Abstract
Avibactam is a new non-β-lactam β-lactamase inhibitor that shows promising restoration of ceftazidime activity against microorganisms producing Ambler class A extended-spectrum β-lactamases (ESBLs) and carbapenemases such as KPCs, class C β-lactamases (AmpC), and some class D enzymes. To determine optimal dosing combinations of ceftazidime-avibactam for treating infections with ceftazidime-resistant Pseudomonas aeruginosa, pharmacodynamic responses were explored in murine neutropenic thigh and lung infection models. Exposure-response relationships for ceftazidime monotherapy were determined first. Subsequently, the efficacy of adding avibactam every 2 h (q2h) or q8h to a fixed q2h dose of ceftazidime was determined in lung infection for two strains. Dosing avibactam q2h was significantly more efficacious, reducing the avibactam daily dose for static effect by factors of 2.7 and 10.1, whereas the mean percentage of the dosing interval that free drug concentrations remain above the threshold concentration of 1 mg/liter (%fT>CT 1 mg/liter) yielding bacteriostasis was similar for both regimens, with mean values of 21.6 (q2h) and 18.5 (q8h). Dose fractionation studies of avibactam in both the thigh and lung models indicated that the effect of avibactam correlated well with %fT>CT 1 mg/liter. This parameter of avibactam was further explored for four P. aeruginosa strains in the lung model and six in the thigh model. Parameter estimates of %fT>CT 1 mg/liter for avibactam ranged from 0 to 21.4% in the lung model and from 14.1 to 62.5% in the thigh model to achieve stasis. In conclusion, addition of avibactam enhanced the effect of ceftazidime, which was more pronounced at frequent dosing and well related with %fT>CT 1 mg/liter. The thigh model appeared more stringent, with higher values, ranging up to 62.5% fT>CT 1 mg/liter, required for a static effect.
INTRODUCTION
All over the world, health care professionals are struggling with the problem of antibiotic resistance, and extended-spectrum β-lactamase (ESBL)- and/or carbapenemase-producing microorganisms especially form a global threat (1–7). Apart from antibiotic stewardship, vaccines, and hygiene measurements, the development of new classes of antibiotics is of life-saving importance. Another, previously successful approach to overcome resistance is to combine a clinically proven β-lactam antibiotic with an inhibitor of the β-lactamases that confer resistance to it.
AstraZeneca and Actavis (formerly Forest-Cerexa) are developing the combination of ceftazidime with avibactam, a new non-β-lactam β-lactamase inhibitor that forms a hydrolytically stable linkage with serine-based β-lactamases to overcome resistance (8, 9). The combination showed in vitro activity against Ambler class A ESBLs, KPC class A enzymes, class C (AmpC) enzymes, and some class D enzymes. Studies in vitro have shown that ceftazidime MIC values against resistant strains were reduced drastically in the presence of this inhibitor, causing the strains to become susceptible to ceftazidime (9, 10). In dose-response and simulated human exposure experiments in mice, it has been shown that the ceftazidime combined with the inhibitor avibactam is active in vivo (see, e.g., references 11, 12, and 13).
In the studies described here, neutropenic mouse infection models (thigh infection and pneumonia) with ceftazidime-resistant Pseudomonas aeruginosa were used to determine the exposure-response relationship of ceftazidime alone and to derive estimates of pharmacodynamic indices (PDI) over 24 h for avibactam in combination with ceftazidime. Since the percentage of the dosing interval that free drug concentrations remain above the MIC (%fT>MIC) is the PDI that correlates best with efficacy for β-lactam antibiotics, as specified in particular for ceftazidime versus P. aeruginosa (14, 15), only every-2-h (q2h) dosing intervals were used for ceftazidime.
To define the minimum-effect concentration of avibactam, a relatively new PDI, introduced recently (16) and based on a notional threshold concentration (CT), was used. This value represents an approximation of the threshold concentration of avibactam during a declining concentration-time curve, below which β-lactamase is no longer effectively inhibited in vivo. Consequently, the exposure of avibactam that is required for pharmacodynamic effects can be expressed using the threshold concentration concept. Thus, the exposure of avibactam is expressed as the pharmacodynamic index of %fT>CT, analogous to %fT>MIC of the β-lactam (which is ceftazidime in this study). Similarly to the case for ceftazidime, the estimate of the %fT>CT depends on the CT itself, but whereas the MIC of the β-lactam is usually known from in vitro data, the CT could in principle be determined in vitro (16) or in vivo. In the experiments presented here, the in vivo approach was used. Three values of CT, i.e., 0.25, 1, and 4 mg/liter, which spanned a CT value of ≤0.5 mg/liter obtained for Enterobacteriaceae in a hollow-fiber model (16, 17), were examined to determine which of these best correlated to efficacy.
(The results of this study were presented in part at the 53rd Interscience Conference on Antimicrobial Agents and Chemotherapy, Denver, CO, 2013 [18, 19].)
MATERIALS AND METHODS
Drugs.
Ceftazidime (lot no. G263770; potency, 77.2%) and avibactam (lot no. AFCH005151 [07113P028]; potency, 91.7%) were provided by AstraZeneca Pharmaceuticals LP, Waltham, MA, USA. The drugs were reconstituted in sterile water to a stock solution of 5,120 mg/liter, and further solutions were prepared in Mueller-Hinton broth (Difco, Brunschwig Chemie, Amsterdam, The Netherlands).
Bacterial strains and susceptibility testing.
