Abstract
Increasing consumption of nitrofurantoin (NIT) for treatment of acute uncomplicated urinary tract infections (UTI) highlights the need to monitor emerging NIT resistance mechanisms. This study investigated the molecular epidemiology of the multidrug-resistant efflux gene oqxAB and its contribution to nitrofurantoin resistance by using Escherichia coli isolates originating from patients with UTI (n = 205; collected in 2004 to 2013) and food-producing animals (n = 136; collected in 2012 to 2013) in Hong Kong. The oqxAB gene was highly prevalent among NIT-intermediate (11.5% to 45.5%) and -resistant (39.2% to 65.5%) isolates but rare (0% to 1.7%) among NIT-susceptible (NIT-S) isolates. In our isolates, the oqxAB gene was associated with IS26 and was carried by plasmids of diverse replicon types. Multilocus sequence typing revealed that the clones of oqxAB-positive E. coli were diverse. The combination of oqxAB and nfsA mutations was found to be sufficient for high-level NIT resistance. Curing of oqxAB-carrying plasmids from 20 NIT-intermediate/resistant UTI isolates markedly reduced the geometric mean MIC of NIT from 168.9 μg/ml to 34.3 μg/ml. In the plasmid-cured variants, 20% (1/5) of isolates with nfsA mutations were NIT-S, while 80% (12/15) of isolates without nfsA mutations were NIT-S (P = 0.015). The presence of plasmid-based oqxAB increased the mutation prevention concentration of NIT from 128 μg/ml to 256 μg/ml and facilitated the development of clinically important levels of nitrofurantoin resistance. In conclusion, plasmid-mediated oqxAB is an important nitrofurantoin resistance mechanism. There is a great need to monitor the dissemination of this transferable multidrug-resistant efflux pump.
INTRODUCTION
Increasing resistance to co-trimoxazole and fluoroquinolone among Enterobacteriaceae, together with a lack of new oral antibiotics, has rekindled interest in nitrofurantoin (1). This drug was approved by the FDA more than 60 years ago for the treatment of lower urinary tract infection (UTI) (2). In a recent meta-analysis of controlled trials, nitrofurantoin was found to have clinical efficacy equivalent to that of co-trimoxazole, ciprofloxacin, and amoxicillin (3). When the treatment duration was short (<14 days), side effects were mild and limited mainly to gastrointestinal ones. Several recent guidelines have repositioned nitrofurantoin as a first-line antibiotic for the treatment of lower urinary tract infection (4). While the prevalence of nitrofurantoin resistance remained low, an exponential increase in the consumption of nitrofurantoin highlights the need to maintain vigilance in resistance surveillance (3, 5, 6).
In Escherichia coli, nitrofurantoin resistance was attributed primarily to mutations in chromosomal nitroreductase genes (nfsA and nfsB) that are involved in converting the drug into toxic intermediate compounds (7). However, nitrofurantoin resistance also may be plasmid encoded (8). While such transferable nitrofurantoin resistance has been recognized for decades, the nature and prevalence of occurrence have never been systematically assessed (1, 7). In 2003, a plasmid-encoded efflux pump, OqxAB, was detected in a multidrug-resistant E. coli isolate originating from swine manure from a Danish farm that used olaquindox (OLA) as a feed additive (9). The isolate was resistant to nitrofurantoin (MIC of 128 μg/ml). OqxAB was identified as a resistance nodulation division (RND)-type efflux pump with broad substrate specificity that included OLA, chloramphenicol, ciprofloxacin, nalidixic acid, trimethoprim, and disinfectants (e.g., benzalkonium, cetrimide, and chlorhexidine) (10). The oqxAB genes, which were flanked by IS26 elements, have been found on plasmids from E. coli and Salmonella enterica and in the native chromosome of Klebsiella spp. without the IS26 elements (11, 12). The high prevalence of oqxAB has been reported among E. coli and Salmonella isolates of animal or meat origins in China (13–17). In the current study, we investigated the association of plasmid-mediated oqxAB and nitrofurantoin resistance in E. coli isolates originating from human and animal sources. The contribution of oqxAB to nitrofurantoin resistance was examined by plasmid curing and mutation prevention concentration (MPC) assays.
MATERIALS AND METHODS
Bacterial strains and susceptibility testing.
Test isolates were drawn from several human clinical and animal intestinal E. coli collections from 2004 to 2013 (see Table S1 in the supplemental material). The clinical isolates were collected from outpatients and inpatients with urinary tract infections from multiple sites in Hong Kong between 2004 and 2013. The inclusion criteria were (i) originating from an adult (aged 18 years or above) and (ii) showing significant growth at ≥105 CFU/ml. The patient demographics and antimicrobial susceptibility of the isolate collections have been reported previously (2, 5, 18, 19). Animal isolates were obtained from an ongoing antimicrobial surveillance program between 2012 and 2013, during which 644 E. coli isolates were recovered from 347 animals, including 159 chickens (10 sampling dates), 111 pigs (32 sampling dates), and 77 cattle (10 sampling dates) (20, 21). For cattle and pigs, rectal swabs were obtained from fresh carcasses in a centralized slaughterhouse in Hong Kong. Specimens from chickens were obtained while the animals were held temporarily for inspection before sale in wet markets in Hong Kong. All cattle were imported from China. Pigs and chickens included animals reared in local farms and those imported from China. On each sampling date, specimens were obtained from 20 chickens, 10 cattle, and two to seven pigs by trained veterinary staff. Specimens (approximately 0.1 g of feces) were seeded with Dacron swabs onto three MacConkey agar plates (one plain and two supplemented with 2 μg/ml cefotaxime or 2 μg/ml ceftazidime) as previously described (21). Isolates from the same animal were considered unique if the resistance profiles for amikacin, tetracycline, co-trimoxazole, ciprofloxacin, gentamicin, chloramphenicol, and nitrofurantoin differ for at least one drug. One isolate from each MacConkey agar plate was included, and up to three isolates per animal were tested. The animal isolates were previously investigated for the presence of blaCTX-M (21).
