Abstract
Isavuconazonium sulfate is a novel triazole prodrug that has been recently approved for the treatment of invasive aspergillosis by the FDA. The active moiety (isavuconazole) has a broad spectrum of activity against many pathogenic fungi. This study utilized a dynamic in vitro model of the human alveolus to describe the pharmacodynamics of isavuconazole against two wild-type and two previously defined azole-resistant isolates of Aspergillus fumigatus. A human-like concentration-time profile for isavuconazole was generated. MICs were determined using CLSI and EUCAST methodologies. Galactomannan was used as a measure of fungal burden. Target values for the area under the concentration-time curve (AUC)/MIC were calculated using a population pharmacokinetics-pharmacodynamics (PK-PD) mathematical model. Isolates with higher MICs required higher AUCs in order to achieve maximal suppression of galactomannan. The AUC/MIC targets necessary to achieve 90% probability of galactomannan suppression of <1 were 11.40 and 11.20 for EUCAST and CLSI, respectively.
INTRODUCTION
Invasive pulmonary aspergillosis (IPA) predominantly affects patients with significant immunological impairment (1). Aspergillus fumigatus is the most common Aspergillus species that is associated with human infection and is a leading cause of opportunistic infection in immunocompromised patients (2). The emergence of triazole resistance in Aspergillus fumigatus is of increasing clinical concern (3, 4). An understanding of the pharmacokinetics and pharmacodynamics (PK-PD) of antifungal agents against Aspergillus spp. is integral to the optimal use of antifungal agents to improve clinical outcomes (5). In particular, such analyses may provide decision support for the design of safe and effective antifungal regimens and provide decision support for setting in vitro susceptibility breakpoints.
Isavuconazonium sulfate (BAL8557) is a novel prodrug of the active triazole moiety, isavuconazole (BAL4815), that has recently been approved for the treatment of invasive aspergillosis and invasive mucormycosis in the United States (6). The water-soluble prodrug is cleaved by plasma esterases to form the active compound isavuconazole (BAL4815) and an inactive cleavage product (BAL8728). Isavuconazole exhibits potent in vitro antifungal activity against the majority of Aspergillus fumigatus isolates (7). Furthermore, the anti-Aspergillus activity of isavuconazole has been demonstrated in murine models of disseminated aspergillosis (8, 9). The pharmacokinetics of isavuconazole in mice is significantly different from that in humans. For example, the terminal half-life in mice is only ∼1 to 2 h, but it is ∼100 h in humans (6). These significant differences in the PK may pose a threat to the validity of any conclusions in attempts to bridge data from mice to humans.
Here, we use a previously described and validated in vitro dynamic model of the human alveolus, which enables human-like pharmacokinetics of isavuconazole to be simulated (10). This in vitro model was used to establish the pharmacokinetics and pharmacodynamics of isavuconazole against two wild-type and two mutant strains of Aspergillus fumigatus. The data obtained were then bridged to humans in order to determine pharmacodynamic targets for isavuconazole against Aspergillus fumigatus isolates.
MATERIALS AND METHODS
Aspergillus isolates and in vitro susceptibility testing.
Four isolates of Aspergillus fumigatus were studied (Table 1). A green fluorescent protein (GFP)-expressing Aspergillus fumigatus transformant and a previously characterized isolate, NIH 4215, were used as representative wild-type organisms (10). Previously described isolates expressing amino acid substitutions within Cyp51A (L98H and G138C) were also studied (10). Isolates were stored at −80°C using a Microbank preservation system (Pro-Lab). Isolates were subcultured onto potato dextrose agar (Sigma-Aldrich) and then incubated at 37°C for 5 days prior to each experiment. Conidial suspensions were prepared by flooding cultures with 20 ml of phosphate-buffered saline (PBS) (Invitrogen) while carefully disturbing the growth surface using a sterile swab. The resulting suspension was then filtered through sterile gauze. Washing was performed twice by centrifuging the filtrate for 10 min at 2,500 rpm and resuspending the pellet in 20 ml of PBS. Endothelial basal medium 2 (EBM-2; Lonza Biologics) was used for the preparation of the final inoculum. A conidial suspension of 1 × 104 to 3 × 104 was prepared using a hemocytometer and was confirmed by quantitative cultures on Sabouraud dextrose agar with chloramphenicol (SAB-C) (Oxoid).
TABLE 1.
MICs used in this study according to EUCAST and CLSI methodologies
| Isolate | Description | EUCAST MIC (mg/liter) |
CLSI MIC (mg/liter) |
||||
|---|---|---|---|---|---|---|---|
| Mode | Range | Geometric mean | Mode | Range | Geometric mean | ||
| GFP | Wild type | 1 | 0.5–2.0 | 1 | 1 | 0.5–1 | 0.81 |
| NIH 4215 | Wild type | 1 | 0.5–2.0 | 1 | 1 | 1–4 | 1.62 |
| F/16216 | L98Ha | 2 | 2–4 | 2.64 | 4 | 1–4 | 2.63 |
| F/11628 | G138Ca | 4 | 4–8 | 4.92 | 4 | 1–8 | 2.82 |
Cyp51A mutation.
MICs for isavuconazole against Aspergillus fumigatus isolates (Table 1) were determined in 10 independent experiments using both the European Committee for Antimicrobial Testing (EUCAST) and Clinical Laboratories Standards Institute (CLSI) methodologies. Concentrations for MIC testing ranged from 0.03 mg/liter to 8 mg/liter. The modal value, range, and geometric mean were determined. The geometric mean was used in the pharmacodynamic analyses for the determination of the values of the area under the concentration-time curve (AUC)/MIC.
Dynamic in vitro model of the human alveolus.
