Abstract
Over a period of 40 months, plasmid-mediated AmpC β-lactamases were detected in Tunis, Tunisia, in 78 isolates (0.59%) of Escherichia coli, Klebsiella pneumoniae, and Proteus mirabilis. In 67 isolates, only one ampC gene was detected, i.e., blaCMY-2-type (n = 33), blaACC (n = 23), blaDHA (n = 6) or blaEBC (n = 5). Multiple ampC genes were detected in 11 isolates, with the following distribution: blaMOX-2, blaFOX-3, and blaCMY-4/16 (n = 6), blaFOX-3 and blaMOX-2 (n = 3), and blaCMY-4 and blaMOX-2 (n = 2). A great variety of plasmids carrying these genes was found, independently of the species and the bla gene. If the genetic context of blaCMY-2-type is variable, that of blaMOX-2, reported in part previously, is unique and that of blaFOX-3 is unique and new.
INTRODUCTION
AmpC β-lactamases are cephalosporinases that are poorly inhibited by clavulanic acid (1). AmpC production is one of the mechanisms of resistance to β-lactams in enterobacteria, conferring resistance to all β-lactams except fourth-generation cephalosporins and carbapenems (2). The genes encoding these enzymes are chromosome or plasmid borne (3).
Most plasmid-borne AmpC (pAmpC) β-lactamase genes are derived from chromosomal genes that have been mobilized onto plasmids. Based on sequence similarities with species-specific AmpC enzymes, pAmpC variants are classified into five evolutionary groups: the CIT variants (CMY-2 types) originating in Citrobacter freundii, the Enterobacter sp. EBC variants (ACT-1 type, MIR-1), the Morganella morganii DHA variants, the Hafnia alvei ACC variants, and the Aeromonas sp. FOX and MOX variants (1, 2). Several genetic elements are involved in the mobilization of pAmpC β-lactamase genes, e.g., the insertion sequences IS26 (CMY-13), ISEcp1 (CMY-2-type, ACC-1-type), and ISCR1, associated with complex integrons (DHA-1 type) (1, 2).
The geographic scattering of the different types of pAmpC shows that the CMY-2 type is the most frequent, particularly in Europe (e.g., in France, Spain, Italy, and Turkey [4–7]), in Canada, Argentina, and Tunisia (8–10), and in Korea and China (11, 12). These pAmpC β-lactamases were detected among Enterobacteriaceae, especially in Escherichia coli, Klebsiella pneumoniae, Klebsiella oxytoca, Salmonella enterica, and Proteus mirabilis, and also in naturally AmpC-producing species such as Enterobacter cloacae, Enterobacter aerogenes, and C. freundii (13–15).
The pAmpC genes have also been reported in animals. Indeed, in the United States, CMY-2-producing E. coli strains have been found among canine clinical isolates (16). Clinical disease associated with AmpC-producing E. coli in dogs in Australia was first reported in 2006 (17). More recently, a survey of clinical isolates of E. coli from dogs and horses in the Netherlands revealed that 2% of them were AmpC producers (8). Furthermore, a Swedish study has described CMY-2-producing E. coli isolated from broilers (18). Such enzymes have been found in Klebsiella isolates from companion animals in Italy (19). In Tunisia, CMY-2-producing E. coli isolates have been recovered from healthy food animals and feces from healthy pets (20, 21).
Cooccurrence of multiple pAmpC β-lactamases in a single strain of Enterobacteriaceae has not been reported previously but has been observed in a Tunisian collection of various species of this family of bacteria. The present study was conducted to characterize the strains and plasmids carrying multiple pAmpC genes and to explore the genetic context of these genes in an attempt to explain this new phenomenon.
MATERIALS AND METHODS
Bacterial strains, antimicrobial susceptibility, and synergy testing.
A total of 11,393 strains from three enterobacterial species. i.e., E. coli (n = 7,504), Klebsiella sp. (n = 2,905) and P. mirabilis (n = 984), were collected at the clinical microbiology laboratory of Charles Nicolle hospital, Tunis, Tunisia, from January 2006 to April 2009. Only 107 nonduplicate isolates (36 E. coli, 58 Klebsiella, and 13 P. mirabilis isolates) resistant to third-generation cephalosporins, with negative double-disc synergy tests (DDST), were investigated. Susceptibility to antibiotics was determined using standard disc diffusion on Mueller-Hinton agar (Bio-Rad, Marnes-la-Coquette, France), and MICs were determined using standard agar dilution on the same medium according to the CLSI recommendations (22). Moreover, all isolates were phenotypically screened for the production of extended-spectrum β-lactamases (ESBL) using DDST (23) with and without cloxacillin (250 mg/liter) (24).
E. coli ATCC 25922, Pseudomonas aeruginosa ATCC 27853, and Staphylococcus aureus ATCC 25923 served as controls for MIC determinations. Rifampin-resistant E. coli J-53 and azide-resistant E. coli C600 were used as recipients in conjugation, and E. coli DH10B (Invitrogen SARL, Cergy-Pontoise, France) was used in cloning and transformation experiments.
E. coli CFT073 (O6 K1 H7) and E. coli J96 (O4 K6 H6) were used as reference strains for the detection of virulence factors (25). Strains carrying FOX-3 and MOX-2 genes (26, 27) were used as positive controls for detection of FOX and MOX producers with PCR.
Molecular characterization of β-lactamases.
Total DNA preparations for PCR were obtained by thermolysis of the isolates. Initial identification was obtained with primers of a multiplex PCR protocol for the detection of ampC families as described by Pérez-Pérez et al. (3). Confirmation was obtained using simplex reactions with primers CF-1/CF-2, FOX MF/MR (20), and MOX MF/MR (MOX-MF, 5′-GCTGCTCAAGGAGCACAGGAT-3′; MOX-MR, 5′-CACATTGACATAGGTGTGGTGC-3′) for blaCMY, blaFOX, and blaMOX, respectively. Amplicons of blaAmpC were purified and sequenced. Primers OT3, OT4, and OXA-1 (forward and reverse) were used to amplify putative blaTEM-1 and blaOXA-1 genes, respectively.
Epidemiological studies.
Total DNA was obtained with the QIAamp DNA minikit (Qiagen, Courtaboeuf, France) to assess epidemiological relationships between isolates producing plasmid-mediated cephalosporinases using repetitive-element PCR (rep-PCR) and enterobacterial repetitive intergenic consensus PCR (ERIC-PCR) as previously described (28, 29) and pulsed-field gel electrophoresis (PFGE) of chromosomal DNA digested with the restriction enzyme XbaI (Biolabs).
