Abstract
Ceftaroline is a fifth-generation cephalosporin with potent antimicrobial activity against Gram-positive and Gram-negative pathogens. Neutropenia is a rare serious adverse event for the class of cephalosporins; however, we observed several cases of severe neutropenia in our outpatient infectious disease practice believed to be associated with ceftaroline use. The aim of this study was to determine the incidence of neutropenia among patients receiving ceftaroline therapy for more than 7 days. We conducted a retrospective cohort analysis of patients admitted to an 800-bed regional medical center between June 2012 and December 2014 who received ceftaroline for more than 7 days to assess the incidence of developing clinically significant neutropenia. Demographic and patient care data points as well as underlying admitting and chronic diagnoses were retrospectively collected from the medical record. Clinically significant neutropenia was defined as an absolute neutrophil count (ANC) less than 1,500 cells/mm3. Analysis was performed to determine the incidence, severity, and outcome of neutropenia following ceftaroline administration. A total of 39 patients were included in the cohort. The median duration of therapy was 27 days. Seven patients (18%) developed neutropenia while on ceftaroline therapy. Four (10%) of the neutropenic patients had an ANC of <500 cells/mm3. The median first neutropenic day was day 17, with the median ANC nadir of 432 cells/mm3 on day 24. We determined that extended ceftaroline infusion is associated with the development of neutropenia. We recommend obtaining a complete blood count (CBC) with differential at the onset of therapy and weekly thereafter. Should the ANC fall below 2,500 cells/mm3, then twice-weekly CBCs should be monitored for the duration of ceftaroline therapy, and therapy should be discontinued if the ANC falls to 1,500 cells/mm3 or less.
INTRODUCTION
Methicillin-resistant Staphylococcus aureus (MRSA) remains a common cause of both hospital- and community-associated infections, with an estimated prevalence of methicillin resistance among community-associated S. aureus isolates in emergency departments as high as 60% (1). With the continued prevalence of MRSA infections and the threat of therapeutic failures in patients receiving vancomycin therapy, new agents with activity against MRSA are needed (2).
Ceftaroline is a fifth-generation cephalosporin with potent antimicrobial activity against Gram-positive and Gram-negative pathogens. It is a novel β-lactam that has a maximum affinity for PBP2a, which confers MRSA resistance via the mecA gene (3, 4). In 2010, the U.S. Food and Drug Administration (FDA) approved ceftaroline for use in the treatment of acute bacterial skin and skin structure infections (SSSI) as well as community-acquired pneumonia (CAP) in patients18 years or older (4–6). Additionally, ceftaroline has bactericidal activity against MRSA, therefore serving as an attractive alternative agent for the treatment of MRSA bacteremia when approved agents are contraindicated or treatment failures have occurred.
Phase III trials proved ceftaroline to be a well-tolerated drug with a favorable safety profile. The mean duration of therapy in the initial ceftaroline clinical trials was nearly 1 week, while the maximum duration of therapy in these trials was 14 days (7–10). The most commonly reported adverse effects were nausea, diarrhea, pruritus, headache, and insomnia (5). In our clinical practice, we identified several patients who developed neutropenia while receiving ceftaroline therapy. Additionally, we believed that following current guideline recommendations for toxicity monitoring while on cephalosporin therapy did not provide for adequate early detection for the development of neutropenia in patients receiving ceftaroline. To determine the incidence, severity, and timing of neutropenia, as well as to explore potential risk factors for the development of neutropenia while on ceftaroline therapy, we conducted a retrospective cohort analysis of patients who received more than 7 days of ceftaroline therapy.
MATERIALS AND METHODS
The cohort included adult patients admitted to an 800-bed academic medical center between June 2012 and December 2014 with an infection for which they received ceftaroline therapy for 7 days or greater. This study was approved by the institutional review board at Louisiana State University Health Sciences Center in New Orleans.
The primary outcome measure was the development of clinically significant neutropenia while receiving ceftaroline therapy. We defined clinically significant neutropenia as an absolute neutrophil count (ANC) of less than 1,500 cells/mm3, as this value is considered abnormal by current laboratory standards.