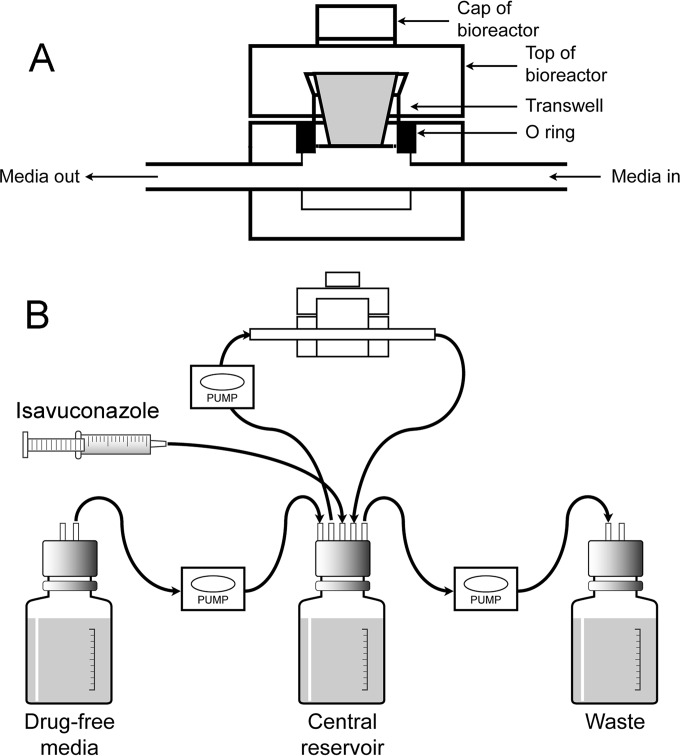
A previously defined cell culture model of the human alveolus using 12-well cell culture plate inserts (Greiner Bio-One) was used (10) (Fig. 1). The cellular bilayer consisted of human pulmonary arterial endothelial cells (HPAECs) (Lonza Biologics) and human alveolar epithelial cells (A549s) (LGC Standards). HPAECs were cultured using endothelial growth medium 2 (EGM-2). This was prepared in accordance with instructions provided by the manufacturer. EBM-2 was supplemented with 2% fetal bovine serum (FBS), ascorbic acid, heparin, hydrocortisone, human endothelial growth factor, vascular endothelial growth factor, human fibroblast factor B, and R-3-insulin-like growth factor 1. Amphotericin B and gentamicin, which are ordinarily components of EGM-2, were excluded due to their antimicrobial activity. A549 cells were cultured using EBM-2 containing 10% FBS. Once 70 to 80% confluence was achieved, cells were washed using Hanks balanced salt solution (HBSS) (Sigma-Aldrich) and harvested with 6 ml of 0.25% trypsin-ethylenediaminetetraacetic solution (Sigma-Aldrich). Cells were then centrifuged for 5 min at 2,000 rpm and resuspended in the appropriate medium to achieve final densities of 1 × 106 and 5.5 × 105 for HPAECs and A549s, respectively. ThinCert cell culture inserts for 12-well plates (Greiner Bio-One) were used for the construction of the cellular bilayer. Cell culture inserts were aseptically inverted and placed in a sterile plate. For the construction of the endothelial cell layer, 400 μl of HPAEC suspension was pipetted on the underside of the membrane. Inserts were then incubated (at 37°C in 5% CO2) for 2 h to ensure cellular adhesion to the membrane. Inserts were then transferred to a 12-well plate containing 1.5 ml of EGM-2, and 400 μl of EBM-2 containing 10% FBS was added to the top compartment (alveolar). The cell culture plate was then incubated (at 37°C in 5% CO2) for 24 h. After 24 h, medium in the alveolar compartment was removed, and inserts were transferred to a new 12-well plate containing fresh EGM-2. At this time, 400 μl of A549 cell suspension was added to the top compartment of each insert and incubated. After 2 h, medium in the alveolar compartment was removed to create an air-liquid interface. EGM-2 in the bottom compartment was replaced every 48 h, and any medium present in the alveolar compartment was also removed. Inserts were used for experiments 5 days after construction. At the end of each 48-h experiment, the integrity of the bilayer was evaluated. Four hundred microliters of 1% dextran blue was added to the top compartment. Inserts were then transferred to a 12-well plate containing 1.5 ml of warmed PBS (endothelial compartment) and incubated for 1 h. The concentration of dextran blue in the endothelial compartment was then determined spectrophotometrically (620 nm).
FIG 1.
The dynamic model of the human alveolus. Panel A shows the bioreactor that houses the cellular bilayer that represents the human alveolar-capillary barrier. Panel B depicts the circuit that is used to generate first-order decay in isavuconazole concentrations. The rate of the pumps determines the rate of decline of isavuconazole in the central compartment. The pumps can be set to mimic a human-like plasma concentration-time profile. (Modified from reference 10.)
Bioreactors and construction of the in vitro dynamic model.
Cell culture inserts were housed in custom-designed stainless steel bioreactors for the duration of the experiment. Bioreactors were specifically engineered to house cell culture inserts while allowing medium to flow past the endothelial layer of cells. Each bioreactor was connected to the circuit using Marprene thermoplastic elastomer tubing (Watson Marlow). Silastic, 1.6-mm bore tubing (Dow Corning) was used to construct the remainder of the circuit. All connections were made using polypropylene-barbed luer adapters (West Group). A central reservoir containing 200-ml of medium warmed to 37°C was connected to the circuit using 1.5-mm bore polytetrafluoroethylene (PTFE) semirigid tubing (Diba Labware) fitted into Omni-Fit Q-series bottle caps (Diba Labware). All central reservoirs contained magnetic stirring bars and were placed on a stirring plate to create a vortex, thereby ensuring continuous mixing of medium components and drug. Duran bottles containing fresh medium and empty bottles for the removal of waste were also connected to the circuit in a similar manner. A three-way tap (Infusion Concepts, Ltd.) connected directly to the central reservoir allowed the administration of drug. An additional three-way tap positioned after the bioreactors was used for the collection of samples. Two, 205-U multichannel cassette peristaltic pumps fitted with 1.52-mm bore Marprene manifold tubing (Watson-Marlow) were used, ensuring flow of medium within the system and allowing multiple circuits to be run simultaneously.