Determination of phylogenetic groups of E. coli was performed using PCR as previously described (30). The E. coli clone O25b-ST131 of phylogenetic group B2 was detected using PCR (31). E. coli isolates were screened for the presence of 18 genes encoding putative virulence factors characteristic of extraintestinal pathogenic E. coli by multiplex PCR as previously described (32, 33, 34). These genes Included fimH, afa, sfa/foc, papG (3 alleles), cnf1, sat, hlyA, iutA, iroN, fyuA, iha, kpsMTII, ompT, malX (pathogenic island marker PAI II of the CFT073 reference strain), traT, and usp.
The clonal structure of the K. pneumoniae strains was determined by multilocus sequence typing (MLST) based on the allelic diversity of seven loci: rpoB, mdh, pgi, phoE, infB, gapA, and tonB. These housekeeping locus fragments of all K. pneumoniae isolates were also sequenced.
The primers used for amplifications were designed by Diancourt et al. (35). Sequence types (STs) were assigned at the http://www.pasteur.fr/recherche/genopole/PF8/mlst website.
The K. pneumoniae isolates were screened for the presence of nine genes encoding putative virulence factors and six capsular serotypes by PCR as previously described (36, 37, 38).
These genes included mrkD, kpn, ycfM, entB, ybtS, iroNKp, rmpA, magA, allS2, wcaG, and genes encoding K2, K5, K20, K54, and K57 (capsular serotypes).
Transfer of resistance and molecular characterization of plasmids.
Mating experiments were performed by mixing equal volumes (1 ml) of exponentially growing cultures of each isolate tested and E. coli J-53 or E. coli C600 in Trypticase soy broth (Bio-Rad) and incubating the mixture for 3 h at 37°C. β-Lactam-resistant E. coli J-53 or E. coli C600 transconjugants were selected on Drigalski agar (Bio-Rad) containing rifampin (250 μg/ml) or azide (100 μg/ml) and cefotaxime (2.5 μg/ml).
E. coli DH10B transformants were obtained by electroporation of plasmid DNA extracted with the High Pure plasmid isolation kit (Roche, Mannheim, Germany) in a Gene Pulser II (Bio-Rad) and selected on Drigalski agar supplemented with cefotaxime (2.5 μg/ml). Transfer of β-lactam resistance markers from all clinical isolates studied was attempted by conjugation or transformation.
Replicon typing of the plasmids of the donor strains and the corresponding transconjugants or transformants was performed by PCR under the conditions previously described (39, 40).
Plasmid DNA of the clinical isolates of P. mirabilis, E. coli, and K. pneumoniae and E. coli transconjugants was extracted by alkaline lysis, as previously described (41), to estimate plasmid number and size. RP4 (54 kb), pCFF04 (85 kb), and pIP173 (126.8 kb) were used as reference plasmids.
Genetic context analysis.
For molecular cloning, we used total DNA of strain Kp10 for the blaMOX gene extracted using the Qiagen DNeasy blood and tissue kit and plasmid DNA from transconjugant J-53 of K. pneumoniae Kp8 for the blaFOX gene extracted with the Qiagen large-construct kit. DNA was partially digested with Sau3A and ligated into the BamHI site of pUC18 (42). The recombinant plasmid was introduced into E. coli DH10B by transformation. Transformants were selected on Drigalski agar supplemented with cefotaxime (2.5 μg/ml). Recombinant plasmids were extracted with the High Pure plasmid isolation kit (Roche), insert sizes were estimated after digestion with EcoRI, and initial genetic environments were determined by sequencing using universal M13 primers the first time for recombinant plasmids obtained. Recombinant plasmids were extracted with the High Pure plasmid isolation kit (Roche), and insert sizes were estimated after digestion with EcoRI. Initial analysis of the genetic environments of the bla genes borne on recombinant plasmids was done by sequencing using universal M13 primers. The genetic context of blaMOX, blaFOX, and blaCMY of recombinant plasmids and all nine remaining strains was further explored by PCR mapping and sequencing using primers designed for blaMOX (A, B, D, E, and G), blaFOX (H and I), and blaCMY (A, B, C, D, E, F, G, H, I, J, K, and L) (see the supplemental material). Two additional primers were tested for blaCMY: CF2/IS26-SL2 up (IS26-SL2 up, 5′-CTGGGTAAAATCCTCAACA-3′) and CF2/ISEcp1 (ISEcp1, 5′-AAAAATGATTGAAAGGTGGT-3′) (see the supplemental material).
Nucleotide sequence accession number.
The accession number of the genetic context of blaFOX-3 is LC072710.
RESULTS AND DISCUSSION
Among the 107 enterobacterial isolates studied, multiplex PCR revealed pampC β-lactamase genes in 78 isolates, whereas in 29 isolates (E. coli), no pampC β-lactamase genes were detected. In 67 isolates, only one pampC gene was detected with blaCMY-2-type (n = 32), blaACC-1-type (n = 23), blaDHA-1-type (n = 6), and blaEBC (n = 5); among these 67 isolates, 48 (K. pneumoniae) also produced CTX-M-15. Multiplex PCR revealed pampC β-lactamase genes in 78 of the 107 isolates studied but not in 29 isolates of E. coli. Single pampC genes were detected in 67 isolates, i.e., blaCMY-2-type (n = 32), blaACC-1-type (n = 23), blaDHA-1-type (n = 6), and blaEBC (n = 5). Among the 67 isolates, 48 K. pneumoniae isolates also produced CTX-M-15.
In the 11 remaining strains (5 E. coli, 4 K. pneumoniae, and 2 P. mirabilis strains), at least two pampC genes were detected. At this stage, our study was focused on these 11 strains that harbored multiple pAmpC β-lactamases, as follows: MOX-type, FOX-type, and CMY-2-type enzymes in six isolates (in three E. coli, one K. pneumoniae, and two P. mirabilis isolates), MOX-type and FOX-type enzymes in three isolates (in one E. coli and two K. pneumoniae isolates), and MOX-type and CMY-2-type enzymes in two isolates (in one E. coli and one K. pneumoniae isolate). The origin and characteristics of clinical interest of these isolates are summarized in Table 1. They were resistant to all β-lactams except ertapenem, imipenem, and cefepime. MICs of ticarcillin and piperacillin ranged from 512 to >2,048 μg/ml, those of cefotaxime from 256 to >2,048 μg/ml, those of ceftazidime from 128 to 1,024 μg/ml, those of cefoxitin from 64 to 512 μg/ml, and those of cefepime 0.5 to 2 μg/ml.
TABLE 1.
