targets other than the LPS. The existence of such structures has been hypothesized before in order to explain the higher anionic zeta potential of stationary-phase lpxA mutants than their parent strains (32). The lower susceptibility of Ab-176R suggests that these structures might be constitutive but lost, modified, or masked as a consequence of lpxD mutation.
Further insight was provided by the observed antagonism between colistin and BP peptides when pmr mutants were challenged (Table 2). Due to the cationic nature of all these compounds, the sequestration of BP peptides by colistin does not appear probable. However, previous studies have shown that, although unable to exert a bactericidal effect, colistin can still effectively bind to the outer membrane of resistant A. baumannii cells (28). A plausible explanation is therefore that colistin and BP peptides compete for binding to the modified LPS, but the former is not able to translate binding into bacterial killing. However, most BP peptides were heavily affected by the presence of colistin, confirming that the latter is also a high-affinity ligand for the modified LPS.
Several observations support this competitive model: (i) thanks to its stronger cationicity and/or nonspecific membrane activity, RW-BP100 appeared unperturbed by the presence of colistin; (ii) the presence of colistin at a high concentration raises the MIC of BP peptides for pmr mutants virtually to the same level as for the LPS-deficient lpx mutants, as in both cases LPS binding is not possible; (iii) this antagonism was generally not observed for lpx mutants. However, the activity of BP214 against Ab-167R again was an exception. This was the only case in which competition between colistin and BP peptides was observed when an lpx mutant was challenged. By definition, a competitive binding implies the presence of a defined target available in limited quantity. This is confirmed by the activity difference between BP214 and its enantiomer. Clearly, for an lpx mutant this target cannot be the LPS.
The competition between BP214 and colistin in the case of Ab-167R leads to additional interesting considerations. To our knowledge, it has never been shown before that colistin can bind other intrinsic membrane targets with high affinity. The prominence of the LPS is presumably the reason why this phenomenon has not been observed before.
From a structural perspective, the advantage of BP214 over colistin might stem from the higher flexibility of linear peptides compared to macrocycles (35). Being more rigid, colistin can bind the LPS with a lower entropic penalty and thus with higher affinity; however, this rigidity prevents it from binding to a modified partner without substantial differences. The more flexible BP214 cannot bind with as high an affinity but is able to modify its conformation easily, resulting in more robust antimicrobial activity. This hypothesis, however, remains to be demonstrated.
In conclusion, under optimal conditions, colistin's activity against susceptible A. baumannii strains remains unrivaled, but its performance drops dramatically in a variety of other relevant scenarios. Colistin-resistant strains are becoming increasingly common and virtually immune to the drug at viable concentrations. Due to its toxicity to kidneys, increasing the dosage entails a serious collateral risk for patients. The advantages of slightly less active but more reliable agents should thus be carefully taken into consideration.
BP214 is one such agent and arguably the most promising member of its family identified to date. Its dual mode of action, both specific and nonspecific, resulted in a potent and very robust antimicrobial activity. Being composed of d-amino acids only, BP214 can be expected to be proteolytically stable and potentially suitable for oral administration (36).
Overall, BP214 displayed attractive antimicrobial properties, and most importantly, its small size and chemical simplicity hold promise of ample improvement potential. These characteristics make BP214 an attractive lead for the development of novel antimicrobials targeting threatening Gram-negative pathogens, especially A. baumannii.
Supplementary Material
ACKNOWLEDGMENTS
We thank Jytte M. Andersen for excellent technical support. J. McConnell of the University of Seville, Spain, and Luis Rivas of the University of Madrid, Spain, are gratefully acknowledged for providing the clinical isolates mentioned in Table 2.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.01966-15.
