Abstract
New regimens based on two or more novel agents are sought to shorten or simplify treatment of tuberculosis (TB). Pretomanid (PMD) is a nitroimidazole in phase 3 trials that has significant bactericidal activity alone and in combination with bedaquiline (BDQ) and/or pyrazinamide (PZA). We previously showed that the novel combination of BDQ+PMD plus the oxazolidinone sutezolid (SZD) had sterilizing activity superior to that of the first-line regimen in a murine model of TB. The present experiments compared the activity of different oxazolidinones in combination with BDQ+PMD with or without PZA in the same model. The 3-drug regimen of BDQ+PMD plus linezolid (LZD) had sterilizing activity approaching that of BDQ+PMD+SZD and superior to that of the first-line regimen. The addition of PZA further enhanced activity. Reducing the duration of LZD to 1 month did not significantly affect the activity of the regimen. Halving the LZD dose or replacing LZD with RWJ-416457 modestly reduced activity over the first month but not after 2 months. AZD5847 and tedizolid also increased the bactericidal activity of BDQ+PMD, but they were less effective than the other oxazolidinones. These results provide optimism for safe, short-course oral regimens for drug-resistant TB that may also be superior to the current first-line regimen for drug-susceptible TB.
INTRODUCTION
Approximately half a million new cases of multidrug-resistant (MDR) tuberculosis (TB) occur annually (1). Current recommendations call for up to 2 years of treatment with second-line drugs that are poorly tolerated, toxic, more difficult to administer, and less effective than the 6-month, so-called short-course regimen for drug-susceptible TB. Regimens containing at least 6 drugs, including newer fluoroquinolones in high doses, an injectable agent, clofazimine, pyrazinamide (PZA), and high-dose isoniazid (INH), have shown potential as effective 9-month regimens in MDR-TB cases with minimal bacillary resistance to second-line drugs (2–4). However, these regimens remain quite cumbersome to administer and are not expected to be as effective in the setting of resistance to fluoroquinolones and/or injectable agents (3). Novel regimens based on 3 or more oral agents with little or no preexisting resistance would provide simpler, more universally active regimens. If such novel regimens are more effective than the current first-line regimen for drug-susceptible TB, they may shorten and simplify treatment for pulmonary TB irrespective of resistance to existing drugs.
Agents from 2 novel classes recently received conditional regulatory approval for use in MDR-TB, the diarylquinoline bedaquiline (BDQ) and the nitroimidazole-derivative delamanid. Aside from some mutations known to confer cross-resistance between BDQ and clofazimine (5, 6), these agents are not known to exhibit cross-resistance with other TB drugs. We recently reported that the 3-drug regimen of BDQ plus pretomanid (PMD; formerly known as PA-824), the second nitroimidazole to enter phase 3 clinical trials, and the oxazolidinone sutezolid (SZD; formerly known as PNU-100480) has greater sterilizing activity than the first-line regimen of rifampin (RIF), INH, and PZA in a murine model of TB (7–10). The 3-drug combo was more active than any of its 2-drug components, indicating that SZD contributes important activity. However, SZD has only completed a single phase 2a trial of its early bactericidal activity (8), and its further development cannot be ensured. Until recently, linezolid (LZD) was the only marketed oxazolidinone antibiotic. It has proven efficacy in salvage therapy for recalcitrant cases of MDR-TB patients (11), but its usage has been curtailed by dose- and duration-dependent toxicity. Tedizolid (TZD) was recently approved for the treatment of acute bacterial skin and skin-structure infections (ABSSSI). Its MIC90 of 0.5 μg/ml against Mycobacterium tuberculosis is the same as its proposed MIC breakpoint for ABSSSI caused by Staphylococcus aureus (12). However, to our knowledge, its activity has never been examined in vivo. Like SZD, AZD5847 (AZD) is in clinical development for treatment of TB and has recently completed a phase 2a study (ClinicalTrials.gov registration no. NCT01516203) evaluating its early bactericidal activity. RWJ-416457 (RWJ) is another oxazolidinone that progressed to phase 1 for other indications (13) but has not previously been evaluated for antituberculosis activity. In the present series of experiments, we sought to compare the contribution of these oxazolidinones to novel drug combinations with BDQ and PMD in the same murine TB model.
MATERIALS AND METHODS
Mycobacterial strain.
M. tuberculosis H37Rv was mouse-passaged, frozen in aliquots, and subcultured in Middlebrook 7H9 broth with 10% oleic acid-albumin-dextrose-catalase (OADC) (Fisher, Pittsburgh, PA) and 0.05% Tween 80 prior to infection.
Antimicrobials.
INH, RIF, PZA, BDQ, PMD, and LZD were obtained and formulated for oral administration as previously described (9, 10, 14, 15). SZD (prepared in a PEG-200/0.5% methylcellulose suspension) and TZD (prepared as phosphate prodrug dissolved in water) were synthesized by WuXi (Hubei, China). AZD (prepared as a disodium phosphate prodrug dissolved in 0.3% dextrose and 0.9% saline) was provided by AstraZeneca. RWJ (prepared in 0.5% methylcellulose) was provided by Johnson and Johnson.
Pharmacokinetics of oxazolidinones.
Uninfected female BALB/c mice weighing approximately 20 g received single doses of SZD (50 mg/kg of body weight), LZD (100 mg/kg), TZD (10 or 20 mg/kg), or AZD (50 or 200 mg/kg), using the same formulations as those used in the efficacy experiments. Three mice per dose per time point were sampled by cardiac puncture at 0.5, 1, 2, 4, 8, and 24 h post-dose (plus an additional 16-h time point for AZD). Plasma drug concentrations were quantified by a validated liquid chromatography-mass spectrometry (LC/MS) method performed at AstraZeneca (for AZD only) (16) or Rutgers New Jersey Medical School (for other oxazolidinones). Standards for the different oxazolidinones were provided by the sponsors. At Rutgers, analytes of interest were extracted by combining 20 μl of mouse plasma with 20 μl of acetonitrile/water (1:1) and with 180 μl of methanol/acetonitrile (1:1) containing 10 ng/ml of verapamil (Sigma-Aldrich) as an internal standard. The mixture was vortexed and centrifuged, and 100 μl of the supernatant was recovered and combined with 100 μl of water for analysis. Liquid chromatography-tandem mass spectrometry (LC-MS/MS) analysis was performed with an Agilent 1260 LC-system coupled with an AB Sciex 4000 QTRAP mass spectrometer (positive mode, electrospray ionization) and with an Agilent column SB-C8 (2.1 × 30 mm, 3.5 μm) with the column temperature fixed at 24°C. Mobile phase A was 0.1% formic acid in 100% H2O, and mobile phase B was 0.1% formic acid in 100% acetonitrile. Injection volumes were routinely 2 μl. The mass selective detector was set to MRM (multiple-reaction monitoring) mode using positive polarity ionization, monitoring for the ions of interest, SZD (m/z 354.12/312.04), SZD-M1 (370.13/238.12), LZD (338.00/235.00), and TZD (371.12/343.00), and the internal standard (m/z 455.4/165.2). The lower limit of quantification for all oxazolidinones was 5 ng/ml. Pharmacokinetics (PK) parameters were determined by noncompartmental analysis using WinNonlin 6.4 (Certara, Princeton, NJ).