Chromosomal characteristics of the 11 Enterobacteriaceae harboring multiple plasmid-borne AmpC β-lactamase genesa
| Isolate | Ward | Specimen | Yr | REP profile | ERIC profile | PFGE profile | PG | ST | Virulence profile | Virulence score | KS |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ec3 | ICU | Blood | 2005 | A1 | A1 | A1 | A | ND | fimH, hlyA, iha, usp, iroN | 5 | ND |
| Ec5 | Med | Urine | 2008 | A2 | A2 | A2 | B2 | ST131 | fimH, pai, fyu A, sfa/foc, pap G, hlyA, cnf1, iha, usp | 9 | ND |
| Ec6 | Chir | Pus | 2008 | A3 | A3 | A3 | D | ND | fimH, iutA, hlyA, iroN | 4 | ND |
| Ec7 | Ped | Urine | 2008 | A4 | A4 | A4 | A | ND | fimH, fyuA, sfa/foc, cnf1 | 4 | ND |
| Ec11 | Uro | Urine | 2007 | A5 | A5 | A5 | B2 | ST131 | fimH, pai, fyu A, sfa/foc, iut A, hlyA, papG, afa/dra, iha, usp | 10 | ND |
| Kp2 | ICU | Blood | 2005 | B1 | B1 | B1 | ND | ST218 | entB, ybtS, mrkD, ycfM, kpn | 5 | K57 |
| Kp4 | Ped | Urine | 2008 | B2 | B2 | B2 | ND | ST11 | entB, ybtS | 2 | ND |
| Kp8 | ICU | Urine | 2005 | B1 | B1 | B1 | ND | ST218 | entB, ybtS, mrkD, ycfM, kpn | 5 | K57 |
| Kp10 | Ortho | Blood | 2005 | B3 | B3 | B1 | ND | ST218 | entB, ybtS, mrkD, ycfM, kpn | 5 | K57 |
| Pm1 | Uro | Urine | 2006 | C1 | C1 | C1 | ND | ND | ND | ND | ND |
| Pm9 | ICU | Catheter | 2007 | C2 | C2 | C2 | ND | ND | ND | ND | ND |
Ec, Escherichia coli; Kp, Klebsiella pneumoniae; Pm, Proteus mirabilis; ICU, intensive care unit; MED, medicine; Surg, surgery; Ped, pediatrics; Uro, urology; Ortho, orthopedics; REP, genomic fingerprinting by repetitive-element PCR profiling; ERIC, enterobacterial repetitive intergenic consensus PCR profile; PFGE, pulsed-field gel electrophoresis profile; PG, phylogenetic groups; ST, sequence type; KS, capsular serotype; ND, not determined.
As determined by sequence analysis, blaCMY genes were blaCMY-4 (n = 6) and blaCMY-16 (n = 2), blaFOX genes were all blaFOX-3 (n = 9), and blaMOX genes were all blaMOX-2 (n = 11). Seven isolates among the pAmpC producers also produced the broad-spectrum TEM-1 β-lactamase, and one of them produced OXA-1 in addition (Table 2).
TABLE 2.
Plasmid characteristics of the 11 Enterobacteriaceae harboring multiple plasmid-borne AmpC genes and exploration by PCR mappinga
| Isolate | Resistance pattern | blaAMPC | blaTEM-1, blaOXA-1 | RT | PCR mapping result |
||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
blaMOX |
blaFOX |
blaCMY |
|||||||||||||||||||||||
| A | B | D | E | G | H | I | A | B | C | F | G | H | I | J | K | L | D | E | CF2/IS26-SL2 up | CF2/ISEcp1 commune | |||||
| Ec3 | GM, TM, NET, TE, C, NA, SXT | FOX-3, MOX-2 | A/C | + | + | + | + | + | + | + | |||||||||||||||
| Ec5 | GM, TM, NA, OFX, CIP | CMY-4, FOX-3, MOX-2 | TEM-1 | FIA, FIB | + | + | + | + | + | + | + | − | − | + | + | − | − | − | − | − | + | + | + | − | − |
| El Ec5 | TM | CMY-4 | FIA | ||||||||||||||||||||||
| Ec6 | TE | CMY-4, FOX-3, MOX-2 | TEM-1 | A/C, FIB | + | + | + | + | + | + | + | − | + | + | + | + | + | + | − | − | − | − | − | − | − |
| El Ec6 | TE | CMY-4 | FIB | ||||||||||||||||||||||
| Ec7 | TE | CMY-4, MOX-2 | TEM-1 | Q | + | + | + | + | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | ||
| Tc Ec7 | CMY-4 | TEM-1 | Q | ||||||||||||||||||||||
| Ec11 | TM, NET, AN, NA, CIP, SXT, OFX | CMY-16, FOX-3, MOX-2 | OXA-1 | FIA | + | + | + | + | + | + | + | − | + | + | − | − | − | − | − | − | − | − | − | − | − |
| El Ec11 | TM, SXT | CMY-16 | OXA-1 | FIA | |||||||||||||||||||||
| Kp2 | GM, TM, NET, TE, C, SXT | FOX-3, MOX-2 | A/C, FIIK | + | + | + | + | + | + | + | |||||||||||||||
| Tc Kp2 | TM, TE, SXT | FOX-3 | A/C | ||||||||||||||||||||||
| Kp4 | TE, SXT | CMY-16, FOX-3, MOX-2 | TEM-1 | Q | + | + | + | + | + | − | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| Tc Kp4A | FOX-3 | Q | |||||||||||||||||||||||
| El Kp4B | TE, SXT | CMY-16 | TEM-1 | - | |||||||||||||||||||||
| Kp8 | GM, TM, NET, TE, SXT | FOX-3, MOX-2 | A/C, FIIK | + | + | + | + | + | + | + | |||||||||||||||
| Tc Kp8 | TM, TE, SXT | FOX-3 | A/C | ||||||||||||||||||||||
| Kp10 | GM, TM, NET, TE, SXT | CMY-4, MOX-2 | TEM-1 | A/C, FIIK | + | + | + | + | + | − | + | + | + | + | − | − | − | − | − | − | − | − | − | ||
| El Kp10 | TE, SXT | CMY-4 | FIIK | ||||||||||||||||||||||
| Pm1 | TE, C, NA, OFX, CIP, SXT | CMY-4, FOX-3, MOX-2 | TEM-1 | A/C, FIA | + | + | + | + | + | + | + | − | + | + | + | + | + | + | − | − | − | − | − | − | |
| Tc Pm1A | C, SXT | CMY-4 | A/C | ||||||||||||||||||||||
| Tc Pm1B | TE | FOX-3 | FIA | ||||||||||||||||||||||
| Pm9 | TM, NET, AN, TE, SXT | CMY-4, FOX-3, MOX-2 | TEM-1 | A/C, FIA | + | + | + | + | + | + | + | − | + | + | + | + | − | − | − | − | − | − | − | − | − |
| Tc Pm9A | TM, TE, SXT | CMY-4 | TEM-1 | A/C | |||||||||||||||||||||
| Tc Pm9B | FOX-3 | FIA | |||||||||||||||||||||||
Ec, Escherichia coli; Kp, Klebsiella pneumoniae; Pm, Proteus mirabilis; Tc, transconjugant; El, electroporant; RT, replicon type; GM, gentamicin; TM, tobramycin; NET, netilmicin; AN, amikacin; C, chloramphenicol; NA, nalidixic acid; OFX, ofloxacin; CIP, ciprofloxacin; SXT, trimethoprim-sulfamethoxazole; TE, tetracycline.
