Abstract
We describe the genome of a penicillinase-producing Kingella kingae strain (KWG1), the first to be isolated in continental Europe, whose blaTEM-1 gene was, for the first time in this species, found to be chromosomally inserted. The blaTEM gene is located in an integrative and conjugative element (ICE) inserted in Met-tRNA and comprising genes that encode resistance to sulfonamides, streptomycin, and tetracycline. This ICE is homologous to resistance-conferring plasmids of K. kingae and other Gram-negative bacteria.
TEXT
Kingella kingae is recognized as the first pathogen causing osteoarticular infections in children younger than 4 years of age (1–4). To date, penicillinase-producing strains harboring the blaTEM-1 gene on a plasmid have been isolated only in the United States, Iceland, and Israel (5). Recently, we described the first penicillinase-producing K. kingae strain to be found in continental Europe, KWG1, isolated from a child with arthritis (6). Using a Southern blot hybridization approach, we showed that the blaTEM-1 gene was chromosomally inserted in KWG1, in contrast to all of the penicillinase-producing strains previously described so far in the literature. Here, we report the complete genome sequence of KWG1.
Sequencing was performed by the Pacific Biosciences SMRT method using P4C2 chemistry. A total of 55,284 reads with a quality of ≥0.8 and a mean length of 4,456 bp were obtained, and a de novo assembly was performed by using the HGAP3 pipeline available through SMRT Analysis (version 2.2) from Pacific Biosciences. The mean depth of coverage was 91×. Polished assembly allowed us to obtain a single unique contig. Annotation was performed by Progenus.
Insertion sequence (IS) annotation was performed with ISfinder (http://www-is.biotoul.fr), and the new IS, ISKki1, was deposited in its database (7). Conjugative system annotation was performed with CONJscan (http://mobyle.pasteur.fr/cgi-bin/portal.py#forms::CONJscan-T4SSscan) (8). Comparisons with other Kingella genomes and with antimicrobial resistance-associated plasmids were performed with the NCBI BLAST software (http://www.ncbi.nlm.nih.gov) and the Synteny Line Plot tool of the MaGe Platform (http://www.genoscope.cns.fr/agc/mage) (9).
The complete and circularized KWG1 genome is 2,140,065 bp long with a GC content of 46.48%. It contains 54 tRNAs, 4 rRNA operons, and 2,250 open reading frames (ORFs) coding for known or putative proteins.
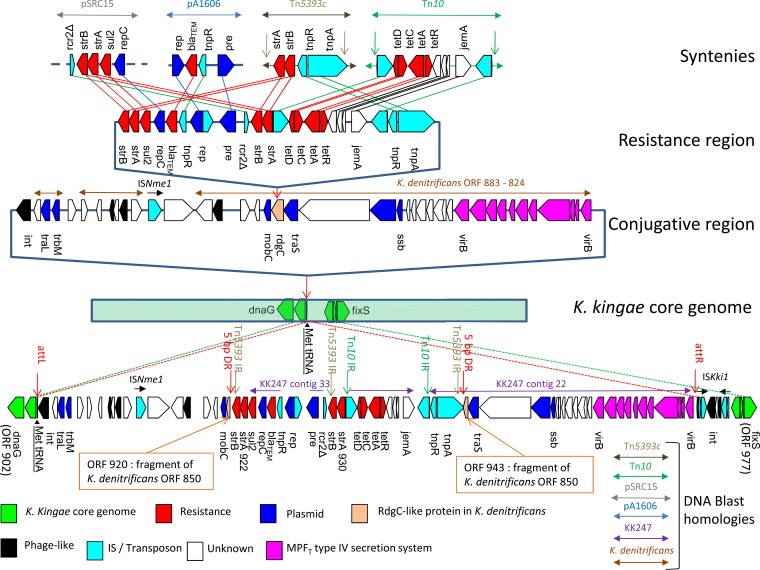
Comparison with the K. kingae type strain ATCC 23330 genome (accession number AFHS00000000) shows that both strains have 1,842 orthologous ORFs in syntons (81.69%) and reveals that the blaTEM gene encoding the K. kingae KWG1 β-lactamase is located on a large (74-kb) genomic island (ORFs 904 to 973) absent from the type strain and inserted in the vicinity of Met-tRNA. A putative phage integrase (ORF 2251) is present at its 3′ end (Fig. 1; Table 1). A second putative phage integrase is found in a 3-kb region (ORFs 967 to 973) located at the 5′ end. This region is composed of phage-like genes surrounded by two inverted copies of the same IS (ISKki1) (Fig. 1; Table 1). The island has a global GC content of 49% and is composed of genes encoding resistance to various antimicrobial compounds, transposase genes, genes of phage origin, and genes associated with plasmid functions (transfer or replication). Among those encoding plasmid functions, CONJscan (8) identified 11 contiguous ORFs (955 to 965) as an MPFT (mating pair formation proteins similar to the archetypal T-DNA conjugation system of Agrobacterium tumefaciens plasmid Ti) type IV secretion system involved in conjugative transfer (10); it also detected a coupling protein (TraG, ORF 946) and a relaxase (ORF 944). All of these colocalizing elements define a full conjugative system, and consequently, the genomic island can be considered an integrative and conjugative element (ICE) (11).