Aerosol infection with M. tuberculosis.
All animal procedures were approved by the Johns Hopkins University Animal Care and Use Committee. High-dose aerosol infection was performed as previously described (17). Briefly, 5-to-6-week-old female BALB/c mice (Charles River, Wilmington, MA) were infected with Mycobacterium tuberculosis H37Rv using the inhalation exposure system (Glas-Col, Terre Haute, IN) and a fresh log-phase broth culture (optical density at 600 nm of 0.8 to 1.0) with the goal of implanting 3.5 to 4.0 log10 CFU in the lungs of each mouse. Two or three mice from each aerosol infection run (experiments 1, 2, and 4) or five mice from the only run (experiments 3a and 3b) were humanely killed 1 day after infection and on the day of treatment initiation (D0) to determine the number of bacteria implanted in the lungs and at the start of treatment, respectively.
Chemotherapy.
Mice were block randomized by aerosol run to experimental arms prior to treatment. Treatment was initiated 14 to 17 days after infection. Treatment was administered once daily, by gavage, 5 days per week. Except for dose-ranging monotherapy experiments, drug doses (in mg/kg) were 10 (INH), 10 (RIF), 150 (PZA), 25 (BDQ), 50 or 100 (PMD), 50 (SZD), 50 or 100 (LZD), 10 (TZD), 125 (AZD), and 100 (RWJ) (14, 16, 18–20). Each drug was administered once daily, by gavage, 5 days per week. For all combinations, PMD was administered immediately after the dose of BDQ with or without PZA, which were formulated together (9, 14). Oxazolidinones were administered at least 4 h later as a precaution against interference with the absorption of companion agents as observed previously with LZD in combination with first-line drugs (9).
In experiment 1, control mice received RIF+INH+PZA for 2 months followed by RIF+INH alone for a total of up to 4 months. To compare the contributions of SZD and LZD, test mice received a 3-drug combination of BDQ and PMD (50 mg/kg) plus either SZD (50 mg/kg) or LZD (100 mg/kg), or they received each 1- or 2-drug component comprising these 3-drug regimens. One- and two-drug regimens were limited to 1 and 2 months of treatment, respectively; however, the BDQ+PMD combination was given for up to 4 months along with the 3-drug combinations. In experiment 2, the contribution of LZD to regimens containing BDQ+PMD plus PZA was assessed. Control mice received BDQ+PZA plus PMD at either 50 or 100 mg/kg. Test mice received BDQ+PZA+PMD (100 mg/kg) plus LZD (100 mg/kg) for up to 2 months. One cohort received LZD for the entire 2 months. Another received LZD for the first month only.
The dose-ranging activities of AZD and RWJ as monotherapies were determined in experiments 3a and 3b, respectively. Control mice received LZD (100 mg/kg) or SZD (30 or 100 mg/kg), and test mice received either AZD (10, 30, 100, or 300 mg/kg) or RWJ (10, 30, or 100 mg/kg) for 1 month. The contributions of all 5 oxazolidinones to the BDQ+PMD combination were studied in experiment 4. Control mice received 2 months of RIF+INH+PZA followed by up to 1 month of RIF+INH, or they received BDQ+PMD (100 mg/kg) alone. Test mice received BDQ+PMD in combination with SZD (50 mg/kg), LZD (50 or 100 mg/kg), TZD (10 mg/kg), AZD (125 mg/kg), or RWJ (100 mg/kg) for a total duration of up to 3 months (in the case of SZD and LZD) or 2 months (for other oxazolidinones). In two additional groups receiving BDQ+PMD+LZD, the LZD component was administered for only the first 1 or 2 months of the 3-month regimen to assess the impact of stopping LZD early.
Assessment of treatment efficacy.
Efficacy was assessed on the basis of lung CFU counts at selected time points during treatment (a measure of bactericidal activity) and on the proportion of mice with culture-positive relapse after treatment completion (a measure of sterilizing activity). Quantitative cultures of lung homogenates were performed in parallel on 7H11 agar enriched with OADC (basic agar) and on basic agar supplemented with 0.4% activated charcoal to reduce drug carryover effects (14). Plates were incubated for up to 42 days at 37°C before final CFU counts were determined. Lung CFU counts were assessed in four or five mice per treatment group at each time point in experiments 1 and 2 or in experiment 3, respectively. The proportion of mice with culture-positive relapse was determined by holding cohorts of 15 to 20 mice for 3 additional months after the completion of treatment and then sacrificing them to determine the proportion with positive lung cultures as defined by ≥1 CFU of M. tuberculosis detected after plating the entire lung homogenate onto five 7H11 plates, at least half of which were supplemented with 0.4% activated charcoal.
Statistical analysis.
CFU counts (x) were log-transformed as (x + 1) before analysis, and group means were compared by one-way analysis of variance with Dunnett's post-test to control for multiple comparisons. Group relapse proportions were compared using Fisher's exact test, adjusting for multiple comparisons. GraphPad Prism version 5 (GraphPad, San Diego, CA) was used for all analyses. Use of 15 mice per group for relapse assessment provides >80% power to detect 40 percentage point differences in the relapse rate, after setting α at 0.01 to adjust for up to 5 simultaneous two-sided comparisons. Smaller differences may not be meaningful in terms of shortening the duration of treatment.
RESULTS
Oxazolidinone pharmacokinetics in BALB/c mice.