Baseline characteristics were obtained by review of the electronic medical record. Gathered variables included demographics (age, sex, and race), renal function, hepatic function, ceftaroline dose, duration of therapy, weight and body mass index (BMI), and selected comorbid illnesses. We considered the baseline ANC to be the ANC on the day prior to or the day of initiation of ceftaroline. In cases in which these data were not available, we considered the earliest ANC while on therapy to be the baseline. Patients were excluded if they had neutropenia at baseline. We considered comorbidities to be present if they were documented in the admission history and physical, discharge summary, or infectious disease consult note.
We calculated the Charlson comorbidity index (11), a scoring system used to assist in prediction of 10-year mortality due to chronic comorbid illnesses, as a measure of chronic health for comparison of those who did and those who did not develop neutropenia. Investigators determined the primary site of infection by reviewing the subjects' medical records for the infection source, categorizing the primary site as skin/skin structure, orthopedic, pulmonary, or endovascular. Orthopedic infections included patients with ostoemyelitis, discitis, and septic arthritis. Endovascular infections included patients with primary bacteremia, endocarditis, or central venous device-related bacteremia. These categories were considered exclusive; a patient could not have more than one source. The investigators reviewed home and inpatient medication lists and recorded concurrent receipt of medications with a >1% risk of neutropenia. Data on risk for neutropenia were gathered from http://www.pdr.net and http://www.wolterskluwercdi.com/.
Statistical analysis was performed with SPSS version 21.0 (IBM Corp., Armonk, NY). Baseline characteristics between those patients with the outcome in question (neutropenia) versus those without this outcome were compared by Wilcoxon's rank sum or chi-square for continuous or dichotomous variables, respectively. Because of the low number of cases, we were unable to perform multivariable analysis to further explore risk factors for the development of neutropenia; thus, all analysis is descriptive in nature.
RESULTS
A total of 49 patients received ceftaroline therapy between June 2012 and December 2014. Of these patients, 39 received greater than 7 days of ceftaroline therapy. Baseline characteristics are listed in Table 1. The median duration of therapy was 27 days, with a range of 9 to 125 days. In patients who did and did not develop neutropenia, the median durations of therapy were 23 days (25th to 75th percentile, 19.5 to 51.5) and 25.5 days (25th to 75th percentile, 14.25 to 38.25), respectively (P = 0.755). Of note, the patient that received 125 days had a complicated disease course with a nonoperable spinal epidural abscess; this patient ultimately did develop neutropenia. The next-longest duration of therapy was 52 days. Figure 1 depicts a box plot of the durations of therapy.
TABLE 1.
Baseline characteristics of patients who did and did not develop neutropenia
| Characteristic | Value for: |
P value | |
|---|---|---|---|
| Nonneutropenic patients (n = 32) | Neutropenic patients (n = 7) | ||
| Age, median (25th–75th percentile) | 50.5 (40.75–60.75) | 44 (26–54) | 0.129a |
| BMI, median (25th–75th percentile) | 31.55 (25.40–38.83) | 23.9 (22.2–26.2) | 0.024a |
| Creatinine clearance, median (25th–75th percentile) | 90 (67.99–141.25) | 105 (93.00–109.00) | 0.360a |
| Male sex, n (%) | 24 (75) | 2 (28.6) | 0.030b |
| White race, n (%) | 28 (87.5) | 7 (100) | 0.614c |
| History of malignancy, n (%) | 2 (6.1) | 1 (14.3) | 0.457b |
| Baseline neutropenic med, n (%) | 13 (41.9) | 3 (42.9) | 1b |
| Charlson comorbidity index, median (25th–75th percentile) | 2 (0.25–3) | 1 (0–3) | 0.359a |
| Primary site of infection, n (%) | 0.249c | ||
| Skin/skin structure | 7 (22) | 1 (14) | |
| Orthopedic | 17 (53) | 3 (43) | |
| Endovascular | 1 (3) | 2 (29) | |
| Pulmonary | 6 (19) | 1 (14) | |
| Other | 1 (3) | 0 | |
| Baseline ANC (thousands), median (25th–75th percentile) | 8.4 (6.3–15.7) | 6.5 (5.4–10.3) | 0.300a |
| Duration of ceftaroline therapy (days), median (25th–75th percentile) | 25.5 (14.25–38.25) | 23 (19.5–51.5) | 0.755a |
Determined by Wilcoxon rank sum test.
Determined by Fisher's exact test.
Determined by Pearson chi-square test.