FIG 1.
Schematic representation of the putative genetic events that led to chromosomal insertion of the blaTEM gene in the K. kingae KWG1 genome and the genetic organization of the genomic region spanning bases 846108 to 926548. ORFs are represented by block arrows oriented according to the reading frame and color coded according to their putative functions or BLAST homologies. Green, K. kingae core genome (based on synteny with the K. kingae type strain ATCC 23330 genome); red, antimicrobial resistance; dark blue, plasmid transfer or replication; light salmon, RdgC-like protein in K. denitrificans strain ATCC 33394 (fragmented in KWG1); black, phage-like gene; light blue, IS or transposon (transposase, resolvase, and other); magenta, MPFT type IV secretion system; white, unknown function without homology or synteny with the K. kingae type strain genome. Transposons Tn10 and Tn5393c, as well as BLAST similarities to Salmonella enterica plasmid pSRC15, H. influenzae plasmid pA1606, K. kingae strain KK247, and K. denitrificans strain ATCC 33394, are indicated by colored horizontal double arrows. Syntenies with KWG1 are indicated by lines connecting ORFs with >50% identity on >80% of the shortest sequence. Isolated ISs (ISNme1 and ISKki1) are indicated by black horizontal arrows. Putative right and left att sites (attR and attL, respectively) and the 5-bp DR generated by insertion of the resistance region in the RgdC-like ORF are indicated by vertical red arrows. Tn5393c IRs (Tn5393 IR) and the 23-bp terminal IRs flanking Tn10 (Tn10 IR) are indicated by vertical green arrows.
TABLE 1.
Predicted ORFs, RNAs, and other genetic structures identified in the region of the K. kingae KWG1 genome spanning bases 846108 to 926548
| Object | Gene | Product | Frame | Start | End | Length (bases) | GC content | Category |
|---|---|---|---|---|---|---|---|---|
| ORF-0902 | dnaG | DNA primase | −2 | 846108 | 847871 | 1,764 | 0.4921 | Core genome |
| ORF-0903 | Conserved protein of unknown function | −2 | 847971 | 849143 | 1,173 | 0.5107 | Core genome | |
| tRNA-15 | Met-tRNA | 1 | 849282 | 849357 | 76 | RNA | ||
| RPT45590902 | DR (putative AttL) | 1 | 849311 | 849357 | 47 | DR | ||
| ORF-2251 | int | Putative site-specific recombinase, phage integrase family | −2 | 849303 | 850427 | 1,125 | 0.5060 | Phage-like |
| ORF-0904 | Conserved protein of unknown function | −3 | 850916 | 851464 | 549 | 0.4699 | ||
| ORF-0905 | traL | TraL protein | −2 | 851451 | 852194 | 744 | 0.5013 | Plasmid |
| ORF-0906 | trbM | TrbM | −1 | 852373 | 852915 | 543 | 0.5378 | Plasmid |
| ORF-0907 | Conserved membrane protein of unknown function | 3 | 853617 | 854255 | 639 | 0.4444 | ||
| ORF-0908 | Conserved protein of unknown function | 1 | 854677 | 855135 | 459 | 0.4597 | ||
| ORF-0909 | Conserved protein of unknown function | −3 | 855839 | 856228 | 390 | 0.4923 | ||
| ORF-0910 | DNA-binding helix-turn-helix protein | −3 | 856895 | 857209 | 315 | 0.4984 | Phage-like | |
| ORF-0911 | Conserved protein of unknown function | −2 | 857193 | 857573 | 381 | 0.4777 | ||
| ORF-0912 | DNA-binding helix-turn-helix protein | −2 | 857673 | 858275 | 603 | 0.4046 | Phage-like | |