All components of the model were sterilized before use, and circuits were assembled in a class II safety cabinet. Once assembled, circuits were transferred to a 37°C incubator for the remainder of the experiment. Dulbecco's modified Eagle medium (DMEM) containing 4,500 mg/liter d-glucose, l-glutamine, and HEPES buffer (Invitrogen) was supplemented with 2% FBS (Lonza Biologics) and penicillin-streptomycin solution (Sigma-Aldrich) to give a final concentration of 100 U/ml penicillin and 0.1 mg/ml streptomycin. This medium was used for all experiments. In order to prime the system, the DMEM was further supplemented with 33% FBS and penicillin-streptomycin solution (Sigma-Aldrich) to the same final concentration as used previously and warmed to 37°C. The circuits were then primed for 2 h at a pump speed of 5 rpm with this medium. After a priming step, the Duran bottles were replaced with those containing DMEM supplemented with 2% FBS and penicillin-streptomycin solution (100 U/ml penicillin and 0.1 mg/ml streptomycin). The bioreactors were then connected to the circuit. The pump responsible for medium flow through the bioreactor was run at 1.9 rpm, producing a flow rate of around 10 ml/h. The pump connected to the fresh medium and the waste Duran bottles was run at 0.5 rpm, generating a flow rate of around 2.5 ml/h.
Inoculation of the dynamic model.
Cell culture inserts were transferred to a 12-well plate containing 1.5 ml of EBM-2 supplemented with 2% FBS per well. A conidial suspension of 1 × 104 to 3 × 104 was prepared as previously described and incubated at 37°C for 20 min. Four hundred microliters of this suspension was then pipetted into the top compartment of each cell culture insert and incubated at 37°C in 5% CO2 for 6 h.
Treatment with isavuconazole.
Isavuconazole pure powder (BAL4815) was dissolved in methanol. Serial dilutions were prepared from a known stock concentration (1,500 mg/liter) in methanol in order to achieve the required concentrations for each dynamic system. The drug was administered directly into the central compartment as a 100-μl bolus followed by 1 ml of DMEM supplemented with 2% FBS and penicillin-streptomycin solution (final concentration of 100 U/ml penicillin and 0.1 mg/ml streptomycin).
Pharmacokinetics and pharmacodynamics of isavuconazole.
The PK and PD of isavuconazole against each isolate of Aspergillus fumigatus were determined in four individual circuits. Each circuit was treated with a different dosage of isavuconazole determined on the basis of preliminary dose-finding experiments. A fifth untreated circuit was used as a control. Isavuconazole was administered 6 h postinoculation as a bolus. Isavuconazole dosages of 0 mg, 0.1 mg, 0.3 mg, 0.5 mg, and 1.5 mg were administered to the central compartment of each corresponding circuit. These dosages were used for all strains. Samples for both PK and PD were taken every 6 h between 6 to 54 h postinoculation. After sampling, medium taken out of the system during sampling was replaced with an equal volume of complete DMEM.
HPLC.
Isavuconazole concentrations in medium were measured using high-performance liquid chromatography (HPLC) with a Prominence system (Shimadzu). The isavuconazole method used a Kinetex 2.6-μm C18 column (75 by 4.6 mm; Phenomenex) and a 40-μl injection volume. A standard curve encompassing 0.005 to 20 mg/liter was constructed from stock solutions of 1,000 mg/liter isavuconazole in dimethyl sulfoxide (DMSO) further diluted in methanol (Fisher Scientific). The internal standard was itraconazole (Sigma-Aldrich). The mobile phase comprised 60% of 0.1% trifluoroacetic acid (TFA) in water and 40% acetonitrile with 0.1% (vol/vol) TFA, with a gradient profile changing to 40% and 60%, respectively, over 4 min and an overall run time of 6 min and flow rate of 1 ml/min. Isavuconazole was detected using fluorescence with an excitation of 250 nm and emission of 380 nm. The retention times of isavuconazole and itraconazole were 3.2 and 4.5 min, respectively. The coefficient of variation (CV; percent) was <6.4% over the concentration range of 0.005 to 20 mg/liter. The limit of detection and quantification was 0.005 mg/liter. The intra- and interday variation was <6.6%.
Galactomannan.
Galactomannan was used as a quantitative biomarker in order to evaluate the antifungal efficacy of isavuconazole. Galactomannan levels were measured using a double-sandwich enzyme linked immunosorbent assay (Platelia Aspergillus; Bio-Rad). The protocol provided by the manufacturer was followed with a single modification. Due to the relatively small volume of sample that can be obtained using this model, 25 μl of sample treatment solution was added to 75 μl of sample as opposed to the recommended volume of 100 μl of sample treatment solution to 300 μl of sample.
Mathematical modeling.
A population methodology was used to fit the mathematical model to the data from each strain. The population pharmacokinetic program nonparametric adaptive grid embedded within Pmetrics was used for all modeling (11). The structural mathematical model consisted of the following two inhomogeneous ordinary differential equations:
| (1) |
| (2a) |
| (2b) |
where X1 is the amount of isavuconazole (in milligrams), B(1) is the bolus input of isavuconazole, SCL is the clearance of isavuconazole from the circuit (liters per hour), Vc is the volume of the circuit (liters), N is the galactomannan concentration, Kgmax is the maximal rate of growth (GMI per h), POPMAX is the theoretical maximal density within the circuit (GMI), Hg is the slope function for the suppression of growth, and C50g is the concentration of isavuconazole in the circuit where there is half-maximal suppression of growth (milligrams per liter).
Equation 1 describes the rate of change of isavuconazole concentrations in the circuit. Equation 2 describes the rate of change of galactomannan in the circuit and contains terms that describe fungal growth in the absence of isavuconazole (equation 2a) and the isavuconazole-induced suppression of growth (equation 2b).
The model was fitted to the data set obtained from each strain (i.e., the data from each of five circuits consisting of a single control and four drug-treated circuits). In this process, each circuit was treated as an individual instance. The Bayesian posterior estimates for each of the parameters in the model were then used to define the concentration-time profile of isavuconazole and the resultant effect on galactomannan concentrations. These parameter estimates were used to estimate the AUC that developed in each circuit and, consequently, the AUC/MIC ratio.
Pharmacodynamic target associated with maximal antifungal activity.