Seven well-characterized ceftazidime-resistant Pseudomonas aeruginosa strains were used in the experiments. All strains were of clinical origin and β-lactamase producers of different origins, with MICs ranging from 32 to 128 mg/liter for ceftazidime alone and 2 to 16 mg/liter for the combination of ceftazidime and avibactam, measured at a concentration of 4 mg/liter avibactam (Table 1). Isolates 1, 3, 5, and 7 were included in human simulated-exposure experiments in mouse thigh and hollow-fiber experiments (13) and mouse lung infections (20) as isolate numbers 1382, 1384, 1386, and 1388, respectively. All 7 isolates in Table 1 were included in the set of 18 isolates of P. aeruginosa studied in ceftazidime-avibactam “checkerboard” MIC experiments by Berkhout et al. (21). MICs were determined as described previously (21) by microdilution according to the ISO guidelines (22). This method is CLSI compatible.
TABLE 1.
P. aeruginosa strains used for pharmacodynamic studies of ceftazidime and avibactam, including magnitude of the PDI %fT>MIC of monotherapy ceftazidime
| Isolate no. | Resistance summarya | MIC (mg/liter) |
Static % fT>MIC (ceftazidime) |
||
|---|---|---|---|---|---|
| Ceftazidime | Ceftazidime-avibactamb | Thigh | Lung | ||
| 1 | Nitrocefinase activity, ++; AmpC transcript, overexpressed; β-lactamase genotype, blaAmpC; class A−, class B− | 128 | 8 | Not done | 0 |
| 3 | Nitrocefinase activity, baseline; AmpC transcript, basal; β-lactamase genotype, blaAmpC blaTEM-24 (CAZ-6); class B− | 64 | 2 | 0 | 0 |
| 5 | Nitrocefinase activity, ++++; AmpC transcript, overexpressed; β-lactamase genotype, blaAmpC; class A−, class B− | 128 | 8 | 0 | 0 |
| 7 | Nitrocefinase activity, +++; AmpC transcript, overexpressed; β-lactamase genotype, blaAmpC; class A−, class B− | 64 | 4 | 0 | 0 |
| 11 | OprD−, AmpCcon, class A−, class B− | 128 | 16 | No stasisc | 0 |
| 18 | OprD−, AmpCind?, class A−, class B− | 32 | 2 | 28.6 | 27.0 |
| 19 | OprD−, AmpCcon, class A−, class B− | 64 | 4 | 29.6 | 0 |
con, constitutive; ind, inducible; OprD−, outer membrane protein deficiency causing resistance to carbapenems in Pseudomonas species; blaAmpC, possesses β-lactamase gene coding for AmpC; blaTEM-24, possesses β-lactamase gene coding for TEM24.
The MIC of ceftazidime-avibactam was the value of the ceftazidime MIC measured in the presence of a fixed concentration of avibactam of 4 mg/liter.
The highest dose used did not result in a bacteriostatic effect.
Animals.
Outbred female CD-1 mice (Charles River, the Netherlands), 7 to 8 weeks old and weighing 20 to 25 g, were used in the experiments. Granulocytopenia was induced by two doses of cyclophosphamide intraperitoneally, one at 4 days (150 mg/kg) and the other at 1 day (100 mg/kg) before the infection experiment.
The animals were housed under standard conditions with drink and feed supplied ad libitum and were examined once daily and after immunosuppression 2 to 3 times per day. The animal studies were conducted in accordance with the recommendations of the European Community (Directive 86/609/EEC, 24 November 1986), and all animal procedures were approved by the Animal Welfare Committee of Radboud University (RU-DEC 2012-003).
Infection.
The infection models included a thigh infection model (two P. aeruginosa strains per animal, one in the left thigh and one in the right thigh) and a lung infection model (one strain per animal). In both cases, 0.05 ml of bacterial suspension consisting of approximately 106 to 107 bacterial CFU was inoculated intramuscularly (thigh model) or intranasally (lung model) with a syringe. For the lung model, animals were under light anesthesia with isoflurane during inoculation.
At t = 0 h, 2 h after infection, 2 mice were humanely sacrificed to determine the initial number of CFU just before start of treatment. In general, the number of CFU at the start of treatment was between 5 × 105 and 107. All other animals were sacrificed at t = 24 h (i.e., 24 h after start of treatment and 26 h after infection) unless the welfare of the animals indicated that earlier termination was necessary in compliance with animal welfare regulations. Thighs and lungs were collected and moved to precooled 10-ml plastic tubes (Transport Tube; Omnilabo, The Netherlands) containing 2 ml phosphate-buffered saline (PBS) (8.00 g/liter NaCl, 1.44 g/liter Na2HPO4 · 2H2O, 0.26 g/liter KH2PO4, pH 7.2 to 7.4). Subsequently thighs and lungs were ground using an Ultra-Turrax (IKA Labortechnik, Germany). The homogenized tissues were diluted in a 10-fold dilution series, and 3 × 10 μl of each dilution was plated (Pseudomonas CFC selective medium, PO5132A; Oxoid, Germany) and incubated at 37°C. The following day, colonies were inspected and counted, and the number of CFU per thigh or lung was calculated. In the thigh infection experiments, if more than 1 strain was inoculated in the same mouse, morphological characteristics of the strains were always distinctive to allow detection of potential cross-contamination between thighs. The drug effect was then determined as the difference between the log10 (CFU/thigh or lung) values at t = 24 h and t = 0 h (mean value for 2 mice) and expressed as “ΔlogCFU” (i.e., algebraically positive for growth, zero for “stasis,” and negative for reduction or “killing”).
Treatment regimens.