PK parameters are presented in Table 1. As previously described (20), oral administration of SZD at 50 mg/kg resulted in rapid and extensive metabolism to the active sulfoxide M1 metabolite (also known as PNU-101603) for which the mean area under the concentration-time curve from 0 to 24 h (AUC0–24) and the maximum concentration of drug in serum (Cmax) were approximately 10 times higher than those of the SZD parent. Overall, the SZD and SZD M1 AUC0–24 values were similar to or, in the case of SZD, modestly lower than the geometric means observed in TB patients receiving 1,200 mg per day (8). LZD showed the highest exposure among the tested compounds with an AUC0–24 of 244 h · μg/ml, which is comparable to the average among TB patients receiving 1,200 mg daily (21, 22). TZD exposure was dose proportional from 10 mg/kg to 20 mg/kg. For the 10-mg/kg dose evaluated in the efficacy study, the total drug TZD AUC0–24 of 40 h · μg/ml was similar to that determined in other infection models (23–25). Interpolating between the mean AZD AUC0–24 values produced by the 50- and 200-mg/kg doses, the predicted AUC0–24 of 160 to 190 h · μg/ml produced by the 125-mg/kg dose used in the combination efficacy study (experiment 4 below) is similar to the steady-state AUC0–24 in healthy human volunteers receiving 800 mg twice daily (26), the highest dose administered in a recent dose-ranging trial of early bactericidal activity. The oxazolidinones were cleared relatively rapidly, with half-life (t1/2) values between 2.8 and 3.9 h. AUC0–24 values from all of the compounds were approximately equal to AUC from 0 h to infinity (AUC0–∞) values (≥98%), suggesting that no accumulation would be expected after multiple doses in BALB/c mice.
TABLE 1.
Pharmacokinetic parameters after single-dose oral administration of sutezolid, linezolid, tedizolid, or AZD5847 in BALB/c mice
| Compound (dose, mg/kg) | Mean (SD) of pharmacokinetics parameter: |
|||
|---|---|---|---|---|
| t1/2 (h) | Tmaxa (h) | Cmax (ng/ml) | AUC0–24 (h · ng/ml) | |
| Sutezolid (50) | 2.8 (0.5) | 0.5 (0.0) | 1,292 (707) | 3,359 (499) |
| Sutezolid M1 | 0.5 (0.0) | 15,233 (7,875) | 31,945 (5,853) | |
| Linezolid (100) | 3.0 (2.5) | 0.5 (0.0) | 81,933 (32,240) | 243,547 (31,577) |
| Tedizolid (10) | 3.0 (0.4) | 0.7 (0.3) | 6,417 (624) | 40,414 (2,942) |
| Tedizolid (20) | 3.9 (1.4) | 0.5 (0.0) | 11,433 (1,665) | 77,698 (6,107) |
| AZD5847 (50) | 2.4 (0.2) | 0.7 (0.3) | 20,433 (2,079) | 74,761 (6,138) |
| AZD5847 (200) | 8.6 (2.9) | 0.7 (0.3) | 31,367 (1,270) | 220,246 (34,953) |
Time to maximum concentration of drug in serum.
Experiment 1. Contribution of LZD and SZD to novel combinations with BDQ+PMD.
Previous work demonstrated the strong sterilizing activity of the novel BDQ+PMD+SZD combination in BALB/c mice (7, 9, 10, 14). Experiment 1 was performed to evaluate whether the marketed oxazolidinone LZD is able to replace SZD in this combination without loss of efficacy and to clarify the contribution of each drug component to the activity of the combination.
(i) Lung CFU counts during treatment.
The mean CFU count (± standard deviation [SD]) at the start of treatment was 6.17 ± 0.27. The lung CFU counts after 1, 2, and 3 months of treatment are presented in Table 2. As expected, the RIF+INH+PZA combination reduced the mean lung CFU count by 2.70 log10 and by 4.58 log10 after 1 and 2 months of treatment, respectively, and left fewer than 10 CFU/mouse after 3 months of treatment. Due to the previously described antagonism of PMD on BDQ activity (7, 14), BDQ+PMD had lower activity than BDQ alone over the first month. However, its activity was virtually identical to that of the first-line regimen over 2 to 3 months. Addition of SZD significantly increased the initial bactericidal activity of BDQ+PMD (P < 0.001), rendering all mice culture negative between 1 and 2 months of treatment. The activity of BDQ+PMD+SZD was significantly greater than that of RIF+INH+PZA after 1 and 2 months of treatment (P < 0.001). Addition of LZD also significantly increased the activity of BDQ+PMD (P < 0.01) and produced activity superior to that of RIF+INH+PZA after 2 and 3 months of treatment (P < 0.001). All 2-drug combinations had inferior activity compared to the activity of the 3-drug combinations of BDQ+PMD plus SZD or LZD at 2 months (P < 0.01), confirming that each component drug contributes to the efficacy of the 3-drug combinations. However, the 3-drug LZD-containing regimen was only superior to BDQ+LZD after 2 months of treatment. LZD-containing regimens produced higher CFU counts than those of their SZD-containing comparator regimen.
TABLE 2.
Lung CFU counts assessed during treatment and proportion of mice relapsing after treatment completion in experiment 1
| Drug regimen | Mean (±SD) log10 CFU count ata: |
Proportion (%) relapsing after treatment for: |
||||||
|---|---|---|---|---|---|---|---|---|
| D13 | D0 | M1 | M2 | M3 | 2 mo | 3 mo | 4 mo | |
| Untreated | 2.69 ± 0.13 | 6.17 ± 0.27 | 6.47 ± 0.06 | |||||
| 2RIF+INH+PZA/RIF+INH | 3.47 ± 0.37 | 1.59 ± 0.25 | 0.50 ± 0.51 | 13/15 (87) | 1/20 (5) | |||
| BDQ | 3.24 ± 0.25 | |||||||
| PMD | 4.57 ± 0.22 | |||||||
| LZD | 4.97 ± 0.26 | |||||||
| SZD | 3.85 ± 0.37 | |||||||
| BDQ+PMD | 4.21 ± 0.40 | 1.62 ± 0.19 | 0.52 ± 0.36 | 15/15 (100) | 10/15 (60) | 2/20 (10) | ||
| BDQ+LZD | 2.82 ± 0.15 | 1.91 ± 0.66 | ||||||
| BDQ+SZD | 2.88 ± 0.07 | 0.65 ± 0.50 | ||||||
| PMD+LZD | 3.23 ± 0.41 | 1.48 ± 0.12 | ||||||
| PMD+SZD | 1.65 ± 0.33 | 0.23 ± 0.40 | ||||||
| BDQ+PMD+LZD | 3.28 ± 0.65 | 0.34 ± 0.41 | 0.00 ± 0.00 | 12/15 (80) | 0/14 (0) | 0/20 (0) | ||
| BDQ+PMD+SZD | 0.94 ± 0.14 | 0.00 ± 0.00 | 14/20 (70) | 1/14 (7) | ||||
Time points are shown in days (e.g., D13, day 13; D0, day 0) or months (e.g., M1, 1 month) of treatment.
(ii) Relapse after treatment completion.