The relationship between the AUC (in this case, the total AUC that developed throughout the experimental period) and the final galactomannan concentration was explored. Galactomannan readings of <1 and <0.5 were used as endpoints, as previously described in this same model for voriconazole (10). Logistic regression was used to link the AUC/MIC value for each strain and the galactomannan concentration quantified as a dichotomous outcome (i.e., success was defined as a galactomannan reading of <0.5 or 1.0, depending on the specific analysis being performed). The statistical program SYSTAT, version 11, was used for the regression. Consistent with a previous study, the drug exposure (AUC/MIC) associated with a 90% probability of a suppressed galactomannan concentration was defined as the drug exposure target associated with maximal antifungal activity (10).
RESULTS
MICs.
MICs (mode, range, and geometric means) for isavuconazole against isolates of Aspergillus fumigatus using EUCAST and CLSI methodologies are summarized in Table 1. The MIC was higher in the isolates with amino acid substitutions in the Cyp51A protein using both methodologies.
Pharmacokinetics and pharmacodynamics of isavuconazole in the dynamic model of the human alveolus.
Human-like plasma concentration-time profiles for isavuconazole were established in the circuit of the in vitro model (Fig. 2 to 5). There was no translocation of 1% dextran blue after 48 h within the circuit, demonstrating the integrity of the cellular bilayer throughout the experimental period.
FIG 2.
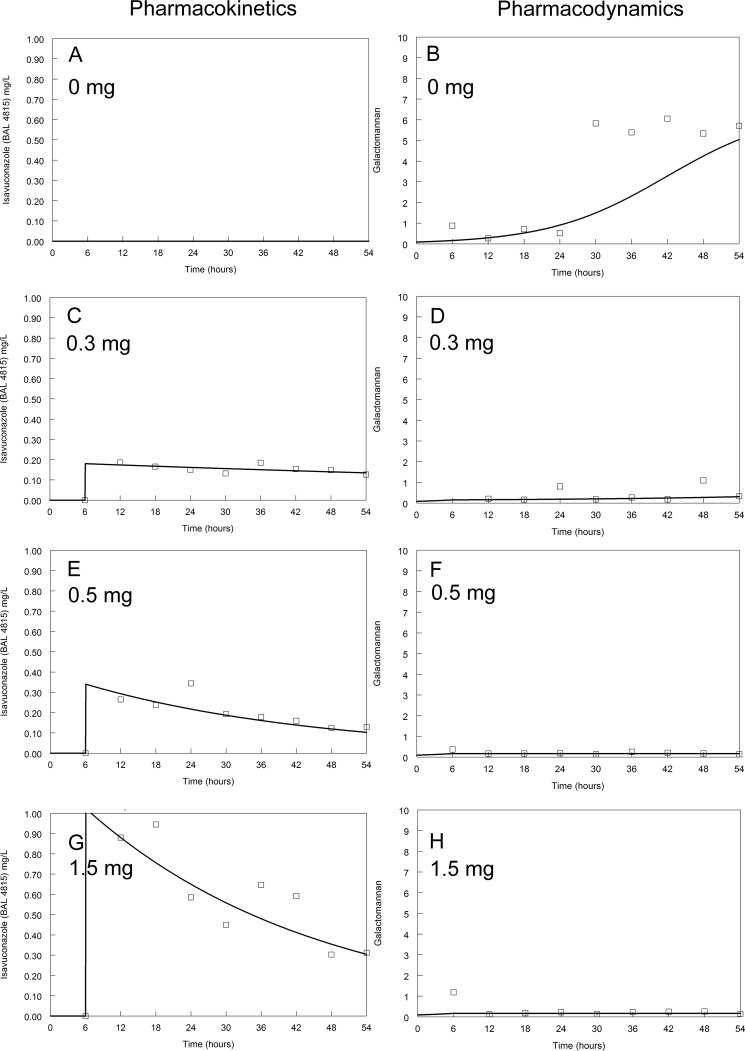
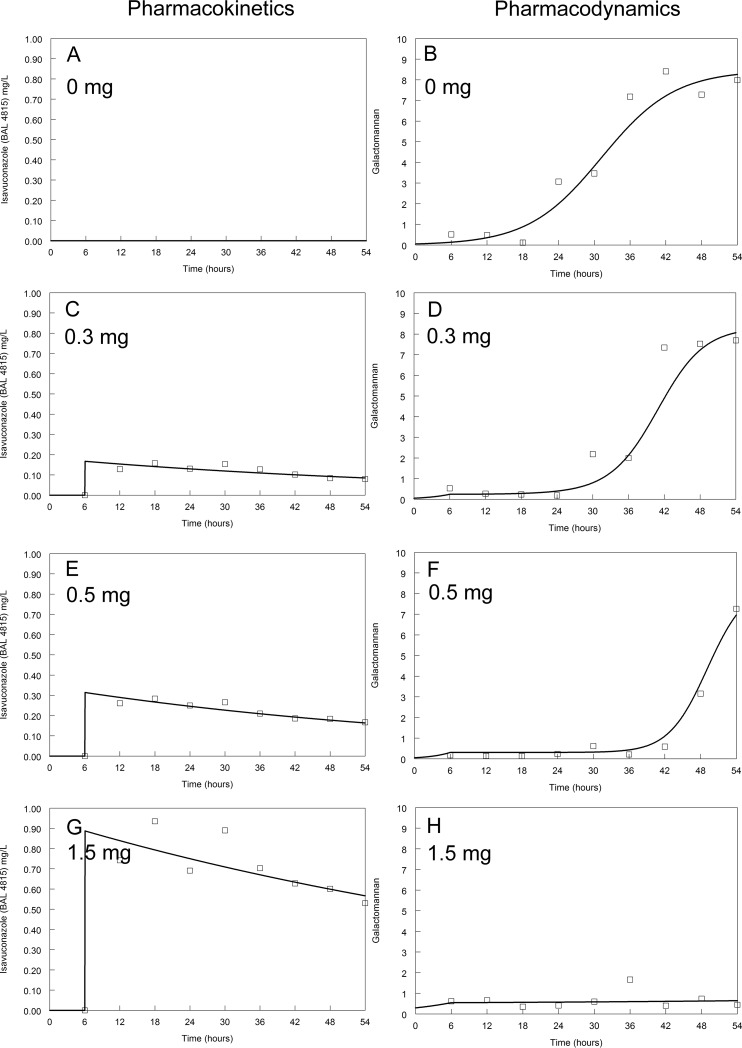
Pharmacokinetics and pharmacodynamics of isavuconazole against the GFP transformant. Dosages are indicated on the figure. The modal MIC of isavuconazole is 1 mg/liter. The solid line is the fit of the mathematical model and the open squares are the raw data.