Experiments with ceftazidime monotherapy were performed initially to determine the exposure-response relationship of each strain without avibactam. Treatment with 0.1 ml of increasing doses of ceftazidime or saline (control) was administered subcutaneously every 2 h, starting at t = 0 h (2 h after inoculation), and continued for a period of 24 h. To determine the PDI of avibactam best correlating with efficacy, infections with two strains of P. aeruginosa (strains 11 and 18) were established in thigh and lung and treated with ceftazidime and avibactam. Avibactam in increasing doses was administered in q2h and q8h regimens, while ceftazidime was dosed in a fixed dosing regimen every 2 h at a dose which resulted in an increase of 1 to 2-log10 CFU in monotherapy treatment. In addition, a full-dose fractionation experiment with avibactam was performed superimposed on the q2h dosing schemes of ceftazidime at a dose level that resulted in an increase of 2 log10 CFU with P. aeruginosa strains 7 and 18 in both infection models. The amounts of avibactam administered varied in frequency and dose (from 4 mg/kg to 64 mg/kg and from q2h to q12h).
For a more precise estimate of the magnitude of the PDI and the variability by strain, codosing experiments were performed for another 2 P. aeruginosa strains in the lung model and 6 strains in the thigh model. All dosing regimens were performed in at least 2 animals per dosing regimen.
Antibiotic concentration measurements.
Antibiotic concentration measurement methods and pharmacokinetics in both infection models in neutropenic female CD-1 mice have been described elsewhere in detail (23). Briefly, the half-lives of ceftazidime and avibactam were 0.28 h and 0.24 h in plasma and 0.39 and 0.34 h in lung epithelial lining fluid (ELF), respectively.
Pharmacodynamic analysis.
Ceftazidime and avibactam free drug concentrations determined as described previously (13) were used in all calculations. Exposures of unbound ceftazidime and avibactam were determined using MicLab 2.36 (Medimatics, Maastricht, The Netherlands) using pharmacokinetic parameter estimates obtained from previous detailed pharmacokinetic studies in infected neutropenic mice (23). The maximum-effect (Emax) model was fit to the exposure and pharmacodynamic responses to determine the PDI values of ceftazidime alone or in combination with avibactam resulting in a static or specified log kill effect. For avibactam %fT>CT, the percentages of the dosing interval above threshold concentration CT were calculated for CTs of 0.25, 1, and 4 mg/liter. In the dose fractionation experiments in the thigh model, regimens with equal total daily areas under the concentration-time curve (AUC) were compared to each other to determine whether doses given more frequently were more effective by establishing the relationship between log of dose frequency and change in log CFU.
RESULTS
The %fT>MICs required for a static effect with ceftazidime monotherapy in thigh- and lung-infected mice are summarized in Table 1. For all strains, an effect of ceftazidime was detectable. The estimates for a static effect varied between 0 and 29.6% fT>MIC, noting that ceftazidime MICs ranged between 32 and 128 mg/liter. All relationships followed a sigmoid pattern, but because of the high MICs, a static effect was not reached even with the highest ceftazidime doses against strain 11 when infecting the thigh.
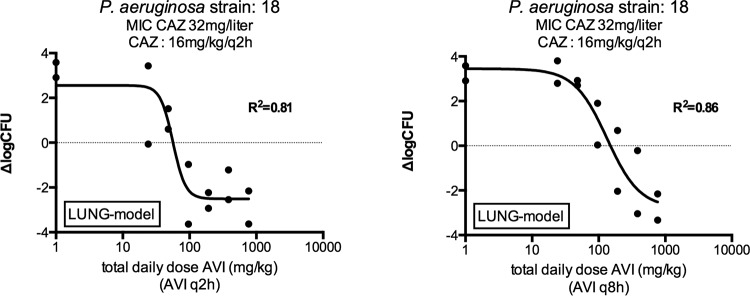
The effect of dosing frequency for avibactam was first determined in the lung model by monitoring the efficacy of avibactam dosed in combination with ceftazidime on a q2h regimen. Figure 1 shows an example of the difference in efficacy between the q2h and the q8h regimens of avibactam in lung-infected mice. The ceftazidime regimen without avibactam was not effective, as shown by outgrowth of the non-avibactam-treated controls plotted on the vertical axis in Fig. 1 at the dose of 1 mg/kg (actually corresponding to no avibactam added). The exposure-response curves indicate that the q2h regimen was more efficacious than the q8h regimen. Analyses of the pharmacokinetics/pharmacodynamics (PK/PD) from Fig. 1 and from a similar experiment with isolate 11 are provided in Table 2. The daily dose of avibactam resulting in a static effect was lower for avibactam q2h than for avibactam q8h by factors of 10.1 and 2.7 for strains 11 and 18, respectively. However, the %fT>CT 1 mg/liter avibactam for a static effect was comparable for both regimens, indicating that the effect of avibactam is related primarily to a time above a certain threshold rather than the total daily dose or AUC. The overall mean %fT>CT 1 mg/liter for avibactam was 20.1% (range, 16.1 to 23.5%, for both dosing regimens combined) for a static effect (Table 2).
FIG 1.
Change in log10 CFU in lung-infected mice treated with ceftazidime dosing q2h and avibactam q2h or q8h., = ceftazidime; AVI, avibactam; ΔlogCFU, change in log10 CFU compared to the initial inoculum The ΔlogCFU values for controls treated with ceftazidime for 24 h but with zero avibactam are plotted on the vertical axis.
TABLE 2.