FIG 1.
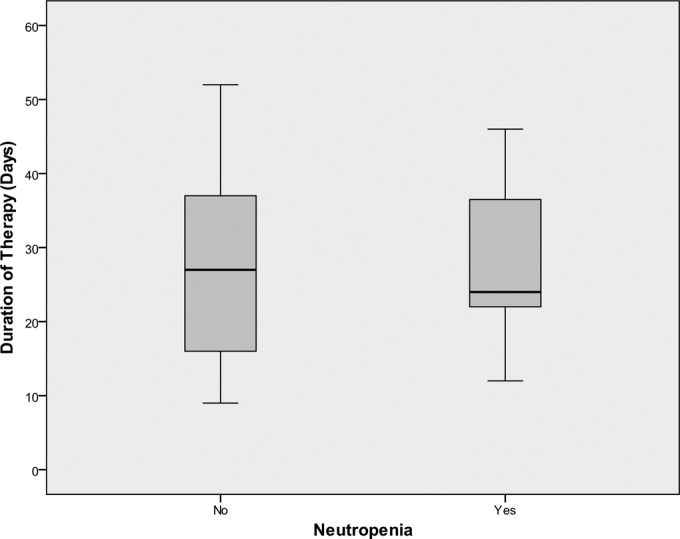
Box plot of duration of therapy in those who became neutropenic and those who did not. P = 0.755.
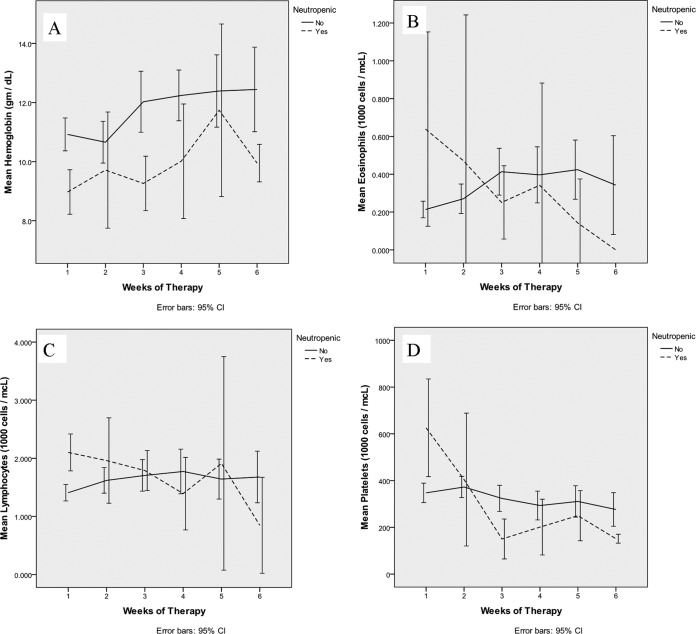
Seven patients developed neutropenia (ANC < 1,500 cells/mm3). We did not detect significant changes in cell lines other than the neutrophil (lymphocytes, basophils, and eosinophils). Figure 2 depicts trends in these cell lines throughout the first 6 weeks of ceftaroline therapy. We noted wide fluctuations in the platelet counts of many patients; however, there was no correlation with ceftaroline therapy or its cessation (some patients developed transient thrombocytopenia [<150,000/mm3] but recovered despite continuation of therapy).
FIG 2.
Graphs of mean hemoglobin concentration (A), mean eosinophil count (B), mean lymphocyte count (C), and mean platelet count (D) by week in patients who did and did not become neutropenic through the course of therapy.
Twenty-six of the 32 patients who did not develop neutropenia initially received 600 mg of ceftaroline every 12 h. One patient, who had an orthopedic infection, received 600 mg every 8 h; the remaining 5 patients received lower doses due to impaired renal function. Three patients required additional dose adjustment during treatment due to changes in renal function. Of the seven patients who did develop neutropenia, six of them received 600 mg of ceftaroline every 12 h; one, again with an orthopedic infection, received 600 mg every 8 h (q8h). None of these patients required dose adjustment or developed significant renal impairment throughout the duration of treatment.