REFERENCES
- 1.Perez F, Hujer AM, Hujer KM, Decker BK, Rather PN, Bonomo RA. 2007. Global challenge of multidrug-resistant Acinetobacter baumannii. Antimicrob Agents Chemother 51:3471–3484. doi: 10.1128/AAC.01464-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hawley JS, Murray CK, Jorgensen JH. 2008. Colistin heteroresistance in Acinetobacter and its association with previous colistin therapy. Antimicrob Agents Chemother 52:351–352. doi: 10.1128/AAC.00766-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li J, Rayner CR, Nation RL, Owen RJ, Spelman D, Tan KE, Liolios L. 2006. Heteroresistance to colistin in multidrug-resistant Acinetobacter baumannii. Antimicrob Agents Chemother 50:2946–2950. doi: 10.1128/AAC.00103-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abbott I, Cerqueira GM, Bhuiyan S, Peleg AY. 2013. Carbapenem resistance in Acinetobacter baumannii: laboratory challenges, mechanistic insights and therapeutic strategies. Expert Rev Anti Infect Ther 11:395–409. doi: 10.1586/eri.13.21. [DOI] [PubMed] [Google Scholar]
- 5.Vila J, Pachon J. 2012. Therapeutic options for Acinetobacter baumannii infections: an update. Expert Opin Pharmacother 13:2319–2336. doi: 10.1517/14656566.2012.729820. [DOI] [PubMed] [Google Scholar]
- 6.Taccone FS, Rodriguez-Villalobos H, De Backer D, De Moor V, Deviere J, Vincent JL, Jacobs F. 2006. Successful treatment of septic shock due to pan-resistant Acinetobacter baumannii using combined antimicrobial therapy including tigecycline. Eur J Clin Microbiol Infect Dis 25:257–260. doi: 10.1007/s10096-006-0123-1. [DOI] [PubMed] [Google Scholar]
- 7.Valencia R, Arroyo LA, Conde M, Aldana JM, Torres MJ, Fernandez-Cuenca F, Garnacho-Montero J, Cisneros JM, Ortiz C, Pachon J, Aznar J. 2009. Nosocomial outbreak of infection with pan-drug-resistant Acinetobacter baumannii in a tertiary care university hospital. Infect Control Hosp Epidemiol 30:257–263. doi: 10.1086/595977. [DOI] [PubMed] [Google Scholar]
- 8.López-Rojas R, Jiménez-Mejías ME, Lepe JA, Pachón J. 2011. Acinetobacter baumannii resistant to colistin alters its antibiotic resistance profile: a case report from Spain. J Infect Dis 204:1147–1148. doi: 10.1093/infdis/jir476. [DOI] [PubMed] [Google Scholar]
- 9.Vaara M. 2013. Novel derivatives of polymyxins. J Antimicrob Chemother 68:1213–1219. doi: 10.1093/jac/dkt039. [DOI] [PubMed] [Google Scholar]
- 10.Tsubery H, Ofek I, Cohen S, Fridkin M. 2000. The functional association of polymyxin B with bacterial lipopolysaccharide is stereospecific: studies on polymyxin B nonapeptide. Biochemistry 39:11837–11844. doi: 10.1021/bi000386q. [DOI] [PubMed] [Google Scholar]
- 11.Beceiro A, Moreno A, Fernández N, Vallejo JA, Aranda J, Adler B, Harper M, Boyce JD, Bou G. 2014. Biological cost of different mechanisms of colistin resistance and their impact on virulence in Acinetobacter baumannii. Antimicrob Agents Chemother 58:518–526. doi: 10.1128/AAC.01597-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beceiro A, Llobet E, Aranda J, Bengoechea JA, Doumith M, Hornsey M, Dhanji H, Chart H, Bou G, Livermore DM, Woodford N. 2011. Phosphoethanolamine modification of lipid A in colistin-resistant variants of Acinetobacter baumannii mediated by the pmrAB two-component regulatory system. Antimicrob Agents Chemother 55:3370–3379. doi: 10.1128/AAC.00079-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moffatt JH, Harper M, Harrison P, Hale JDF, Vinogradov E, Seemann T, Henry R, Crane B, St Michael F, Cox AD, Adler B, Nation RL, Li J, Boyce