Abstract
ASP2397 is a new compound with a novel and as-yet-unknown target different from that of licensed antifungal agents. It has activity against Aspergillus and Candida glabrata. We compared its in vitro activity against wild-type and azole-resistant A. fumigatus and A. terreus isolates with that of amphotericin B, itraconazole, posaconazole, and voriconazole. Thirty-four isolates, including 4 wild-type A. fumigatus isolates, 24 A. fumigatus isolates with alterations in CYP51A TR/L98H (5 isolates), M220 (9 isolates), G54 (9 isolates), and HapE (1 isolate), and A. terreus isolates (2 wild-type isolates and 1 isolate with an M217I CYP51A alteration), were analyzed. EUCAST E.Def 9.2 and CLSI M38-A2 MIC susceptibility testing was performed. ASP2397 MIC50 values (in milligrams per liter, with MIC ranges in parentheses) determined by EUCAST and CLSI were 0.5 (0.25 to 1) and 0.25 (0.06 to 0.25) against A. fumigatus CYP51A wild-type isolates and were similarly 0.5 (0.125 to >4) and 0.125 (0.06 to >4) against azole-resistant A. fumigatus isolates, respectively. These values were comparable to those for amphotericin B, which were 0.25 (0.125 to 0.5) and 0.25 (0.125 to 0.25) against wild-type isolates and 0.25 (0.125 to 1) and 0.25 (0.125 to 1) against isolates with azole resistance mechanisms, respectively. In contrast, MICs for the azole compounds were elevated and highest for itraconazole: >4 (1 to >4) and 4 (0.5 to >4) against isolates with azole resistance mechanisms compared to 0.125 (0.125 to 0.25) and 0.125 (0.06 to 0.25) against wild-type isolates, respectively. ASP2397 was active against A. terreus CYP51A wild-type isolates (MIC 0.5 to 1), whereas MICs of both azole and ASP2397 were elevated for the mutant isolate. ASP2397 displayed in vitro activity against A. fumigatus and A. terreus isolates which was independent of the presence or absence of azole target gene resistance mutations in A. fumigatus. The findings are promising at a time when azole-resistant A. fumigatus is emerging globally.
INTRODUCTION
Aspergillus causes invasive aspergillosis, which is a life-threatening infection, as well as a variety of less acute though still devastating chronic infections. The cornerstone in management of the disease is azole treatment due to the superiority of voriconazole in a randomized clinical trial and in postmarketing studies for treatment of invasive aspergillosis and the oral availability of azole drugs for outpatient treatment of chronic aspergillosis (1–8). However, increasing numbers of reports document emerging azole resistance in Europe, Africa, and the Asia-Pacific region, which highlights the importance of safe and efficacious alternatives (9–15). Amphotericin B is active but is associated with significant toxicity even in its lipid formulations, and echinocandins have only static activity against Aspergillus.
ASP2397 is a new compound with activity against Aspergillus species, including A. fumigatus, A. terreus, A. flavus, and A. nidulans, with an MIC range of 1 to 4 mg/liter in human serum (16). It is actively incorporated into A. fumigatus through the membrane siderophore transporter Sit1. The mode of action is novel and is as yet unknown but is different from that of the azoles and amphotericin. Hence, it may be a promising alternative option for the treatment of azole-resistant Aspergillus infections.
The purpose of this study was to evaluate the in vitro activity of ASP2397 in comparison with that of itraconazole, posaconazole, voriconazole, and amphotericin against a well-characterized panel of clinical Aspergillus species isolates with or without azole resistance mechanisms. Preliminary studies performed elsewhere had raised a potential concern that full inhibition might not be achieved if the higher inoculum and glucose concentration recommended by EUCAST were used. Therefore, the in vitro activity was examined using both the EUCAST and CLSI reference methodologies and modified EUCAST (mod-EUCAST) methods with either a 10-fold-lower inoculum or a spectrophotometer 50% growth inhibition endpoint.