CMY-4 was first identified in a clinical P. mirabilis isolate from Tunisia (43), and since then, it has been found throughout the world. CMY-16, a variant of the CMY lineage, which differs from its closest homologue, CMY-4, by a single amino acid substitution (A171S), was first identified in a P. mirabilis isolate from Italy (6).
FOX-3 and MOX-2 β-lactamases have been detected in K. pneumoniae isolates from Italy (26) and Greece (27), respectively. However, these enzymes were not previously reported in Tunisia; hence, this is the first report of FOX-3 and MOX-2 β-lactamase in this country. To our knowledge, this is also the first report of cooccurrence of various plasmid-borne blaAmpC genes in a single strain and of such cooccurrence in several species of Enterobacteriaceae.
Based on the distinct patterns observed with molecular typing (rep-PCR, ERIC-PCR, and PFGE), there was no epidemiological link between the five E. coli isolates harboring multiple pampC genes. The phylogenetic study showed that the five E. coli isolates belonged to phylogenetic groups B2 (n = 2), A (n = 2), and D (n = 1). We found that the two B2 isolates belonged to the ST131 clone and harbored many virulence determinants leading to the highest virulence scores, i.e., 9 and 10 (Table 1). The ST131 isolates harbored an extended virulence profile and presented a virulence gene panel similar to that of other previously described members of ST131 (44), an ST often involved in the dissemination of CTX-M-15 genes (31). Moreover, a recent study showed that this clone has enhanced metabolic capacities, acts as a potent colonizer of the intestine, and displays the typical features of virulent E. coli strains (45).
ERIC and Rep-PCR typing revealed a similarity in the patterns of two K. pneumoniae isolates (Kp2 and Kp8) indicating that they are genetically related. In addition, PFGE analysis confirmed the existence of an epidemic clone represented by these strains, distinct from Kp10, while all of them belonged to ST218 (Table 1). This ST has been described recently in K. pneumoniae isolates from patients with bacteremia in China (46). Moreover, we found that the clonal K. pneumoniae strains had the capsular serotype K57 and showed important virulence profiles including five genes, i.e., entB, mrkD, ycfM, kpn, and ybtS. The latter is considered a major virulence determinant in K. pneumoniae and is required for the synthesis of yersiniabactin, which promotes biofilm formation and a reduction in the killing capacity of innate immune cells by blocking the production of reactive oxygen species (47, 48). Kp4, on the other hand, was a sporadic isolate of ST11, reported to be involved in the dissemination of various β-lactamases, including carbapenemases and pAmpC (49).
Plasmids carrying pAmpC genes were successfully transferred via conjugation and/or electroporation from 10 of the 11 isolates tested. The pAmpC gene transfer experiments showed that no more than one gene was transferred at one time, either blaCMY or blaFOX, indicating that they are probably located on different plasmids. However, blaMOX could not be transferred from any isolate, suggesting its location on the chromosome.
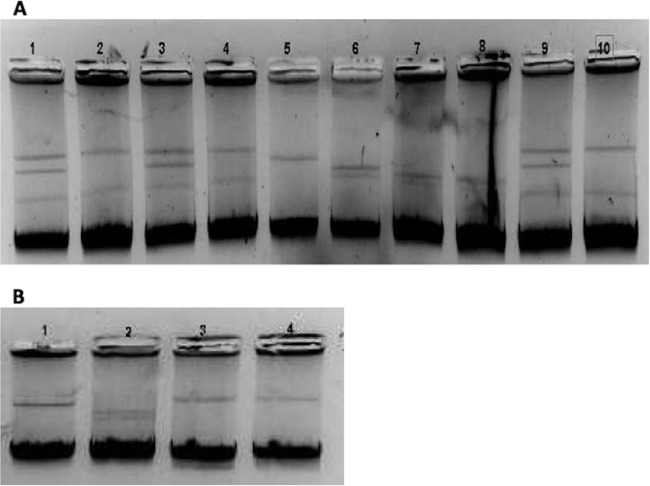
We found that pAmpC genes were carried on plasmids of different incompatibility groups. The blaCMY genes were carried on conjugative Inc A/C (n = 2), Inc FIA (n = 2), Inc FIB (n = 1), Inc Q (n = 1), or Inc FIIK (n = 1) plasmids, and blaFOX genes were located on Inc FIA (n = 2), Inc A/C (n = 2), or Inc Q (n = 1) plasmids (Table 2). Our findings are consistent with previous reports noting the predominance of Inc A/C replicons among plasmids carrying blaCMY (34). Accordingly, the Inc A/C replicon is the most commonly reported worldwide (39). The fact that pAmpC genes are carried by different plasmid types promotes their rapid spread among Enterobacteriaceae (28, 50). Furthermore, we found that pAmpC genes were generally located on plasmids of various sizes. Analysis of DNA extracted according to Kado and Liu (41) revealed that K. pneumoniae isolates Kp2, Kp4, and Kp8 harbored two large plasmids (>130 kb) and a third smaller one (ca. 50 kb), while E. coli isolate Ec7 harbored only one large plasmid (>130 kb) and one of smaller one (50 kb). The Kp10 isolate and its corresponding transformant harbored one large plasmid (>130 kb) (Fig. 1). For the remaining E. coli and P. mirabilis isolates, we obtained no clear results with the procedure of Kado and Liu (41). Our results are in agreement with reports of pAmpC genes found on plasmids of sizes varying from 7 to 180 kb (2).
FIG 1.
Plasmid analysis. (A) Lane 1, Kp2; lane 2, transconjugant (Tc) Kp2; lane 3, Kp4; lane 4, Tc Kp4 B; lane 5, pIP173 (126 kb); lane 6, RP4 (55 kb) and pCFF04 (85 kb); lane 7, Ec7; lane 8, Tc Ec7; lane 9, Kp8; lane 10, Tc Kp8. (B) Lane 1, pIP173 (126 kb); lane 2, RP4 (55 kb) and pCFF04 (85 kb); lane 3, Kp10; lane 4, transformant (Tf) Kp10.
Two plasmids of the present study carrying CMY-4 and FOX-3 genes belonged to the Q1 group. These replicons are characterized by their relatively small size (5 to 10 kb), their ability to be mobilized by several self-transmissible plasmids, and their broad host range, making them highly promiscuous (51).