FIG 5.
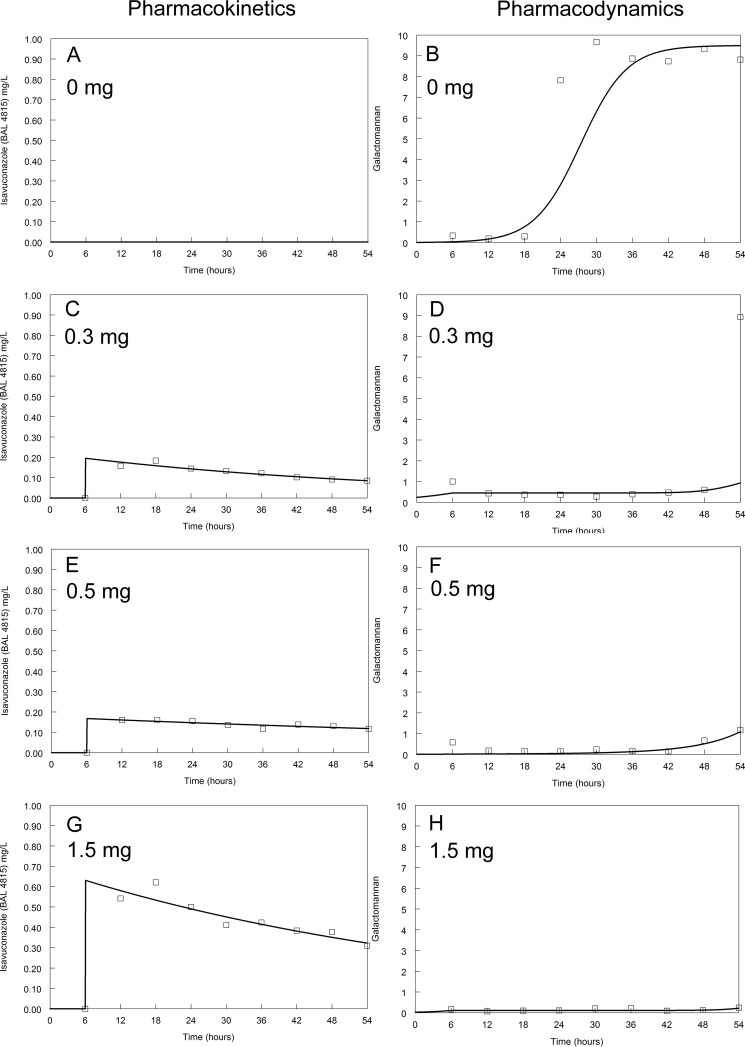
Pharmacokinetics and pharmacodynamics of isavuconazole against F/11628. Dosages are indicated on the figure. The modal MIC of isavuconazole is 8 mg/liter. The solid line is the fit of the mathematical model and the open squares are the raw data.
FIG 3.
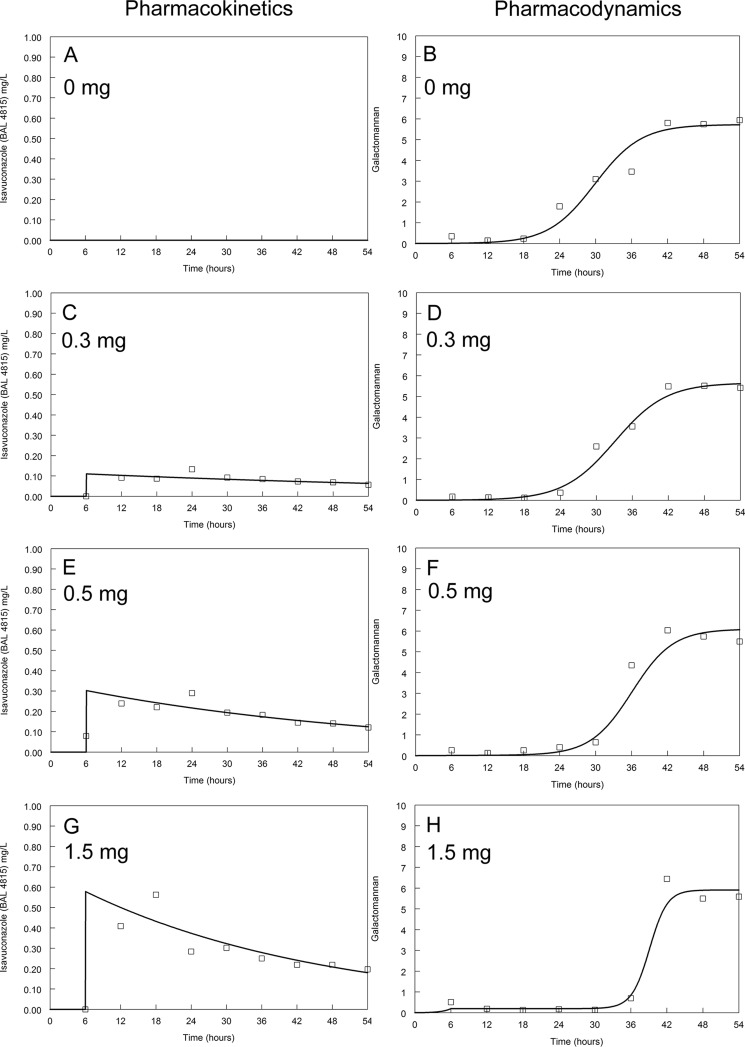
Pharmacokinetics and pharmacodynamics of isavuconazole against NIH 4215. Dosages are indicated on the figure. The modal MIC of isavuconazole is 1 mg/liter. The solid line is the fit of the mathematical model and the open squares are the raw data.