The relapse results are displayed in Table 2. Treatment with the first-line regimen for 3 and 4 months resulted in relapse of 13 (87%) of 15 and 1 (5%) of 20 mice, respectively. The higher cure rate compared to that of previous experiments is due to the lower-than-usual bacterial burden at the initiation of treatment. Consistent with the CFU count results, treatment with BDQ+PMD produced relapse results similar to those of the first-line regimen after 3 and 4 months but did not prevent relapse after just 2 months of treatment. However, unlike the more rapid decline in CFU counts with the 3-drug regimen containing SZD compared to that containing LZD, the two BDQ+PMD plus oxazolidinone regimens had similar sterilizing activity. While only the difference between the SZD-containing regimen and BDQ+PMD alone was statistically significant after 2 months of treatment, the two oxazolidinone-containing regimens resulted in significantly fewer relapses after 3 months of treatment compared to the two BDQ+PMD regimens and the first-line regimen.
Experiment 2. Contribution of LZD to BDQ+PZA+PMD.
We previously reported the potent sterilizing activity of BDQ+PZA+SZD in mice and the additive sterilizing activity of PMD (7, 9, 10, 14). After observing that LZD had comparable sterilizing activity to SZD in combination with BDQ+PMD in experiment 1, we hypothesized that LZD could also replace SZD in the BDQ+PZA+PMD+SZD combination. The mean CFU count at the start of treatment was 7.92 ± 0.26. The lung CFU count and relapse results are displayed in Table 3. As previously observed, BDQ+PZA+PMD had significantly greater bactericidal activity compared to that of RIF+INH+PZA (P < 0.001). Greater activity was observed when PMD was dosed at 100 rather than 50 mg/kg, but the difference was only noted after 2 months of treatment (P < 0.01). The higher PMD dose helped render all mice but one culture negative at this time point. The addition of LZD had a dramatic effect on efficacy, significantly reducing the CFU count at 1 month (P < 0.001) and rendering all mice but one culture negative 1 month earlier. The addition of LZD to BDQ+PZA+PMD also significantly reduced the proportion of mice relapsing after 1.5 months of treatment from 60% to 0% (P < 0.001) irrespective of whether LZD was discontinued after the first month of treatment.
TABLE 3.
Lung CFU counts assessed during treatment and proportion of mice relapsing after treatment completion in experiment 2
| Regimen | Mean (±SD) log10 CFU count ata: |
Proportion (%) relapsing after treatment for: |
||||
|---|---|---|---|---|---|---|
| D13 | D0 | M1 | M2 | 1.5 mo | 2 mo | |
| Untreated | 4.42 ± 0.15 | 7.92 ± 0.26 | ||||
| RIF+INH+PZA | 2.06 ± 0.37 | |||||
| BDQ+PZA+PMD50 | 2.91 ± 0.33 | 0.95 ± 0.38 | ||||
| BDQ+PZA+PMD100 | 2.93 ± 0.31 | 0.06 ± 0.13 | 9/15 (60) | 1/15 (7) | ||
| 1BDQ+PZA+PMD100+LZD/1BDQ+PZA+PMD100 | 0.11 ± 0.24 | 0/15 (0) | 0/15 (0) | |||
| BDQ+PZA+PMD100+LZD | 0/15 (0) | 1/15 (7) | ||||
Time points are shown in days (e.g., D13, day 13; D0, day 0) or months (e.g., M1, 1 month) of treatment.
Experiments 3a and 3b. Dose-ranging activity of AZD5847 and RWJ-416457.
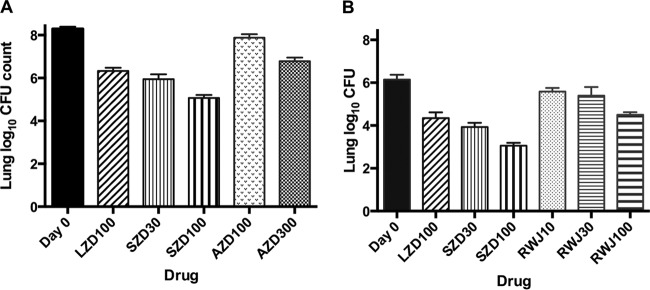
Because the dose of AZD or RWJ that could be advanced for TB treatment remains in question, we evaluated their dose-ranging activity as monotherapies before combining them with BDQ+PMD. In experiment 3a, the mean lung log10 CFU count (±SD) was 4.50 ± 0.09 on the day after aerosol infection. By D0, the mean CFU count had increased to 8.29 ± 0.10. Untreated mice and mice receiving AZD at 10 and 30 mg/kg were euthanized during the first week of the treatment phase due to their moribund status. Mean lung CFU counts were 9.21 ± 0.27, 9.00 ± 0.35, and 8.88 ± 0.22, respectively. The remaining mice survived the 1-month treatment phase. Treatment with LZD at 100 mg/kg and with SZD at 30 mg/kg produced a roughly 2-log kill, compared to a 3-log kill with SZD at 100 mg/kg. Treatment with AZD 100 mg/kg produced a bacteriostatic effect, whereas AZD at 300 mg/kg produced a roughly 1.5-log kill (Fig. 1A). In experiment 3b, the number of CFU implanted by aerosol was lower (2.60 ± 0.18 log10), resulting in a lower mean CFU count at D0 (6.15 ± 0.22). Nevertheless, treatment with LZD at 100 mg/kg and with SZD at 30 mg/kg again produced a roughly 2-log kill compared to a 3-log kill with SZD at 100 mg/kg (Fig. 1B). Treatment with RWJ at 10 and 30 mg/kg reduced the lung CFU counts by approximately 0.5 and 0.75 log CFU, respectively, whereas RWJ at 100 mg/kg produced a roughly 1.5-log kill that was not significantly different from that of LZD at 100 mg/kg.
FIG 1.
Mean lung log10 CFU counts (±SD) after 1 month of treatment with LZD, SZD, and either AZD (A) or RWJ (B) in experiment 3. Number beside drug abbreviation indicates dose (in milligrams per kilogram body weight).
Experiment 4. Contribution of oxazolidinones to novel combinations with BDQ+PMD.
Experiment 4 was performed to confirm the additive sterilizing activity of SZD and LZD when added to BDQ+PMD in experiment 1 and to evaluate whether reducing the LZD dose by 50% or limiting the duration of LZD to the first 1 to 2 months would significantly affect its contribution. Additional arms were included to assess whether AZD, RWJ, or TZD can replace SZD or LZD in the combination without loss of efficacy over the first 2 months of treatment.
Lung CFU counts during treatment.