Magnitudes of avibactam exposures associated with stasis and bacterial killing of P. aeruginosa in lungs of mice for avibactam dosing regimens of q2h and q8h, codosed with ceftazidime at 16 mg/kg q2h
| Pneumoniaa | Strain | Ceftazidime MIC (mg/liter) | Avibactam dose (mg/kg) |
%fT>CT 1b |
||
|---|---|---|---|---|---|---|
| q2h | q8h | q2h | q8h | |||
| Stasis | 11 | 128 | 3.8 | 154.4 | 19.7 | 20.9 |
| 18 | 32 | 4.7 | 50.2 | 23.5 | 16.1 | |
| 1-log kill | 11 | 128 | 9.0 | 183.6 | 34.9 | 21.6 |
| 18 | 32 | 5.7 | 74.3 | 26.7 | 17.8 | |
| 2-log kill | 11 | 128 | 29.6 | 225.6 | 55.3 | 22.5 |
| 18 | 32 | 7.6 | 132.5 | 31.8 | 20.2 | |
Stasis, no growth of microorganisms compared to the initial inoculum; 1-log kill, 1-log10 kill of microorganisms compared to the initial inoculum.
CT, threshold concentration (virtual in vivo inhibitory concentration) in mg/liter; %fT>CT 1, percentage of time that free concentrations of avibactam stay above a CT of 1 mg/liter.
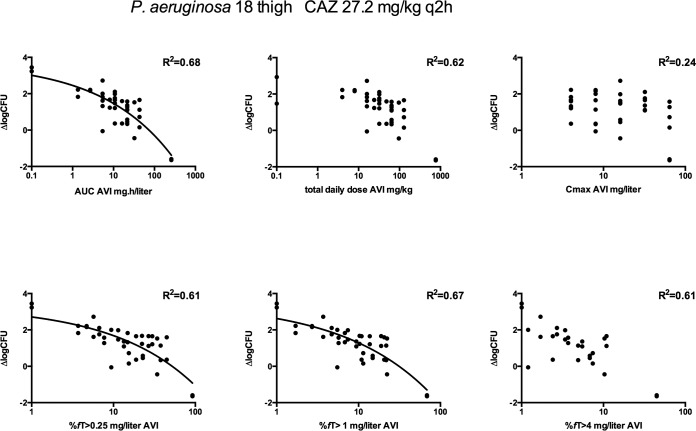
The effect of dosing frequency was also determined in both lung- and thigh-infected mice by full-dose fractionation studies of avibactam, varying from 4 mg/kg to 64 mg/kg per dose and from a q2h to a q12h dosing scheme. In these experiments, ceftazidime was concomitantly administered in a fixed q2h dosing regimen that had resulted in an increase of about 2 log10 CFU compared to the initial inoculum in the absence of avibactam. Figure 2 shows an example for P. aeruginosa strain 18 in thigh-infected mice. The various avibactam dosing regimens showed considerable variation in outcome. There was clearly no significant correlation with maximum concentration (Cmax). The %fT>CT as well as AUC and dose show a reasonable correlation with the change in log CFU, but visual inspection of the plots as well as the r2 values of the different relationships confirmed that %fT>CT 1 mg/liter avibactam was the best predictor, with the highest r2 values of the three threshold concentrations, although r2 values for AUC and %fT>CT 1 mg/liter were comparable, being 0.68 and 0.67, respectively (r2 was 0.61 for both %fT>CT 0.25 mg/liter and %fT>CT 4 mg/liter). For thigh-infected animals, a %fT>CT 1 mg/liter of 30.2% (strain 18) or 74.1% (strain 7) was required to result in a bacteriostatic effect. In the thigh infection model, there were three sets of regimens with the same total daily AUC that allowed a regression analysis between log frequency and change in log CFU. A significant relationship (P < 0.05) was found for both strains 7 and 18, indicating that frequency of dosing and therefore %fT>CT is an important factor in the overall effect and confirming the results in the lung model described above.
FIG 2.
Example of exposure-response, dose fractionation studies of avibactam in thigh-infected neutropenic mice treated with ceftazidime q2h. CAZ, ceftazidime; AVI, avibactam; ΔlogCFU, change in log10 CFU compared to the initial inoculum.
For lung-infected animals, similar patterns were observed (data not shown) except that lower exposures of avibactam were required to result in a bacteriostatic effect, with a %fT>CT 1 mg/liter of 24.5% (strain 18) and 6.7% (strain 7). There was no clear reason for the magnitudes of %fT>CT 1 mg/liter being higher in thigh-infected animals.
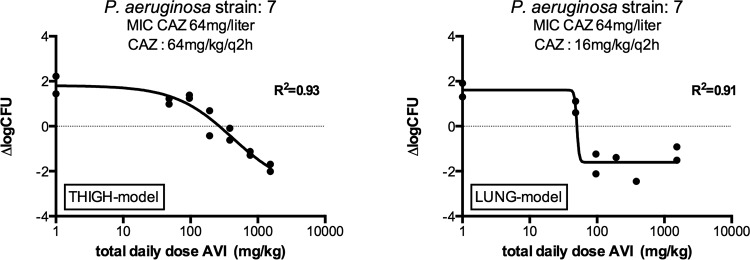
The %fT>CT of avibactam was determined for an additional two strains in the lung model and six in the thigh model using various doses of avibactam codosed with ceftazidime in a fixed regimen of q2h. In general, the %fT>CT required for stasis was again somewhat lower in the lung model. Figure 3 shows an example of the exposure-response relationship for avibactam and P. aeruginosa strain 7 in the two infection models. In Table 3, a summary of the results of the static doses, MICs of ceftazidime in the presence of 4 mg/liter avibactam, 1-log10 kill, and the associated %fT>CT or %fT>MIC to reach those specific effects in ceftazidime-avibactam codosing experiments are provided for the lung model.