| ORF-0913 | Conserved protein of unknown function | 1 | 858604 | 859422 | 819 | 0.5678 | ||
| ORF-0914 | Transposase, IS5 family, ISNme1 | 1 | 859948 | 860955 | 1,008 | 0.5179 | Transposon | |
| ORF-0915 | Conserved exported protein of unknown function | 1 | 861187 | 863475 | 2,289 | 0.5457 | ||
| ORF-0916 | Conserved protein of unknown function | −3 | 863675 | 864952 | 1,278 | 0.4030 | ||
| ORF-0917 | Phage protein Gp37/Gp68 | −1 | 864991 | 865734 | 744 | 0.4382 | Phage-like | |
| ORF-0918 | Conserved protein of unknown function | 1 | 867187 | 867918 | 732 | 0.5601 | ||
| ORF-2252 | Conserved protein of unknown function | −2 | 868137 | 868511 | 375 | 0.4880 | ||
| ORF-0919 | mobC | Bacterial mobilization protein MobC | −3 | 869015 | 869545 | 531 | 0.4953 | Plasmid |
| ORF-0920 | Recombination-associated protein RdgC (fragment 1) | −1 | 869617 | 869997 | 381 | 0.5696 | ||
| 5-bp DR flanking resistance region | 1 | 870124 | 870128 | 5 | DR | |||
| misc_feature870129D | IR of Tn5393 | 1 | 870129 | 870208 | 80 | Transposon | ||
| ORF-0921 | strB | Streptomycin phosphotransferase B | −1 | 870235 | 871071 | 837 | 0.5591 | Resistance |
| ORF-0922 | strA | Streptomycin 3″-kinase | −2 | 871071 | 871874 | 804 | 0.5609 | Resistance |
| ORF-0923 | sul2 | Dihydropteroate synthase type-2 | −2 | 871935 | 872750 | 816 | 0.6078 | Resistance |
| ORF-0924 | repC | RepC | −2 | 873060 | 873869 | 810 | 0.6556 | Plasmid |
| ORF-0925 | blaTEM | TEM-1 β-lactamase | −1 | 873985 | 874845 | 861 | 0.4925 | Resistance |
| ORF-0926 | tnpR | Tn3-like transposon resolvase, transposon Tn2a | −2 | 875028 | 875585 | 558 | 0.5269 | Transposon |
| ORF-0927 | rep | Replication protein | −2 | 875919 | 876848 | 930 | 0.3527 | Plasmid |
| ORF-0928 | Integrase core genome domain protein | 1 | 876898 | 877704 | 807 | 0.5279 | Transposon | |
| ORF-0929 | pre | Plasmid recombination enzyme | −2 | 878208 | 879479 | 1,272 | 0.3852 | Plasmid |
| ORF-2255 | Putative transposase zinc-binding domain, fragment of ISCR2 | +3 | 880263 | 880544 | 282 | 0.5890 | Transposon | |
| misc_feature880548D | IR of Tn5393 | 1 | 880548 | 880627 | 80 | Transposon | ||
| ORF-2253 | strB | Streptomycin phosphotransferase B | −1 | 880654 | 881490 | 837 | 0.5591 | Resistance |
| ORF-0930 | strA | Streptomycin 3″-kinase | −2 | 881490 | 882293 | 804 | 0.5609 | Resistance |
| 9-bp repeat of Tn10 insertion | 1 | 882338 | 882346 | 9 | Transposon | |||
| misc_feature882340D | Terminal IR of transposon Tn10 | 1 | 882340 | 882362 | 23 | Transposon | ||
| misc_RNA_3 | RNA-OUT | −1 | 882385 | 882446 | 62 | RNA | ||
| ORF-0931 | Transposase, IS4 family, IS10 | 3 | 882447 | 883655 | 1,209 | 0.4475 | Transposon | |
| ORF-0932 | tetD | Transposon Tn10 TetD protein | −2 | 883665 | 884081 | 417 | 0.3597 | Resistance |
| ORF-0933 | tetC | Transposon Tn10 TetC protein | 3 | 884094 | 884762 | 669 | 0.3259 | Resistance |
| ORF-0934 | tetA | Class B tetracycline resistance protein | −1 | 884875 | 886080 | 1,206 | 0.4328 | Resistance |
| ORF-0935 | tetR | Class B tetracycline repressor protein from transposon Tn10 | 1 | 886162 | 886737 | 576 | 0.4097 | Resistance |
| ORF-0936 | jemC | Conserved protein of unknown function | −1 | 886762 | 887385 | 624 | 0.4119 | |