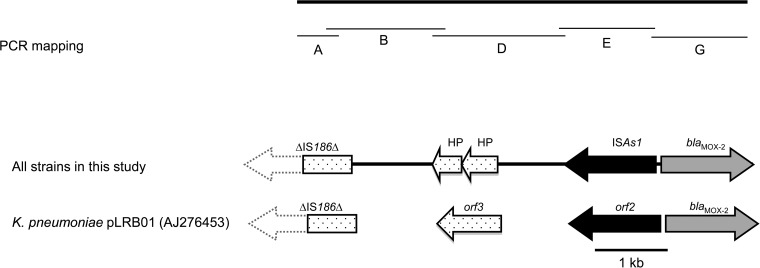
Analysis of the genetic environment of blaMOX-2 by cloning and mapping experiments showed that it was very closely related to that of the single reference strain K. pneumoniae KOL (accession number AJ276453) reported in 2002 (27). The DNA insert carrying blaMOX-2 was sequenced and four additional open reading frames (ORFs) were identified (Fig. 2). The ORFs are on the opposite DNA strand. The first (ORF2, accession number AJ276453) is located 212 bp upstream from blaMOX-2 and encodes a putative 375-amino-acid protein 99% identical to a transposase (ISA1 type) of the IS4 family. Nine copies of this ORF are found on the chromosome of Aeromonas media strain WS (accession number CP007567) and on the multiresistance-encoding plasmid pP2G1 from an Aeromonas sp. (accession number HE616910). Two other ORFs are located upstream from ISA1 and encode two hypothetical proteins of 150 and 129 amino acids, respectively; they are organized in tandem in many plasmids from Enterobacteriaceae, Vibrio spp., and Aeromonas spp. A 612-bp ORF lacking 5′ sequences was identified 1,010 bp upstream from ORF3. It encodes a 204-amino-acid product 100% identical to the putative transposase A of IS186 of E. coli K-12 (accession number X03123).
FIG 2.
Genetic context of blaMOX-2 beta-lactamase genes.
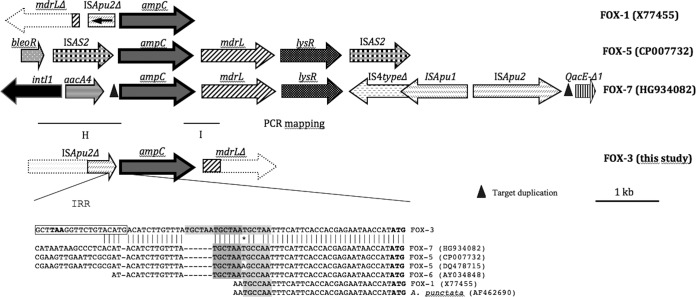
Exploration of the genetic environment of blaFOX-3 of Kp8 revealed the presence of ISApu2 upstream from blaFOX-3 (Fig. 3). The genetic organization was different from that of the previously published genes blaFOX-1, blaFOX-5, blaFOX-6, and blaFOX-7 (accession numbers X77455, CP007732 and DQ478715, AY034848, and HG934082, respectively), suggesting different recombination events, although the insertion sequence ISApu2 also seems to play a role in the mobilization of FOX-1 and FOX-7. We noted the presence of a repeat sequence of unknown function (TGCTAA) upstream from blaFOX-3. Using PCR mapping and sequencing, the genetic environment of blaFOX-3 in the other strains carrying this gene was found to be the same. To our knowledge, this is the first report of the genetic environment of blaFOX-3.
FIG 3.
Genetic context of blaFOX-3 beta-lactamase genes.
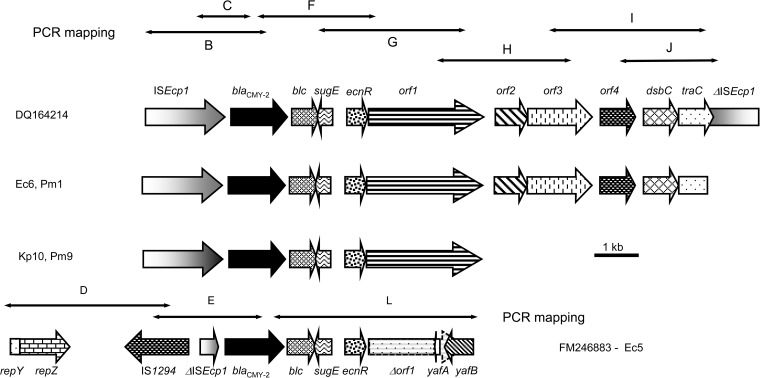
In six isolates (Ec6, Pm1, Kp10, Pm9, Ec5, and Ec11) of eight in the present study which carried blaCMY, at least part of ISEcp1 was present upstream from the blaCMY-2-like gene (Fig. 4). The region between ISEcp1 and the blaCMY-2-like gene had the same structure in only five isolates (Table 2; Fig. 4).
FIG 4.
Genetic context of blaCMY-2-type beta-lactamase genes.
Our findings are consistent with those concerning the genetic environment of blaCMY-2-type genes described previously in which ISEcp1 is present (42). It was suggested that ISEcp1 could be involved in mobilizing the blaCMY-2-like gene from the C. freundii chromosome to plasmids (52, 53).
All plasmids carrying blaCMY described here shared various structural similarities with the 13-kb type I structure described in Salmonella enterica serovar Newport (accession number DQ164214) (Fig. 4) in which blaCMY-2 is duplicated. The second copy is adjacent to a partial copy of ISEcp1 in the opposite orientation (53). Downstream from the blaCMY-2-like gene in this 13-kb type I structure, several ORFs were detected between the two copies of blaCMY-2 (53). Very similar structures were found in isolates Ec6, Pm1, Pm9, and Kp10. However, no isolate harbored two copies of the blaCMY-2-like gene. For Ec6, Pm1, Kp10, and Pm9, the sequence either downstream from traC or downstream from orf1 remains unknown (Fig. 4). In Ec5, as opposed to Ec6, Pm1, Pm9, and Kp10, there were fewer similarities with the 13-kb type I structure. Downstream from the blaCMY-2-like gene were blc, sugE, ecnR, and orf1 only, with a truncated yafA adjacent in reverse orientation, followed by yafB. Both yaf genes were part of a segment which is 98% identical to a region of S. enterica plasmid pNF1358 (accession number DQ017661) (Fig. 4). This segment is located directly upstream from ISEcp1−blaCMY-2 on pNF1358. In Ec5, truncation of ISEcp1 was due to the insertion of IS1294 in the reverse orientation. IS1294 belongs to the IS91 family and is a putative transposable element capable of mediating one-ended transposition (www-is.biotoul.fr). Downstream from IS1294, a segment comprising the repY and repZ genes involved in replication initiation is 95% identical to a region of S. enterica pNF1358. In Ec11, only ISEcp1 was present upstream from the blaCMY-2-like gene, while the rest of the structure remains unknown, suggesting a new genetic environment.