JD. 2010. Colistin resistance in Acinetobacter baumannii is mediated by complete loss of lipopolysaccharide production. Antimicrob Agents Chemother 54:4971–4977. doi: 10.1128/AAC.00834-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Velkov T, Thompson PE, Nation RL, Li J. 2010. Structure-activity relationships of polymyxin antibiotics. J Med Chem 53:1898–1916. doi: 10.1021/jm900999h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saugar JM, Alarcón T, López-Hernández S, López-Brea M, Andreu D, Rivas L. 2002. Activities of polymyxin B and cecropin A-melittin peptide CA(1-8)M(1-18) against a multiresistant strain of Acinetobacter baumannii. Antimicrob Agents Chemother 46:875–878. doi: 10.1128/AAC.46.3.875-878.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saugar JM, Rodríguez-Hernández MJ, de la Torre BG, Pachón-Ibañez ME, Fernández-Reyes M, Andreu D, Pachón J, Rivas L. 2006. Activity of cecropin A-melittin hybrid peptides against colistin-resistant clinical strains of Acinetobacter baumannii: molecular basis for the differential mechanisms of action. Antimicrob Agents Chemother 50:1251–1256. doi: 10.1128/AAC.50.4.1251-1256.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Badosa E, Ferre R, Planas M, Feliu L, Besalu E, Cabrefiga J, Bardaji E, Montesinos E. 2007. A library of linear undecapeptides with bactericidal activity against phytopathogenic bacteria. Peptides 28:2276–2285. doi: 10.1016/j.peptides.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 18.Güell I, Cabrefiga J, Badosa E, Ferre R, Talleda M, Bardají E, Planas M, Feliu L, Montesinos E. 2011. Improvement of the efficacy of linear undecapeptides against plant-pathogenic bacteria by incorporation of d-amino acids. Appl Environ Microbiol 77:2667–2675. doi: 10.1128/AEM.02759-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Torcato IM, Huang Y-H, Franquelim HG, Gaspar D, Craik DJ, Castanho MARB, Troeira Henriques S. 2013. Design and characterization of novel antimicrobial peptides, R-BP100 and RW-BP100, with activity against Gram-negative and Gram-positive bacteria. Biochim Biophys Acta 1828:944–955. doi: 10.1016/j.bbamem.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 20.Zuckermann RN, Kerr JM, Kent SBH, Moos WH. 1992. Efficient method for the preparation of peptoids [oligo(N-substituted glycines)] by submonomer solid-phase synthesis. J Am Chem Soc 114:10646–10647. doi: 10.1021/ja00052a076. [DOI] [Google Scholar]
- 21.Fernandez-Cuenca F, Pascual A, Ribera A, Vila J, Bou G, Cisneros JM, Rodriguez-Bano J, Pachon J, Martinez-Martinez L. 2004. Clonal diversity and antimicrobial susceptibility of Acinetobacter baumannii isolated in Spain. A nationwide multicenter study: GEIH-Ab project (2000). Enferm Infecc Microbiol Clin 22:267–271. [DOI] [PubMed] [Google Scholar]
- 22.García-Quintanilla M, Pulido MR, Moreno-Martínez P, Martín-Peña R, López-Rojas R, Pachón J, McConnell MJ. 2014. Activity of host antimicrobials against multidrug-resistant Acinetobacter baumannii acquiring colistin resistance through loss of lipopolysaccharide. Antimicrob Agents Chemother 58:2972–2975. doi: 10.1128/AAC.02642-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fernandez-Reyes M, Rodriguez-Falcon M, Chiva C, Pachon J, Andreu D, Rivas L. 2009. The cost of resistance to colistin in Acinetobacter baumannii: a proteomic perspective. Proteomics 9:1632–1645. doi: 10.1002/pmic.200800434. [DOI] [PubMed] [Google Scholar]
- 24.Lewis K. 2007. Persister cells, dormancy and infectious disease. Nat Rev Microbiol 5:48–56. doi: 10.1038/nrmicro1557. [DOI] [PubMed] [Google Scholar]
