Abstract
This was an observational study comparing methicillin-resistant Staphylococcus aureus (MRSA) transmission with no decolonization of medical patients to required decolonization of all MRSA carriers during two consecutive periods: baseline with no decolonization of medical patients (16 months) and universal MRSA carrier decolonization (13 months). The setting was a one-hospital, 156-bed facility with 9,200 annual admissions. Regression models were used to compare rates of MRSA acquisition. The chi-square test was used to compare event frequencies. We used rates of MRSA clinical disease as an outcome monitor of the program. Analysis was done on 15,666 patients who had admission and discharge tests; 27.9% of inpatient days were occupied by a MRSA-positive patient (colonized patient-days) who received decolonization while hospitalized during the baseline period (this 27.9% represented those who had planned surgery) compared to 76.0% during the intervention period (P < 0.0001). The rate of MRSA transmission was 97 events (1.0%) for 9,415 admissions (2.0 transmission events/1,000 patient-days) during baseline and was 87 (1.4%) for 6,251 admissions (2.7 transmission events/1,000 patient-days) during intervention (P = 0.06; rate ratio, 0.74; 95% confidence interval [CI], 0.55 to 1.00). The MRSA nosocomial clinical disease rate was 5.9 infections/10,000 patient-days in the baseline period and was 7.2 infections/10,000 patient-days for the intervention period (rate ratio, 0.82; 95% CI, 0.46 to 1.45; P = 0.49). Decolonization of MRSA patients does not add benefit when contact precautions are used for patients colonized with MRSA in acute (hospital) care.
INTRODUCTION
Control of infection from methicillin-resistant Staphylococcus aureus (MRSA) has been a health care focus for more than 50 years (1). Importantly, by 2005, MRSA-related mortality in the United States was more than that from infections due to salmonella, tuberculosis, influenza, and HIV combined (2, 3). Since that time, efforts to reduce MRSA infection have had impacts in both Europe and the United States (4–6). However, the improvement has not been uniform, with some medical centers not realizing a reduction in disease (7). At least one large state (Illinois) reported an increase in overall rates of MRSA infection during the most recently monitored 5 years, with 11.7 MRSA infections/1,000 hospital discharges in 2009, 11.6 in 2010, 10.7 in 2011, and increasing to 14.2 in 2012 and 13.3 in 2013 (8).
While debate continues over the role of active surveillance testing (AST) for the prevention of MRSA infection (9, 10), it is clear that this approach is widely and successfully used in pragmatic settings (11, 12). One of the reasons that active surveillance testing for MRSA should be expected to be effective is that the greatest risk factor for developing clinical disease is to first become colonized in the nares with MRSA (13). Critically, persons who are either colonized or infected with MRSA are equally likely to have their skin and surrounding environment contaminated with this organism, so it can be readily contracted by others coming close to or touching them (14). Therefore, strategies to limit transmission that consider both colonized and infected persons are most likely to be successful at reducing disease (15).
The recent REDUCE MRSA (“Randomized Evaluation of Decolonization versus Universal Clearance to Eliminate MRSA”) trial suggested that universal decolonization of patients hospitalized in the intensive care unit (ICU) was the optimal approach for preventing MRSA and other multidrug-resistant (MDR) infections (based on assessment of clinical cultures) in that environment (16). One of the concerns raised in the report was the potential development of mupirocin resistance with widespread use (16). We have decolonized inpatients as part of our successful universal admission surveillance program for reducing MRSA infection and have found nearly a 3-fold increase in mupirocin resistance, rising from 3.9% in 2005 to 10.9% in 2012 (I. K. Dusich, et al., presented at the 113th Annual Meeting of the American Society for Microbiology, Denver, CO, 18 to 21 May 2013) (17).
The impact of inpatient decolonization for methicillin-resistant Staphylococcus aureus carriers on transmission of MRSA when isolation (contact precautions) is practiced is not well studied. Our objective was to examine the impact of inpatient decolonization as an adjunct to contact precautions by determining if inpatient nasal decolonization with mupirocin added benefit when contact precautions were used once a person was determined to be colonized with MRSA. Based on the sustained reduction in MRSA clinical disease realized by the U.S. Department of Veterans Affairs health care system using surveillance and contact precautions only, with no decolonization (12, 18), our hypothesis was that, if contact precautions were applied in a timely manner, then decolonization added no benefit. Our study was a quality improvement project designed to test that hypothesis by measuring transmission when decolonization of medical patients was and was not used as an adjunct measure to contact precautions (isolation).