The results of the present study are consistent with those of Barlow and Hall with respect to Ec6, Pm1, Kp10, Pm9, Ec5, and Ec11, in which ISEcp1 could be involved in the mobilization of the blaCMY-2-like gene, irrespective of the replicon type and the plasmid backbone (54).
According to Hopkins et al., each replicon type reflects the genetic organization surrounding the blaCMY-2-like gene, with several minor rearrangements, including small insertions or deletions (55). Our findings are not consistent with those of Hopkins et al., since we found in our collection diverse plasmids carrying blaCMY-2-like genes in which the structure of the genetic environment of blaCMY is different even in cases of the same replicon type. In Ec7, blaCMY was carried on an IncQ plasmid. This is the first observation of the gene on such an extremely rare plasmid. The study of the genetic environment of blaCMY carried on IncQ by mapping PCR was negative, suggesting a new genetic structure.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the Ministry of Scientific Research, Technology and Competence Development of Tunisia and by the Faculté de Médecine Pierre et Marie Curie, Université Pierre et Marie Curie, Paris VI.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.00828-15.
REFERENCES
- 1.Jacoby GA. 2009. AmpC β-lactamases. Clin Microbiol Rev 22:161–182. doi: 10.1128/CMR.00036-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Philippon A, Arlet G, Jacoby GA. 2002. Plasmid-mediated AmpC-type β-lactamases. Antimicrob Agents Chemother 46:1–11. doi: 10.1128/AAC.46.1.1-11.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pérez-Pérez FJ, Hanson ND. 2002. Detection of plasmid-mediated AmpC beta-lactamase genes in clinical isolates by using multiplex PCR. J Clin Microbiol 40:2153–2162. doi: 10.1128/JCM.40.6.2153-2162.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Verdet C, Benzarara Y, Gautier V, Adam O, Ould-Hocine Z, Arlet G. 2006. Emergence of DHA-1 producing Klebsiella spp. in the Parisian region: genetic organization of the ampC and ampR genes originating from Morganella morganii. Antimicrob Agents Chemother 50:607–617. doi: 10.1128/AAC.50.2.607-617.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miró E, Aguero J, Larrosa MN, Fernández A, Conejo MC, Bou G, González-López JJ, Lara N, Martínez-Martínez L, Oliver A, Aracil B, Oteo J, Pascual A, Rodríguez-Baño J, Zamorano L, Navarro F. 2013. Prevalence and molecular epidemiology of acquired AmpC beta-lactamases and carbapenemases in Enterobacteriaceae isolates from 35 hospitals in Spain. Eur J Clin Microbiol Infect Dis 32:253–259. doi: 10.1007/s10096-012-1737-0. [DOI] [PubMed] [Google Scholar]
- 6.D'Andrea MM, Nucleo E, Luzzaro F, Giani T, Migliavacca R, Vailati F, Kroumova V, Pagani L, Rossolini GM. 2006. CMY-16, a novel acquired AmpC-type β-lactamase of the CMY/LAT lineage in multifocal monophyletic isolates of Proteus mirabilis from northern Italy. Antimicrob Agents Chemother 50:618–624. doi: 10.1128/AAC.50.2.618-624.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yilmaz NO, Agus N, Bozcal E, Oner O, Uzel A. 2013. Detection of plasmid-mediated AmpC beta-lactamase in Escherichia coli and Klebsiella pneumoniae. Indian J Med Microbiol 31:53–59. doi: 10.4103/0255-0857.108723. [DOI] [PubMed] [Google Scholar]
- 8.Denisuik AJ, Lagacé-Wiens PR, Pitout JD, Mulvey MR, Simner PJ, Tailor F, Karlowsky JA, Hoban DJ, Adam HJ, Zhanel GG. 2013. Molecular epidemiology of extended-spectrum beta-lactamase, AmpC beta-lactamase and carbapenemase producing Escherichia coli and Klebsiella pneumoniae isolated from Canadian hospitals over a 5 year period: CANWARD 2007-11. J Antimicrob Chemother 68(Suppl 1):i57–i65. doi: 10.1093/jac/dkt027. [DOI] [PubMed] [Google Scholar]
- 9.Cejas D, Fernandez CL, Quinteros M, Giovanakis M, Vay C, Lascialandare S, Mutti D, Pagniez G, Almuzara M, Gutkind G, Radice M. 2012. Plasmid-encoded AmpC (pAmpC) in Enterobacteriaceae: epidemiology of microorganisms and resistance markers. Rev Argent Microbiol 44:182–186. [PubMed] [Google Scholar]
- 10.Mnif B, Ktari S, Chaari A, Medhioub F, Rhimi F, Bouaziz M, Hammami A. 2013. Nosocomial dissemination of Providencia stuartii isolates carrying blaOXA-48, blaPER-1, blaCMY-4 and qnrA6 in a Tunisian hospital. J Antimicrob Chemother 68:329–332. doi: 10.1093/jac/dks386. [DOI] [PubMed] [Google Scholar]
- 11.Park MJ, Kim TK, Song W, Kim JS, Kim HS, Lee J. 2013. An increase in the clinical isolation of acquired AmpC beta-lactamase-producing Klebsiella pneumoniae in Korea from 2007 to 2010. Ann Lab Med 33:353–355. doi: 10.3343/alm.2013.33.5.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sheng WH, Badal RE, Hsueh PR. 2013. Distribution of extended-spectrum beta-lactamases, AmpC beta-lactamases, and carbapenemases among Enterobacteriaceae isolates causing intra-abdominal infections in the Asia-Pacific region: results of the Study for Monitoring Antimicrobial Resistance Trends (SMART). Antimicrob Agents Chemother 57:2981–2988. doi: 10.1128/AAC.00971-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iabadene H, Messai Y, Ammari H, Alouache S, Verdet C, Bakour R, Arlet G. 2009. Prevalence of plasmid-mediated AmpC β-lactamases among Enterobacteriaceae in Algiers hospitals. Int J Antimicrob Agents 34:340–342. doi: 10.1016/j.ijantimicag.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 14.Lee SH, Jeong SH, Park YM. 2003. Characterization of blaCMY-10 a novel, plasmid-encoded AmpC-type beta-lactamase gene in a clinical isolate of Enterobacter aerogenes. J Appl Microbiol 95:744–752. doi: 10.1046/j.1365-2672.2003.02040.x. [DOI] [PubMed] [Google Scholar]
- 15.Yim G, Kwong W, Davies J, Miao V. 2013. Complex integrons containing qnrB4-ampC (blaDHA-1) in plasmids of multidrug-resistant Citrobacter freundii from wastewater. Can J Microbiol 59:110–116. doi: 10.1139/cjm-2012-0576. [DOI] [PubMed] [Google Scholar]