All nitrofurantoin-intermediate (NIT-I) and nitrofurantoin-resistant (NIT-R) isolates in the collections were included (see Table S1 in the supplemental material). Of 202 such isolates, 180 (89.1%) were available. A subset of 161 nitrofurantoin-susceptible (NIT-S) isolates were included as controls. All of the NIT-I/R animal isolates were included irrespective of the MacConkey plate source. Approximately 10% of the available NIT-S isolates from chickens, pigs, and cattle were chosen randomly. The NIT-S isolates from human were chosen randomly to provide representation from different time periods. In total, 341 isolates, including 205 clinical isolates and 136 animal intestinal isolates (69 isolates from chickens, 56 from pigs, and 11 from cattle) were investigated retrospectively. All bacteria were identified as E. coli by the Vitek GNI system (bioMérieux Vitek Inc., Hazelwood, MO) or a Bruker matrix-assisted laser desorption ionization (MALDI) Biotyper system (Bruker Daltonics, Bremen, Germany). The antimicrobial susceptibility of the isolates was determined by the disc diffusion method and interpreted according to CLSI guidelines (22). The double-disc synergy test was used for phenotypic detection of extended-spectrum β-lactamases (ESBL) (23). Aerobic and anaerobic MICs of nitrofurantoin for selected isolates also were determined by Etest and interpreted according to the CLSI guidelines: ≤32 μg/ml, susceptible; 64 μg/ml intermediate; and ≥128 μg/ml, resistant (7, 22). Susceptibility to OLA was determined by spot inoculation from a 0.5 McFarland standard bacterial suspension onto Mueller-Hinton (MH) agar supplemented with 32 and 64 μg/ml of OLA. In previous studies, OLA MICs of isolates carrying oqxAB often were ≥128 μg/ml (10, 24).
MPC assays.
Plasmids pLOW2 and pLOW2::oqxAB (kindly provided by S. J. Sørensen) were introduced into E. coli J53 by transformation (24). Three isogenic strains were tested: (i) wild-type E. coli J53, (ii) E. coli J53 with plasmid vector (J53/pLOW2), and (iii) E. coli J53 with plasmid vector containing a 6-kb ApaLI insert that included IS6010-associated oqxAB from pOLA52 (J53/pLOW2::oqxAB) (25). Etest was used to determine the MICs of E. coli J53, J53/pLOW2, and J53/pLOW2::oqxAB. MPC assays were performed as previously described (26). Approximately 1010 organisms and appropriate dilutions were applied to Mueller-Hinton plates containing a range of nitrofurantoin concentrations (0, 2, 4, 6, 8, 16, 32, 48, 64, 128, 256, and 512 μg/ml). Surviving colonies were counted after incubation for 72 h at 37°C. Each strain was tested in duplicate in two independent experiments.
Detection of oqxAB and plasmid curing.
Previously reported primers were used to detect oqxA and oqxB (13). PCR mapping was used to detect the presence of IS26 elements on the two sides of oqxAB (see Table S2 in the supplemental material). A heat technique was used for plasmid curing (27). In brief, 250 μl of an overnight growth of the bacteria were inoculated into 5 ml of a fresh brain-heart infusion broth and incubated for 24 h at 44°C. The process described above was repeated every 24 h for a maximum of 21 passages. After each passage, a sample of the culture was diluted and spread onto a MacConkey plate. One hundred colonies were picked and replica inoculated onto a pair of plain and OLA-containing (64 μg/ml) MH agar plates. Colonies that failed to grow on the OLA-containing plate were investigated further. PCR for oqxAB was used to confirm successful curing of the oqxAB-carrying plasmid.
Detection of mutations in nfsA and nfsB genes.
The full lengths of nfsA and nfsB genes were amplified and sequenced by using previously described primers (7). Target gene mutations in the test strains were identified by comparison to the wild-type J53 sequences (GenBank accession number AICK00000000). Only mutations that are likely to contribute to nitrofurantoin resistance were considered significant. These include (i) gene-inactivating mutations (deletion, insertion, and nonsense mutation that results in a premature stop codon), (ii) nonsynonymous mutations involving residues in the active-site pocket, and (iii) nonsynonymous mutations involving other residues that have been demonstrated to reduce nitroreductase activity (7, 28, 29).
Detection of mutations in ribE gene.
The full length of the ribE gene, encoding lumazine synthase, was amplified and sequenced by using forward (ribE-FW; GCA TTT AGT GGG TGC ATG ATC) and reverse (ribE-RV; GGA ACT GGT ATT CAA CAT CAG CG) primers, and the sequence was compared to the wild-type J53 sequence (GenBank accession number AICK00000000). Nonsynonymous mutations involving active-site residues and loss-of-function mutations were considered significant (30, 31).
Strain typing.
Multilocus sequence typing (MLST) was carried out according to the Warwick scheme (32) (http://mlst.warwick.ac.uk/mlst/dbs/Ecoli). Sequence types (STs) were assigned to an ST complex (STC) when there were at least three isolates. STs with one or two isolates were designated singletons. The major E. coli phylogroups were determined by multiplex PCRs (32).
Plasmid studies.
The transferability of the oqxAB-carrying plasmids was investigated by filter mating using E. coli J53 (azide resistant) as the recipient (33). Transconjugants were selected on Luria-Bertani plates containing 150 μg/ml sodium azide and 32 μg/ml OLA. Plasmids were sized by the S1 nuclease pulsed-field gel electrophoresis (PFGE) method (21, 34). The replicon types for the E. coli transconjugants or parents with oqxAB-carrying plasmids were determined by an expanded PCR-based replicon typing scheme which recognizes HI1, HI2, I1, I2, L/M, N1, N2, FIA, FIB, FIC, FIIA, F, K, B/O, W, Y, P, A/C, A/C2, T, X1, X2, X3, and X4 replicons (21, 34, 35). The identification of the plasmid replicons was confirmed by sequencing the PCR products. In all isolates, the plasmid locations of oqxAB and replicons were confirmed by hybridization using specific PCR products as probes (21, 35, 36). Resistance genes cocarried on the oqxAB plasmids were identified by PCR assays (19, 33, 36).
Statistical analysis.
Fisher's exact test, one-way analysis of variance (ANOVA), or Wilcoxon signed-rank test was used for statistical analysis. A two-tailed P value of <0.05 was considered significant. All analyses were performed using commercial software packages (GraphPad Prism 5 [GraphPad, La Jolla, CA] and SPSS version 23.0 [SPSS Inc., Chicago, IL]).
RESULTS
Prevalence of oqxAB.