MATERIALS AND METHODS
The study was performed as a before-and-after observational trial at one of four hospitals in the NorthShore University HealthSystem (NorthShore; demographics for patients at this hospital are listed in Table 1). Paired nasal swabs were collected on all inpatients at admission and discharge from April 2010 through August 2012. One swab was used for real-time PCR, following the manufacturer's recommendation (19). The second swab (swab 2) was used for culture, and the result of this was used to determine the rate of MRSA transmission. Swab 2 was plated onto CHROMagar MRSA (CMRSA; BBL, Becton Dickinson, Sparks, MD) and then placed into tryptic soy broth for enrichment that was incubated for 24 h; then the broth was plated to another CMRSA. Mauve colonies were subcultured to a blood agar plate (BBL). S. aureus was confirmed using a Staphaurex agglutination test (Remel, Lenexa, KS). Colonies that were latex positive and mauve color were considered MRSA.
TABLE 1.
Demographics of patients at the study hospital sitea
| Sex | Age (yr) |
No. (%) of patients by race/ethnicityb |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | Median (SD) | African American | American Indian or Alaska Native | Asian | Caucasian | Other | Pacific Islander/Hawaiian Native | Total | |
| Male (44%) | 68.7 | 71.0 (16.7) | |||||||
| Hispanic/Latino | 12 (0) | 89 (1) | 1 (0) | 102 (1.1) | |||||
| Non-Hispanic | 172 (1.9) | 8 (0.1) | 242 (2.6) | 2,837 (30.9) | 716 (7.8) | 3,975 (43.3) | |||
| Total of those who reported race/ethnicity | 172 (1.9) | 8 (0.1) | 242 (2.6) | 2,849 (30.9) | 805 (8.8) | 1 (0) | 4,077 (44.4) | ||
| Female (56%) | 71.1 | 75.0 (17.4) | |||||||
| Hispanic/Latino | 23 (0.3) | 100 (1.1) | 3 (0) | 126 (1.4) | |||||
| Non-Hispanic | 230 (2.5) | 10 (0.1) | 355 (3.9) | 3,380 (36.8) | 994 (10.8) | 1 (0) | 4,970 (54.2) | ||
| Total of those who reported race/ethnicity | 230 (2.5) | 10 (0.1) | 355 (3.9) | 3,403 (37.1) | 1,094 (11.9) | 4 (0) | 5,096 (55.6) | ||
| Grand total | 402 (4.4) | 18 (0.2) | 597 (6.5) | 6,252 (68.2) | 1,899 (20.7) | 5 (0) | 9,173 (100) | ||
Annual admission data for 2011.
No. (%) data are from the 9,173 total.
Mupirocin ointment (2%) applied to the anterior nares (twice daily for 5 days) plus chlorhexidine (4%; used as a liquid soap) bathing on days 1, 3, and 5 was the decolonization regimen. Before August 2011, inpatient decolonization was not recommended for MRSA colonization, except in presurgical patients (baseline period of 16 months). Starting in August 2011, decolonization was required for all MRSA-positive inpatients (intervention period of 13 months). An MRSA transmission event (TE) was defined as a patient having a negative MRSA culture on admission and a positive MRSA culture at discharge; only those patients with both an admission and discharge sample were included. Patients who had no history of culture-proven MRSA in the prior 2 years and a negative admission test (i.e., those eligible for acquisition) and who had a positive discharge culture were considered to have newly acquired MRSA. The sample size was powered to detect a 50% reduction in the transmission rate between the baseline and intervention periods. An infection preventionist monitored testing and decolonization therapy throughout the study and immediately intervened whenever compliance with either recommended measure was below 90%.
We also determined the inpatient length of stay (LOS) for those with a positive MRSA admission test and those with a negative test. The inpatient admission MRSA prevalence was determined by dividing the number of patients with a positive culture by the number of tests performed; discharge prevalence was calculated by dividing the number of positive culture patients at discharge plus those with a positive discharge PCR test who were culture positive at admission and decolonized during their stay, divided by the number of tests performed. Finally, a measurement of clinical MRSA disease was performed using the nosocomial infection marker (CareFusion Corporation, San Diego, CA), an electronic surveillance program we previously validated as a reliable tool for monitoring changes in MRSA clinical disease rate (20). Compliance with infection control practices was monitored by the NorthShore infection preventionists, and immediate education given to any hospital staff not following the correct practice.
The chi-square test was used to compare event frequencies between the baseline and intervention groups. A P value of <0.05 was considered significant. Analyses were conducted in R version 3.1.1 (21). The two-sample Poisson test was used to determine the difference in MRSA transmission between the two study periods. For our literature review, we used PubMed.gov (Medline) with the search terms MRSA, transmission, and prevention to search the medical literature from 1999 through August 2015. This study was approved by the institutional review board at NorthShore University HealthSystem (protocol EH10-148).