- 16.Shaheen BW, Nayak R, Foley SL, Kweon O, Deck J, Park M, Rafii F, Boothe DM. 2011. Molecular characterization of resistance to extended spectrum cephalosporins in clinical Escherichia coli isolates from companion animals in the United States. Antimicrob Agents Chemother 55:5666–5675. doi: 10.1128/AAC.00656-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sidjabat HE, Townsend KM, Hanson ND, Bell JM, Stokes HW, Gobius KS, Moss SM, Trott DJ. 2006. Identification of blaCMY and associated plasmid-mediated resistance genes in multidrug-resistant Escherichia coli isolated from dogs at a veterinary teaching hospital in Australia. J Antimicrob Chemother 57:840–848. doi: 10.1093/jac/dkl057. [DOI] [PubMed] [Google Scholar]
- 18.Börjesson S, Jernberg C, Brolund A, Edquist P, Finn M, Landén A, Olsson-Liljequist B, Tegmark Wisell K, Bengtsson B, Englund S. 2013. Characterization of plasmid-mediated AmpC-producing E. coli from Swedish broilers and association with human clinical isolates. Clin Microbiol Infect 19:309–311. doi: 10.1111/1469-0691.12192. [DOI] [PubMed] [Google Scholar]
- 19.Donati V, Feltrin F, Hendriksen RS, Svendsen CA, Cordaro G, García-Fernández A, Lorenzetti S, Lorenzetti R, Battisti A, Franco A. 2014. Extended-spectrum beta-lactamases, AmpC beta-lactamases and plasmid mediated quinolone resistance in Klebsiella spp. from companion annimals in Italy. PLoS One 3:e90564. doi: 10.1371/journal.pone.0090564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mnif B, Ktari S, Rhimi FM, Hammami A. 2012. Extensive dissemination of CTX-M-1- and CMY-2-producing Escherichia coli in poultry farms in Tunisia. Lett Appl Microbiol 55:407−413. doi: 10.1111/j.1472-765X.2012.03309.x. [DOI] [PubMed] [Google Scholar]
- 21.Sallem RB, Gharsa H, Slama KB, Rojo-Bezares B, Estepa V, Porres-Osante N, Jouini A, Klibi N, Sáenz Y, Boudabous A, Torres C. 2013. First detection of CTX-M-1, CMY-2, and QnrB19 resistance mechanisms in fecal Escherichia coli isolates from healthy pets in Tunisia. Vector Borne Zoonotic Dis 13:98–102. doi: 10.1089/vbz.2012.1047. [DOI] [PubMed] [Google Scholar]
- 22.CLSI. 2005. Performance standards for antimicrobial susceptibility testing: 15th informational supplement. M100-S15. CLSI, Wayne, Pa. [Google Scholar]
- 23.Jarlier V, Nicolas MH, Fournier G, Philippon A. 1988. Extended broad-spectrum β-lactamases conferring transferable resistance to newer β-lactam agents in Enterobacteriaceae: hospital prevalence and susceptibility patterns. Rev Infect Dis 10:867–778. doi: 10.1093/clinids/10.4.867. [DOI] [PubMed] [Google Scholar]
- 24.Tan TY, Nq LS, He J, Kah TH, Hsu LY. 2009. Evaluation of screening methods to detect plasmid-mediated AmpC in Escherichia coli, Klebsiella pneumoniae, and Proteus mirabilis. Antimicrob Agents Chemother 53:146–149. doi: 10.1128/AAC.00862-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Welch RA, Burland V, Plunkett G, Redford P, Roesch P, Rasko D, Buckles EL, Liou SR, Boutin A, Hackett J, Stroud D, Mayhew GF, Rose DJ, Zhou S, Schwartz DG, Perna NT, Mobley HL, Donnenberg MS, Blattner FR. 2002. Extensive mosaic structure revealed by the complete genome sequence of uropathogenic Escherichia coli. Proc Natl Acad Sci U S A 99:17020–17024. doi: 10.1073/pnas.252529799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marchese A, Arlet G, Schito GC, Lagrange PH, Philippon A. 1998. Characterization of FOX-3, an AmpC-type plasmid-mediated β-lactamase from an Italian isolate of Klebsiella oxytoca. Antimicrob Agents Chemother 42:464−467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Raskine L, Borrel I, Barnaud G, Boyer S, Hanau-Beṛcot B, Gravisse J, Labia R, Arlet G, Sanson-Le-Pors MJ. 2002. Novel plasmid-encoded class C β-lactamase (MOX-2) in Klebsiella pneumoniae from Greece. Antimicrob Agents Chemother 46:2262–2265. doi: 10.1128/AAC.46.7.2262-2265.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Decré D, Gachot B, Lucet JC, Arlet G, Bergogne-Bérézin E, Régnier B. 1998. Clinical and bacteriological epidemiology of extended-spectrum β-lactamase-producing strains of Klebsiella pneumoniae in a medical intensive care unit. Clin Infect Dis 27:834–844. doi: 10.1086/514938. [DOI] [PubMed] [Google Scholar]
- 29.Versalovic J, Koeuth T, Lupski JR. 1991. Distribution of repetitive DNA sequences in eubacteria and application to fingerprinting of bacterial genomes. Nucleic Acids Res 19:6823–6831. doi: 10.1093/nar/19.24.6823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clermont O, Bonacorsi S, Bingen E. 2000. Rapid and simple determination of the Escherichia coli phylogenetic group. Appl Environ Microbiol 66:4555−4558. doi: 10.1128/AEM.66.10.4555-4558.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clermont O, Dhanji H, Upton M, Gibreel T, Fox A, Boyd D, Mulvey MR, Nordmann P, Ruppé E, Sarthou JL, Frank T, Vimont S, Arlet G, Branger C, Woodford N, Denamur E. 2009. Rapid detection of the O25b-ST131 clone of Escherichia coli encompassing the CTX-M-15-producing strains. J Antimicrob Chemother 64:274−277. doi: 10.1093/jac/dkp194. [DOI] [PubMed] [Google Scholar]
- 32.Johnson JR, Stell AL. 2000. Extended virulence genotypes of Escherichia coli strains from patients with urosepsis in relation to phylogeny and host compromise. J Infect Dis 181:261−272. doi: 10.1086/315217. [DOI] [PubMed] [Google Scholar]