Of the 39 patients included in the analysis, 36 had ANC data available from the day of or 1 day prior to the initiation of therapy. The remaining 3 patients had their first ANC documented after the first day therapy. Table 1 includes the baseline characteristics of those patients who developed neutropenia and those patients who did not. Patients who developed neutropenia were more likely to be females with a lower BMI; however, in the total cohort, women did not have a statistically significantly lower BMI than men, with a median of 28.3 (25th to 75th percentile, 22.3 to 33.5) versus 31.6 (25th to 75th percentile, 24.8 to 39.2) (P = 0.153 by Wilcoxon rank sum test). One patient who developed neutropenia had a BMI greater than 30 (34.1), while the remainder had a BMI of 26.2 or below.
Orthopedic infections were the most common indications for more than 7 days of ceftaroline therapy. We identified no association between the site of infection and the subsequent development of neutropenia. Furthermore, there was no difference in median baseline ANC between the two groups: the median baseline ANC of those who developed neutropenia was 6,489 cells/mm3, and the median baseline ANC was 8,410 cells/mm3 in those who did not develop neutropenia (Table 1).
The median first neutropenic day was day 17; three of the patients who developed neutropenia did so by day 14. Of the 7 patients who developed neutropenia, 4 of them had a nadir ANC of <500 cells/mm3. The median treatment day of the ANC nadir was day 24, with a median ANC nadir of 432 cells/mm3 in those patients who developed neutropenia. Analysis of the 32 patients who did not develop neutropenia revealed that their ANC nadir occurred at a time similar to that in those in the neutropenic group, with their lowest ANC on day 21. Figure 3 depicts trends over time in the ANC in those who did and did not develop neutropenia.
FIG 3.
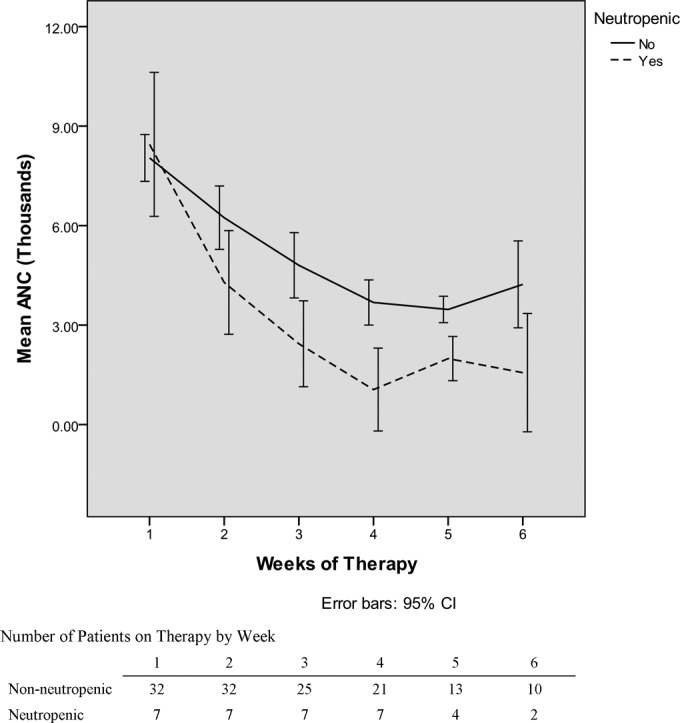
Graph of mean absolute neutrophil count (ANC) by week in those who became neutropenic and those who did not. CI, confidence interval.
Because neutropenia was not an anticipated finding, we did not routinely collect posttherapy data that would document the likelihood and timing of recovery. Despite this, we were able to retrieve posttherapy complete blood counts (CBCs) for five of seven patients who did develop neutropenia. Four of these patient exhibited recovery to an ANC greater than 1,500 cells/μl within 14 days of cessation of therapy; the remaining patient had a CBC 25 days posttherapy which revealed an ANC of >1,500 cells/μl. We documented no case of persistent neutropenia after cessation of therapy. Only one patient was hospitalized for a condition believed to be a result of neutropenia. This patient had several ANC's beginning on day one posttherapy. The first ANC of >1,500 cells/mm3 occurred on posttherapy day 13. We confirmed that all of the neutropenic patients were alive at least 30 days posttreatment.
DISCUSSION
In general, antibiotics are a well-tolerated class of medications; however, their administration is not without adverse effects. From anaphylaxis to photosensitivity, from benign to life threatening, these adverse effects are numerous and diverse, affecting nearly every organ system. One of the most commonly affected systems affected by antimicrobial therapy is that of hematopoiesis.