The kinetics of galactomannan release into the circulating medium in untreated (control) circuits differed between isolates. Concentrations of galactomannan in all untreated circuits started to increase at around 18 to 24 h (Fig. 2 to 5). A maximum galactomannan concentration was observed at approximately 24 h for the GFP transformant, F/11628, and NIH 4215. The strain F/16216 appeared to grow more slowly, and maximal galactomannan concentrations were reached at around 36 h postinoculation (Fig. 4). The maximum galactomannan concentrations were similar between strains, ranging from an index of approximately 6 to 9.
FIG 4.
Pharmacokinetics and pharmacodynamics of isavuconazole against F/16216. Dosages are indicated on the figure. The modal MIC of isavuconazole is 4 mg/liter. The solid line is the fit of the mathematical model and the open squares are the raw data.
There were clear differences in exposure-response relationships for isavuconazole against each of the isolates. A trough concentration of approximately 0.2 to 0.5 mg/liter resulted in near-maximal suppression of galactomannan release for the wild-type GFP transformant. Similar findings were apparent for NIH 4215. In contrast, the exposure-response relationships for the two non-wild-type isolates were different. Only the highest concentrations of isavuconazole against F/16216 resulted in suppression of galactomannan. None of the isavuconazole concentrations were able to suppress the release of galactomannan for strain F/11628. Thus, the MIC (and genotype) appeared to account for at least some differences in the observed drug exposure-response relationships.
Mathematical modeling.
The fit of the mathematical model to the data was acceptable. The fit of the Bayesian posterior estimates for each circuit is shown in each of the figures (i.e., the solid continuous lines).
Pharmacodynamic target associated with maximal antifungal activity.
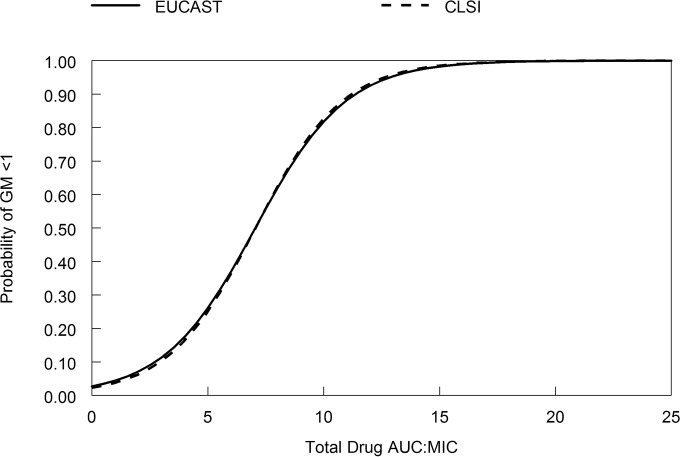
The logistic regression model is shown in Fig. 6. The regression was statistically significant (intercept, −3.578; P = 0.017, coefficient [i.e., AUC/MIC], 0.508 [P = 0.038]; odds ratio, 1.66 [95% confidence interval, 1.03 to 2.68]), with a McFadden's rho-squared value of 0.538. An AUC/MIC target of 11.40 resulted in a 90% probability of galactomannan suppression of <1 using MICs from the EUCAST methodology. For the CLSI methodology an AUC/MIC target of 11.20 is necessary to achieve 90% probability of galactomannan suppression of <1. The results were identical if an endpoint of 0.5 was used to define a successful outcome.
FIG 6.
The relationship between the AUC/MIC and the probability of galactomannan (GM) suppression. AUC/MIC values of 7.03 and 11.40 are associated with 50 and 90% probabilities of galactomannan suppression, respectively, using the EUCAST methodology. AUC/MIC values of 7.06 and 11.20 are associated with 50 and 90% probabilities of galactomannan suppression, respectively, using the CLSI methodology.
DISCUSSION
Invasive pulmonary aspergillosis is associated with relatively high morbidity and mortality (2). Pathogenic isolates of Aspergillus fumigatus that are resistant to existing antifungal drugs are increasingly seen (12). A pipeline of novel antifungal agents is required to improve clinical outcomes and meet evolving clinical requirements (13). Isavuconazole is a novel triazole antifungal agent that is administered as the water-soluble prodrug isavuconazonium sulfate (BAL8557) (6). The efficacy of isavuconazole has recently been established in a phase III clinical trial (SECURE), which compared the clinical response and mortality of patients with invasive aspergillosis receiving isavuconazole or voriconazole. An understanding of the pharmacokinetic and pharmacodynamic relationships of isavuconazole is central to the optimal use of this agent. Here, we describe the use of an in vitro dynamic model of early invasive pulmonary aspergillosis to define the PK-PD relationships of isavuconazole against clinical isolates of Aspergillus fumigatus with a range of triazole resistance mechanisms.
In the current study, there are clear pharmacodynamic differences between isolates of Aspergillus fumigatus that are treated with isavuconazole. These differences can largely be understood and quantified in terms of the MIC. Higher MIC values for isavuconazole are observed against isolates of Aspergillus fumigatus carrying Cyp51A amino acid substitutions L98H and G138C (7). Relatively more isavuconazole (quantified in terms of the AUC) is required to “treat” these strains in this in vitro dynamic model, suggesting that both the MIC and the genotype account for a considerable portion of system variance. This observation further suggests that the MIC may be a reliable in vitro measure to guide antifungal therapy in patients in the setting of invasive aspergillosis. A standardized testing methodology of isavuconazole has been developed by CLSI and EUCAST, and epidemiological surveys detailing MICs in medically important opportunistic fungal pathogens have been published (14, 15). The EUCAST epidemiological cutoff (ECOFF) of isavuconazole (i.e., the MIC value that defines the upper limit of the wild-type distribution) is 2 mg/liter for Aspergillus fumigatus, Aspergillus flavus, and Aspergillus terreus and 4 mg/liter for Aspergillus niger (15). According to the CLSI methodology, the ECOFF is 1 mg/liter for Aspergillus fumigatus, Aspergillus flavus, and Aspergillus terreus. The CLSI ECOFF is 4 mg/liter for Aspergillus niger (14). Preliminary results from the phase III clinical trial (SECURE) suggest that the majority of strains that belong to the wild-type distribution are likely to be treatable (using EUCAST terminology, wild-type Aspergillus fumigatus is likely to be a “good target” for isavuconazole). There is still some uncertainty as to the clinical breakpoint for isavuconazole using either the EUCAST or CLSI methodology.