The mean CFU count at the start of treatment was 7.74 ± 0.20. The lung CFU counts observed after 1, 2, and 3 months of treatment are presented in Table 4. RIF+INH+PZA reduced the mean lung CFU count by almost 6 log10 over 2 months of treatment. BDQ+PMD alone had modestly lower activity over this period. As previously observed, addition of 50 mg/kg SZD conferred superior activity compared to that of 100 mg/kg LZD, although the two oxazolidinones significantly increased the initial bactericidal activity of BDQ+PMD. However, results with BDQ+PMD+LZD at 100 mg/kg were similar whether LZD was discontinued after 1 month or continued for 2 months. Similarly, use of LZD at 50 mg/kg rather than at 100 mg/kg resulted in higher CFU counts at 1 month but not 2 months, suggesting that the total LZD dose achieved with 100 mg/kg for 1 month or 50 mg/kg for 2 months sufficiently maximized the contribution of LZD to the regimen. RWJ at 100 mg/kg performed very similarly to LZD at 50 mg/kg, whereas AZD and TZD were somewhat less effective. Compared to BDQ+PMD alone, all oxazolidinones significantly increased bactericidal activity at 1 and 2 months, except for TZD at the 1-month time point (P < 0.05 for AZD and for TZD at M2; P < 0.001 for other oxazolidinones).
TABLE 4.
Lung CFU counts assessed during treatment and proportion of mice relapsing after treatment completion in experiment 4
| Regimen | Mean (±SD) log10 CFU count ata: |
Proportion (%) relapsing after treatment for: |
||||
|---|---|---|---|---|---|---|
| D13 | D0 | M1 | M2 | 2 mo | 3 mo | |
| Untreated | 3.96 ± 0.08 | 7.74 ± 0.20 | ||||
| 2RIF+INH+PZA/1RIF+INH | 1.94 ± 0.27 | 8/14 (57) | ||||
| BDQ+PMD | 4.48 ± 0.20 | 2.33 ± 0.30 | 3/14 (21) | |||
| BDQ+PMD+TZD | 4.20 ± 0.13 | 1.67 ± 0.41 | ||||
| BDQ+PMD+AZD | 4.07 ± 0.36 | 1.43 ± 0.36 | ||||
| BDQ+PMD+RWJ | 3.63 ± 0.18 | 0.54 ± 0.41 | ||||
| BDQ+PMD+LZD50 | 3.48 ± 0.36 | 0.39 ± 0.26 | ||||
| 1BDQ+PMD+LZD100/BDQ+PMD | 2.69 ± 0.37 | 0.93 ± 0.49 | 9/15 (60) | 0/15 (0) | ||
| 2BDQ+PMD+LZD100/BDQ+PMD | 0.66 ± 0.39 | 6/15 (40) | 0/15 (0) | |||
| 2BDQ+PMD+LZD100/BDQ+PMD+LZD50 | 0/12 (0) | |||||
| BDQ+PMD+LZD100 | 0/15 (0) | |||||
| BDQ+PMD+SZD | 1.88 ± 0.22 | 0.00 ± 0.00 | 1/14 (7) | 0/14 (0) | ||
Time points are shown in days (e.g., D13, day 13; D0, day 0) or months (e.g., M1, 1 month) of treatment.
Relapse after treatment completion.
The relapse results are displayed in Table 4. Treatment with the first-line regimen for 3 months resulted in the relapse of 8 (57%) of 14 mice. Treatment with BDQ+PMD produced numerically superior results, with just 3 (21%) of 14 mice relapsing at 3 months although this difference was not statistically significant. Addition of SZD to BDQ+PMD resulted in only one relapse after just 2 months of treatment, a result that was at least as effective as 3 months of BDQ+PMD and more effective than 3 months of the first-line regimen. Addition of SZD at 50 mg/kg also resulted in fewer relapses than did the addition of LZD at 100 mg/kg for 2 months; however, the difference did not reach statistical significance. Use of LZD at 100 mg/kg for the first month only was less effective than SZD for 2 months but was not significantly different from LZD at 100 mg/kg for 2 months. Similarly, among regimens administered for 3 months, no relapse was observed irrespective of whether LZD at 100 mg/kg was continued throughout or discontinued after 1 or 2 months or replaced with LZD at 50 mg/kg after 2 months. In a post hoc analysis comparing BDQ+PMD to all BDQ+PMD+LZD regimens, the addition of LZD at 100 mg/kg for at least 1 month resulted in fewer relapses after 3 months of treatment.
DISCUSSION
The recent regulatory approvals of agents from two new classes of TB drugs, the diarylquinoline BDQ and the bicyclic nitroimidazole delamanid, open the door to novel regimens to shorten or otherwise simplify the treatment of drug-resistant TB. Addition of a third agent to which little or no preexisting resistance exists may provide the basis for novel regimens with nearly universal activity irrespective of resistance to existing TB drugs. The present study evaluated the contributions of various oxazolidinones to novel regimens based on BDQ plus another bicyclic nitroimidazole in clinical development, PMD, in a BALB/c mouse model of TB. The results confirm our prior work (7, 9) showing that BDQ+PMD+SZD has sterilizing activity superior to that of the current first-line regimen while also demonstrating for the first time that the addition of SZD is necessary to achieve superiority. Furthermore, analysis of the contribution of each 2-drug component in experiment 1 indicates that each of the SZD-containing drug pairs contributes more than BDQ+PMD to the activity of the 3-drug combination over the first 2 months of treatment. Thus, an oxazolidinone might lend important activity to novel regimens containing BDQ+PMD.
Although SZD demonstrated early bactericidal activity in pulmonary TB patients more than 2 years ago (27), the clinical development of SZD has stalled. Therefore, we examined whether LZD, the only marketed oxazolidinone at the time that our studies began, could replace SZD in the 3-drug regimen without sacrificing efficacy. Our results demonstrate that, although it contributes less than SZD to the initial bactericidal activity of the regimen, LZD does add bactericidal and sterilizing activities. Its additive sterilizing activity was indistinguishable from that of SZD in experiment 1. In experiment 4, in which the bacterial burden and the dose of PMD were higher, LZD did not appear quite as effective as SZD, although the difference was not statistically significant. Thus, the impact of substituting LZD for SZD is small in terms of the duration needed to cure mice. This finding constitutes the experimental basis for moving the evaluation of the BDQ+PMD+LZD regimen into patients with extensively drug-resistant TB (XDR-TB) or treatment-intolerant or nonresponsive MDR-TB in the recently initiated NiX-TB trial (ClinicalTrials.gov registration no. NCT02333799).