Hematologic consequences of antimicrobial use have been described for nearly all classes of antibiotics. The effect of antimicrobials on hematopoiesis can be fairly benign, such as the transient thrombocytopenia seen with the ureidopenicillins (12), or tragic, as seen in the aplastic anemia precipitated by chloramphenicol (13). Fortunately, the broadest class of antimicrobials—the β-lactam class—is among the most well tolerated of all, with few adverse hematologic reactions (14).
Though no hematologic toxicity is without concern, antibiotic-induced neutropenia is especially worrisome, as neutrophils are required to combat the very process for which the antibiotic is prescribed. Fortunately, drug-induced neutropenia is a rare event. Andrès and Maloisel reported 6 cases per million population per year, and the incidence of drug-induced neutropenia increases with age. The authors also noted that women appear twice as likely to experience the complication as men, an observation consistent with our findings (15).
The development of neutropenia is associated with an increased risk of infection. This risk is stratified by the degree of neutropenia, a patient's underlying immune status, and the adequacy of bone marrow stores. In the case of patients with chemotherapy-induced neutropenia, febrile patients are further risk stratified as high-risk and low-risk individuals. High-risk patients are those who are anticipated to have profound neutropenia (ANC of <100 cells/mm3) for a prolonged period (>7 days). Low-risk patients are patients who are expected to have a brief period (<7 days) of neutropenia followed by recovery of cells. Definitions of neutropenia vary by source. The WHO defines neutropenia as an ANC of <1,800 cells/mm3. The lower limit of the reference range for ANC is 1,500 cells/mm3. In patients with neutropenia due to chemotherapy, an ANC of <500 cells/mm3 is the point at which the risk of admission and complications increases; therefore, this value stratifies febrile neutropenic patients in the guidelines for the treatment of this condition (16).
Many drug classes have been described to cause neutropenia, including the penicillin (17) and cephalosporin members of the β-lactam class; however, the risk of cephalosporin-induced neutropenia appears to be small compared to the risk with more commonly associated drugs, such as methimazole, clozapine, sulfasalazine, and trimethoprim-sulfamethoxazole. Most consider the cephalosporin class of β-lactam drugs to have a favorable safety profile, with hypersensitivity reactions being the most common adverse events (17). Few members of the cephalosporin class of antibiotics have been associated with the development of neutropenia, with less than 1% of patients receiving a cephalosporin having developed the complication (18). Although rare, neutropenia has been described for patients receiving prolonged therapy with the fourth-generation cephalosporin cefepime for osteomyelitis. In these cases, neutropenia resolved after discontinuation of the drug (19).
The mechanism underlying β-lactam-induced neutropenia is not completely understood, but it likely occurs due to an immune-mediated event or a direct toxic mechanism (20). Bone marrow examination in patients with drug induced neutropenia may show decreased or absent myeloid precursor cells. The term myeloid maturation arrest refers to the disease state in which the patient remains neutropenic but immature forms of the myelocyte are present on bone marrow biopsy. Recovery of cells is faster in those patients with myeloid precursors present (2 to 7 days) on bone marrow biopsy than in those without precursor cells (>14 days) (15).
Although a review of the four major phase III ceftaroline clinical trials did not reveal an increased incidence of neutropenia in patients who received ceftaroline (7, 10, 21), there are documented cases of neutropenia thought to be secondary to its use reported in the aftermarket literature. The first case was a 90-year-old woman being treated with ceftaroline for MRSA health care-associated pneumonia, with secondary bacteremia and possible vertebral osteomyelitis. The patient developed neutropenia after 25 days of ceftaroline. Recovery occurred within 1 week of discontinuation of the drug (22). In a second case, a 67-year-old man treated with high-dose ceftaroline (600 mg every 8 h q8h) for MRSA septic arthritis developed profound neutropenia after 21 days of therapy (23). Of note, both of these cases occurred after the patients received therapy far longer than that administered in the phase III studies (7–10).
More recently, Jain et al. performed a retrospective chart review of 12 patients who received ceftaroline therapy for refractory MRSA and coagulase-negative Staphylococcus infections; ceftaroline was discontinued in 7 of those patients due to hematologic toxicities. The median time of discontinuation was 22 days (24).