In this model of early invasive pulmonary aspergillosis, total drug AUC/MIC targets of 11.40 and 11.20 using the EUCAST and CLSI methodologies, respectively, are associated with near-maximal antifungal activity. The pharmacodynamic target from our analyses is lower than that in two recently published studies (8, 9). In the first of these studies, Lepak et al. (8) used a profoundly neutropenic murine model of invasive pulmonary aspergillosis and reported that a much higher median AUC/MIC target of approximately 500 is associated with a static antifungal effect (i.e., prevents net growth or kill as assessed using a quantitative PCR [qPCR] endpoint). This pharmacodynamic target increases almost 2-fold to ∼1,000 if orders of logarithmic killing are used as the pharmacodynamic endpoint (9). A second study by Seyedmousavi et al. (9) suggests that a much lower pharmacodynamic AUC/MIC target of 24.73 is reasonable. This target is closer to the value in our study and was estimated using a nonneutropenic model of disseminated aspergillosis. The differences in these estimates of the pharmacodynamic target for isavuconazole are probably related to the degree of severity of each model, the stage of disease that is being mimicked, and the background immunosuppression. For example, the neutropenic model of IPA used by Lepak et al. (8) is a severe model and an extremely rigorous test of antifungal efficacy. In this murine model there is rapid progression of disease, which is uniformly fatal without prompt therapeutic intervention. In contrast, the nonneutropenic model of disseminated aspergillosis has a much longer time course, and estimates of drug exposure effect relationships are necessarily confounded by additional antifungal activity of immunological effectors. The in vitro dynamic model in this study is a model of early invasive disease before there is significant tissue damage. Drug exposure-response relationships are estimated before the onset of hemorrhagic infarction and pyogranulomatous inflammation that are characteristic of invasive aspergillosis in neutropenic and nonneutropenic hosts, respectively (1). The three experimental models represent the spectrum of invasive aspergillosis that is observed in the clinic, which ranges from very early disease right through to established disease in profoundly immunosuppressed hosts. The estimates for pharmacodynamic targets required for therapeutic efficacy from the three models vary accordingly. The differences in pharmacodynamic targets emphasize the potential folly of selecting a “one size fits all” approach for establishing breakpoints.
An understanding of the PK-PD of isavuconazole and the relevant pharmacodynamic targets is important for decision support in setting in vitro susceptibility breakpoints (5). An AUC/MIC target from the neutropenic mouse model of IPA (i.e., 500 or 1,000) may be difficult to achieve for the majority of wild-type isolates, given what is currently known about the population pharmacokinetics of isavuconazole in patients and the effective regimens used in clinical studies. A loading dose of 200 mg every 8 h (q8h) on days 1 and 2 followed by 200 mg/day produces a mean AUC of approximately 100 mg · h/liter (16–18). An AUC/MIC target of 500 would therefore likely be achievable for strains with an MIC of 0.125 mg/liter (i.e., 100 divided by 0.125 = 800, which is greater than a nominal target of 500) but becomes progressively less likely with strains with MICs of >0.125 mg/liter. Using an AUC/MIC target of 500 would therefore mean that the breakpoint would bisect the wild-type population. This is not consistent with observations from the randomized phase III clinical trial where there was not a tendency for clinical failure with strains with elevated MICs. In contrast, using a lower pharmacodynamic target of 11 to 25 would enable the wild-type population to be adequately covered in the majority of patients. The most profoundly immunosuppressed patients with a high burden of infection may require higher drug exposure for a successful outcome, as suggested by Lepak et al. (8). Whether this is ultimately the case will require further experience and clinical studies with isavuconazole.
In summary, therefore, the MIC of isavuconazole for Aspergillus fumigatus is an important determinant of the exposure-response relationships. The preclinical PK-PD data and analyses from three different laboratories are somewhat discordant, which likely reflects significant differences in the experimental models that are used to characterize these relationships. The results from this study suggest that the majority of wild-type isolates can be treated with the currently used regimens, at least for early invasive pulmonary aspergillosis. A definitive breakpoint analysis will require the development of a population PK model, which is currently in progress. The population PK model and PK-PD bridging studies will then help define the highest MIC that can be treated with the current isavuconazole regimen and indicate the potential benefit of dosage escalation to treat strains that may otherwise be classified as having intermediate susceptibility.
ACKNOWLEDGMENTS
William Hope is supported by a Clinician Scientist Award from the National Institute of Health Research (NIHR) in the United Kingdom (CS_08_08).
William Hope has acted as a consultant and/or received research grant support from Pfizer, Inc., Astellas Pharma, Gilead Sciences, F2G, and Pulmocide.
Funding Statement
This study was supported by Astellas Pharma.