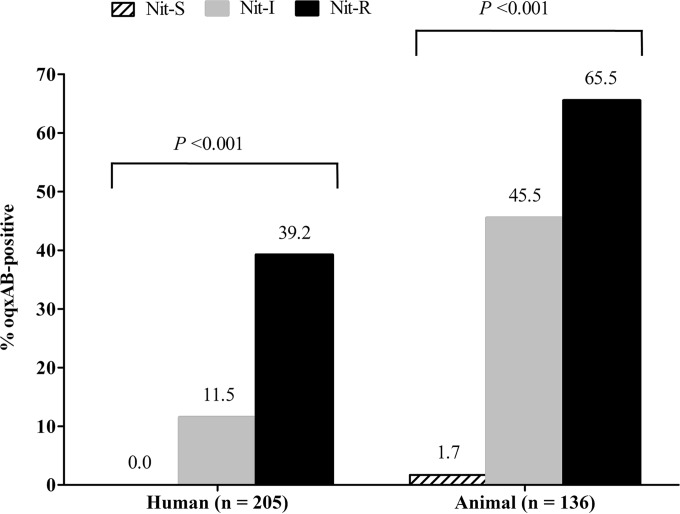
Among the 341 E. coli isolates, oqxAB was detected in 73 isolates (26 from patients, 30 from pigs, and 17 from chickens). The prevalence of oqxAB differed significantly according to nitrofurantoin susceptibility category, with the highest rate being among NIT-R isolates, followed by NIT-I isolates and then NIT-S isolates (Fig. 1). Except for one NIT-S isolate from a pig (strain P0452), all other NIT-S isolates were oqxAB negative. Among nitrofurantoin-intermediate and -resistant (NIT-I/R) isolates (n = 180), the oqxAB-positive rate was highest among isolates originating from pigs (80.6%; 29/36), followed by chickens (41.5%; 17/41) and patients (25.2%; 26/103) (P < 0.001). The prevalence of oqxAB among NIT-I/R isolates from patients in different time periods were similar, 26.8% (19/71) in 2004 to 2005, 20.0% (2/10) in 2006 to 2008, and 22.7% (5/22) in 2013. Overall, the prevalence of oqxAB was higher in ESBL-positive isolates (33.9%; 41/121) than in ESBL-negative isolates (14.6%; 32/220) (P < 0.001). Given that ESBL production was more common among animal isolates (70.8%) than human patient isolates (12.2%) (see Table S2 in the supplemental material), we further compared the prevalence of oqxAB after stratification by ESBL status. The analysis showed that the prevalence of oqxAB was significantly higher in animal isolates than in patient isolates, both for ESBL-positive (38.5% versus 16.0%; P = 0.036) and ESBL-negative isolates (25.0% versus 12.2%; P = 0.047).
FIG 1.
Prevalence of oqxAB stratified by host source and nitrofurantoin susceptibility, Hong Kong. The number of isolates is shown in parentheses. NIT, nitrofurantoin; S, susceptible; I, intermediate; R, resistant.
Characteristics of oqxAB-carrying E. coli isolates and plasmids.
MLST analysis revealed 34 different STs in eight ST complexes (STCs) and 22 singletons. STC10/phylogroup A (28.8%; 21/73), STC155/phylogroup B1 (6.8%; 5/73), and STC156/phylogroup B1 (6.8%; 5/73) were the most common (Table 1). The proportions of oqxAB-positive isolates distributed into the three major STCs by host sources were 82.4% (14/17) for chickens, 30.0% (9/30) for pigs, and 30.8% (8/26) for humans.
TABLE 1.
Characteristics of oqxAB-carrying E. coli isolates and plasmids
| Characteristic | n | No. (%) of isolates from: |
||
|---|---|---|---|---|
| Chicken | Pig | Human | ||
| E. coli clonea | ||||
| STC10/phylogroup A | 21 | 10 (58.8) | 5 (16.7) | 6 (23.1) |
| STC155/phylogroup B1 | 5 | 2 (11.8) | 2 (6.7) | 1 (3.8) |
| STC156/phylogroup B1 | 5 | 2 (11.8) | 2 (6.7) | 1 (3.8) |
| STC165/phylogroup A | 4 | 4 (13.3) | ||
| STC23/phylogroup C | 4 | 1 (3.3) | 3 (11.5) | |
| STC101/phylogroup B1 | 3 | 2 (6.7) | 1 (3.8) | |
| STC446/phylogroup B1 | 3 | 1 (5.9) | 2 (7.7) | |
| STC95/phylogroup B2 | 3 | 3 (11.5) | ||
| Singletonsb | 25 | 2 (11.8) | 14 (46.7) | 9 (34.6) |
| Total | 73 | 17 (100) | 30 (100) | 26 (100) |
| Plasmid replicon type(s) | ||||
| FIB | 20 | 3 (17.6) | 5 (16.7) | 12 (46.2) |
| FII | 8 | 4 (13.3) | 4 (15.4) | |
| FIB, FII | 3 | 1 (5.9) | 1 (3.3) | 1 (3.8) |
| HI2 and FIB-FII-N | 7 | 7c (41.2) | ||
| N1 | 5 | 1 (5.9) | 3 (10.0) | 1 (3.8) |
| X1 | 2 | 1 (3.3) | 1 (3.8) | |
| Otherd | 28 | 5 (29.4) | 16 (53.3) | 7 (26.9) |
| Total | 73 | 17 (100) | 30 (100) | 26 (100) |
STC, sequence type complex.
Including ST1642/phylogroup B1 (n = 3), ST117/phylogroup F (n = 2), and one each of the following 20 types: ST46/phylogroup A, ST127/phylogroup B2, ST162/phylogroup B1, ST393/phylogroup D, ST453/phylogroup B1, ST457/phylogroup F, ST641/phylogroup B1, ST746/phylogroup A, ST793/phylogroup A, ST877/phylogroup B1, ST1114/phylogroup A, ST1968/phylogroup A, ST2172/phylogroup A, ST2309/phylogroup D, ST3902/phylogroup A, ST4540/phylogroup B1, ST4541/phylogroup B2, ST4542/phylogroup A, ST4543/phylogroup A, and ST5070/phylogroup B1.
In these seven isolates, two plasmids of HI2 and FIB-FII-N were found to carry oqxAB.
In one isolate (strain 13QMH071), the plasmid location of oqxAB cannot be confirmed by hybridization because of DNA autodigestion and smearing. A total of 36 plasmids were found to harbor oqxAB in the remaining 27 isolates, of which 18 isolates had one oqxAB-carrying plasmid and nine isolates had two oqxAB-carrying plasmids. The 36 plasmids belong to the following replicon types: A/C2 (n = 3), FIA-FIB (n = 1), FIA-FIB-FII (n = 1), FIB-FII-P (n = 2), FIB-FIC-FII (n = 1), FIA-FIB-FIC-Y (n = 1), FIB (n = 1), FIB-N (n = 1), FII-FIC (n = 1), FII-B/O-K (n = 2), FII-K (n = 2), FII-X2 (n = 1), I1 (n = 3), I2 (n = 1), K (n = 1), N (n = 1), X2 (n = 1), X4 (n = 1), and nontypeable (n = 11).