While neutropenia is not a commonly reported serious adverse event (<2%) associated with ceftaroline use, in our clinical practice, nearly 18% of patients who received more than 7 days of ceftaroline therapy developed laboratory-confirmed neutropenia defined as an ANC of <1,500 cells/mm3. The timing of the development of neutropenia in our study is consistent with the previously reported cases of neutropenia, developing early in the fourth week of therapy. Both the neutropenic group and those that did not develop neutropenia had a fall in their ANC with a nadir near the same time (day 24 versus day 21, respectively). This finding may reflect a decline due to treatment of an infection, but the similarity in timing is concerning for a drug reaction that continues only in a selected group of people.
Although our sample size is not large enough to determine specific risk factors for the development of neutropenia, we noted that patients who developed neutropenia had a lower BMI and were more likely to be female. This finding may implicate pharmacodynamic principles as a possible etiology of the development of neutropenia; it is possible that in patients with low body mass, there is a cumulative bone marrow toxicity manifested over time as suppression in the neutrophil population. Further studies should explore this finding, as dose reduction in lean patients could potentially avoid this complication. Although in the overall cohort, we did not find a statistical difference in the BMI of men versus women, there was a trend for women to have a lower BMI. The association between female sex and the development of neutropenia may be due, in large part, to the lower BMI rather than other gender-related differences.
Ceftaroline is currently FDA approved for the treatment of acute bacterial skin and skin structure infections (ABSSSI) and community-acquired pneumonia (CAP). CANVAS and FOCUS included treatment courses with durations between 5 and 14 days. As noted in our study, only 25% of those patients who would develop neutropenia did so by day 14. The recovery from neutropenia may be rapid and therefore may not have been noted as clinically significant during the shorter duration of therapy in the clinical trials.
Our patient population consisted primarily of patients receiving ceftaroline for off-label indications such as bacteremia and osteomyelitis. Off-label use of medications is very common and accounts for up to 20% of all prescription drug use in some studies (25). While vancomycin and daptomycin are suggested treatment modalities for infective endocarditis and osteomyelitis in the Infectious Diseases Society of America (IDSA) guidelines on the management of patients with MRSA infections, the use of these medications for these indications is largely off-label (26). The risk of use for these diagnoses has long been accepted as the standard of care, as a bactericidal antibacterial agent is desired for these disease processes. In our study population, ceftaroline was administered as a third-line agent for those patients unable to complete therapy with either vancomycin or daptomycin. Adverse drug reactions to vancomycin and daptomycin are commonly encountered in clinical practice, and we anticipate further off-label use, including that of longer duration, of ceftaroline as practitioners look for alternative bactericidal agents to treat MRSA infections.
Patients receiving outpatient parenteral antibiotic therapy (OPAT) require a heightened awareness for complications, as these patients are no longer close at hand and monitoring occurs intermittently. The IDSA practice guideline for OPAT recommends monitoring once-weekly complete blood count (CBC) and basic metabolic panel to monitor for adverse events in patients receiving cephalosporin therapy. The guideline also suggests more frequent monitoring of laboratory data if an adverse trend is identified during laboratory monitoring; however, the guideline does not give recommendations on how frequently (27).
This investigation represents the first study to describe the experience of all patients receiving long-term ceftaroline therapy at a single institution and the adverse effect of neutropenia seen in such a cohort. Based on our findings, a CBC with a differential should be obtained prior to the initiation of therapy and weekly during therapy. In our study, 4 (10%) of our 39 patients receiving ceftaroline developed an ANC of <500 cells/mm3 during therapy. To avoid the complications associated with the development of neutropenia, intense monitoring should begin prior to the development of the complication of neutropenia. Seventy percent of our patients who had an ANC fall below 2,500 cells/mm3 went on to develop laboratory-confirmed neutropenia. Due to our findings, we now recommend twice-weekly CBCs once a fall in the ANC below 2,500 cells/mm3 is seen. Clinicians should continue this intense monitoring for the duration of ceftaroline therapy, and therapy should be discontinued if the ANC falls below 1,500 cells/mm3.
Study limitations.