RESULTS
There were 22,548 patients admitted during the entire study period; 13,051 were admitted during the baseline period, and 9,497 were admitted during the intervention period. A total of 19,593 patients were tested at discharge (86.9%). The final analysis was performed on 15,666 patients who had both admission and discharge tests. The rate of admission testing compliance was >90%. However, in January 2012, an electronic prediction rule was implemented that determined who should be screened for MRSA upon admission that captured >90% of potential carriers (22); this resulted in testing of approximately 66% of admissions beginning in January 2012. The proportion (percentage) of MRSA-positive patients who had decolonization was 34% during the baseline period (April 2010 through June 2011) and was 82% during the intervention period (July 2011 through August 2012); 100% were decolonized when the length of stay was ≥3 days during the intervention period. The 34% decolonized during baseline represented those who had planned surgery. Since our hospital practice is to decolonize preoperative patients to prevent surgical site infection from S. aureus (23), this practice was continued, even though medical patients with MRSA colonization were not decolonized. Decolonization was enforced as a required practice by infection control. Compliance was monitored during the first 6 months of the intervention period by one of the infection preventionists, and of 300 patients who tested positive at admission, 54 were not decolonized; most (50 of 54) of those not decolonized were hospitalized for <1 day, so their positive tests were reported after discharge. The remaining 246 patients were virtually all decolonized (244 of 246; 99.2%) during their hospitalization. Eight patients were colonized with mupirocin-resistant MRSA and 50% of these (four patients) were treated with retapamulin applied in the same manner as mupirocin. The mean and median LOS during baseline were 5.6 and 4.0 days for MRSA-colonized patients and 4.1 and 3.0 days for noncolonized patients, respectively; the mean and median LOS during the intervention period were 5.1 and 4.0 days for MRSA-colonized patients and 4.0 and 3.0 days for noncolonized patients. The combination of this LOS data with the decolonization compliance indicates that, for those colonized, patients received decolonization therapy while hospitalized for 27.9% of inpatient MRSA days (1,226 of 4,396 days) during the baseline period (representing those who had planned surgery) compared to 76.0% of inpatient MRSA-colonized days (1,818 of 2,392 days) during the intervention period (P < 0.0001).
The rate of MRSA transmission was 97 (1%) transmission events for 9,415 admissions (2 transmission events/1,000 patient-days) during the baseline period, and it was 87 (1.4%) transmission events for 6,251 of admissions (2.7 transmission events/1,000 patient-days) during the intervention period (P = 0.06; rate ratio, 0.74; 95% confidence interval [CI], 0.55 to 1.00). MRSA acquisition data are listed in Table 2. The admission prevalences for MRSA colonization were 6.1% during baseline and 5.5% in the intervention period; the discharge prevalences during baseline and the intervention period were 5.5% (636 positive of 11,555 tests) and 4.7% (371 positive of 7,972 tests), respectively. The MRSA nosocomial clinical disease rate was 5.9 infections/10,000 patient-days in the baseline period and 7.2 infections/10,000 patient-days for the intervention period (rate ratio, 0.82; 95% CI, 0.46 to 1.45; P = 0.49). These data, along with the poststudy disease rate, are depicted in Fig. 1; there was no decolonization of medical patients in the poststudy period, and the clinical disease rate continued to decline. During 15 months of observation after the end of the study (in the poststudy period), the compliance with infection control precautions was 83.0% (1,433 of 1,727 observations) across all NorthShore hospitals. At the facility where this project took place, the compliance was 80.8% (319 of 395 observations).
TABLE 2.
MRSA acquisition data for patients with and without decolonization
| Period | % of patients MRSA positive at admission | No. of patients eligible for acquiring MRSA | Patients who acquired MRSA |
Eligible patient-days | Acquisition/1,000 eligible patient-days |
|||
|---|---|---|---|---|---|---|---|---|
| No. | % | Pa | No. | Pa | ||||
| No decolonization of medical patients | 6.7 | 9,415 | 97 | 1.0 | 0.04 | 47,784 | 2.0 | 0.06 |
| Decolonization of medical patients | 6.0 | 6,251 | 87 | 1.4 | 32,298 | 2.7 | ||
| Total | 6.5 | 15,666 | 184 | 1.2 | 80,082 | 2.3 | ||
P value for no decolonization versus decolonization.
FIG 1.