In 70 isolates, PCR mapping revealed that the oqxAB genes were flanked by two copies of IS26 in the same orientation, comprising Tn6010. The remaining three isolates had only one copy of IS26 in the upstream (n = 2) or the downstream (n = 1) end. In conjugation experiments, the plasmids carrying oqxAB could be transferred in 24.7% (18/73) of the isolates at frequencies of 10−4 to 10−9 per donor cell. PCR and hybridization probes were used to determine the replicon types of the oqxAB-carrying plasmids in the 18 transconjugants and the remaining 55 parent strains. Despite repeated testing, the plasmid location of the Tn6010-associated oqxAB for one human isolate (strain 13QMH071) cannot be confirmed because of plasmid DNA autodigestion and smearing. No hybridization signal was obtained in the chromosome of this isolate. In 16 isolates, oqxAB was carried by two plasmids. Hence, a total of 88 oqxAB-carrying plasmids (with sizes of 50 to 200 kb) were detected in 72 isolates (Table 1).
Sixteen different replicons (IncA/C2, IncB/O, IncFIA, IncFIB, IncFIC, IncFII, IncHI2, IncK, IncI1, IncI2, IncN1, IncP, IncX1, IncX2, IncX4, and IncY), either alone or in combination, were found among the oqxAB-carrying plasmids (Table 1). The two most common replicon types, alone or in combination with other replicons, were IncFIB (52.1%; 38/73) and IncFII (34.2%; 25/73). The replicon types of the oqxAB-carrying plasmids in the 18 transconjugants were the following: IncFIB (n = 7), IncFIB, FII (n = 2), IncFII (n = 3), IncFIA, FIB-FIC-Y (n = 1), IncN1 (n = 1), IncX1 (n = 1), IncX4 (n = 1), and nontypeable (n = 2). All transconjugants exhibited reduced susceptibility or resistance to nitrofurantoin and other agents that are substrates of the OqxAB pump, including OLA, chloramphenicol, nalidixic acid, and trimethoprim (see Table S3 in the supplemental material). In 12 transconjugants with the oqxAB-carrying plasmid as the only plasmid, there was cotransfer of resistance to ampicillin (n = 10), cefotaxime (n = 1), fosfomycin (n = 1), gentamicin (n = 2), and tetracycline (n = 8). PCR revealed that the resistance genes cotransferred were blaTEM (n = 10), blaCTX-M (n = 1), fosA3 (n = 1), aacC2 (n = 1), aacC4 (n = 1), tet(A) (n = 5), and tet(B) (n = 3).
In general, oqxAB-carrying plasmids of different replicon types and sizes were detected in isolates of the same STs, indicating limited clonal spread. An exception was noted for seven isolates originating from chickens sampled on different dates from January to August 2013. In the seven isolates, all of which were ST48/phylogroup A, oqxAB was cocarried on two plasmids of IncHI2 (size, ∼200 kb) and IncFIB-FII-N (size, ∼120 kb).
Phenotypic detection of oqxAB.
The CLSI disc diffusion breakpoints for nalidixic acid, chloramphenicol, and nitrofurantoin nonsusceptibility displayed high sensitivities of 98.6%, 94.5%, and 98.6%, respectively, and high negative predictive values of 98.9%, 97.4%, and 99.4%, respectively, for oqxAB detection (see Table S2 in the supplemental material). The lowest sensitivities were displayed by trimethoprim (87.7%) and ciprofloxacin (71.2%). These five compounds displayed low specificities (32.5% to 59.7%) and positive predictive values (28.5% to 40.0%). The sensitivity and negative predictive value of OLA resistance were 97.3% and 99.2% (for both 32 μg/ml and 64 μg/ml), respectively. The specificity/positive predictive value of OLA at 32 μg/ml (90.3%/73.2%) was lower than that for OLA at 64 μg/ml (95.6%/86.6%).
Effect of oqxAB on MPC.
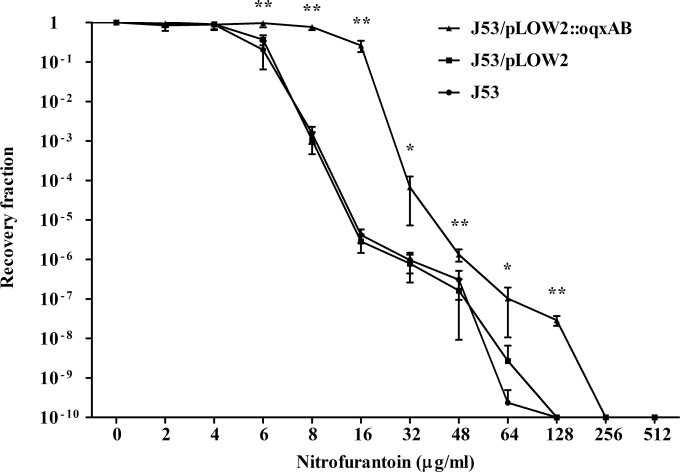
MICs of nitrofurantoin for E. coli J53, J53/pLOW2, and J53/pLOW2::oqxAB were 8 μg/ml, 8 μg/ml, and 32 μg/ml, respectively. MPC testing revealed that NIT-R mutants were recovered over a wide range of drug concentrations (Fig. 2). The MPCs of nitrofurantoin for J53 and J53/pLOW2 both were 128 μg/ml. The presence of oqxAB increased the MPC of J53/pLOW2::oqxAB to 256 μg/ml. At concentrations ranging from 6 to 128 μg/ml, the counts of resistant mutants were 10- to 105-fold higher for J53/pLOW2::oqxAB than for E. coli J53 or J53/pLOW2 (Fig. 2).
FIG 2.
Mutation prevention concentration (MPC) assay. Approximately 1010 organisms were applied to Mueller-Hinton agar plates containing 0 to 512 μg/ml of nitrofurantoin. Surviving colonies were counted after a 72-h incubation at 37°C. All bacterial strains were tested in duplicate in two independent experiments. The mean recovery fraction and standard deviations are shown. Recovery fractions of J53, J53/pLOW2, and J53/pLOW2::oqxAB strains were compared by ANOVA. *, P < 0.05; **, P < 0.001.
Contribution of oqxAB to nitrofurantoin resistance.
To assess the contribution of oqxAB to nitrofurantoin resistance, 44 out of 103 available NIT-I/R clinical isolates were randomly chosen for nfsA and nfsB sequencing, and 20 NIT-S clinical isolates were included as controls. Of the 44 NIT-I/R isolates, 20 (45.5%) were oqxAB positive. Significant mutations in nfsA and nfsB were detected in 15 (34.1%) and 2 (4.5%) of the 44 NIT-I/R isolates, respectively (Table 2). No significant mutations were detected in the NIT-S isolates. The ribE genes in the 44 NIT-I/R isolates were sequenced. In total, 15 isolates had one or more synonymous substitutions (C57T, T165A, C267T, G345T, G318A, and G345T). Nonsynonymous substitutions at non-active-site residues were found in four of the 15 isolates (one had Asn2Asp and three had Val51Ile). The four isolates (two NIT-I and two NIT-R) with non-active-site residue substitutions had wild-type nfsA and nfsB and were oqxAB positive. The remaining 29 isolates had no mutation. Therefore, no significant ribE mutation was found.