The size of the cohort precluded us from performing a multivariable statistical analysis to determine if there were any specific patient characteristics that increased the risk for developing neutropenia while on ceftaroline. We recommend that analysis be performed on a larger cohort, as this additional information would also assist providers in determining those who are at a higher risk for this complication of therapy.
REFERENCES
- 1.Boucher HW, Corey GR. 2008. Epidemiology of methicillin-resistant Staphylococcus aureus. Clin Infect Dis 46(Suppl 5):S344–S349. [DOI] [PubMed] [Google Scholar]
- 2.Magana M, Ioannidis A, Magiorkinis E, Ursu O, Bologa CG, Chatzipanagiotou S, Hamblin MR, Tegos GP. 2015. Therapeutic options and emerging alternatives for multidrug resistant staphylococcal infections. Curr Pharm Des 21:2058–2072. doi: 10.2174/1381612821666150310101851. [DOI] [PubMed] [Google Scholar]
- 3.Drusano GL. 2010. Pharmacodynamics of ceftaroline fosamil for complicated skin and skin structure infection: rationale for improved anti-methicillin-resistant Staphylococcus aureus activity. J Antimicrob Chemother 65(Suppl 4):iv33–iv39. [DOI] [PubMed] [Google Scholar]
- 4.Jacqueline C, Amador G, Batard E, LeMabecque V, Potel G, Caillon J. 2012. Antibiotics against endocarditis—past, present and future (experimental data), p 97–124. In Breijo-Marquez PFR. (ed), Endocarditis. InTech, Rijeka, Croatia. [Google Scholar]
- 5.Bazan JA, Martin SI, Kaye KM. 2011. Newer beta-lactam antibiotics: doripenem, ceftobiprole, ceftaroline, and cefepime. Med Clin North Am 95:743–760, viii. doi: 10.1016/j.mcna.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 6.Gould IM, David MZ, Esposito S, Garau J, Lina G, Mazzei T, Peters G. 2012. New insights into meticillin-resistant Staphylococcus aureus (MRSA) pathogenesis, treatment and resistance. Int J Antimicrob Agents 39:96–104. doi: 10.1016/j.ijantimicag.2011.09.028. [DOI] [PubMed] [Google Scholar]
- 7.Corey GR, Wilcox MH, Talbot GH, Thye D, Friedland D, Baculi T. 2010. CANVAS 1: the first phase III, randomized, double-blind study evaluating ceftaroline fosamil for the treatment of patients with complicated skin and skin structure infections. J Antimicrob Chemother 65(Suppl 4):iv41–iv51. [DOI] [PubMed] [Google Scholar]
- 8.File TM Jr, Low DE, Eckburg PB, Talbot GH, Friedland HD, Lee J, Llorens L, Critchley IA, Thye DA. 2011. FOCUS 1: a randomized, double-blinded, multicentre, phase III trial of the efficacy and safety of ceftaroline fosamil versus ceftriaxone in community-acquired pneumonia. J Antimicrob Chemother 66(Suppl 3):iii19–iii32. [DOI] [PubMed] [Google Scholar]
- 9.Low DE, File TM Jr, Eckburg PB, Talbot GH, David Friedland H, Lee J, Llorens L, Critchley IA, Thye DA. 2011. FOCUS 2: a randomized, double-blinded, multicentre, phase III trial of the efficacy and safety of ceftaroline fosamil versus ceftriaxone in community-acquired pneumonia. J Antimicrob Chemother 66(Suppl 3):iii33–iii44. [DOI] [PubMed] [Google Scholar]
- 10.Wilcox MH, Corey GR, Talbot GH, Thye D, Friedland D, Baculik T. 2010. CANVAS 2: the second phase III, randomized, double-blind study evaluating ceftaroline fosamil for the treatment of patients with complicated skin and skin structure infections. J Antimicrob Chemother 65(Suppl 4):iv53–iv65. [DOI] [PubMed] [Google Scholar]