MATERIALS AND METHODS
Thirty-four Aspergillus isolates and reference strains were included, specifically, (i) 24 A. fumigatus isolates with well-known azole resistance CYP51A target protein alterations (involving TR/L98H [5 isolates], alterations at the M220 codon [9 isolates] and at the G54 codon [9 isolates], and an HapE mutation [1 isolate]), (ii) 4 CYP51A wild-type A. fumigatus isolates, (iii) 1 A. terreus isolate with an M217I Cyp51Ap alteration, and (iv) 2 CYP51A wild-type A. terreus isolates. Finally, one EUCAST Aspergillus reference strain (A. fumigatus ATCC 204305), one CLSI Aspergillus reference strain (A. fumigatus ATCC MYA-2636), and the Candida krusei ATCC 6258 control strain recommended by both EUCAST and CLSI were included as controls.
CLSI and EUCAST testing was performed according to the EUCAST E.Def 9.2 and CLSI (M38-A2) methodologies (17, 18). In addition, a modified EUCAST method that uses a 10-fold lower fungal inoculum concentration (final inoculum concentration, 1 × 104 to 2.5 × 104 CFU/ml) was included. All isolates were cultured twice on Sabouraud agar (SSI Diagnostika, Hillerød, Denmark) before susceptibility testing was performed to ensure viability. The antifungal agents (manufacturers) used in stock solutions (5,000 mg/liter) in dimethyl sulfoxide (DMSO) (D8779; Sigma-Aldrich, Vallensbæk Strand, Denmark) were the following: ASP2397 (Astellas Pharma Inc., Tokyo, Japan), amphotericin B (Sigma-Aldrich), posaconazole (Merck, Ballerup, Denmark), and voriconazole (Pfizer A/S, Ballerup, Denmark). The drug concentration range studied was 0.03 to 4 mg/liter for all compounds. For both methods, plates were made in one batch, immediately frozen (−80°C), and used as soon as they were thawed. Inoculated plates were incubated at 35°C and read visually (blind to the species identity) at day 2. The primary endpoint was complete inhibition (MIC) for all drugs and methods as specified by CLSI and EUCAST. In addition, a spectrophotometer reading of the EUCAST plates using a 50% endpoint was performed. Finally, an additional visual endpoint reading for ASP2397 was performed for the EUCAST plates with a partial-inhibition endpoint (allowing weak growth) due to an initial concern that full inhibition would not be obtained for this agent in performing EUCAST susceptibility testing. The EUCAST MICs for the A. fumigatus ATCC 204305 and C. krusei ATCC 6258 reference strains were all within the recommended ranges for the licensed compounds (see Table S1 in the supplemental material). The CLSI MICs for ATCC MYA-2636 were also within the limits for voriconazole but were 1 dilution step lower than the recommended range for amphotericin B and itraconazole (ranges are not established for posaconazole). The quality control (QC) results confirmed the good performance of the susceptibility tests and the characteristic low-end MIC results as expected with freshly made trays.
RESULTS
ASP2397 and amphotericin B were equally efficacious in vitro against A. fumigatus isolates with or without Cyp51A mutations independently of the method or endpoint used (Fig. 1 and Table 1). The MICs obtained with the EUCAST method were in general 1 dilution step higher than those obtained with the other methods, and those obtained using a spectrophotometer 50% endpoint for the EUCAST plates were slightly lower (Table 1). The endpoints for ASP2397 were clear, with full inhibition at the high end concentrations even for the EUCAST method (see Fig. S1 in the supplemental material). A single A. fumigatus isolate with a G54E alteration was found to be consistently resistant to ASP2397 across all methods used, and one A. fumigatus isolate with a M220K alteration was found to be resistant by the EUCAST method due to only partial inhibition in the 1-to-4-mg/liter concentration range (Table 1 and Fig. 2).
FIG 1.