TABLE 2.
Nitrofurantoin resistance determinants in 64 clinical E. coli isolates
| Resistance determinant | No. (%) of isolates |
||
|---|---|---|---|
| NIT-S | NIT-Ic | NIT-Rc | |
| nfsA mutationsa | 0 | 3 (25.0) | 6 (18.8) |
| nfsA mutations and oqxABa | 0 | 0 | 6 (18.8) |
| nfsB mutationsb | 0 | 0 | 2 (6.3) |
| oqxAB | 0 | 6 (50.0) | 14 (43.8) |
| None | 20 (100) | 3 (25.0) | 4 (12.5) |
| Total | 20 (100) | 12 (100) | 32 (100) |
The 15 nfsA mutations include six deletions (three had a deletion of the first 26 amino acids, one had a deletion of amino acids 191 to 197, one had deletion T25 [frameshift C9], and one had deletion C605 [frameshift R203]), three insertions (IS1; one each at nucleotide positions −24, 70, and 413), four premature stop mutations (one Q44Stop, one K141Stop, and two E233Stop), and two substitutions in active-site residues (one R133S and one R203C).
The two nfsB mutations include a premature stop (E137stop) and an IS5 insertion.
No loss-of-function or other significant mutations of ribE were detected in the 44 NIT-I/R isolates.
To assess the contribution of oqxAB to nitrofurantoin resistance, 29 oqxAB-carrying NIT-I/R isolates (including 26 clinical isolates and three in vitro J53 mutants from the MPC experiments) were subjected to heat curing of plasmids. Plasmid-cured variants (PCVs) were successfully obtained from the three mutants and 20 of the 26 clinical isolates. All PCVs were PCR negative for oqxAB genes. Curing of pLOW2::oqxAB from the three in vitro mutants lowered the aerobic MIC of nitrofurantoin from ≥512 μg/ml to 32 to 64 μg/ml. In view of the elevated MICs compared with that for the wild-type J53 strain (MIC of 8 μg/ml), we sequenced the nfsA, nfsB, and ribE genes in the three PCVs of the mutants. Unexpectedly, no mutation was found to be common to all three genes.
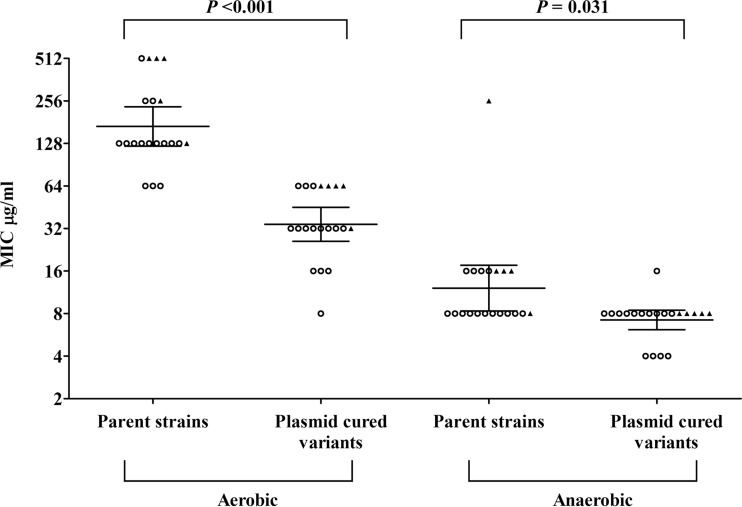
Among the 20 PCVs of the clinical parent strains, five had nfsA mutations (two deletions, one insertion, and two premature stops). None had nfsB mutation. The geometric mean MIC of nitrofurantoin was higher in oqxAB-carrying isolates with nfsA mutations (388.0 μg/ml) than in those with oqxAB but no nfsA mutations (134.1 μg/ml) (Fig. 3). Plasmid curing significantly reduced the geometric mean aerobic MIC of nitrofurantoin for the 20 clinical isolates, from 168.9 μg/ml to 34.3 μg/ml. Except for one strain (with no change in MIC), plasmid curing reduced the MIC by 2- to 16-fold. In the PCVs, 20% (1/5) of isolates with nfsA mutations were NIT-S, while 80% (12/15) of isolates without nfsA mutations were NIT-S (P = 0.031). Even greater MIC reductions were observed following anaerobic incubation. Geometric means of anaerobic MICs for parent strains and PCVs were 12.1 μg/ml and 7.2 μg/ml, respectively (Fig. 3).
FIG 3.
Comparison of nitrofurantoin geometric mean MICs for parent strains and plasmid-cured variants. The scatterplot shows MIC data for the parent strains and plasmid-cured variants following aerobic and anaerobic incubation. Solid triangles and open circles were used to represent the presence and absence of nfsA mutations, respectively. No strain had an nfsB mutation. Geometric means and 95% confidence intervals were indicated by horizontal lines in the groups. The geometric mean MICs for parent strains and plasmid-cured variants were compared by Wilcoxon signed-rank test.
DISCUSSION
There were variations in the prevalence of oqxAB by host source of the isolates, with the highest prevalence for pigs followed by chickens and human, and none were detected from cattle. He et al. investigated 1,123 E. coli isolates originating from 19 farms in north and south China from the 1970s to 2013 (14). Following the initial detection of oqxAB in the early 1990s, the prevalence of this resistance mechanism among E. coli isolates of swine and poultry origin increased rapidly, from 12.7 to 19.4% between 1990 and 2004 to 25.6% in 2005, and it has remained at >40% since 2010 (14). Among the veterinary antimicrobials, OLA and carbadox are OqxAB pump substrates (10). Since the 2000s, the veterinary use of OLA and carbadox in many countries has been banned, because they are both mutagenic and genotoxic (37). In China, other less toxic and chemically related compounds, namely, mequindox and quinocetone, are approved for use in animal feed for pigs and poultry (14, 37). The widespread use of these compounds may exert selection pressure for the rapid emergence of oqxAB in swine and poultry isolates. These chemical compounds are seldom used in cattle (13). Humans may be exposed to these resistant bacteria through visits to farms, wet markets, and contacts with contaminated meats (17, 38).