- 11.Charlson ME, Pompei P, Ales KL, MacKenzie CR. 1987. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 12.Lang R, Lishner M, Ravid M. 1991. Adverse reactions to prolonged treatment with high doses of carbenicillin and ureidopenicillins. Rev Infect Dis 13:68–72. doi: 10.1093/clinids/13.1.68. [DOI] [PubMed] [Google Scholar]
- 13.Wallerstein RO, Condit PK, Kasper CK, Brown JW, Morrison FR. 1969. Statewide study of chloramphenicol therapy and fatal aplastic anemia. JAMA 208:2045–2050. [PubMed] [Google Scholar]
- 14.Cunha BA. 2001. Antibiotic side effects. Med Clin North Am 85:149–185. doi: 10.1016/S0025-7125(05)70309-6. [DOI] [PubMed] [Google Scholar]
- 15.Andrès E, Maloisel F. 2008. Idiosyncratic drug-induced agranulocytosis or acute neutropenia. Curr Opin Hematol 15:15–21. doi: 10.1097/MOH.0b013e3282f15fb9. [DOI] [PubMed] [Google Scholar]
- 16.Freifeld AG, Bow EJ, Sepkowitz KA, Boeckh MJ, Ito JI, Mullen CA, Raad II, Rolston KV, Young JA, Wingard JR, Infectious Diseases Society of America. 2011. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis 52:e56–e93. doi: 10.1093/cid/cir073. [DOI] [PubMed] [Google Scholar]
- 17.Wright AJ. 1999. The penicillins. Mayo Clin Proc 74:290–307. doi: 10.4065/74.3.290. [DOI] [PubMed] [Google Scholar]
- 18.Mandell GL, Bennett JE, Dolin R. 2010. Mandell, Douglas, and Bennett's principles and practice of infectious diseases, 7th ed Churchill Livingstone/Elsevier, Philadelphia, PA. [Google Scholar]
- 19.Wong BB, Ko GJ. 2003. Neutropenia in patients receiving long-term cefepime therapy for osteomyelitis. Am J Health Syst Pharm 60:2229–2232. [DOI] [PubMed] [Google Scholar]
- 20.Murphy MF, Chapman JF, Metcalfe P, Waters AH. 1985. Antibiotic-induced neutropenia. Lancet ii:1306–1307. [DOI] [PubMed] [Google Scholar]
- 21.File TM Jr, Low DE, Eckburg PB, Talbot GH, Friedland HD, Lee J, Llorens L, Critchley I, Thye D. 2010. Integrated analysis of FOCUS 1 and FOCUS 2: randomized, doubled-blinded, multicenter phase 3 trials of the efficacy and safety of ceftaroline fosamil versus ceftriaxone in patients with community-acquired pneumonia. Clin Infect Dis 51:1395–1405. doi: 10.1086/657313. [DOI] [PubMed] [Google Scholar]
- 22.Rimawi RH, Frenkel A, Cook PP. 2013. Ceftaroline—a cause for neutropenia. J Clin Pharm Ther 38:330–332. doi: 10.1111/jcpt.12062. [DOI] [PubMed] [Google Scholar]
- 23.Yam FK, Kwan BK. 2014. A case of profound neutropenia and agranulocytosis associated with off-label use of ceftaroline. Am J Health Syst Pharm 71:1457–1461. doi: 10.2146/ajhp130474. [DOI] [PubMed] [Google Scholar]
- 24.Jain R, Chan JD, Rogers L, Dellit TH, Lynch JB, Pottinger PS. 2014. High incidence of discontinuations due to adverse events in patients treated with ceftaroline. Pharmacotherapy 34:758–763. doi: 10.1002/phar.1435. [DOI] [PubMed] [Google Scholar]
- 25.Radley DC, Finkelstein SN, Stafford RS. 2006. Off-label prescribing among office-based physicians. Arch Intern Med 166:1021–1026. doi: 10.1001/archinte.166.9.1021. [DOI] [PubMed] [Google Scholar]
- 26.Liu C, Bayer A, Cosgrove SE, Daum RS, Fridkin SK, Gorwitz RJ, Kaplan SL, Karchmer AW, Levine DP, Murray BE, Rybak MJ, Talan DA, Chambers HF. 2011. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children: executive summary. Clin Infect Dis 52:1–38. [DOI] [PubMed] [Google Scholar]
- 27.Tice AD, Rehm SJ, Dalovisio JR, Bradley JS, Martinelli LP, Graham DR, Gainer RB, Kunkel MJ, Yancey RW, Williams DN. 2004. Practice guidelines for outpatient parenteral antimicrobial therapy. IDSA guidelines. Clin Infect Dis 38:1651–1672. doi: 10.1086/420939. [DOI] [PubMed] [Google Scholar]