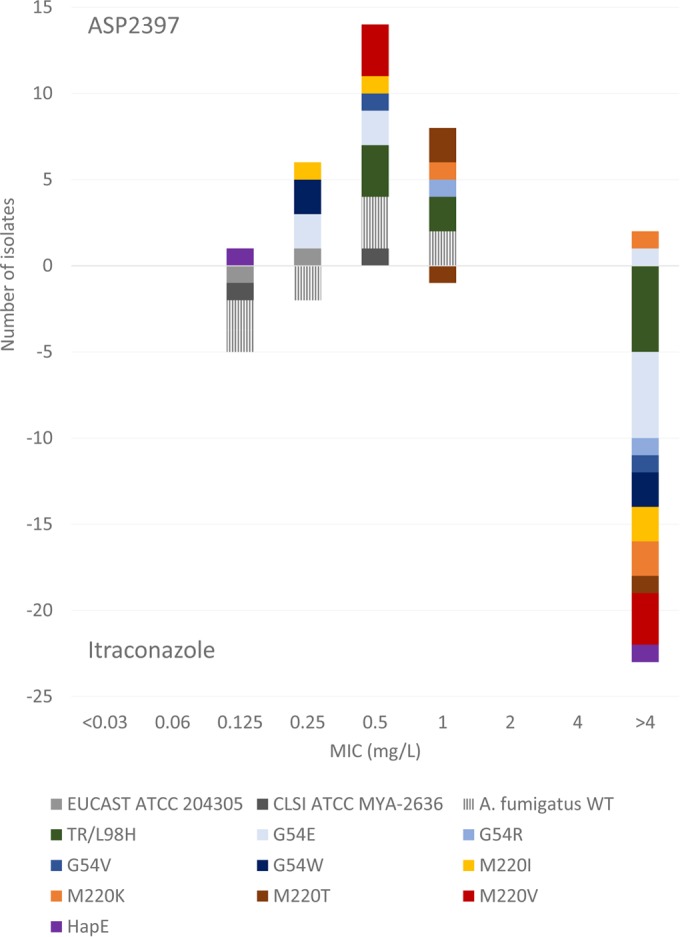
Histogram illustrating the uniform activity of ASP2397 (EUCAST MICs are shown above the x axis) across wild-type and azole-resistant A. fumigatus isolates compared to that of itraconazole (shown below the x axis). The strains are named according to the genotype, with “wt” indicating the wild-type strain versus the strains harboring different CYP51A alterations at position G54, M220, or TR/L98H. Reference strains are indicated with official strain numbers preceded by “EUCAST” or “CLSI.”
TABLE 1.
MIC50 and MIC ranges for ASP2397 in comparison with amphotericin B, itraconazole, posaconazole, and voriconazole against A. fumigatus with or without CYP51A mutationsa
| Antifungal agent and isolate category | MIC50 (range) (mg/liter) obtained by the specified method |
||||
|---|---|---|---|---|---|
| CLSI | EUCAST | EUCAST—partial inhib. | Modified EUCAST | EUCAST spec-50% | |
| ASP2397 | |||||
| Wild-type isolates | 0.25 (0.06 to 0.25) | 0.5 (0.25 to 1) | 0.25 (0.25 to 0.5) | 0.25 (0.125 to 0.5) | 0.25 (0.25) |
| Cyp51A mutants | 0.125 (0.06 to >4)b | 0.5 (0.125 to >4)c | 0.25 (0.15 to >4)b | 0.25 (0.06 to >4)b | 0.25 (0.06 to >4)b,d |
| Amphotericin B | |||||
| Wild-type isolates | 0.25 (0.125 to 0.25) | 0.25 (0.125 to 0.5) | ND | 0.25 (0.125 to 0.25) | 0.25 (0.125 to 0.5) |
| Cyp51A mutants | 0.25 (0.125 to 1) | 0.25 (0.125 to 1) | ND | 0.25 (0.125 to 1) | 0.25 (0.125 to 1) |
| Itraconazole | |||||
| Wild-type isolates | 0.125 (0.06 to 0.25) | 0.125 (0.125 to 0.25) | ND | 0.125 (0.06 to 0.25) | 0.06 (≤0.03 to 0.125) |
| Cyp51A mutants | 4 (0.5 to >4) | >4 (1 to >4) | ND | >4 (0.5 to >4) | >4 (0.5 to >4) |
| Posaconazole | |||||
| Wild-type isolates | ≤0.03 (≤0.03 to 0.06) | ≤0.03 (≤0.03 to 0.06) | ND | ≤0.03 (≤0.03) | ≤0.03 (≤0.03) |
| Cyp51A mutants | 0.5 (≤0.06 to 4) | 0.5 (0.06 to >4) | ND | 0.5 (0.125 to >4) | 0.25 (≤0.03 to >4) |
| Voriconazole | |||||
| Wild-type isolates | 0.25 (0.25 to 0.5) | 0.5 (0.5 to 1) | ND | 0.25 (0.25 to 0.5) | 0.25 (0.125 to 0.5) |
| Cyp51A mutants | 0.5 (0.125 to 4) | 0.5 (0.25 to >4) | ND | 0.5 (0.125 to 4) | 0.25 (0.125 to 4) |
partial inhib., partial-inhibition endpoint; spec-50%, spectrophotometer reading using a 50% growth inhibition endpoint; ND, not done.