Given the superior sterilizing activity of BDQ+PMD plus an oxazolidinone compared to that of the first-line regimen, it is also reasonable to consider whether regimens based on this combination can shorten the treatment of drug-susceptible TB. We previously showed that the addition of PZA to the BDQ+PMD+SZD combination produced even greater sterilizing activity, curing nearly all mice after just 6 weeks of treatment (7). However, we did not confirm the contribution of SZD to the 4-drug combination. In experiment 2 of the present study, we confirmed that addition of LZD to BDQ+PZA+PMD also significantly increases the bactericidal and sterilizing activities of the regimen, curing all mice after 6 weeks of treatment, while at least 5 months of treatment with the first-line regimen is typically required to produce this result under the same conditions in our model.
The 100-mg/kg dose of LZD used in our study produces a mean plasma AUC0–24 comparable to that produced by a dose of 600 mg twice daily in humans (21, 22), the same dose at which treatment is initiated in the NiX-TB trial. This dose has produced unacceptably high rates of myelotoxicity and neuropathy when administered for long durations in salvage regimens to treat MDR- and XDR-TB (28). However, these toxicities are dose and duration dependent. If the superior efficacy of BDQ+PMD+LZD, with or without PZA, compared to that of the first-line regimen, as demonstrated here, translates even generally to the clinical setting, then the much shorter treatment durations necessary for cure may enable safer usage of 1,200 mg of LZD daily. Moreover, our results with abbreviated durations of LZD within these regimens in the current study further suggest that the sterilizing activity of these regimens is not adversely affected by reducing the duration of LZD. The potent sterilizing activity of the 4-drug regimen containing PZA in experiment 2 was such that all mice were cured after 1.5 months of treatment. Nevertheless, limiting the duration of LZD at 100 mg/kg to the first month did not adversely affect LZD's contribution to the sterilizing activity. In the absence of PZA, limiting LZD at 100 mg/kg to the first month of treatment did not significantly increase the rate of relapse after 2 or 3 months of treatment. Whereas reducing the LZD dose to 50 mg/kg, which produces a mean AUC0–24 equivalent to a 600-mg daily dose in humans, reduced the bactericidal activity over the first month of treatment, only small numbers of CFU were recovered after 2 months of treatment, irrespective of whether LZD was administered as 100 mg/kg for 1 or 2 months or as 50 mg/kg for 2 months. Taken together, these results suggest that the use of LZD in doses of 600 to 1,200 mg daily for the first few months of treatment may enable it to contribute important bactericidal and sterilizing activity to these regimens before the risk of potentially irreversible neuropathy becomes significant (11, 29).
The ability of additional oxazolidinones to replace SZD and LZD was evaluated in the present study. Like SZD, AZD is in phase 2 of clinical development for TB. While its anti-TB activity was less potent than that of SZD and LZD on a mg/kg basis in BALB/c mice, the addition of AZD at 125 mg/kg did increase the bactericidal activity of BDQ+PMD. The AUC0–24 produced by this dose is expected to be between 150 and 200 μg · h/ml, which is comparable to the exposure observed with 800 mg twice daily in a phase 1 multiple ascending dose trial (26). As this was the highest dose tested in a recent phase 2 trial of early bactericidal activity (ClinicalTrials.gov registration no. NCT01516203), testing higher AZD doses in mice does not appear justified at this time.
The recent approval of TZD for a non-TB indication makes it a potential candidate for novel TB regimens. At a dose producing plasma AUC values comparable to those observed in humans at the 200-mg daily dose approved for treatment of acute bacterial skin and skin-structure infection (18), TZD had additive activity comparable to that of AZD. If pharmacodynamic comparisons translate to results in humans, any advantage of using AZD or TZD over LZD may depend on demonstrating superior safety over reduced doses (e.g., 300 to 600 mg) of LZD. Whether TZD at 200 mg is safer than LZD at 600 mg twice daily for durations greater than 1 week in humans is not well studied, although animal studies are promising in this regard (30, 31).
At a dose of 100 mg/kg, RWJ has similar activity to LZD at 100 mg/kg, alone and in combination with BDQ+PMD. Although it progressed to phase 1 for other indications (13), data on the human pharmacokinetics are not publicly available. The results of the present study suggest that if comparable exposures are safe, this oxazolidinone may have potential in the treatment of TB.
In the present study, we evaluated the oxazolidinones at doses that produce comparable plasma exposures compared to those reported from clinical studies. As we have discussed recently (9, 14), experiments in the BALB/c mouse model of TB have potential limitations related to differences in the pathology of M. tuberculosis infection between mice and humans. Perhaps most significant for the present work is the fact that, because TB in BALB/c mice does not produce caseation, the vast majority of tubercle bacilli reside intracellularly in cellular granulomas. Compared to activity against extracellular bacilli residing within caseous lesions, which predominate in human TB, oxazolidinone efficacy in BALB/c mice may be unduly influenced by penetration into, and activity within, infected host cells. For example, the SZD parent molecule has much greater potency against intracellular M. tuberculosis than that of LZD or the principal SZD metabolite (PNU-101603) that comprises over 80% of the SZD species measured in vivo. It is the SZD parent molecule that likely drives the superior bactericidal activity of SZD in BALB/c mice (32, 33). Because it remains unclear whether the tubercle bacilli that persist and ultimately determine the duration of treatment needed to cure human TB reside intracellularly, extracellularly, or in both locales, studies of the relative efficacy of oxazolidinones in animal models exhibiting caseation might provide very useful information. Work evaluating the bactericidal and sterilizing activities of BDQ+PMD with or without oxazolidinones and the effect of adding PZA is under way in C3HeB/FeJ mice, which develop caseous lung lesions. These experiments are expected to add value given the differing pathology and apparently differing relative potency of SZD, LZD, and PZA in these mice compared to BALB/c mice (34).
Oxazolidinones also differ in their penetration into the alveolar epithelial lining fluid (ELF) of the lung. For example, work in other pneumonia infection models suggests that TZD has better ELF penetration than that of LZD and that this penetration is greater in humans than in mice (35). To better reproduce TZD exposures in human ELF, these investigators recommend using a 20-mg/kg dose of TZD rather than the 10-mg/kg dose used here. However, the significance of ELF exposures in the treatment of TB remains undefined. The significance is even less clear for the predominantly intracellular infection in BALB/c mice. Therefore, we chose the oxazolidinone doses used here on the basis of plasma exposures observed with clinical doses.
In conclusion, these experiments have confirmed and extended prior studies in demonstrating the potential for truly novel drug combinations containing BDQ, PMD, and an oxazolidinone to provide a strong new foundation for universally active short-course regimens capable of effectively treating TB irrespective of resistance to existing drugs. When infecting isolates remain susceptible to PZA, the inclusion of this sterilizing agent is expected to shorten treatment further. While the remarkable treatment-shortening potential of these regimens in mice cannot be assumed to translate directly to human TB, the results presented here certainly support clinical trials to investigate the efficacy of these and other similar regimens.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to Mark Macielag, Darren Abbanat, and Simon Lynch (all of Janssen Research & Development LLC) for provision of the RWJ-416457 material and related information.