FIG 3.
Example of exposure response of avibactam in neutropenic thigh- and lung-infected mice treated with ceftazidime and various doses of avibactam q2h. CAZ, ceftazidime; AVI, avibactam; ΔlogCFU, change in log10 CFU compared to the initial inoculum. MIC of ceftazidime-avibactam versus this strain, 4 mg/liter.
TABLE 3.
Magnitudes of avibactam %fT>CT associated with stasis and bacterial killing of P. aeruginosa in lungs of mice codosed with ceftazidime and various amounts of avibactam
| Strain | MIC (mg/liter) |
Ceftazidime dose (mg/kg) q2h | Avibactam %fT>CT 1b |
CAZ-AVI %fT>MICc | ||
|---|---|---|---|---|---|---|
| Ceftazidime | CAZ-AVIa | Stasis | 1-log kill | |||
| 1 | 128 | 8 | 32 | 0.0 | 0.0 | 34.6 |
| 5 | 128 | 8 | 64 | 19.4 | 20.6 | 49.3 |
| 7 | 64 | 4 | 16 | 21.4 | 22.4 | 63.5 |
| Median | 19.4 | 20.6 | 49.3 | |||
| Mean | 13.6 | 14.3 | 49.1 | |||
| SD | 11.8 | 12.4 | 14.5 | |||
MIC of ceftazidime measured with a fixed avibactam concentration of 4 mg/liter.
Stasis, no growth of microorganisms compared to the initial inoculum; 1-log kill, = 1-log10 kill of microorganisms compared to the initial inoculum; CT, threshold concentration (virtual in vivo inhibitory concentration) in mg/liter; %fT > CT 1, percentage of time that free concentrations of avibactam stay above a CT of 1 mg/liter.
Percentage of time that free concentrations of ceftazidime stay above the MIC of CAZ-AVI (the MIC of ceftazidime as determined in the presence of a fixed avibactam concentration of 4 mg/liter).
For two isolates (1 and 19) the bactericidal effect was stronger than expected, and relatively low doses of avibactam resulted in more than 1-log10 kill. In addition, the effect of ceftazidime without avibactam was too strong for strain 19. Static doses could therefore not be estimated reliably for isolate 19, and the %fT>CT 1 mg/liter avibactam estimates for the change in log10 CFU/tissue sample for both isolates was 0.
Table 4 shows a summary of the results of the static doses, MICs of ceftazidime in the presence of 4 mg/liter avibactam, 1-log10 kill, and the associated %fT>CT or %fT>MIC to reach a specific effect in the thigh model.
TABLE 4.
Magnitudes of avibactam %fT>CT associated with stasis and bacterial killing of P. aeruginosa in thighs of mice
| Strain | MIC (mg/liter) |
Ceftazidime dose (mg/kg) q2h | Avibactam %fT>CT 1b |
CAZ-AVI %f T>MICc | ||
|---|---|---|---|---|---|---|
| Ceftazidime | CAZ-AVIa | Stasis | 1-log kill | |||
| 1 | 128 | 8 | 32 | 37.2 | 65.7 | 34.6 |
| 5 | 128 | 8 | 64 | 14.1 | 32.9 | 49.3 |
| 7 | 64 | 4 | 64 | 50.4 | 65.3 | 63.5 |
| 11 | 128 | 16 | 64 | 29.1 | 37.5 | 34.6 |
| 18 | 32 | 2 | 64 | 24.2 | 33.2 | 77.5 |
| 19 | 64 | 4 | 32 | 62.5 | 67.2 | 49.3 |
| Mean | 31.0 | 46.9 | 51.9 | |||
| SD | 13.7 | 17.1 | 18.7 | |||
MIC of ceftazidime measured with a fixed avibactam concentration of 4 mg/liter.
Stasis, no growth of microorganisms compared to the initial inoculum; 1-log kill, 1-log10 kill of microorganisms compared to the initial inoculum; CT, threshold concentration (virtual in vivo inhibitory concentration) in mg/liter; %fT>CT 1, percentage of time that free concentrations of avibactam stay above a CT of 1 mg/liter.
Percentage of time that free concentrations of ceftazidime stay above the MIC of CAZ-AVI (the MIC of ceftazidime as determined in the presence of a fixed avibactam concentration of 4 mg/liter).
As can be derived from Table 4, the mean %fT>CT 1 mg/liter for a static effect was 31.0% (14.1 to 62.5%; standard deviation [SD] = 13.7). For a 1-log10 kill compared to the initial inoculum, the mean %fT>CT 1 mg/liter was 46.9% (32.9 to 67.2%; SD = 17.1). The relation to %fT>MIC of ceftazidime-avibactam (i.e., as measured in vitro in the presence of 4 mg/liter avibactam) is displayed as well, showing a mean of 51.9% (range, 34.6 to 77.5%; SD = 18.7).