One G54E isolate was found to be resistant (drug MIC of >4 mg/liter) to Asp2397 by all susceptibility tests methods used.
One G54E isolate and one M220K isolate had drug MICs of >4 mg/liter using the full-inhibition endpoint—the drug MIC for the M220K isolate was >4 only using the full-inhibition endpoint (a week of growth at concentrations of 1 to 4 mg/liter) and not using the other methods.
For one isolate, the growth curve crossed the 50% endpoint several times (trailing); thus, an automated endpoint could not be determined.
FIG 2.
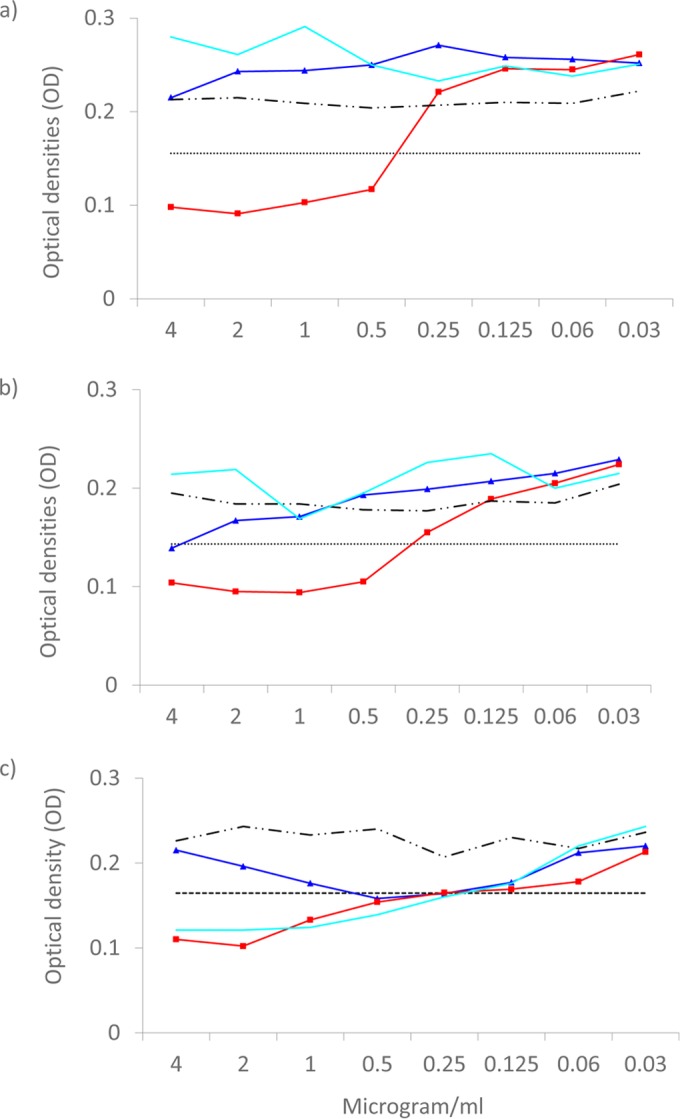
ASP2397 growth inhibition curves (light blue) for the atypical G54E and M220K mutant A. fumigatus isolates compared to those for itraconazole (dark blue) and posaconazole (red). (a and b) Growth curves for the G54E isolate determined by the EUCAST method (a) and a modified EUCAST method using a lower inoculum (b) show the fully resistant phenotype of this specific isolate. (c) In contrast, the phenotype of partial growth inhibition of the M220K isolate tested using the EUCAST method is shown. Results of growth in 8 growth control wells are shown by the upper broken black line. The horizontal dotted lines indicate 50% growth inhibition. Background absorbance in the medium corresponds to an optical density (OD at 490 nm) of approximately 0.1.