REFERENCES
- 1.Hope WW, Walsh TJ, Denning DW. 2005. The invasive and saprophytic syndromes due to Aspergillus spp. Med Mycol 43(Suppl 1):S207–S238. [DOI] [PubMed] [Google Scholar]
- 2.Nivoix Y, Velten M, Letscher-Bru V, Moghaddam A, Natarajan-Ame S, Fohrer C, Lioure B, Bilger K, Lutun P, Marcellin L, Launoy A, Freys G, Bergerat JP, Herbrecht R. 2008. Factors associated with overall and attributable mortality in invasive aspergillosis. Clin Infect Dis 47:1176–1184. doi: 10.1086/592255. [DOI] [PubMed] [Google Scholar]
- 3.Vermeulen E, Lagrou K, Verweij PE. 2013. Azole resistance in Aspergillus fumigatus: a growing public health concern. Curr Opin Infect Dis 26:493–500. doi: 10.1097/QCO.0000000000000005. [DOI] [PubMed] [Google Scholar]
- 4.Verweij PE, Ananda-Rajah M, Andes D, Arendrup MC, Brüggemann RJ, Chowdhary A, Cornely OA, Denning DW, Groll AH, Izumikawa K, Kullberg BJ, Lagrou K, Maertens J, Meis JF, Newton P, Page I, Seyedmousavi S, Sheppard DC, Viscoli C, Warris A, Donnelly JP. 2015. International expert opinion on the management of infection caused by azole-resistant Aspergillus fumigatus. Drug Resist Updat 21–22:30–40. doi: 10.1016/j.drup.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 5.Hope WW, Drusano GL. 2009. Antifungal pharmacokinetics and pharmacodynamics: bridging from the bench to bedside. Clin Microbiol Infect 15:602–612. doi: 10.1111/j.1469-0691.2009.02913.x. [DOI] [PubMed] [Google Scholar]
- 6.Livermore J, Hope W. 2012. Evaluation of the pharmacokinetics and clinical utility of isavuconazole for treatment of invasive fungal infections. Expert Opin Drug Metab Toxicol 8:759–765. doi: 10.1517/17425255.2012.683859. [DOI] [PubMed] [Google Scholar]
- 7.Gregson L, Goodwin J, Johnson A, McEntee L, Moore CB, Richardson M, Hope WW, Howard SJ. 2013. In vitro susceptibility of Aspergillus fumigatus to isavuconazole: correlation with itraconazole, voriconazole, and posaconazole. Antimicrob Agents Chemother 57:5778–5780. doi: 10.1128/AAC.01141-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lepak AJ, Marchillo K, Vanhecker J, Andes DR. 2013. Isavuconazole (BAL4815) pharmacodynamic target determination in an in vivo murine model of invasive pulmonary aspergillosis against wild-type and cyp51 mutant isolates of Aspergillus fumigatus. Antimicrob Agents Chemother 57:6284–6289. doi: 10.1128/AAC.01355-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seyedmousavi S, Brüggemann RJM, Meis JF, Melchers WJG, Verweij PE, Mouton JW. 2015. Pharmacodynamics of isavuconazole in an Aspergillus fumigatus mouse infection model. Antimicrob Agents Chemother 59:2855–2866. doi: 10.1128/AAC.04907-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jeans AR, Howard SJ, Al-Nakeeb Z, Goodwin J, Gregson L, Majithiya JB, Lass-Florl C, Cuenca-Estrella M, Arendrup MC, Warn PA, Hope WW. 2012. Pharmacodynamics of voriconazole in a dynamic in vitro model of invasive pulmonary aspergillosis: implications for in vitro susceptibility breakpoints. J Infect Dis 206:442–452. doi: 10.1093/infdis/jis372. [DOI] [PubMed] [Google Scholar]
- 11.Neely MN, van Guilder MG, Yamada WM, Schumitzky A, Jelliffe RW. 2012. Accurate detection of outliers and subpopulations with Pmetrics, a nonparametric and parametric pharmacometric modeling and simulation package for R. Ther Drug Monit 34:467–476. doi: 10.1097/FTD.0b013e31825c4ba6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van der Linden JWM, Arendrup MC, Warris A, Lagrou K, Pelloux H, Hauser PM, Chryssanthou E, Mellado E, Kidd SE, Tortorano AM, Dannaoui E, Gaustad P, Baddley JW, Uekötter A, Lass-Flörl C, Klimko N, Moore CB, Denning DW, Pasqualotto AC, Kibbler C, Arikan-Akdaqli S, Andes D, Meletiadis J, Naumiuk L, Nucci M, Melchers WJ, Verweij PE. 2015. Prospective multicenter international surveillance of azole resistance in Aspergillus fumigatus. Emerg Infect Dis 21:1041–1044. doi: 10.3201/eid2106.140717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Denning DW, Hope WW. 2010. Therapy for fungal diseases: opportunities and priorities. Trends Microbiol 18:195–204. doi: 10.1016/j.tim.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 14.Espinel-Ingroff A, Chowdhary A, Gonzalez GM, Lass-Flörl C, Martin-Mazuelos E, Meis J, Peláez T, Pfaller MA, Turnidge J. 2013. Multicenter study of isavuconazole MIC distributions and epidemiological cutoff Values for Aspergillus spp. for the CLSI M38-A2 broth microdilution method. Antimicrob Agents Chemother 57:3823–3828. doi: 10.1128/AAC.00636-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Howard SJ, Lass-Flörl C, Cuenca-Estrella M, Gomez-Lopez A, Arendrup MC. 2013. Determination of isavuconazole susceptibility of Aspergillus and Candida species by the EUCAST method. Antimicrob Agents Chemother 57:5426–5431. doi: 10.1128/AAC.01111-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cornely OA, Böhme A, Schmitt-Hoffmann A, Ullmann AJ. 2015. Safety and pharmacokinetics of isavuconazole as antifungal prophylaxis in acute myeloid leukemia patients with neutropenia: results of a phase 2, dose escalation study. Antimicrob Agents Chemother 59:2078–2085. doi: 10.1128/AAC.04569-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schmitt-Hoffmann A, Roos B, Heep M, Schleimer M, Weidekamm E, Brown T, Roehrle M, Beglinger C. 2006. Single-ascending-dose pharmacokinetics and safety of the novel broad-spectrum antifungal triazole BAL4815 after intravenous infusions (50, 100, and 200 milligrams) and oral administrations (100, 200, and 400 milligrams) of its prodrug, BAL8557, in healthy volunteers. Antimicrob Agents Chemother 50:279–285. doi: 10.1128/AAC.50.1.279-285.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schmitt-Hoffmann A, Roos B, Maares J, Heep M, Spickerman J, Weidekamm E, Brown T, Roehrle M. 2006. Multiple-dose pharmacokinetics and safety of the new antifungal triazole BAL4815 after intravenous infusion and oral administration of its prodrug, BAL8557, in healthy volunteers. Antimicrob Agents Chemother 50:286–293. doi: 10.1128/AAC.50.1.286-293.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]