Funding Statement
The Global Alliance for TB Drug Development provided funding to Johns Hopkins University under grant number 90036107. The National Institutes of Health provided funding to Johns Hopkins University under grant number R01-AI090820.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.01691-15.
REFERENCES
- 1.World Health Organization. 2014. Multidrug-resistant tuberculosis (MDR-TB) 2014 update. World Health Organization, Geneva, Switzerland: http://www.who.int/tb/challenges/mdr/mdr_tb_factsheet.pdf?ua=1. [Google Scholar]
- 2.Aung KJ, Van Deun A, Declercq E, Sarker MR, Das PK, Hossain MA, Rieder HL. 2014. Successful ‘9-month Bangladesh regimen’ for multidrug-resistant tuberculosis among over 500 consecutive patients. Int J Tuberc Lung Dis 18:1180–1187. doi: 10.5588/ijtld.14.0100. [DOI] [PubMed] [Google Scholar]
- 3.Nuermberger E, Yew WW. 2015. Expanding the evidence base supporting shorter treatment durations for multidrug-resistant tuberculosis. Int J Tuberc Lung Dis 19:497–498. doi: 10.5588/ijtld.15.0110. [DOI] [PubMed] [Google Scholar]
- 4.Van Deun A, Maug AK, Salim MA, Das PK, Sarker MR, Daru P, Rieder HL. 2010. Short, highly effective, and inexpensive standardized treatment of multidrug-resistant tuberculosis. Am J Respir Crit Care Med 182:684–692. doi: 10.1164/rccm.201001-0077OC. [DOI] [PubMed] [Google Scholar]
- 5.Andries K, Villellas C, Coeck N, Thys K, Gevers T, Vranckx L, Lounis N, de Jong BC, Koul A. 2014. Acquired resistance of Mycobacterium tuberculosis to bedaquiline. PLoS One 9:e102135. doi: 10.1371/journal.pone.0102135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hartkoorn RC, Uplekar S, Cole ST. 2014. Cross-resistance between clofazimine and bedaquiline through upregulation of MmpL5 in Mycobacterium tuberculosis. Antimicrob Agents Chemother 58:2979–2981. doi: 10.1128/AAC.00037-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tasneen R, Williams K, Amoabeng O, Minkowski A, Mdluli KE, Upton AM, Nuermberger EL. 2015. Contribution of the nitroimidazoles PA-824 and TBA-354 to the activity of novel regimens in murine models of tuberculosis. Antimicrob Agents Chemother 59:129–135. doi: 10.1128/AAC.03822-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wallis RS, Dawson R, Friedrich SO, Venter A, Paige D, Zhu T, Silvia A, Gobey J, Ellery C, Zhang Y, Eisenach K, Miller P, Diacon AH. 2014. Mycobactericidal activity of sutezolid (PNU-100480) in sputum (EBA) and blood (WBA) of patients with pulmonary tuberculosis. PLoS One 9:e94462. doi: 10.1371/journal.pone.0094462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Williams K, Minkowski A, Amoabeng O, Peloquin CA, Taylor D, Andries K, Wallis RS, Mdluli KE, Nuermberger EL. 2012. Sterilizing activities of novel combinations lacking first- and second-line drugs in a murine model of tuberculosis. Antimicrob Agents Chemother 56:3114–3120. doi: 10.1128/AAC.00384-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Williams KN, Brickner SJ, Stover CK, Zhu T, Ogden A, Tasneen R, Tyagi S, Grosset JH, Nuermberger EL. 2009. Addition of PNU-100480 to first-line drugs shortens the time needed to cure murine tuberculosis. Am J Respir Crit Care Med 180:371–376. doi: 10.1164/rccm.200904-0611OC. [DOI] [PubMed] [Google Scholar]
- 11.Lee M, Lee J, Carroll MW, Choi H, Min S, Song T, Via LE, Goldfeder LC, Kang E, Jin B, Park H, Kwak H, Kim H, Jeon HS, Jeong I, Joh JS, Chen RY, Olivier KN, Shaw PA, Follmann D, Song SD, Lee JK, Lee D, Kim CT, Dartois V, Park SK, Cho SN, Barry CE III. 2012. Linezolid for treatment of chronic extensively drug-resistant tuberculosis. N Engl J Med 367:1508–1518. doi: 10.1056/NEJMoa1201964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vera-Cabrera L, Gonzalez E, Rendon A, Ocampo-Candiani J, Welsh O, Velazquez-Moreno VM, Choi SH, Molina-Torres C. 2006. In vitro activities of DA-7157 and DA-7218 against Mycobacterium tuberculosis and Nocardia brasiliensis. Antimicrob Agents Chemother 50:3170–3172. doi: 10.1128/AAC.00571-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hilliard JJ, Fernandez J, Melton J, Macielag MJ, Goldschmidt R, Bush K, Abbanat D. 2009. In vivo activity of the pyrrolopyrazolyl-substituted oxazolidinone RWJ-416457. Antimicrob Agents Chemother 53:2028–2033. doi: 10.1128/AAC.00833-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tasneen R, Li S-Y, Peloquin CA, Taylor D, Williams KN, Andries K, Mdluli KE, Nuermberger EL. 2011. Sterilizing activity of novel TMC207- and PA-824-containing regimens in a murine model of tuberculosis. Antimicrob Agents Chemother 55:5485–5492. doi: 10.1128/AAC.05293-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tyagi S, Nuermberger E, Yoshimatsu T, Williams K, Rosenthal I, Lounis N, Bishai W, Grosset J. 2005. Bactericidal activity of the nitroimidazopyran PA-824 in a murine model of tuberculosis. Antimicrob Agents Chemother 49:2289–2293. doi: 10.1128/AAC.49.6.2289-2293.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Balasubramanian V, Solapure S, Shandil R, Gaonkar S, Mahesh KN, Reddy J, Deshpande A, Bharath S, Kumar N, Wright L, Melnick D, Butler SL. 2014. Pharmacokinetic and pharmacodynamic evaluation of AZD5847 in a mouse model of tuberculosis. Antimicrob Agents Chemother 58:4185–4190. doi: 10.1128/AAC.00137-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nuermberger E, Tyagi S, Tasneen R, Williams KN, Almeida D, Rosenthal I, Grosset JH. 2008. Powerful bactericidal and sterilizing activity of a regimen containing PA-824, moxifloxacin, and pyrazinamide in a murine model of tuberculosis. Antimicrob Agents Chemother 52:1522–1524. doi: 10.1128/AAC.00074-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Drusano GL, Liu W, Kulawy R, Louie A. 2011. Impact of granulocytes on the antimicrobial effect of tedizolid in a mouse thigh infection model. Antimicrob Agents Chemother 55:5300–5305. doi: 