The activities of the three azoles are also summarized in Table 1. All wild-type isolates were classified as susceptible (S), adopting the EUCAST clinical breakpoints for the EUCAST results (itraconazole, ≤1 mg/liter; posaconazole, ≤0.125 mg/liter; voriconazole, ≤1 mg/liter). The most notable MIC increase against Cyp51A mutants was observed for itraconazole (Table 1). For example, the EUCAST itraconazole MIC50 increased from 0.125 to >4 mg/liter, and all mutant isolates except one (an M220T isolate for which the MIC was 1 mg/liter) were classified as resistant (Fig. 1). Similarly, all isolates, with the exception of the two isolates harboring the M220T alteration (drug MICs of 0.06 mg/liter), were classified as intermediate or resistant to posaconazole. Finally, voriconazole retained activity against isolates with alterations at the G54 codon and slightly reduced activity against isolates with alterations at the M220 codon, whereas the TR34/L98H and the HapE mutant isolates were classified as resistant. Again, the EUCAST MICs were slightly higher than the MICs obtained by the other methods (≤1 dilution step), but an overall excellent agreement was found between the MICs obtained by the different susceptibility methods.
Three A. terreus isolates, namely, two wild-type isolates and one isolate with a M217I alteration (corresponding to the M220I alteration in A. fumigatus), were included. The antifungal activity of ASP2397 against A. terreus appeared lower than against A. fumigatus, with CLSI MICs of 0.5 to 2 mg/liter, EUCAST MICs of 0.5 to >4 mg/liter, and mod-EUCAST MICs of 0.5 to 4 mg/liter (Table 1 and 2). Noticeably, the MICs were slightly higher against the M217I mutant isolate than against the wild-type isolates. This was also the case when either a less stringent visual minimum effective concentration (MEC) endpoint or a spectrophotometer reading with a 50% endpoint was used for the EUCAST tray (0.5 to 4 mg/liter) (Table 2). The amphotericin B MICs were within the expected range (1 to 4 mg/liter) for this species (Table 2). The efficacy of itraconazole and voriconazole against the M217I mutant was reduced, with MICs that were approximately 2 dilution steps higher than those against the wild-type isolates (the exact MIC could not be determined for posaconazole, as all endpoints were ≤0.03 mg/liter) (Table 2).
TABLE 2.
MICs for ASP2397 in comparison with amphotericin B, itraconazole, posaconazole, and voriconazole against A. terreus with or without CYP51A mutations
| Antifungal agent and isolate category | MIC (mg/liter) obtained by the specified method |
||||
|---|---|---|---|---|---|
| CLSI | EUCAST | EUCAST—partial inhib.a | Modified EUCAST | EUCAST spec-50% | |
| ASP2397 | |||||
| Wild-type isolates | 0.5–1 | 0.5–1 | 0.5 | 0.5–4 | 0.25–0.5 |
| Cyp51A mutant | 2 | >4 | 4 | 4 | 4 |
| Amphotericin B | |||||
| Wild-type isolates | 1–4 | 2–4 | ND | 2–4 | 1–2 |
| Cyp51A mutant | 2 | 1 | ND | 1 | 1 |
| Itraconazole | |||||
| Wild-type isolates | ≤0.03 | ≤0.03 | ND | ≤0.03–0.06 | ≤0.03 |
| Cyp51A mutant | 0.125 | 0.125 | ND | 0.125 | 0.06 |
| Posaconazole | |||||
| Wild-type isolates | ≤0.03 | ≤0.03 | ND | ≤0.03 | ≤0.03 |
| Cyp51A mutant | ≤0.03 | ≤0.03 | ND | ≤0.03 | ≤0.03 |
| Voriconazole | |||||
| Wild-type isolates | 0.25 | 0.5 | ND | 0.25–0.5 | 0.125–0.25 |
| Cyp51A mutant | 2 | 2 | ND | 2 | 1 |
partial inhib., partial-inhibition endpoint; spec-50%, spectrophotometer reading using a 50% growth inhibition endpoint; ND, not done.
DISCUSSION
The in vitro testing of ASP2397 showed excellent in vitro efficacy against wild-type strains as well as azole-resistant mutants of A. fumigatus. Acknowledging the caveat that in vitro efficacy should always be confirmed in vivo, this is obviously a finding with promising implications at a time when azole-resistant A. fumigatus strains are emerging and the only fungicidal alternative is amphotericin B, with infusion-related side effects and renal toxicity (9–15). In this context, it is noteworthy that trailing growth was not a common phenomenon at the higher concentrations such as is found for the Aspergillus fungistatic echinocandins.