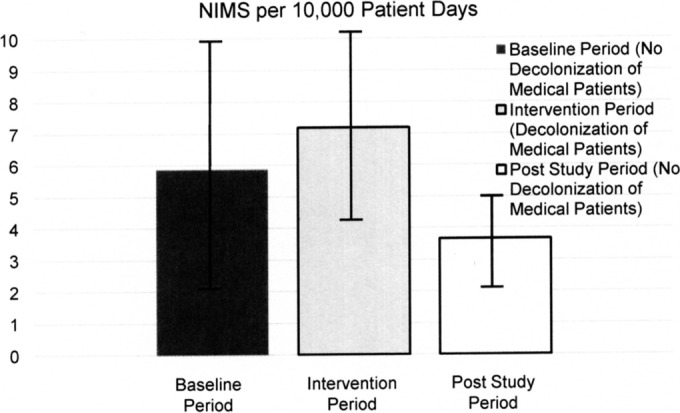
MRSA clinical disease rate over 4.75 years (April 2010 to January 2015) measured using the nosocomial infection marker (NIM) in the baseline period (16 months), the intervention period (13 months), and the poststudy period (28 months). The data are depicted as MRSA clinical disease (NIM) with ± 1 standard error range per 10,000 patient-days.
DISCUSSION
The goal of the NorthShore admission MRSA testing program is to identify patients who are colonized with MRSA and then implement infection control practices, minimizing new MRSA acquisition in order to reduce disease. We assessed the adjunctive benefit of decolonization using mupirocin and chlorhexidine for reducing MRSA transmission in a setting where newly admitted patients undergo rapid screening followed by placement into contact precautions (gown and glove isolation in a private room) for those colonized. Decolonization added no benefit to the placement of patients into contact precautions when evaluated on over 15,600 persons. This indicates that, if one is following the recommended practice of placing MRSA-colonized and/or infected patients into isolation, there is no need to accept the added cost of decolonization or risk the development of antimicrobial resistance by adding antibiotics.
The institution of contact precautions, when adequately followed, raised a sufficient barrier to MRSA dissemination, such that the addition of a decolonizing regimen did not positively supplement this effect. The finding is important, since an adverse consequence of using mupirocin for all hospitalized persons would be the risk of development of commonplace mupirocin resistance not only in MRSA but also in methicillin-susceptible Staphylococcus aureus strains, which would impair the ability to decolonize preoperative patients carrying this potential pathogen. Decolonizing all S. aureus carriers in presurgical patients is an important infection prevention tool, where it has been demonstrated to have a severalfold impact on cost-effectively reducing postoperative infection (23–25). With the deployment of universal MRSA screening in a newly acquired health care facility, we implemented discharge testing in order to determine if mupirocin decolonization was necessary. The data from our evaluation demonstrate that mupirocin does not significantly add to the reduced transmission of MRSA above that achieved from rapid testing and contact precautions. As can be seen from Fig. 1, clinical disease continued to decrease in the postintervention period, when decolonization of medical patients was not done for the hospitalized patients who were not anticipating surgery.
There are three reports that are particularly useful in supporting our conclusion that contact precautions alone are sufficient to impede the spread of MRSA. The first is by Jernigan et al., who found a 15.6-fold reduction in MRSA transmission when contact precautions were applied versus no infection prevention intervention (26). The second study addresses the sustained impact of rapid testing and contact precautions (gown and glove isolation) for the Department of Veterans Affairs hospital system, which monitors the impact of their MRSA control program on 1 million patients per year across the United States (12, 18). Their reported transmission rates of 2 to 2.5 cases per 1,000 patient-days are very similar to our finding, and decolonization is not used in that program. The final report is by Harris et al., who studied a universal contact precaution intervention (without decolonization) in 20 ICUs within 20 hospitals across the United States (27). With an admission colonization pressure somewhat higher than ours (10.5% versus 6.5%), they found a transmission rate of 6 MRSA transmissions per 1,000 patient-days at risk, which is also slightly higher than our rate (Table 2) and the Veterans Affairs rate. Thus, we conclude that decolonization to reduce the spread of MRSA in acute care hospitals is not necessary when contact precautions are rapidly applied. Importantly, for this approach to infection control, the first randomized trial to study adverse events from contact precautions found no association between this practice and additional risk to patients (28).