| ORF-0937 | Conserved protein of unknown function | −3 | 887456 | 887842 | 387 | 0.3902 | ||
| ORF-0938 | jemB | Conserved protein of unknown function | −2 | 887835 | 888134 | 300 | 0.3533 | |
| ORF-0939 | jemA | Glutamate transporter | 2 | 888599 | 889804 | 1,206 | 0.3947 | |
| ORF-0940 | Transposase, IS4 family, IS10 | −1 | 890170 | 891378 | 1,209 | 0.4508 | Transposon | |
| misc_RNA_4 | RNA-OUT | 1 | 891379 | 891440 | 62 | RNA | ||
| misc_feature891463R | Terminal IR of transposon Tn10 | −1 | 891463 | 891485 | 23 | Transposon | ||
| 9-bp repeat of Tn10 insertion | −1 | 891479 | 891487 | 9 | Transposon | |||
| ORF-0941 | tnpR | Putative resolvase for transposon Tn5393 | −1 | 891514 | 892128 | 615 | 0.6065 | Transposon |
| ORF-0942 | tnpA | Transposase TnpA for transposon Tn5393 | 3 | 892254 | 895139 | 2,886 | 0.6195 | Transposon |
| misc_feature895093R | IR of Tn5393 | −1 | 895093 | 895172 | 80 | Transposon | ||
| 5-bp DR flanking resistance region | 1 | 895173 | 895177 | 5 | DR | |||
| ORF-0943 | Recombination-associated protein RdgC (fragment 2) | −2 | 895173 | 895565 | 393 | 0.5838 | ||
| ORF-0944 | traS | TraS relaxase | −3 | 895562 | 896680 | 1,119 | 0.5621 | Plasmid |
| ORF-0945 | Toprim domain protein | −1 | 896779 | 902313 | 5,535 | 0.5429 | ||
| ORF-0946 | TraG/TraD family protein | −3 | 902333 | 904372 | 2,040 | 0.5520 | Plasmid | |
| ORF-0947 | ssb | Single-stranded DNA-binding protein 1 | −2 | 904467 | 904880 | 414 | 0.5845 | Plasmid |
| ORF-2254 | Putative metalloprotease | +2 | 904925 | 905263 | 339 | 0.5580 | ||
| ORF-0948 | Conserved protein of unknown function | −2 | 905223 | 905486 | 264 | 0.5568 | ||
| ORF-0949 | Conserved protein of unknown function | −3 | 905618 | 905983 | 366 | 0.5519 | ||
| ORF-0950 | Conserved protein of unknown function | −1 | 905980 | 906753 | 774 | 0.5581 | ||
| ORF-0951 | Conserved protein of unknown function | −2 | 906798 | 907319 | 522 | 0.4540 | ||
| ORF-0952 | Conserved protein of unknown function | −3 | 907316 | 908230 | 915 | 0.5607 | ||
| ORF-0953 | Conserved membrane protein of unknown function | −1 | 907999 | 908325 | 327 | 0.5199 | ||
| ORF-0954 | Conserved exported protein of unknown function | −1 | 908380 | 908844 | 465 | 0.4409 | ||
| ORF-0955 | virB11 | P-type DNA transfer ATPase VirB11 | −1 | 909004 | 910104 | 1,101 | 0.5595 | MPFT |
| ORF-0956 | virB10 | Bacterial conjugation TrbI-like protein | −3 | 910190 | 911464 | 1,275 | 0.5137 | MPFT |
| ORF-0957 | virB9 | Putative P-type conjugative transfer protein VirB9 | −1 | 911461 | 912300 | 840 | 0.5417 | MPFT |
| ORF-0958 | virB8 | VirB8 protein | −1 | 912313 | 913056 | 744 | 0.4449 | MPFT |
| ORF-0959 | virB7 | Conserved protein of unknown function | −2 | 913275 | 913754 | 480 | 0.4812 | MPFT |
| ORF-0960 | virB6 | TrbL/VirB6 plasmid conjugal transfer protein | −1 | 913786 | 914748 | 963 | 0.4798 | MPFT |
| ORF-0961 | virB5 | Type IV secretion system protein | −2 | 914808 | 915446 | 639 | 0.4507 | MPFT |
| ORF-0962 | virB4 | Type IV secretion/conjugal transfer ATPase, VirB4 family | −3 | 915548 | 918103 | 2,556 | 0.5082 | MPFT |
| ORF-0963 | virB3 | Type IV secretory pathway, VirB3-like protein | −2 | 918015 | 918443 | 429 | 0.4452 | MPFT |