Overall, the efficacy of ASP2397 was method independent in the sense that clear endpoints were observed for the CLSI method, the EUCAST method, and the modified EUCAST method using a reduced inoculum concentration. However, the MICs were 1 dilution step higher for the EUCAST method than for the CLSI method. EUCAST has adopted a higher glucose and inoculum concentration, which facilitate increased growth, and higher endpoints have also been seen for other substances, including the new compound isavuconazole, when A. fumigatus isolates have been susceptibility tested using both methods (19, 20). Similarly, MICs obtained using a 50% growth inhibition endpoint read by a spectrophotometer yielded lower MIC values, as expected when a less stringent endpoint criterion is adopted. Therefore, clinical breakpoints would need to be method specific in order to obtain a reproducible classification of isolates as susceptible, intermediate, or resistant across the two reference methods.
One A. fumigatus isolate harboring the M220K mutation was categorized as ASP2397 resistant by the EUCAST method. This was due to weak growth in the EUCAST wells containing 1 to 4 mg/liter ASP2397. This isolate was, however, susceptible in testing using the partial-inhibition endpoint or 50% spectrophotometer endpoint as well as by the CLSI and the modified EUCAST methodology. The clinical significance of this finding remains unclear. Notably, however, another A. fumigatus isolate harboring the G54E alteration was found to be resistant to ASP2397 by all methods and using all endpoints. Some cryptic species of A. fumigatus have differential levels of susceptibility to azoles. Although the susceptibility of such species to ASP2397 has not been studied, CYP51A as well as β-tubulin sequencing was undertaken and confirmed the species identification as A. fumigatus sensu stricto (data not shown). ASP2397 was originally isolated from an isolate of Acremonium (16). Acremonium and A. fumigatus are both ubiquitous in the environment, and, hypothetically, a resistance selection could take place in the environment if these fungi were found in close proximity of each other and ASP2397 was secreted extracellularly. Future studies of ASP2397 resistance epidemiology and the underlying molecular genes and mechanisms involved in acquired resistance to this new compound are warranted. Nevertheless, it is comforting that no other isolates of A. fumigatus were found to be ASP2392 resistant either in our study or in a previous study of 53 isolates (16).
The in vitro activities of ASP2397 against A. terreus were higher than those found against A. fumigatus, which may suggest that ASP2397 is in general less efficacious against this organism, a trend that has been observed previously (16). Moreover, the MICs against the isolate harboring the M217I Cyp51A azole resistance mechanism were elevated. This finding was slightly surprising, as CYP51A is not seen as the target for ASP2397. Further studies are needed to explore the underlying mechanisms behind the MIC elevation against this isolate and whether it is a coincidence that the two isolates in this study that were ASP2397 resistant both harbored a CYP51A alteration, particularly if the molecular resistance mechanism is somehow related to CYP51A mutations or to compensatory molecular changes induced by the CYP51A mutations. However, a greater number of isolates and efficacy studies in animal models would be needed to understand the clinical implications of this preliminary finding.
In conclusion, the results of this study support the notion that ASP2397 exhibits good in vitro activity against a broad range of clinically relevant azole-resistant mutants. This is truly interesting in the context of increasing azole resistance.
Supplementary Material
ACKNOWLEDGMENTS
We thank Birgit Brandt for excellent technical assistance.
Outside this study, M.C.A. has received research grants and travel grants from and has been paid for talks on behalf of Astellas Pharma, Basilea, Gilead, Merck Sharp & Dohme, and Pfizer. She has been on the advisory board for Merck and Gilead. R.H.J. has received grant support from Gilead Sciences and travel grants from Gilead, Merck Sharp & Dohme, Pfizer, and Astellas Pharma. M.C.-E. has received grant support from Astellas Pharma, bioMérieux, Gilead Sciences, Merck Sharp and Dohme, Pfizer, Schering Plough, Soria Melguizo SA, Ferrer International, the European Union, the ALBAN program, the Spanish Agency for International Cooperation, the Spanish Ministry of Culture and Education, the Spanish Health Research Fund, the Instituto de Salud Carlos III, the Ramon Areces Foundation, and the Mutua Madrileña Foundation. He has been an advisor/consultant to the Panamerican Health Organization, Astellas Pharma, Gilead Sciences, Merck Sharp and Dohme, Pfizer, and Schering Plough. He has been paid for talks on behalf of Gilead Sciences, Merck Sharp and Dohme, Pfizer, Astellas Pharma, and Schering Plough.