DISCUSSION
When adding avibactam in different dosing schemes to a ceftazidime treatment regimen that was not effective given alone, we found that the most important pharmacodynamic index that determined outcome was the time above a certain threshold concentration, CT. In the dose fractionation experiments in the lung, as well as in the experiments with dosing schemes of avibactam q2h and q8h in lung-infected animals, a %fT>CT 1 mg/liter of around 20% in plasma was required to achieve a static effect in vivo. For 1- and 2-log kills, this increased to about 24% and 30%, respectively. A significantly lower total daily dose of avibactam was needed to reach a static effect if avibactam was given more frequently. For the two strains submitted to a q2h and q8h dosing regimen of avibactam, the total daily dose of avibactam resulting in a static effect was lower by factors of 10.1 and 2.7, respectively, for strains 11 and 18, a considerable difference between the two strains in both cases. A similar effect was observed in dose fractionation experiments in the thigh, where efficacy increased if the frequency of dosing increased for similar total daily doses.
From these findings, two important conclusions can be drawn. The first is that the effect of avibactam was not dependent on the peak concentration Cmax. The second is that, although there was some relationship between the bacteriostatic or bactericidal response and the total daily dose of avibactam administered (i.e., equivalent to fAUC), the more efficacious therapy was obtained with the more frequent dosing regimen for any given AUC. This fits well with the efficacy of ceftazidime, the principal PK/PD index of which is %fT>MIC (14, 15). From a practical point of view, this PK/PD property has its advantages if patients are treated with the combination of these two drugs because their human PK are also similar, both being renally eliminated with similar half-lives (9).
Still, the amount of avibactam required for effect was also dependent on other factors. In the lung infection experiments, a relatively lower exposure of avibactam was required to result in efficacy for strains 1 and 19 (Table 3). In vitro results could not fully explain this; in a checkerboard experiment with these strains, the concentration of avibactam required to reduce the MIC was not less than for other strains (results not shown). There might also be some dependency of the ceftazidime dose. In the lung model, additional dose fractionation experiments were performed with strain 7 using two different total daily doses of ceftazidime (1.87 mg/kg and 16 mg/kg) (data not shown). The results indicated that a four-times-higher value of avibactam was needed with the lowest daily dose of ceftazidime to reach stasis, thus supporting the idea that the amount of avibactam necessary is (partly) dependent on the dose of ceftazidime administered. This is also in agreement with the in vitro effects of avibactam, showing an increased reduction in MIC if the concentration increases. Comparable effects were observed earlier with the β-lactamase inhibitor MK7655 combined with imipenem (24).
Similar to the findings in the lung model, %fT>CT 1 mg/liter was the pharmacodynamic index that best described the effect of avibactam in the thigh infections. However, for most strains, higher doses and a higher %fT>CT appeared to be necessary to reach a static effect and 1-log10 kill than with lung infections. There is no immediate explanation for this observation, but it could be related to the infection models. Similar inocula were used for establishing thigh or lung infections, and the total number of bacteria in both models appears to be similar after infection. However, the thigh infection is limited to a small inoculum-to-surface-area ratio, whereas the bacteria instilled to cause lung infection distribute over the lungs. It could be hypothesized that drug penetration to each bacterial cell is somewhat better in the lung. Potentially, less inhibitor would be required to render all strains susceptible. In addition, the total amount of inhibitor present in the mouse exceeds by far what is required for inactivation of β-lactamases. For some other antimicrobials, more drug is required in lung infections because penetration into ELF is not significant, but this is, for example, not the case for aminoglycosides (25). A second possibility is a lower expression of β-lactamases in lung tissue, resulting in greater susceptibility to avibactam in the lung. This might also explain the observation of a greater sensitivity to ceftazidime monotherapy in the lung than in the thigh.
Although there appeared to be a reasonable fit between avibactam effect and %fT>CT 1 mg/liter in both the thigh and lung infections, the exact threshold value cannot be determined from the analysis performed, and a different magnitude close to CT 1 mg/liter being superior cannot be excluded. The best approximation of the threshold in our studies is 1 mg/liter, but in reality it might be 1.5 or 0.8 mg/liter. However, from visual inspection as well as the r2 of the fits, it is reasonably close to 1 mg/liter, and this is a number that in practice is easy to use. Besides, to determine the exact threshold would require a significant number of experiments to be performed. In addition, it may well turn out that there is strain-to-strain variation. In the hollow-fiber model, a range of CT of >0.25 and <0.5 mg/liter was described recently for β-lactamase-producing strains of Enterobacteriaceae tested in that model (16).
As has been known since the early days of therapy with β-lactam antibiotics (26) and since confirmed in numerous studies (25, 27, 28), the efficacy of this class is dependent on the frequency of administration, and the %fT>MIC is the most important pharmacodynamic index for treatment success. This was also the case in our mouse models with P. aeruginosa infection and ceftazidime monotherapy. However, in contrast to the %fT>MIC of 40% to 50% usually required for a static effect, we found that for most strains a much lower value was required, particularly those with very high MICs. Thus, relatively lower doses of ceftazidime monotherapy were required for these strains to result in stasis.
All strains used were quite resistant, based on different resistance mechanisms, and it seems very plausible that the reason for our observations is associated with this. It thus appears that the in vitro MIC for resistant strains is not fully predictive of the effect in vivo, and the amount of ceftazidime required for a static effect might be overestimated based on MIC alone for some very resistant strains. We have found this phenomenon for virtually every β-lactamase inhibitor combination that we have studied in our model, and this phenomenon was also reported earlier with imipenem in this model (24).
We are not the only study group reporting these kind of results. For example, MacVane et al. (29) found in the same type of infection model with NDM-producing Enterobacteriaceae a moderate reduction in bacterial density, although the %fT>MIC was 0% (MIC of >128 in vitro).