| ORF-0964 | virB2 | Conserved membrane protein of unknown function | −3 | 918464 | 918784 | 321 | 0.5109 | MPFT |
| ORF-0965 | virB1 | Type IV secretion system protein VirB1 | −3 | 918941 | 919747 | 807 | 0.5502 | MPFT |
| RPT45590902 | DR (putative AttR) | 1 | 919949 | 919995 | 47 | DR | ||
| ORF-0966 | Protein of unknown function | −1 | 920122 | 920250 | 129 | 0.4496 | ||
| ORF-0967 | Transposase, ISKki1 ORF A | 2 | 920342 | 920578 | 237 | 0.4093 | Transposon | |
| ORF-0968 | Transposase, ISKki1 ORF B | 2 | 920606 | 921172 | 567 | 0.3369 | Transposon | |
| ORF-0969 | int | Putative site-specific recombinase, phage integrase family | −3 | 921179 | 922090 | 912 | 0.4748 | Phage-like |
| ORF-0970 | Conserved protein of unknown function | −3 | 922112 | 922399 | 288 | 0.5104 | ||
| ORF-0971 | Replication protein A (fragment) | −1 | 922408 | 922743 | 336 | 0.5060 | Phage-like | |
| ORF-0972 | Transposase, ISKki1 ORF B | −2 | 922761 | 923327 | 567 | 0.3351 | Transposon | |
| ORF-0973 | Transposase, ISKki1 ORF A | −2 | 923355 | 923591 | 237 | 0.4093 | Transposon | |
| ORF-0974 | Conserved membrane protein of unknown function | −2 | 923856 | 924728 | 873 | 0.5074 | Core genome | |
| ORF-0975 | Conserved protein of unknown function | 3 | 924783 | 925094 | 312 | 0.4679 | Core genome | |
| ORF-0976 | fixS | Cytochrome oxidase maturation protein, cbb3 type | 1 | 925096 | 925272 | 177 | 0.4407 | Core genome |
| ORF-0977 | Major facilitator superfamily MFS_1 transporter | 2 | 925229 | 926548 | 1,320 | 0.5136 | Core genome |
ICEs are mobile genetic elements found in about 18% of prokaryotic chromosomes and display both plasmid and phage features (8, 11). Like plasmids, they harbor a conjugative system composed of a type IV secretion system (MPFT, ORFs 955 to 965), a coupling protein (ORF 946), and a relaxase (ORF 944). Like phages, they integrate into tRNA (tRNA-15) genes with integrases (ORF 2251). Evolutionary analyses suggest that ICEs derive from conjugative plasmids that acquired the phage-like ability to integrate into the chromosomes of bacteria (8). Of note, the KWG1 ICE harbors two putative plasmid replication genes (ORFs 924 and 927).
The presence of a 47-bp direct repeat (DR) sequence (RPT45590902; GAC TCA TAA TCC CTT GGT CGT GGG TTC GAA ACC CAC CCG ACC CAC CA) within the Met-tRNA gene and at the 5′ end of the MPFT cluster could represent an att site for the insertion of bacteriophages and/or ICEs (Table 1; Fig. 1). Resistance genes for streptomycin (strB and strA), sulfonamides (sul2), penicillin (blaTEM), and tetracycline (tetR, tetA, tetC, tetD) explaining the resistance profile of KWG1 are grouped together with genes encoding transposases and integrases in a small 25-kb region surrounded by two fragments of a truncated ORF encoding a putative exonuclease of the RdgC family (ORFs 920 and 943). Within this resistance region, streptomycin resistance-associated genes strA and strB are duplicated (ORFs 922 and 930 for strA, ORFs 921 and 2253 for strB). The resistance region is flanked by the inverted repeat (IR) of Tn5393 and a 5-bp (ATAAT) DR, suggesting direct insertion into the RdgC family ORF (Fig. 1).