Expanding the use of universal decolonization for MRSA control in the acute care setting, as suggested in the REDUCE MRSA trial (16), has the potential to limit future use of mupirocin through the development of resistance in staphylococci toward this agent. Presentation of the mupirocin susceptibility trend from this study indicates this is a meaningful risk (M. Hayden et al., presented at ID Week, Philadelphia, PA, 8 to 12 October 2014). During the relatively short 18-month period of study, high-level mupirocin resistance declined for MRSA acquired in the ICU between baseline and intervention periods in both the contact isolation and targeted decolonization arms (from 12% to 9% and from 7% to 5%, respectively) but rose in the universal decolonization arm (from 11% to 16%). The overall difference in differences between arms was significant, at a P value of <0.001. This finding should raise concern if mupirocin decolonization were to be used for all inpatients. Furthermore, as reported by Evans et al. (29), the screening and isolation arm (group 1) had already achieved a 39% decrease in catheter-associated bloodstream infection and a 54% reduction in ventilator-associated pneumonia prior to the start of the REDUCE MRSA trial, demonstrating the impact of screening with targeted application of contact precautions.
Our study has some limitations. Since our investigation was primarily a quality improvement quasiexperimental intervention, there were changes in practice that took place that potentially confounded the measurements of nosocomial MRSA transmission. Specifically, beginning in January, we no longer tested all patients admitted but, rather, applied an automated algorithm to select those patients to be tested (22). This resulted in 34% of admissions not being tested for the final 8 months of the intervention period. Fewer patients tested on admission meant that we could monitor transmission only in those patients actually tested and found to be negative, which could have led to less detected transmission in the intervention (required decolonization of all MRSA-positive patients) arm. In fact, just the opposite was observed: there was a slight, but significant, rise in transmission when measured as patients who acquired MRSA colonization, which demonstrated that the study was not biased by this change in admission testing practice. This observation was interesting and may suggest that decolonizing inpatients could predispose them to new MRSA acquisition. We decolonized based on PCR results that have a 2% to 3% false-positive rate, and our transmission data were based on culture-positive samples; it is possible that some patients were decolonized because of a false-positive PCR and later acquired MRSA during hospitalization, but our study was not designed to measure this. There is growing evidence that the microbiota of the nares is usually colonized by organisms distinct from other regions of the body surface and that reduction of this phylum Actinobacteria flora is associated with increased numbers of S. aureus and Staphylococcus epidermidis (30). Thus, disruption of the usual flora could lead to some risk for MRSA colonization even with targeted decolonization and to a much greater chance for unintended consequences with universal decolonization of the nares. Finally, in order to ensure that any diminished testing did not have a negative clinical impact, we monitored clinical disease development in the baseline, intervention, and poststudy periods. There was no significant change in clinical MRSA disease between the various periods. Importantly, the infection rate continued to slowly decline in the poststudy time frame, as is expected when a new MRSA control program is introduced (12); this decline occurred during a period when no inpatient medical patients were decolonized, further indicating that the change in testing or decolonization practice did not negatively impact the program results or patient care.
A recent report by Schora et al., demonstrated reduced MRSA colonization prevalence (e.g., less transmission) when only targeted decolonization was used for new admissions to long-term care (31). Another report by Das et al. found that targeted decolonization with no isolation prevented MRSA transmission in an inpatient psychiatry unit (32). These studies, taken with our data, suggest the next step in MRSA control for health care organizations. Since our current study indicates that both decolonization and contact precautions (isolation) are not needed for MRSA control, the next logical step would be to perform targeted decolonization without isolation in acute care (throughout the hospital) using an agent that has a low potential for resistance development to evaluate if this could have the same impact as application of contact precautions in a universal AST setting. If, as suspected, targeted decolonization is as effective as mandated wearing of gowns and gloves for MRSA-colonized or -infected patients, moving to targeted decolonization as a replacement for contact precautions could lower health care cost, improving patient care and health care worker satisfaction. Thus, there is a need for an effective decolonization agent with a low potential for resistance. The active search for such an agent is under way, with some initial success as suggested by experience with a novel synthesized, peptide-mimetic compound causing cell lysis and membrane disruption in S. aureus (33); future studies with novel compounds such as this are to be expected.
The quality improvement investigation described in this report demonstrated that mupirocin and chlorhexidine decolonization does not add benefit to an active surveillance testing program where patients found to be positive for MRSA colonization are placed into contact precautions (gown and glove isolation). The addition of decolonization raises the risk of unnecessary antibiotic resistance development, increases cost, and adds one more task for the health care workers in caring for their patients. At the conclusion of this investigation, the hospitals of NorthShore University HealthSystem discontinued inpatient decolonization for all patients who were not anticipated to undergo surgery.
ACKNOWLEDGMENTS
We thank the Department of Nursing for their dedication to the patients at NorthShore and for advancing the practice of medicine by performing the test sample collection needed for this investigation.
Agency for Healthcare Research and Quality (AHRQ) award R18 HS19968 to L.R.P. supported a portion of the discharge testing. An investigator-initiated grant from Roche Diagnostics Corporation to L.R.P. supported the discharge testing of this project.