In our isolates, plasmid-mediated oqxAB was contained within the IS26-flanked Tn6010 transposon, as in the prototype plasmid pOLA52 (25). This transposon is unstable and prone to excision via IS26-mediated recombination (14, 39). Hall et al. further demonstrated that the replicative transposition of IS26 can result in a cointegrate plasmid with replicon fusions from donor and target plasmids (39). Such properties of IS26 may explain the high prevalence (26.1%) (Table 1) of multireplicon status among the oqxAB-carrying plasmids in this study. In comparison, only 8.8% of the E. coli plasmids carrying ISEcp1-blaCTX-M from similar bacterial collections were of multireplicon status (33). In our bacterial collection, oqxAB most often was carried by IncF-type plasmids. We interpret this to reflect the high prevalence of IncF plasmids in E. coli populations and their availability for Tn6010 integration (35). In Guangdong, IncF plasmids also were commonly found to carry Tn6010-associated oqxAB (15). Our findings and previous literature indicated that Tn6010, rather than bacterial clones and epidemic plasmids, is involved mainly in the dissemination of oqxAB in bacteria (11, 13, 15, 17).
It is notable that oqxAB was detected in a substantial proportion (39.2%) of the NIT-R isolates from patients with urinary tract infections. In E. coli, nitrofurantoin resistance was previously considered to be caused mainly by inactivating mutations in the oxygen-insensitive nitroreductase genes (nfsA and nfsB) (7, 29). Of the 32 NIT-R isolates that were investigated for nfsA and nfsB mutations, inactivating nfsA mutations were detected in 12 isolates, one of which had mutations in both nfsA and nfsB genes. This is unexpected, because nfsA inactivation causes only low-level resistance (7). The inactivation of nfsB following nfsA inactivation is required for the development of high-level resistance (MIC of ≥128 μg/ml) (7, 29). Our data showed that this level of nitrofurantoin resistance also could be caused by the combination of the presence of oqxAB and an nfsA mutation. Previous studies showed that MICs for susceptible clinical isolates usually ranged from 8 to 16 μg/ml (40). Although plasmid curing was associated with the reversion of the resistance phenotype in clinical isolates without nfsA mutations, some of the PCVs did not exhibit wild-type MICs. Hence, it remains unclear whether oqxAB alone is sufficient to cause high-level nitrofurantoin resistance. The curing of oqxAB-carrying plasmids did not restore wild-type nitrofurantoin susceptibility in the three J53 in vitro mutants. Since the nfsA and nfsB genes were wild type in the mutants, we postulate the nitrofurantoin resistance is caused by other novel mechanisms that developed during the MPC experiments. This also would explain why many of the PCVs without nfsA and nfsB mutations had MICs of 32 to 64 μg/ml. Besides nfsA and nfsB, the inactivation of the ribE gene also could contribute to nitrofurantoin resistance (31). However, ribE genes in all of the NIT-I/R clinical isolates and J53 in vitro mutants were wild type. In eight NIT-I/R isolates (wild-type nfsA and nfsB, wild-type ribE, and oqxAB negative), no resistance determinants were found (Table 2). These data also imply novel nitrofurantoin resistance mechanisms.
The nitrofurantoin resistance phenotype associated with plasmid-mediated oqxAB resulted in a 2- to 16-fold increase in nitrofurantoin MIC (Fig. 3; also see Table S3 in the supplemental material). However, the elevated nitrofurantoin MICs may still be classified as nitrofurantoin susceptible at the current CLSI breakpoint. An example is the NIT-S, oqxAB-positive strain P0452. It had a nitrofurantoin MIC of 32 μg/ml. Sequencing of the oqxAB operon in the strain confirmed that the genes are not inactivated (data not shown). In both parental and PCV strains, anaerobic incubation yielded nitrofurantoin MICs that were substantially lower than those obtained after aerobic incubation. This is because oxygen-sensitive nitroreductase in E. coli can generate bioreactive intermediates from nitrofurantoin under anaerobic conditions (7, 41). However, the anaerobic MIC of oqxAB-positive parents remained significantly higher than those for PCVs. The data suggested that oqxAB pump activities are not completely inhibited under anaerobic conditions. As demonstrated by the MPC experiments, the pump activities may enable the organism to temporarily withstand nitrofurantoin and facilitates the emergence of higher-level resistance in the presence of nitrofurantoin at therapeutic levels (50 to 200 μg/ml) that were reached in urine after a standard 100-mg dose (42). It is concerning that one of the oqxAB plasmids also cocarried fosA3. Besides nitrofurantoin, fosfomycin is another important oral antibiotic for the treatment of uncomplicated urinary tract infections (1). In several Asian countries, fosA3 currently is the main mechanism of fosfomycin resistance in E. coli (19, 34). The cocirculation of plasmid-mediated oqxAB and fosA3 should be closely monitored. For clinical laboratories, our findings show that screening for OLA resistance would facilitate the identification of Tn6010-associated oqxAB.
In conclusion, this study documented plasmid-mediated, Tn6010-associated oqxAB to be an important nitrofurantoin resistance determinant. The acquisition of oqxAB in E. coli could mediate resistance to nitrofurantoin while plasmid curing caused marked MIC reductions. The efflux pump also could facilitate the development of clinically important levels of nitrofurantoin resistance. Emerging nitrofurantoin resistance in clinical E. coli isolates is worrisome, because this drug is a key drug for the treatment of uncomplicated urinary tract infection.
Supplementary Material
ACKNOWLEDGMENTS
We thank the staff at the Food and Environmental Hygiene Department (FHED) of the Hong Kong Special Administrative Region (HKSAR) for assistance with specimen collection and the research staff (Connie Chan, Jane Chan, and Andes Lau) in the Department of Microbiology, University of Hong Kong, for assistance with the microbiological investigations.
This work was supported by grants from the Consultancy Service for Enhancing Laboratory Surveillance of Emerging Infectious Disease for the HKSAR, Department of Health, and the Health and Medical Research Fund of the Food and Health Bureau of the Government of the HKSAR.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.02156-15.