- 33.Ruiz J, Simon K, Horcajada JP, Velasco M, Barranco M, Roig G, Moreno-Martínez A, Martínez JA, Jiménez de Anta T, Mensa J, Vila J. 2002. Differences in virulence factors among clinical isolates of Escherichia coli causing cystitis and pyelonephritis in women and prostatitis in men. J Clin Microbiol 40:4445−4449. doi: 10.1128/JCM.40.12.4445-4449.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takahashi A, Kanamaru S, Kurazono H, Kunishima Y, Tsukamoto T, Ogawa O, Yamamoto S. 2006. Escherichia coli isolates associated with uncomplicated and complicated cystitis and asymptomatic bacteriuria possess similar phylogenies, virulence genes, and O-serogroup profiles. J Clin Microbiol 44:4589−4592. doi: 10.1128/JCM.02070-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Diancourt L, Passet V, Verhoef J, Grimont PA, Brisse S. 2005. Multilocus sequence typing of Klebsiella pneumoniae nosocomial isolates. J Clin Microbiol 43:4178−4182. doi: 10.1128/JCM.43.8.4178-4182.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brisse S, Fevre C, Passet V, Issenhuth-Jeanjean S, Tournebize R, Diancourt L, Grimont P. 2009. Virulent clones of Klebsiella pneumoniae: identification and evolutionary scenario based on genomic and phenotypic characterization. PLoS One 4:e4982. doi: 10.1371/journal.pone.0004982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Turton JF, Baklan H, Siu LK, Kaufmann ME, Pitt TL. 2008. Evaluation of a multiplex PCR for detection of serotypes K1, K2 and K5 in Klebsiella spp. and comparison of isolates within these serotypes. FEMS Microbiol Lett 284:247−252. doi: 10.1111/j.1574-6968.2008.01208.x. [DOI] [PubMed] [Google Scholar]
- 38.Yu WL, Fung CP, Ko WC, Cheng KC, Lee CC, Chuang YC. 2007. Polymerase chain reaction analysis for detecting capsule serotypes K1 and K2 of Klebsiella pneumoniae causing abscesses of the liver and other sites. J Infect Dis 195:1235–1236. doi: 10.1086/512686. [DOI] [PubMed] [Google Scholar]
- 39.Carattoli A, Bertini A, Villa L, Falbo V, Hopkins KL, Threlfall EJ. 2005. Identification of plasmids by PCR-based replicon typing. J Microbiol Methods 63:219− 228. doi: 10.1016/j.mimet.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 40.Gotz A, Pukall R, Smit E, Tietze E, Prager R, Tschäpe H, van Elsas JD, Smalla K. 1996. Detection and characterization of broad-host-range plasmids in environmental bacteria by PCR. Appl Environ Microbiol 62:2621−2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kado CI, Liu ST. 1981. Rapid procedure for detection and isolation of large and small plasmids. J Bacteriol 145:1365–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Verdet C, Gautier V, Chachaty E, Ronco E, Hidri N, Decré D, Arlet G. 2009. Genetic context of plasmid-carried blaCMY-2 like genes in Enterobacteriaceae. Antimicrob Agents Chemother 53:4002–4006. doi: 10.1128/AAC.00753-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Verdet C, Arlet G, Ben Redjeb S, Ben Hassen A, Lagrange PH, Philippon A. 1998. Characterisation of CMY-4 an ampC-type plasmid-mediated β-lactamase in a Tunisian clinical isolate of Proteus mirabilis. FEMS Microbiol Lett 169:235–240. [DOI] [PubMed] [Google Scholar]
- 44.Chanoine MHN, Blanco J, Leflon-Guibout V, Demarty R, Pilar Alonso M, Manuela Canic M, Park YJ, Lavigne JP, Pitout J, Johnson JR. 2008. Intercontinental emergence of Escherichia coli clone O25:H4-ST131 producing CTX-M-15. J Antimicrob Chemother 61:273−281. [DOI] [PubMed] [Google Scholar]
- 45.Vimont S, Boyd A, Bleibtreu A, Bens M, Goujon JM, Garry L, Clermont O, Denamur E, Arlet G, Vandewalle A. 2012. The CTX-M-15-producing Escherichia coli clone O25b: H4-ST131 has high intestine colonization and urinary tract infection abilities. PLoS One 7:e46547. doi: 10.1371/journal.pone.0046547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liao CH, Huang YT, Chang CY, Hsu HS, Hsueh PR. 2014. Capsular serotypes and multilocus sequence types of bacteremic Klebsiella pneumoniae isolates associated with different types of infections. Eur J Clin Microbiol Infect Dis 33:365–369. doi: 10.1007/s10096-013-1964-z. [DOI] [PubMed] [Google Scholar]
- 47.Bachman MA, Oyler JE, Burns SH, Caza M, Lépine F, Dozois CM, Weiser JN. 2011. Klebsiella pneumoniae yersiniabactin promotes respiratory tract infection through evasion of lipocalin 2. Infect Immun 79:3309−3316. doi: 10.1128/IAI.05114-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Paauw A, Leverstein-van Hall MA, van Kessel KP, Verhoef J, Fluit AC. 2009. Yersiniabactin reduces the respiratory oxidative stress response of innate immune cells. PLoS One 4:e8240. doi: 10.1371/journal.pone.0008240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shin SY, Bae IK, Kim J, Jeong SH, Yong D, Kim JM, Lee K. 2012. Resistance to carbapenems in sequence type 11 Klebsiella pneumoniae is related to DHA-1 and loss of OmpK35 and/or OmpK36. J Med Microbiol 61:239–245. doi: 10.1099/jmm.0.037036-0. [DOI] [PubMed] [Google Scholar]
- 50.Mata C, Miro E, Rivera A, Mirelis B, Coll P, Navarro F. 2010. Prevalence of acquired AmpC β-lactamases in Enterobacteriaceae lacking inducible chromosomal ampC genes at a Spanish hospital from 1999 to 2007. Clin Microbiol Infect 16:472–476. doi: 10.1111/j.1469-0691.2009.02864.x. [DOI] [PubMed] [Google Scholar]
- 51.Frey J, Bagdasarian M. 1989. The biology of IncQ plasmids, p 79− 93 In Thomas CM. (ed), Promiscuous plasmids of Gram-negative bacteria. Academic Press Ltd., London, United Kingdom. [Google Scholar]
- 52.Giles WP, Benson AK, Olson ME, Hutkins RW, Whichard JM, Winokur PL, Fey PD. 2004. DNA sequence analysis of regions surrounding blaCMY-2 from multiple Salmonella plasmid backbones. Antimicrob Agents Chemother 48:2845−2852. doi: 10.1128/AAC.48.8.2845-2852.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kang MS, Besser TE, Call DR. 2006. Variability in the region downstream of the blaCMY-2 β-lactamase gene in Escherichia coli and Salmonella enterica plasmids. Antimicrob Agents Chemother 50:1590−1593. doi: 10.1128/AAC.50.4.1590-1593.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Barlow M, Hall BG. 2002. Origin and evolution of the AmpC β-lactamases of Citrobacter freundii. Antimicrob Agents Chemother 46:1190−1198. doi: 10.1128/AAC.46.5.1190-1198.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hopkins KL, Liebana E, Villa L, Batchelor M, Threlfall EJ, Carattoli A. 2006. Replicon typing of plasmids carrying CTX-M or CMY β-lactamases circulating among Salmonella and Escherichia coli isolates. Antimicrob Agents Chemother 50:3203−3206. doi: 10.1128/AAC.00149-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.