Funding Statement
The funders had no role in study design or data collection and interpretation.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.02336-15.
REFERENCES
- 1.Herbrecht R, Denning DW, Patterson TF, Bennett JE, Greene RE, Oestmann J-W, Kern WV, Marr KA, Ribaud P, Lortholary O, Sylvester R, Rubin RH, Wingard JR, Stark P, Durand C, Caillot D, Thiel E, Chandrasekar PH, Hodges MR, Schlamm HT, Troke PF, de Pauw B. 2002. Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N Engl J Med 347:408–415. doi: 10.1056/NEJMoa020191. [DOI] [PubMed] [Google Scholar]
- 2.Upton A, Kirby KA, Carpenter P, Boeckh M, Marr KA. 2007. Invasive aspergillosis following hematopoietic cell transplantation: outcomes and prognostic factors associated with mortality. Clin Infect Dis 44:531–540. doi: 10.1086/510592. [DOI] [PubMed] [Google Scholar]
- 3.Greene RE, Schlamm HT, Oestmann JW, Stark P, Durand C, Lortholary O, Wingard JR, Herbrecht R, Ribaud P, Patterson TF, Troke PF, Denning DW, Bennett JE, de Pauw BE, Rubin RH. 2007. Imaging findings in acute invasive pulmonary aspergillosis: clinical significance of the halo sign. Clin Infect Dis 44:373–379. doi: 10.1086/509917. [DOI] [PubMed] [Google Scholar]
- 4.Nivoix Y, Velten M, Letscher-Bru V, Moghaddam A, Natarajan-Amé S, Fohrer C, Lioure B, Bilger K, Lutun P, Marcellin L, Launoy A, Freys G, Bergerat J-P, Herbrecht R. 2008. Factors associated with overall and attributable mortality in invasive aspergillosis. Clin Infect Dis 47:1176–1184. doi: 10.1086/592255. [DOI] [PubMed] [Google Scholar]
- 5.Pagano L, Fianchi L, Fanci R, Candoni A, Caira M, Posteraro B, Morselli M, Valentini CG, Farina G, Mitra ME, Offidani M, Sanguinetti M, Tosti ME, Nosari A, Leone G, Viale P. 2010. Caspofungin for the treatment of candidaemia in patients with haematological malignancies. Clin Microbiol Infect 16:298–301. doi: 10.1111/j.1469-0691.2009.02832.x. [DOI] [PubMed] [Google Scholar]
- 6.Baddley JW, Andes DR, Marr KA, Kontoyiannis DP, Alexander BD, Kauffman CA, Oster RA, Anaissie EJ, Walsh TJ, Schuster MG, Wingard JR, Patterson TF, Ito JI, Williams OD, Chiller T, Pappas PG. 2010. Factors associated with mortality in transplant patients with invasive aspergillosis. Clin Infect Dis 50:1559–1567. doi: 10.1086/652768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lortholary O, Gangneux J-P, Sitbon K, Lebeau B, de Monbrison F, Le Strat Y, Coignard B, Dromer F, Bretagne S. 2011. Epidemiological trends in invasive aspergillosis in France: the SAIF network (2005–2007). Clin Microbiol Infect 17:1882–1889. doi: 10.1111/j.1469-0691.2011.03548.x. [DOI] [PubMed] [Google Scholar]
- 8.Herbrecht R, Patterson TF, Slavin MA, Marchetti O, Maertens J, Johnson EM, Schlamm HT, Donnelly JP, Pappas PG. 2015. Application of the 2008 definitions for invasive fungal diseases to the trial comparing voriconazole versus amphotericin B for therapy of invasive aspergillosis: a collaborative study of the Mycoses Study Group (MSG 05) and the European Organization for Research and Treatment of Cancer Infectious Diseases Group. Clin Infect Dis 60:713–720. doi: 10.1093/cid/ciu911. [DOI] [PubMed] [Google Scholar]
- 9.Walsh TJ, Anaissie EJ, Denning DW, Herbrecht R, Kontoyiannis DP, Marr KA, Morrison VA, Segal BH, Steinbach WJ, Stevens DA, van Burik J-A, Wingard JR, Patterson TF. 2008. Treatment of aspergillosis: clinical practice guidelines of the Infectious Diseases Society of America. Clin Infect Dis 46:327–360. doi: 10.1086/525258. [DOI] [PubMed] [Google Scholar]