- 25.Hiramatsu K, Aritaka N, Hanaki H, Kawasaki S, Hosoda Y, Hori S, Fukuchi Y, Kobayashi I. 1997. Dissemination in Japanese hospitals of strains of Staphylococcus aureus heterogeneously resistant to vancomycin. Lancet 350:1670–1673. doi: 10.1016/S0140-6736(97)07324-8. [DOI] [PubMed] [Google Scholar]
- 26.Clements JM, Coignard F, Johnson I, Chandler S, Palan S, Waller A, Wijkmans J, Hunter MG. 2002. Antibacterial activities and characterization of novel inhibitors of LpxC. Antimicrob Agents Chemother 46:1793–1799. doi: 10.1128/AAC.46.6.1793-1799.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Evans BA, Hamouda A, Amyes SG. 2013. The rise of carbapenem-resistant Acinetobacter baumannii. Curr Pharm Des 19:223–238. doi: 10.2174/138161213804070285. [DOI] [PubMed] [Google Scholar]
- 28.Soon RL, Nation RL, Hartley PG, Larson I, Li J. 2009. Atomic force microscopy investigation of the morphology and topography of colistin-heteroresistant Acinetobacter baumannii strains as a function of growth phase and in response to colistin treatment. Antimicrob Agents Chemother 53:4979–4986. doi: 10.1128/AAC.00497-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jacobs AC, Sayood K, Olmsted SB, Blanchard CE, Hinrichs S, Russell D, Dunman PM. 2012. Characterization of the Acinetobacter baumannii growth phase-dependent and serum responsive transcriptomes. FEMS Immunol Med Microbiol 64:403–412. doi: 10.1111/j.1574-695X.2011.00926.x. [DOI] [PubMed] [Google Scholar]
- 30.Fiester SE, Actis LA. 2013. Stress responses in the opportunistic pathogen Acinetobacter baumannii. Future Microbiol 8:353–365. doi: 10.2217/fmb.12.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pescaretti MdlM, López FE, Morero RD, Delgado MA. 2011. The PmrA/PmrB regulatory system controls the expression of the wzzfepE gene involved in the O-antigen synthesis of Salmonella enterica serovar Typhimurium. Microbiology 157:2515–2521. doi: 10.1099/mic.0.050088-0. [DOI] [PubMed] [Google Scholar]
- 32.Soon RL, Nation RL, Cockram S, Moffatt JH, Harper M, Adler B, Boyce JD, Larson I, Li J. 2011. Different surface charge of colistin-susceptible and -resistant Acinetobacter baumannii cells measured with zeta potential as a function of growth phase and colistin treatment. J Antimicrob Chemother 66:126–133. doi: 10.1093/jac/dkq422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wade D, Boman A, Wahlin B, Drain CM, Andreu D, Boman HG, Merrifield RB. 1990. All-D amino acid-containing channel-forming antibiotic peptides. Proc Natl Acad Sci U S A 87:4761–4765. doi: 10.1073/pnas.87.12.4761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bulet P, Urge L, Ohresser S, Hetru C, Otvos L Jr. 1996. Enlarged scale chemical synthesis and range of activity of drosocin, an O-glycosylated antibacterial peptide of Drosophila. Eur J Biochem 238:64–69. doi: 10.1111/j.1432-1033.1996.0064q.x. [DOI] [PubMed] [Google Scholar]
- 35.Liu L, Fang Y, Wu J. 2013. Flexibility is a mechanical determinant of antimicrobial activity for amphipathic cationic α-helical antimicrobial peptides. Biochim Biophys Acta 1828:2479–2486. doi: 10.1016/j.bbamem.2013.06.017. [DOI] [PubMed] [Google Scholar]
- 36.Navab M, Anantharamaiah GM, Reddy ST, Hama S, Hough G, Grijalva VR, Yu N, Ansell BJ, Datta G, Garber DW, Fogelman AM. 2005. Apolipoprotein A-I mimetic peptides. Arterioscler Thromb Vasc Biol 25:1325–1331. doi: 10.1161/01.ATV.0000165694.39518.95. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.