The 74-kb-long ICE is 97% similar to a region of the Kingella denitrificans ATCC 33394 genome (GenBank accession number NZ_GL870929). This region contains the same conjugative system and thus also corresponds to an ICE but notably lacks two features, the 25-kb region encoding antimicrobial resistance and the 3-kb region at the 5′ end corresponding to ORFs 967 to 973 (Fig. 1). In K. denitrificans, this ICE is inserted in the vicinity of Asn-tRNA, with bases 68111 to 69268 of contig 15 (GenBank accession number AEWV01000015) probably encoding an undetected phage-like integrase that is close to a Kingella oralis putative phage integrase (GenBank accession number EEP68425; 80% identity on 86% coverage) but clearly different from KWG1 ORF 2251 (52% identity on 92% coverage). Interestingly, in K. denitrificans, the putative exonuclease of the RdgC family (ORF 850) corresponding to fragmented ORFs 920 and 943 of KWG1 is complete and undisrupted (Fig. 1); this confirms that the resistance region of KWG1 has been inserted into this putative exonuclease.
The resistance region appears as a complex structure combining elements of plasmids and transposons. Its 5′ part (ORFs 921 to 924) is nearly (99%) identical to the repC-sul2-strAB-rcr2Δ cluster of pSRC15 and other similar plasmids found in Gram-negative bacteria (12) (Fig. 1). The sul2-strAB-rcr2Δ cluster is supposed to result from the insertion of transposon Tn5393c into a CR2-sul2 region (12). Interestingly, a fragment of mobile element CR2 (rcr2Δ, ORF 2255) is found adjacent to the second copy of strAB (ORFs 2253 and 930) (Table 1; Fig. 1). Thus, the cluster of genes described by Yau et al. is present in two pieces separated by the central part carrying the blaTEM gene (Fig. 1) (12). The 3′ part of the resistance region (ORFs 2255 to 942) results from the insertion of transposon Tn10 (flanked by the 9-bp repeat CCCTGATGA and 23-bp 5′ terminal IRs) (13) into a Tn5393-like transposon (Tn5393c) (Fig. 1) (14). Finally, the central part carrying the blaTEM gene from ORF 925 to ORF 929 is substantially similar (98% over 82% coverage) to a small blaTEM-1-bearing plasmid in Haemophilus influenzae (pA1606; GenBank accession number JQ611726), as well as similar in organization (rep, replication gene; blaTEM-1, TEM-1 β-lactamase gene; pre, plasmid recombination enzyme gene; tnpR, Tn3-like transposon resolvase gene) (Fig. 1) (15). However, unlike pA1606, the tnpR resolvase gene of KWG1 (ORF 926) and its res sites (I, II, and III) display the closest similarity to those of transposon Tn2a (16); moreover, the blaTEM-1 gene (ORF 925) is the TEM-1c variant (17), while pA1606 harbors the TEM-1b variant.
Fourteen contigs of the blaTEM-harboring KKC2005004457 plasmid sequencing project (GenBank accession number AMPT00000000), with lengths ranging from 37 to 1,684 bases, are highly (99 to 100%) similar to the whole resistance region (18). However, because of the heavily fragmented nature of these contigs, we cannot tell if the architecture of this K. kingae resistance plasmid is similar to that of the KWG1 resistance region.
Two contigs of the β-lactamase-producing K. kingae KK247 strain sequencing project (GenBank accession number CCJT00000000) (19) are similar to parts of the ICE: contig 33 with the resistance region (98%) and contig 22 with the MPFT cluster and the 3′ end of the resistance region (99%) (Fig. 1).
The strong similarity observed between the ICE and contigs of K. kingae strains carrying resistance plasmids suggests that transitions between the episomal and plasmidic forms of blaTEM-encoding mobile genetic elements in this species do exist. This observation is in line with recent work that blurs the distinction between ICEs and plasmids (20).