- 10.Astvad KMT, Jensen RH, Hassan TM, Mathiasen EG, Thomsen GM, Pedersen UG, Christensen M, Hilberg O, Arendrup MC. 2014. First detection of TR46/Y121F/T289A and TR34/L98H alterations in Aspergillus fumigatus isolates from azole-naive patients in Denmark despite negative findings in the environment. Antimicrob Agents Chemother 58:5096–5101. doi: 10.1128/AAC.02855-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Steinmann J, Hamprecht A, Vehreschild MJGT, Cornely OA, Buchheidt D, Spiess B, Koldehoff M, Buer J, Meis JF, Rath P-M. 2015. Emergence of azole-resistant invasive aspergillosis in HSCT recipients in Germany. J Antimicrob Chemother 70:1522–1526. doi: 10.1093/jac/dku566. [DOI] [PubMed] [Google Scholar]
- 12.van der Linden JWM, Snelders E, Kampinga GA, Rijnders BJA, Mattsson E, Debets-Ossenkopp YJ, Kuijper EJ, van Tiel FH, Melchers WJG, Verweij PE. 2011. Clinical implications of azole resistance in Aspergillus fumigatus, The Netherlands, 2007–2009. Emerg Infect Dis 17:1846–1854. doi: 10.3201/eid1710.110226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Der Linden JWM, Camps SMT, Kampinga GA, Arends JPA, Debets-Ossenkopp YJ, Haas PJA, Rijnders BJA, Kuijper EJ, Van Tiel FH, Varga J, Karawajczyk A, Zoll J, Melchers WJG, Verweij PE. 2013. Aspergillosis due to voriconazole highly resistant Aspergillus fumigatus and recovery of genetically related resistant isolates from domiciles. Clin Infect Dis 57:513–520. doi: 10.1093/cid/cit320. [DOI] [PubMed] [Google Scholar]
- 14.Chowdhary A, Sharma C, van den Boom M, Yntema JB, Hagen F, Verweij PE, Meis JF. 2014. Multi-azole-resistant Aspergillus fumigatus in the environment in Tanzania. J Antimicrob Chemother 69:2979–2983. doi: 10.1093/jac/dku259. [DOI] [PubMed] [Google Scholar]
- 15.Lockhart SR, Frade JP, Etienne KA, Pfaller MA, Diekema DJ, Balajee SA. 2011. Azole resistance in Aspergillus fumigatus isolates from the ARTEMIS global surveillance study is primarily due to the TR/L98H mutation in the cyp51A gene. Antimicrob Agents Chemother 55:4465–4468. doi: 10.1128/AAC.00185-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakamura I, Ohsumi K, Yoshikawa K, Kanasaki R, Masaki T, Takase S, Hashimoto M, Fujie A, Nakai T, Matsumoto S, Takeda S, Akamatsu S, Uchida S, Maki K. 2014. ASP2397: a novel natural product with potent fungicidal activity against Aspergillus spp. (1)—a new mode of action and in vitro activity, abstr F1590 Abstr Intersci Conf Antimicrob Agents Chemother (ICAAC). [Google Scholar]
- 17.Arendrup MC, Hope W, Howard SJ. 2014. EUCAST definitive document E. Def 9.2 method for the determination of broth dilution minimum inhibitory concentrations of antifungal agents for conidia forming moulds. EUCAST. [Google Scholar]
- 18.CLSI. 2008. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi; approved standard—2nd ed. CLSI document M38-A2 Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 19.Howard SJ, Lass-Flörl C, Cuenca-Estrella M, Gomez-Lopez A, Arendrup MC. 2013. Determination of isavuconazole susceptibility of Aspergillus and Candida species by the EUCAST method. Antimicrob Agents Chemother 57:5426–5431. doi: 10.1128/AAC.01111-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Espinel-Ingroff A, Chowdhary A, Gonzalez GM, Lass-Flörl C, Martin-Mazuelos E, Meis J, Peláez T, Pfaller MA, Turnidge J. 2013. Multicenter study of isavuconazole MIC distributions and epidemiological cutoff values for Aspergillus spp. for the CLSI M38-A2 broth microdilution method. Antimicrob Agents Chemother 57:3823–3828. doi: 10.1128/AAC.00636-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