REFERENCES
- 1.Giske CG. 2015. Contemporary resistance trends and mechanisms for the old antibiotics colistin, temocillin, fosfomycin, mecillinam and nitrofurantoin. Clin Microbiol Infect 21:899–905. doi: 10.1016/j.cmi.2015.05.022. [DOI] [PubMed] [Google Scholar]
- 2.Ho PL, Wong RC, Yip KS, Loke SL, Leung MS, Mak GC, Chow FK, Tsang KW, Que TL. 2007. Antimicrobial resistance in Escherichia coli outpatient urinary isolates from women: emerging multidrug resistance phenotypes. Diagn Microbiol Infect Dis 59:439–445. doi: 10.1016/j.diagmicrobio.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 3.Huttner A, Verhaegh EM, Harbarth S, Muller AE, Theuretzbacher U, Mouton JW. 2015. Nitrofurantoin revisited: a systematic review and meta-analysis of controlled trials. J Antimicrob Chemother 70:2456–2464. doi: 10.1093/jac/dkv147. [DOI] [PubMed] [Google Scholar]
- 4.Gupta K, Hooton TM, Naber KG, Wullt B, Colgan R, Miller LG, Moran GJ, Nicolle LE, Raz R, Schaeffer AJ, Soper DE. 2011. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis 52:e103–e120. doi: 10.1093/cid/ciq257. [DOI] [PubMed] [Google Scholar]
- 5.Ho PL, Chu YP, Lo WU, Chow KH, Law PY, Tse CW, Ng TK, Cheng VC, Que TL. 2015. High prevalence of Escherichia coli sequence type 131 among antimicrobial-resistant E. coli isolates from geriatric patients. J Med Microbiol 64:243–247. doi: 10.1099/jmm.0.000012. [DOI] [PubMed] [Google Scholar]
- 6.Sanchez GV, Baird AM, Karlowsky JA, Master RN, Bordon JM. 2014. Nitrofurantoin retains antimicrobial activity against multidrug-resistant urinary Escherichia coli from US outpatients. J Antimicrob Chemother 69:3259–3262. doi: 10.1093/jac/dku282. [DOI] [PubMed] [Google Scholar]
- 7.Sandegren L, Lindqvist A, Kahlmeter G, Andersson DI. 2008. Nitrofurantoin resistance mechanism and fitness cost in Escherichia coli. J Antimicrob Chemother 62:495–503. doi: 10.1093/jac/dkn222. [DOI] [PubMed] [Google Scholar]
- 8.Breeze AS, Obaseiki-Ebor EE. 1983. Transferable nitrofuran resistance conferred by R-plasmids in clinical isolates of Escherichia coli. J Antimicrob Chemother 12:459–467. doi: 10.1093/jac/12.5.459. [DOI] [PubMed] [Google Scholar]
- 9.Sorensen AH, Hansen LH, Johannesen E, Sorensen SJ. 2003. Conjugative plasmid conferring resistance to olaquindox. Antimicrob Agents Chemother 47:798–799. doi: 10.1128/AAC.47.2.798-799.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hansen LH, Jensen LB, Sorensen HI, Sorensen SJ. 2007. Substrate specificity of the OqxAB multidrug resistance pump in Escherichia coli and selected enteric bacteria. J Antimicrob Chemother 60:145–147. doi: 10.1093/jac/dkm167. [DOI] [PubMed] [Google Scholar]
- 11.Guillard T, Lebreil AL, Hansen LH, Kisserli A, Berger S, Lozniewski A, Alauzet C, de Champs C. 2015. Discrimination between native and Tn6010-associated oqxAB in Klebsiella spp., Raoultella spp. and other Enterobacteriaceae using a two-step strategy. Antimicrob Agents Chemother 59:5838–5840. doi: 10.1128/AAC.00669-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li L, Liao X, Yang Y, Sun J, Li L, Liu B, Yang S, Ma J, Li X, Zhang Q, Liu Y. 2013. Spread of oqxAB in Salmonella enterica serotype Typhimurium predominantly by IncHI2 plasmids. J Antimicrob Chemother 68:2263–2268. [DOI] [PubMed] [Google Scholar]
- 13.Chen X, Zhang W, Pan W, Yin J, Pan Z, Gao S, Jiao X. 2012. Prevalence of qnr, aac(6′)-Ib-cr, qepA, and oqxAB in Escherichia coli isolates from humans, animals, and the environment. Antimicrob Agents Chemother 56:3423–3427. doi: 10.1128/AAC.06191-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.He T, Wang Y, Qian M, Wu C. 2015. Mequindox resistance and in vitro efficacy in animal-derived Escherichia coli strains. Vet Microbiol 177:341–346. doi: 10.1016/j.vetmic.2015.04.007. [DOI] [PubMed] [Google Scholar]
- 15.Liu BT, Yang QE, Li L, Sun J, Liao XP, Fang LX, Yang SS, Deng H, Liu YH. 2013. Dissemination and characterization of plasmids carrying oqxAB-blaCTX-M genes in Escherichia coli isolates from food-producing animals. PLoS One 8:e73947. doi: 10.1371/journal.pone.0073947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Z, Meng X, Wang Y, Xia X, Wang X, Xi M, Meng J, Shi X, Wang D, Yang B. 2014. Presence of qnr, aac(6′)-Ib, qepA, oqxAB, and mutations in gyrase and topoisomerase in nalidixic acid-resistant Salmonella isolates recovered from retail chicken carcasses Foodborne. Pathog Dis 11:698–705. doi: 10.1089/fpd.2014.1736. [DOI] [PubMed] [Google Scholar]
- 17.Zhao J, Chen Z, Chen S, Deng Y, Liu Y, Tian W, Huang X, Wu C, Sun Y, Sun Y, Zeng Z, Liu JH. 2010. Prevalence and dissemination of oqxAB in Escherichia coli isolates from animals, farmworkers, and the environment. Antimicrob Agents Chemother 54:4219–4224. doi: 10.1128/AAC.00139-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ho PL, Yip KS, Chow KH, Lo JY, Que TL, Yuen KY. 2010. Antimicrobial resistance among uropathogens that cause acute uncomplicated cystitis in women in Hong Kong: a prospective multicenter study in 2006 to 2008. Diagn Microbiol Infect Dis 66:87–93. doi: 10.1016/j.diagmicrobio.2009.03.027. [DOI] [PubMed] [Google Scholar]
- 19.Ho PL, Chan J, Lo WU, Lai EL, Cheung YY, Lau TC, Chow KH. 2013. Prevalence and molecular epidemiology of plasmid-mediated fosfomycin resistance genes among blood and urinary Escherichia coli isolates. J Med Microbiol 62:1707–1713. doi: 10.1099/jmm.0.062653-0. [DOI] [PubMed] [Google Scholar]
- 20.Ho PL, Chow KH, Lai EL, Lo WU, Yeung MK, Chan J, Chan PY, Yuen KY. 2011. Extensive dissemination of CTX-M-producing Escherichia coli with multidrug resistance to “critically important” antibiotics among food animals in Hong Kong, 2008-10. J Antimicrob Chemother 66:765–768. doi: 10.1093/jac/dkq539. [DOI] [PubMed] [Google Scholar]
- 21.Ho PL, Liu MC, Lo WU, Lai EL, Lau TC, Law OK, Chow KH. 2015. Prevalence and characterization of hybrid blaCTX-M among Escherichia coli isolates from livestock and other animals. Diagn Microbiol Infect Dis 82:148–153. [DOI] [PubMed] [Google Scholar]