10.1128/AAC.00502-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rouan M-C, Lounis N, Gevers T, Dillen L, Gilissen R, Raoof A, Andries K. 2012. Pharmacokinetics and pharmacodynamics of TMC207 and its N-desmethyl metabolite in a murine model of tuberculosis. Antimicrob Agents Chemother 56:1444–1451. doi: 10.1128/AAC.00720-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Williams KN, Stover CK, Zhu T, Tasneen R, Tyagi S, Grosset JH, Nuermberger E. 2009. Promising antituberculosis activity of the oxazolidinone PNU-100480 relative to that of linezolid in a murine model. Antimicrob Agents Chemother 53:1314–1319. doi: 10.1128/AAC.01182-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alffenaar JW, van Altena R, Harmelink IM, Filguera P, Molenaar E, Wessels AM, van Soolingen D, Kosterink JG, Uges DR, van der Werf TS. 2010. Comparison of the pharmacokinetics of two dosage regimens of linezolid in multidrug-resistant and extensively drug-resistant tuberculosis patients. Clin Pharmacokinet 49:559–565. doi: 10.2165/11532080-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 22.McGee B, Dietze R, Hadad DJ, Molino LP, Maciel EL, Boom WH, Palaci M, Johnson JL, Peloquin CA. 2009. Population pharmacokinetics of linezolid in adults with pulmonary tuberculosis. Antimicrob Agents Chemother 53:3981–3984. doi: 10.1128/AAC.01378-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Keel RA, Crandon JL, Nicolau DP. 2012. Pharmacokinetics and pulmonary disposition of tedizolid and linezolid in a murine pneumonia model under variable conditions. Antimicrob Agents Chemother 56:3420–3422. doi: 10.1128/AAC.06121-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Keel RA, Tessier PR, Crandon JL, Nicolau DP. 2012. Comparative efficacies of human simulated exposures of tedizolid and linezolid against Staphylococcus aureus in the murine thigh infection model. Antimicrob Agents Chemother 56:4403–4407. doi: 10.1128/AAC.00122-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Louie A, Liu W, Kulawy R, Drusano GL. 2011. In vivo pharmacodynamics of torezolid phosphate (TR-701), a new oxazolidinone antibiotic, against methicillin-susceptible and methicillin-resistant Staphylococcus aureus strains in a mouse thigh infection model. Antimicrob Agents Chemother 55:3453–3460. doi: 10.1128/AAC.01565-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reele S, Xiao AJ, Das S, Balasubramanian V, Melnick D. 2011. A 14-day multiple ascending dose study: AZD5847 is well tolerated at predicted exposure for treatment of tuberculosis (TB), abstr A1-1735. Abstr 51st Intersci Conf Antimicrob Agents Chemother. American Society for Microbiology, Washington, DC. [Google Scholar]
- 27.Wallis RS, Diacon AH, Dawson R, Venter A, Friedrich SO, Paige D, Zhu T, Silvia A, Gobey J, Ellery C, Zhang Y, Kadyszewski E. 2012. Safety, tolerability and early bactericidal activity in sputum of PNU-100480 (sutezolid) in patients with pulmonary tuberculosis, abstr THLBB02 Abstr XIX International AIDS Conference, Washington, DC. [Google Scholar]
- 28.Sotgiu G, Centis R, D'Ambrosio L, Alffenaar JW, Anger HA, Caminero JA, Castiglia P, De Lorenzo S, Ferrara G, Koh WJ, Schecter GF, Shim TS, Singla R, Skrahina A, Spanevello A, Udwadia ZF, Villar M, Zampogna E, Zellweger JP, Zumla A, Migliori GB. 2012. Efficacy, safety and tolerability of linezolid containing regimens in treating MDR-TB and XDR-TB: systematic review and meta-analysis. Eur Respir J 40:1430–1442. doi: 10.1183/09031936.00022912. [DOI] [PubMed] [Google Scholar]
- 29.Chang KC, Yew WW, Tam CM, Leung CC. 2013. WHO group 5 drugs and difficult multidrug-resistant tuberculosis: a systematic review with cohort analysis and meta-analysis. Antimicrob Agents Chemother 57:4097–4104. doi: 10.1128/AAC.00120-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Flanagan S, McKee EE, Das D, Tulkens PM, Hosako H, Fiedler-Kelly J, Passarell J, Radovsky A, Prokocimer P. 2015. Nonclinical and pharmacokinetic assessments to evaluate the potential of tedizolid and linezolid to affect mitochondrial function. Antimicrob Agents Chemother 59:178–185. doi: 10.1128/AAC.03684-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schlosser MJ, Hosako H, Radovsky A, Butt MT, Draganov D, Vija J, Oleson F. 2015. Lack of neuropathological changes in rats administered tedizolid phosphate for nine months. Antimicrob Agents Chemother 59:475–481. doi: 10.1128/AAC.03950-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Converse PJ, Lee J, Williams KN, Tasneen R, Dionne K, Parish N, Wallis RS, Nuermberger E. 2012. Activity of PNU-100480 and its major metabolite in whole blood and broth culture models of tuberculosis, abstr 1563. Abstr 112th American Society for Microbiology General Meeting. American Society for Microbiology, Washington, DC. [Google Scholar]
- 33.Zhu T, Friedrich SO, Diacon A, Wallis RS. 2014. Population pharmacokinetic/pharmacodynamic analysis of the bactericidal activities of sutezolid (PNU-100480) and its major metabolite against intracellular Mycobacterium tuberculosis in ex vivo whole-blood cultures of patients with pulmonary tuberculosis. Antimicrob Agents Chemother 58:3306–3311. doi: 10.1128/AAC.01920-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lanoix JP, Lenaerts AJ, Nuermberger EL. 2015. Heterogeneous disease progression and treatment response in a C3HeB/FeJ mouse model of tuberculosis. Dis Model Mech 8:603–610. doi: 10.1242/dmm.019513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tessier PR, Keel RA, Hagihara M, Crandon JL, Nicolau DP. 2012. Comparative in vivo efficacies of epithelial lining fluid exposures of tedizolid, linezolid, and vancomycin for methicillin-resistant Staphylococcus aureus in a mouse pneumonia model. Antimicrob Agents Chemother 56:2342–2346. doi: 10.1128/AAC.06427-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.