The amount of ceftazidime present relative to the amount of β-lactamase, with the latter being much higher in vitro than in vivo because in vivo the infection is localized, might be the significant factor here, in particular if ceftazidime hydrolysis by the β-lactamase is rate limited and the excess of ceftazidime present is therefore significant. Alternatively, the growth rate may be significantly lower in vivo for these resistant strains, where the balance between kill rate and growth rate changes in favor of kill rate (30) and relatively lower %fT>MIC values are required for a static effect.
We conclude that the effect of avibactam in combination with ceftazidime is dependent on the time above threshold %fT>CT 1 mg/liter. The results found in this study are proposed to be applicable to designing and assessing human dosing regimens.
ACKNOWLEDGMENTS
This study was supported by an unrestricted grant from AstraZeneca and by Forest Laboratories Inc. (now a subsidiary of Actavis PLC).
Virna J. Schuck is a former employee of AstraZeneca, and Wright W. Nichols is a current employee of AstraZeneca. Johan W. Mouton has been a consultant and/or received research funding from Angelini, Astra-Zeneca, Basilea, Jansen-Cilag, Merck & Co, Cubist, Pfizer, Polyphor, and Roche. Johanna Berkhout, Maria J. Melchers, Anita C. van Mil, Seyedmojtaba Seyedmousavi, and Claudia M. Lagarde declare no conflicts.
Funding Statement
This study was supported by an unrestricted grant from AstraZeneca and by Forest Laboratories, Inc. (now a subsidiary of Actavis PLC).
REFERENCES
- 1.Canton R, Akova M, Carmeli Y, Giske CG, Glupczynski Y, Gniadkowski M, Livermore DM, Miriagou V, Naas T, Rossolini GM, Samuelsen O, Seifert H, Woodford N, Nordmann P. 2012. Rapid evolution and spread of carbapenemases among Enterobacteriaceae in Europe. Clin Microbiol Infect 18:413–431. doi: 10.1111/j.1469-0691.2012.03821.x. [DOI] [PubMed] [Google Scholar]
- 2.Patel G, Bonomo RA. 2013. “Stormy waters ahead”: global emergence of carbapenemases. Front Microbiol 4:48. doi: 10.3389/fmicb.2013.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bradford PA. 2001. Extended-spectrum beta-lactamases in the 21st century: characterization, epidemiology, and detection of this important resistance threat. Clin Microbiol Rev 14:933–951. doi: 10.1128/CMR.14.4.933-951.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dhillon RH, Clark J. 2012. ESBLs: a clear and present danger? Crit Care Res Pract 2012:625170. doi: 10.1155/2012/625170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bush K. 2013. Carbapenemases: partners in crime. J Global Antimicrob Resist 1:7–16. doi: 10.1016/j.jgar.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 6.Hirsch EB, Tam VH. 2010. Detection and treatment options for Klebsiella pneumoniae carbapenemases (KPCs): an emerging cause of multidrug-resistant infection. J Antimicrob Chemother 65:1119–1125. doi: 10.1093/jac/dkq108. [DOI] [PubMed] [Google Scholar]
- 7.Poirel L, Potron A, Nordmann P. 2012. OXA-48-like carbapenemases: the phantom menace. J Antimicrob Chemother 67:1597–1606. doi: 10.1093/jac/dks121. [DOI] [PubMed] [Google Scholar]
- 8.Coleman K. 2011. Diazabicyclooctanes (DBOs): a potent new class of non-beta-lactam beta-lactamase inhibitors. Curr Opin Microbiol 14:550–555. doi: 10.1016/j.mib.2011.07.026. [DOI] [PubMed] [Google Scholar]
- 9.Zhanel GG, Lawson CD, Adam H, Schweizer F, Zelenitsky S, Lagace-Wiens PR, Denisuik A, Rubinstein E, Gin AS, Hoban DJ, Lynch JP III, Karlowsky JA. 2013. Ceftazidime-avibactam: a novel cephalosporin/beta-lactamase inhibitor combination. Drugs 73:159–177. doi: 10.1007/s40265-013-0013-7. [DOI] [PubMed] [Google Scholar]
- 10.Lagace-Wiens PR, Tailor F, Simner P, DeCorby M, Karlowsky JA, Walkty A, Hoban DJ, Zhanel GG. 2011. Activity of NXL104 in combination with beta-lactams against genetically characterized Escherichia coli and Klebsiella pneumoniae isolates producing class A extended-spectrum beta-lactamases and class C beta-lactamases. Antimicrob Agents Chemother 55:2434–2437. doi: 10.1128/AAC.01722-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Endimiani A, Hujer KM, Hujer AM, Pulse ME, Weiss WJ, Bonomo RA. 2011. Evaluation of ceftazidime and NXL104 in two murine models of infection due to KPC-producing Klebsiella pneumoniae. Antimicrob Agents Chemother 55:82–85. doi: 10.1128/AAC.01198-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levasseur P, Girard AM, Lavallade L, Miossec C, Pace J, Coleman K. 2014. Efficacy of a ceftazidime-avibactam combination in a murine model of septicemia caused by Enterobacteriaceae species producing AmpC or extended-spectrum beta-lactamases. Antimicrob Agents Chemother 58:6490–6495. doi: 10.1128/AAC.03579-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crandon JL, Schuck VJ, Banevicius MA, Beaudoin ME, Nichols WW, Tanudra MA, Nicolau DP. 2012. Comparative in vitro and in vivo efficacies of human simulated doses of ceftazidime and ceftazidime-avibactam against Pseudomonas aeruginosa. Antimicrob Agents Chemother 56:6137–6146. doi: 10.1128/AAC.00851-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Craig WA. 2003. Basic pharmacodynamics of antibacterials with clinical applications to the use of beta-lactams, glycopeptides, and linezolid. Infect Dis Clin North Am 17:479–501. doi: 10.1016/S0891-5520(03)00065-5. [DOI] [PubMed] [Google Scholar]
- 15.Mouton JW, Punt N, Vinks AA. 2007. Concentration-effect relationship of ceftazidime explains why the time above the MIC is 40 percent for a static effect in vivo. Antimicrob Agents Chemother 51:3449–3451. doi: 10.1128/AAC.01586-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coleman K, Levasseur P, Girard AM, Borgonovi M, Miossec C, Merdjan H, Drusano G, Shlaes D, Nichols WW. 2014. Activities of ceftazidime and avibactam against beta-lactamase-producing Enterobacteriaceae in a hollow-fiber pharmacodynamic model. Antimicrob Agents Chemother 58:3366–3372. doi: 10.1128/AAC.00080-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nichols W, Levasseur P, Li J, Das S. 2012. Abstr 52nd Intersci Conf Antimicrob Agents Chemother, San Francisco, CA. http://www.icaac.org/.