- 22.CLSI. 2015. Performance standards for antimicrobial susceptibility testing: 25th informational supplement M100-S25. CLSI, Wayne, PA. [Google Scholar]
- 23.Ho PL, Chow KH, Yuen KY, Ng WS, Chau PY. 1998. Comparison of a novel, inhibitor-potentiated disc-diffusion test with other methods for the detection of extended-spectrum beta-lactamases in Escherichia coli and Klebsiella pneumoniae. J Antimicrob Chemother 42:49–54. doi: 10.1093/jac/42.1.49. [DOI] [PubMed] [Google Scholar]
- 24.Hansen LH, Johannesen E, Burmolle M, Sorensen AH, Sorensen SJ. 2004. Plasmid-encoded multidrug efflux pump conferring resistance to olaquindox in Escherichia coli. Antimicrob Agents Chemother 48:3332–3337. doi: 10.1128/AAC.48.9.3332-3337.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Norman A, Hansen LH, She Q, Sorensen SJ. 2008. Nucleotide sequence of pOLA52: a conjugative IncX1 plasmid from Escherichia coli which enables biofilm formation and multidrug efflux. Plasmid 60:59–74. doi: 10.1016/j.plasmid.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 26.Robicsek A, Strahilevitz J, Jacoby GA, Macielag M, Abbanat D, Park CH, Bush K, Hooper DC. 2006. Fluoroquinolone-modifying enzyme: a new adaptation of a common aminoglycoside acetyltransferase. Nat Med 12:83–88. doi: 10.1038/nm1347. [DOI] [PubMed] [Google Scholar]
- 27.Schaufler K, Wieler LH, Semmler T, Ewers C, Guenther S. 2013. ESBL-plasmids carrying toxin-antitoxin systems can be “cured” of wild-type Escherichia coli using a heat technique. Gut Pathog 5:34. doi: 10.1186/1757-4749-5-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kobori T, Sasaki H, Lee WC, Zenno S, Saigo K, Murphy ME, Tanokura M. 2001. Structure and site-directed mutagenesis of a flavoprotein from Escherichia coli that reduces nitrocompounds: alteration of pyridine nucleotide binding by a single amino acid substitution. J Biol Chem 276:2816–2823. doi: 10.1074/jbc.M002617200. [DOI] [PubMed] [Google Scholar]
- 29.Whiteway J, Koziarz P, Veall J, Sandhu N, Kumar P, Hoecher B, Lambert IB. 1998. Oxygen-insensitive nitroreductases: analysis of the roles of nfsA and nfsB in development of resistance to 5-nitrofuran derivatives in Escherichia coli. J Bacteriol 180:5529–5539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fischer M, Haase I, Kis K, Meining W, Ladenstein R, Cushman M, Schramek N, Huber R, Bacher A. 2003. Enzyme catalysis via control of activation entropy: site-directed mutagenesis of 6,7-dimethyl-8-ribityllumazine synthase. J Mol Biol 326:783–793. doi: 10.1016/S0022-2836(02)01473-0. [DOI] [PubMed] [Google Scholar]
- 31.Vervoort J, Xavier BB, Stewardson A, Coenen S, Godycki-Cwirko M, Adriaenssens N, Kowalczyk A, Lammens C, Harbarth S, Goossens H, Malhotra-Kumar S. 2014. An in vitro deletion in ribE encoding lumazine synthase contributes to nitrofurantoin resistance in Escherichia coli. Antimicrob Agents Chemother 58:7225–7233. doi: 10.1128/AAC.03952-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Clermont O, Gordon D, Denamur E. 2015. Guide to the various phylogenetic classification schemes for Escherichia coli and the correspondence among schemes. Microbiology 161:980–988. doi: 10.1099/mic.0.000063. [DOI] [PubMed] [Google Scholar]
- 33.Ho PL, Lo WU, Yeung MK, Li Z, Chan J, Chow KH, Yam WC, Tong AH, Bao JY, Lin CH, Lok S, Chiu SS. 2012. Dissemination of pHK01-like incompatibility group IncFII plasmids encoding CTX-M-14 in Escherichia coli from human and animal sources. Vet Microbiol 158:172–179. doi: 10.1016/j.vetmic.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 34.Ho PL, Chan J, Lo WU, Law PY, Li Z, Lai EL, Chow KH. 2013. Dissemination of plasmid-mediated fosfomycin resistance fosA3 among multidrug-resistant Escherichia coli from livestock and other animals. J Appl Microbiol 114:695–702. doi: 10.1111/jam.12099. [DOI] [PubMed] [Google Scholar]
- 35.Lo WU, Chow KH, Law PY, Ng KY, Cheung YY, Lai EL, Ho PL. 2014. Highly conjugative IncX4 plasmids carrying blaCTX-M in Escherichia coli from humans and food animals. J Med Microbiol 63:835–840. doi: 10.1099/jmm.0.074021-0. [DOI] [PubMed] [Google Scholar]
- 36.Ho PL, Wong RC, Lo SW, Chow KH, Wong SS, Que TL. 2010. Genetic identity of aminoglycoside-resistance genes in Escherichia coli isolates from human and animal sources. J Med Microbiol 59:702–707. doi: 10.1099/jmm.0.015032-0. [DOI] [PubMed] [Google Scholar]
- 37.Ihsan A, Wang X, Zhang W, Tu H, Wang Y, Huang L, Iqbal Z, Cheng G, Pan Y, Liu Z, Tan Z, Zhang Y, Yuan Z. 2013. Genotoxicity of quinocetone, cyadox and olaquindox in vitro and in vivo. Food Chem Toxicol 59:207–214. doi: 10.1016/j.fct.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 38.Economou V, Gousia P. 2015. Agriculture and food animals as a source of antimicrobial-resistant bacteria. Infect Drug Resist 8:49–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harmer CJ, Moran RA, Hall RM. 2014. Movement of IS26-associated antibiotic resistance genes occurs via a translocatable unit that includes a single IS26 and preferentially inserts adjacent to another IS26. mBio 5:e01801–e01814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhanel GG, Karlowsky JA, Harding GK, Carrie A, Mazzulli T, Low DE, Hoban DJ. 2000. A Canadian national surveillance study of urinary tract isolates from outpatients: comparison of the activities of trimethoprim-sulfamethoxazole, ampicillin, mecillinam, nitrofurantoin, and ciprofloxacin. The Canadian Urinary Isolate Study Group. Antimicrob Agents Chemother 44:1089–1092. doi: 10.1128/AAC.44.4.1089-1092.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peterson FJ, Mason RP, Hovsepian J, Holtzman JL. 1979. Oxygen-sensitive and -insensitive nitroreduction by Escherichia coli and rat hepatic microsomes. J Biol Chem 254:4009–4014. [PubMed] [Google Scholar]
- 42.Komp Lindgren P, Klockars O, Malmberg C, Cars O. 2015. Pharmacodynamic studies of nitrofurantoin against common uropathogens. J Antimicrob Chemother 70:1076–1082. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.