Although no circularized complete sequence of a K. kingae resistance plasmid harboring the blaTEM gene is available to date, we may hypothesize the history of the insertion process with a reasonable degree of confidence (Fig. 1).
First, a small blaTEM-harboring plasmid, similar (but not identical) to pA1606, inserted in a larger region associated with resistance to tetracycline, sulfonamides, and streptomycin, near strAB, with duplication of these genes. Second, the whole resistance region is later inserted in a larger MPFT conjugative plasmid, causing disruption of a rdgC-like gene. Finally, the conjugative plasmid, or a part of it, inserted itself into the chromosome at Met-tRNA via a phage-like integration process. The precise order of these three events is only postulated and may have differed.
The fact that a similar plasmid is also inserted into the chromosome of K. denitrificans suggests that it is an integrative element of the Kingella genus. However, this plasmid inserted into another tRNA (Asn-tRNA) with a different integrase lacks several insertion sequences (ISNme1 and ISKki1) and does not carry any resistance gene. Recombination and transposition events have thus modified the architecture of this mobile genetic element during its transfer from one species to another.
Interestingly, KK247, described as a strain with plasmid-borne blaTEM, displays significant sequence similarity and a similar architecture for two of its contigs, suggesting that the ICE still exists in its plasmid form in K. kingae (19).
As KK247 belongs to clone A, a clone composed mainly of oropharyngeal commensal isolates different from the one to which KWG1 belongs, and as we have previously shown that some K. kingae strains belonging to clone A also carry an integrated blaTEM gene on their chromosome, we can conclude that the integration process of the resistance plasmid occurred at least twice in different phylogenetic groups (21).
Episomal integration confers on bacteria the advantage that resistance to antimicrobials is automatically transmitted to daughter cells without the need for plasmid replication. In the era of massive antibiotic use, bacteria harboring stable mechanisms of resistance to various antimicrobials have a selective advantage in colonizing the oropharynxes of children frequently treated with antibiotics. Strains of K. kingae clone A have been associated with asymptomatic carriage in Israel and are rarely involved in invasive infections (22). Conversely, KWG1 belongs to a clone involved in osteoarticular infections. The fact that the same plasmid can insert itself into different genetic backgrounds of Kingella raises concerns that another, more virulent, clone may also integrate the resistance genes in the future.
Nucleotide sequence accession number.
The genome project described here was deposited in the European Nucleotide Archive under accession number LN869922.
ACKNOWLEDGMENTS
We acknowledge the Laboratory of Bioinformatics Analyses for Genomics and Metabolism and the National Infrastructure France Genomique for their technical support of the expert annotation and comparative genomic tools (MicroScope platform). We thank Harry Kemble for his proofreading.
This work was supported in part by the Booster Innovation Fund of the Département de la Recherche Clinique et du Développement, DIRC Ile-de-France, Assistance Publique—Hôpitaux de Paris. The funders had no role in the study design, data collection and analysis, publication decision, or preparation of the manuscript.
We declare no conflicts of interest.
REFERENCES
- 1.Ceroni D, Cherkaoui A, Ferey S, Kaelin A, Schrenzel J. 2010. Kingella kingae osteoarticular infections in young children: clinical features and contribution of a new specific real-time PCR assay to the diagnosis. J Pediatr Orthop 30:301–304. doi: 10.1097/BPO.0b013e3181d4732f. [DOI] [PubMed] [Google Scholar]
- 2.Chometon S, Benito Y, Chaker M, Boisset S, Ploton C, Berard J, Vandenesch F, Freydiere AM. 2007. Specific real-time polymerase chain reaction places Kingella kingae as the most common cause of osteoarticular infections in young children. Pediatr Infect Dis J 26:377–381. doi: 10.1097/01.inf.0000259954.88139.f4. [DOI] [PubMed] [Google Scholar]