We declare no conflict of interest that pertains to this study and report. L.R.P. has received speaking honoraria from Becton Dickinson, Cepheid, Roche, and CareFusion. L.R.P. has received research funding from Becton Dickinson, Cepheid, Nanosphere, 3M, GeneWeave, and Roche. The other authors declare no potential conflicts of interest.
The opinions are those of the authors and do not reflect the official position of AHRQ or the U.S. Department of Health and Human Services.
L.R.P., M.O.W., V.K., P.A.P., D.M.S., B.H.S., and A.R. contributed equally to the study design. All authors contributed equally to interpretation of data, and approval of final report. J.L.B. did the statistical analysis. L.R.P. had primary responsibility for drafting the report.
Funding Statement
Funding through an investigator-initiated grant from Roche Diagnostics Corporation supported the discharge testing of this project. Award R18 HS19968 from the Agency for Healthcare Research and Quality also supported a portion of the discharge testing. The opinions are those of the authors and do not reflect the official position of AHRQ or the U.S. Department of Health and Human Services. Only the authors had a role in the study design, data analysis, data interpretation, or writing of this report. The corresponding authors had full access to all the data and had final responsibility for the decision to submit for publication.
REFERENCES
- 1.Wise RI, Ossman EA, Littlefield DR. 1989. Personal reflections on nosocomial staphylococcal infections and the development of hospital surveillance. Rev Infect Dis 11:1005–1019. doi: 10.1093/clinids/11.6.1005. [DOI] [PubMed] [Google Scholar]
- 2.DeLeo FR, Chambers HF. 2009. Reemergence of antibiotic-resistant Staphylococcus aureus in the genomics era. J Clin Invest 119:2464–2474. doi: 10.1172/JCI38226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klevens RM, Morrison MA, Nadle J, Petit S, Gershman K, Ray S, Harrison LH, Lynfield R, Dumyati G, Townes JM, Craig AS, Zell ER, Fosheim GE, McDougal LK, Carey RB, Fridkin SK, for the Active Bacterial Core surveillance (ABCs) MRSA Investigators. 2007. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA 298:1763–1771. doi: 10.1001/jama.298.15.1763. [DOI] [PubMed] [Google Scholar]
- 4.Johnson AP, Davies J, Guy R, Abernethy J, Sheridan E, Pearson A, Duckworth G. 2012. Mandatory surveillance of methicillin-resistant Staphylococcus aureus (MRSA) bacteremia in England: the first 10 years. J Antimicrob Chemother 67:802–809. doi: 10.1093/jac/dkr561. [DOI] [PubMed] [Google Scholar]
- 5.de Kraker ME, Jarlier V, Monen JC, Heuer OE, van de Sande N, Grundmann H. 2013. The changing epidemiology of bacteremias in Europe: trends from the European Antimicrobial Resistance Surveillance System. Clin Microbiol Infect 19:860–868. doi: 10.1111/1469-0691.12028. [DOI] [PubMed] [Google Scholar]
- 6.Dantes R, Mu Y, Belflower R, Aragon D, Dumyati G, Harrison LH, Lessa FC, Lynfield R, Nadle J, Petit S, Ray SM, Schaffner W, Townes J, Fridkin S, Emerging Infections Program—Active Bacterial Core Surveillance MRSA Surveillance Investigators. 2013. National burden of invasive methicillin-resistant Staphylococcus aureus infections, United States, 2011. JAMA Intern Med 173:1970–1978. doi: 10.1001/jamainternmed.2013.10423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.David MZ, Daum RS, Bayer AS, Chambers HF, Fowler VG Jr, Miller LG, Ostrowsky B, Baesa A, Boyle-Vavra S, Eells SJ, Garcia-Houchins S, Gialanella P, Macias-Gil R, Rude TH, Ruffin F, Sieth JJ, Volinski J, Spellberg B. 2014. Staphylococcus aureus bacteremia at 5 U.S. academic medical centers, 2008-2011: significant geographic variation in community-onset infections. Clin Infect Dis 59:798–807. doi: 10.1093/cid/ciu410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Illinois Department of Public Health Trends in methicillin-resistant Staphylococcus aureus (MRSA) in Illinois based on hospital discharge data, 2009-2012. Illinois Department of Public Health, Chicago, IL: http://www.healthcarereportcard.illinois.gov/files/pdf/mrsa_2012_Trends.pdf Accessed 7 August 2015. [Google Scholar]