- 18.Berkhout J, Melchers MJ, van Mil AC, Seyedmousavi S, Lagarde CM, Schuck V, Nichols WW, Mouton JW. 2013. Exposure response relationships of ceftazidime and avibactam in a neutropenic thigh model, abstr A-1023 Abstr 53rd Intersci Conf Antimicrob Agents Chemother, Denver, CO. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berkhout J, Melchers MJ, van Mil AC, Seyedmousavi S, Lagarde CM, Schuck V, Nichols WW, Mouton JW. 2013. Pharmacodynamics of ceftazidime and avibactam in a neutropenic mouse lung model, abstr A-1022 Abstr 53rd Intersci Conf Antimicrob Agents Chemother, Denver, CO. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Housman ST, Crandon JL, Nichols WW, Nicolau DP. 2014. Efficacies of ceftazidime-avibactam and ceftazidime against Pseudomonas aeruginosa in a murine lung infection model. Antimicrob Agents Chemother 58:1365–1371. doi: 10.1128/AAC.02161-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berkhout J, Melchers MJ, van Mil AC, Nichols WW, Mouton JW. 2015. In vitro activity of combinations of ceftazidime-avibactam in in vitro checkerboard assays. Antimicrob Agents Chemother 59:1138–1144. doi: 10.1128/AAC.04146-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.ISO. 2006. ISO 20776-1:2006. Clinical laboratory testing and in vitro diagnostic test systems—susceptibility testing of infectious agents and evaluation of performance of antimicrobial susceptibility test devices, part 1. Reference method for testing the in vitro activity of antimicrobial agents against rapidly growing aerobic bacteria involved in infectious diseases. International Standards Organisation, Geneva, Switzerland. [Google Scholar]
- 23.Berkhout J, Melchers MJ, van Mil AC, Seyedmousavi S, Lagarde CM, Nichols WW, Mouton JW. 2015. Pharmacokinetics and penetration of ceftazidime and avibactam into epithelial lining fluid in thigh- and lung-infected mice. Antimicrob Agents Chemother 59:2299–2304. doi: 10.1128/AAC.04627-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mavridou E, Melchers RJ, van Mil AC, Mangin E, Motyl MR, Mouton JW. 2015. Pharmacodynamics of imipenem in combination with beta-lactamase inhibitor MK7655 in a murine thigh model. Antimicrob Agents Chemother 59:790–795. doi: 10.1128/AAC.03706-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leggett JE, Fantin B, Ebert S, Totsuka K, Vogelman B, Calame W, Mattie H, Craig WA. 1989. Comparative antibiotic dose-effect relations at several dosing intervals in murine pneumonitis and thigh-infection models. J Infect Dis 159:281–292. doi: 10.1093/infdis/159.2.281. [DOI] [PubMed] [Google Scholar]
- 26.Eagle H, Fleischman R, Levy M. 1953. “Continuous” vs. “discontinuous” therapy with penicillin; the effect of the interval between injections on therapeutic efficacy. N Engl J Med 248:481–488. doi: 10.1056/NEJM195303192481201. [DOI] [PubMed] [Google Scholar]
- 27.Roosendaal R, Bakker-Woudenberg IA, van den Berg JC, Michel MF. 1985. Therapeutic efficacy of continuous versus intermittent administration of ceftazidime in an experimental Klebsiella pneumoniae pneumonia in rats. J Infect Dis 152:373–378. doi: 10.1093/infdis/152.2.373. [DOI] [PubMed] [Google Scholar]
- 28.Andes D, Craig WA. 1998. In vivo activities of amoxicillin and amoxicillin-clavulanate against Streptococcus pneumoniae: application to breakpoint determinations. Antimicrob Agents Chemother 42:2375–2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.MacVane SH, Crandon JL, Nichols WW, Nicolau DP. 2014. Unexpected in vivo activity of ceftazidime alone and in combination with avibactam against New Delhi metallo-beta-lactamase-producing Enterobacteriaceae in a murine thigh infection model. Antimicrob Agents Chemother 58:7007–7009. doi: 10.1128/AAC.02662-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mouton JW, Vinks AA. 2005. Pharmacokinetic/pharmacodynamic modelling of antibacterials in vitro and in vivo using bacterial growth and kill kinetics: the minimum inhibitory concentration versus stationary concentration. Clin Pharmacokinet 44:201–210. doi: 10.2165/00003088-200544020-00005. [DOI] [PubMed] [Google Scholar]