- 9.Peterson LR, Diekema DJ. 2010. To screen or not to screen for methicillin-resistant Staphylococcus aureus. J Clin Microbiol 48:683–689. doi: 10.1128/JCM.02516-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kavanagh KT, Calderon LE, Saman DM. 2015. Viewpoint: a response to “Screening and isolation to control methicillin-resistant Staphylococcus aureus: sense, nonsense, and evidence.” Antimicrob Resist Infect Control 4:4. doi: 10.1186/s13756-015-0044-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kavanagh KT, Saman DM, Yu Y. 2013. A perspective on how the United States fell behind Northern Europe in the battle against methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 57:5789–5791. doi: 10.1128/AAC.01839-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Evans ME, Kralovic SM, Simbartl LA, Freyberg RW, Obrosky DS, Roselle GA, Jain R. 2013. Veterans Affairs methicillin-resistant Staphylococcus aureus prevention initiative associated with a sustained reduction in transmissions and health care-associated infections. Am J Infect Control 41:1093–1095. doi: 10.1016/j.ajic.2013.04.015. [DOI] [PubMed] [Google Scholar]
- 13.Ridgway JP, Peterson LR, Brown EC, Du H, Hebert C, Thomson RB Jr, Kaul KL, Robicsek A. 2013. Clinical significance of methicillin-resistant Staphylococcus aureus colonization on hospital admission: one-year infection risk. PLoS One 8:e79716. doi: 10.1371/journal.pone.0079716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang S, Sethi AK, Eckstein BC, Stiefel U, Cadnum JL, Donskey CJ. 2009. Skin and environmental contamination with methicillin-resistant Staphylococcus aureus among carriers identified clinically versus through active surveillance. Clin Infect Dis 48:1423–1428. doi: 10.1086/598505. [DOI] [PubMed] [Google Scholar]
- 15.Milstone AM, Goldner BW, Ross T, Shepard JW, Carroll KC, Perl TM. 2011. Methicillin-resistant Staphylococcus aureus colonization and risk of subsequent infection in critically ill children: importance of preventing nosocomial methicillin-resistant Staphylococcus aureus transmission. Clin Infect Dis; 53:853–859. doi: 10.1093/cid/cir547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang SS, Septimus E, Kleinman K, Moody J, Hickok J, Avery TR, Lankiewicz J, Gombosev A, Terpstra L, Hartford F, Hayden MK, Jernigan JA, Weinstein RA, Fraser VJ, Haffenreffer K, Cui E, Kaganov RE, Lolans K, Perlin JB, Platt R, the CDC Prevention Epicenters Program, the AHRQ DECIDE Network and Healthcare-Associated Infections Program. 2013. Targeted versus universal decolonization to prevent ICU infection. N Engl J Med 368:2255–2265. doi: 10.1056/NEJMoa1207290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Robicsek A, Beaumont JL, Paule SM, Hacek DM, Thomson RB Jr, Kaul KL, King P, Peterson LR. 2008. Universal surveillance for methicillin-resistant Staphylococcus aureus in 3 affiliated hospitals. Ann Intern Med 148:409–418. doi: 10.7326/0003-4819-148-6-200803180-00003. [DOI] [PubMed] [Google Scholar]
- 18.Jain R, Kralovic SM, Evans ME, Ambrose M, Simbartl LA, Obrosky DS, Render ML, Freyberg RW, Jernigan JA, Muder RR, Miller LJ, Roselle GA. 2011. Veterans Affairs initiative to prevent methicillin-resistant Staphylococcus aureus infections. N Engl J Med 364:1419–1430. doi: 10.1056/NEJMoa1007474. [DOI] [PubMed] [Google Scholar]
- 19.Patel PA, Ari Robicsek A, Grayes A, Schora DM, Peterson KE, Wright MO, Peterson LR. 2015. Evaluation of multiple real-time PCR tests on nasal samples in a large MRSA surveillance program. Am J Clin Pathol 143:652–658. doi: 10.1309/AJCPMDY32ZTDXPFC. [DOI] [PubMed] [Google Scholar]
- 20.Peterson KE, Hacek DM, Robicsek A, Thomson RB Jr, Peterson LR. 2012. Electronic surveillance for infectious disease trend analysis following a quality improvement intervention. Infect Control Hosp Epidemiol 33:790–795. doi: 10.1086/666625. [DOI] [PubMed] [Google Scholar]
- 21.R Core Team. 2014. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria: http://www.R-project.org/ Accessed 10 July 2015. [Google Scholar]
- 22.Robicsek A, Beaumont JL, Wright MO, Thomson RB Jr, Kaul KL, Peterson LR. 2011. Electronic prediction rules for methicillin-resistant Staphylococcus aureus colonization. Infect Control Hosp Epidemiol 32:9–19. doi: 10.1086/657631. [DOI] [PubMed] [Google Scholar]
- 23.Hacek DM, Robb WJ, Paule SM, Kudrna JC, Stamos VP, Peterson LR. 2008. Staphylococcus aureus nasal decolonization in joint replacement surgery reduces infection. Clin Orthop Relat Res 466:1349–1355. doi: 10.1007/s11999-008-0210-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bode LG, Kluytmans JA, Wertheim HF, Bogaers D, Vandenbroucke-Grauls CM, Roosendaal R, Troelstra A, Box AT, Voss A, van der Tweel I, van Belkum A, Verbrugh HA, Vos MC. 2010. Preventing surgical-site infections in nasal carriers of Staphylococcus aureus. N Engl J Med 362:9–17. doi: 10.1056/NEJMoa0808939. [DOI] [PubMed] [Google Scholar]
- 25.van Rijen MM, Bode LG, Baak DA, Kluytmans JA, Vos MC. 2012. Reduced costs for Staphylococcus aureus carriers treated prophylactically with mupirocin and chlorhexidine in cardiothoracic and orthopedic surgery. PLoS One 7:e43065. doi: 10.1371/journal.pone.0043065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jernigan JA, Titus MG, Gröschel DH, Getchell-White S, Farr BM. 1996. Effectiveness of contact isolation during a hospital outbreak of methicillin-resistant Staphylococcus aureus. Am J Epidemiol 143:496–504. doi: 10.1093/oxfordjournals.aje.a008770. [DOI] [PubMed] [Google Scholar]
- 27.Harris AD, Pineles L, Belton B, Johnson JK, Shardell M, Loeb M, Newhouse R, Dembry L, Braun B, Perencevich EN, Hall KK, Morgan DJ, Benefits of Universal Glove and Gown (BUGG) Investigators, Shahryar SK, Price CS, Gadbaw JJ, Drees M, Kett DH, Muñoz-Price LS, Jacob JT, Herwaldt LA, Sulis CA, Yokoe DS, Maragakis L, Lissauer ME, Zervos MJ, Warren DK, Carver RL, Anderson DJ, Calfee DP, Bowling JE, Safdar N. 2013. Universal glove and gown use and acquisition of antibiotic-resistant bacteria in the ICU: a randomized trial. JAMA 310:1571–1580. doi: 10.1001/jama.2013.277815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Croft LD, Liquori M, Ladd J, Day H, Pineles L, Lamos E, Arnold R, Mehrotra P, Fink JC, Langenberg P, Simoni-Wastila L, Perencevich E, Harris AD, Morgan DJ. 2015. The effect of contact precautions on frequency of hospital adverse events. Infect Control Hosp Epidemiol 36:1268–1274. doi: 10.1017/ice.2015.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Evans ME, Roselle G, Jain R. 2013. Targeted decolonization to prevent ICU infections. N Engl J Med 369:1468–1469. doi: 10.1056/NEJMc1309704. [DOI] [PubMed] [Google Scholar]
- 30.Frank DN, Feazel LM, Bessesen MT, Price CS, Janoff EN, Pace NR. 2010. The human nasal microbiota and Staphylococcus aureus carriage. PLoS One 5:e10598. doi: 10.1371/journal.pone.0010598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schora DM, Boehm S, Das S, Patel PA, O'Brien J, Hines C, Burdsall D, Beaumont J, Peterson K, Fausone M, Peterson LR. 2014. Impact of Detection, Education, Research and Decolonization without Isolation in Long-term care (DERAIL) on methicillin-resistant Staphylococcus aureus colonization and transmission at 3 long-term care facilities. Am J Infect Control 42(10 Suppl):S269–S273. doi: 10.1016/j.ajic.2014.05.011. [DOI] [PubMed] [Google Scholar]
- 32.Das S, Harazin M, Wright MO, Dusich I, Robicsek A, Peterson LR. 2014. Active surveillance and decolonization without isolation is effective in preventing methicillin-resistant Staphylococcus aureus transmission in the psychiatry unit. Open Forum Infect Dis 1:ofu067. doi: 10.1093/ofid/ofu067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nilsson AC, Janson H, Wold H, Fugelli A, Andersson K, Håkangård C, Olsson P, Olsen WM. 2015. LTX-109 is a novel agent for nasal decolonization of methicillin-resistant and-sensitive Staphylococcus aureus. Antimicrob Agents Chemother 59:145–151. doi: 10.1128/AAC.03513-14. [DOI] [PMC free article] [PubMed] [Google Scholar]