- 3.Ilharreborde B, Bidet P, Lorrot M, Even J, Mariani-Kurkdjian P, Liguori S, Vitoux C, Lefevre Y, Doit C, Fitoussi F, Pennecot G, Bingen E, Mazda K, Bonacorsi S. 2009. New real-time PCR-based method for Kingella kingae DNA detection: application to samples collected from 89 children with acute arthritis. J Clin Microbiol 47:1837-1841. doi: 10.1128/JCM.00144-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yagupsky P. 2004. Kingella kingae: from medical rarity to an emerging paediatric pathogen. Lancet Infect Dis 4:358–367. doi: 10.1016/S1473-3099(04)01046-1. [DOI] [PubMed] [Google Scholar]
- 5.Basmaci R, Bonacorsi S, Bidet P, Balashova NV, Lau J, Munoz-Almagro C, Gene A, Yagupsky P. 2014. Genotyping, local prevalence and international dissemination of beta-lactamase-producing Kingella kingae strains. Clin Microbiol Infect 20:O811–O817. doi: 10.1111/1469-0691.12648. [DOI] [PubMed] [Google Scholar]
- 6.Basmaci R, Bidet P, Bercot B, Jost C, Kwon T, Gaumetou E, Bonacorsi S. 2014. First identification of a chromosomally located penicillinase gene in Kingella kingae species isolated in continental Europe. Antimicrob Agents Chemother 58:6258–6259. doi: 10.1128/AAC.03562-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Siguier P, Perochon J, Lestrade L, Mahillon J, Chandler M. 2006. ISfinder: the reference centre for bacterial insertion sequences. Nucleic Acids Res 34:D32–D36. doi: 10.1093/nar/gkj014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guglielmini J, Quintais L, Garcillan-Barcia MP, de la Cruz F, Rocha EP. 2011. The repertoire of ICE in prokaryotes underscores the unity, diversity, and ubiquity of conjugation. PLoS Genet 7:e1002222. doi: 10.1371/journal.pgen.1002222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vallenet D, Belda E, Calteau A, Cruveiller S, Engelen S, Lajus A, Le Fevre F, Longin C, Mornico D, Roche D, Rouy Z, Salvignol G, Scarpelli C, Thil Smith AA, Weiman M, Medigue C. 2013. MicroScope—an integrated microbial resource for the curation and comparative analysis of genomic and metabolic data. Nucleic Acids Res 41:D636–D647. doi: 10.1093/nar/gks1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smillie C, Garcillan-Barcia MP, Francia MV, Rocha EP, de la Cruz F. 2010. Mobility of plasmids. Microbiol Mol Biol Rev 74:434–452. doi: 10.1128/MMBR.00020-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wozniak RA, Waldor MK. 2010. Integrative and conjugative elements: mosaic mobile genetic elements enabling dynamic lateral gene flow. Nat Rev Microbiol 8:552–563. doi: 10.1038/nrmicro2382. [DOI] [PubMed] [Google Scholar]
- 12.Yau S, Liu X, Djordjevic SP, Hall RM. 2010. RSF1010-like plasmids in Australian Salmonella enterica serovar Typhimurium and origin of their sul2-strA-strB antibiotic resistance gene cluster. Microb Drug Resist 16:249–252. doi: 10.1089/mdr.2010.0033. [DOI] [PubMed] [Google Scholar]
- 13.Halling SM, Kleckner N. 1982. A symmetrical six-base-pair target site sequence determines Tn10 insertion specificity. Cell 28:155–163. doi: 10.1016/0092-8674(82)90385-3. [DOI] [PubMed] [Google Scholar]
- 14.L'Abée-Lund TM, Sorum H. 2000. Functional Tn5393-like transposon in the R plasmid pRAS2 from the fish pathogen Aeromonas salmonicida subspecies salmonicida isolated in Norway. Appl Environ Microbiol 66:5533–5535. doi: 10.1128/AEM.66.12.5533-5535.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Søndergaard A, San Millan A, Santos-Lopez A, Nielsen SM, Gonzalez-Zorn B, Norskov-Lauritsen N. 2012. Molecular organization of small plasmids bearing blaTEM-1 and conferring resistance to β-lactams in Haemophilus influenzae. Antimicrob Agents Chemother 56:4958–4960. doi: 10.1128/AAC.00408-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bailey JK, Pinyon JL, Anantham S, Hall RM. 2011. Distribution of the blaTEM gene and blaTEM-containing transposons in commensal Escherichia coli. J Antimicrob Chemother 66:745–751. doi: 10.1093/jac/dkq529. [DOI] [PubMed] [Google Scholar]
- 17.Goussard S, Courvalin P. 1999. Updated sequence information for TEM beta-lactamase genes. Antimicrob Agents Chemother 43:367–370. doi: 10.1093/jac/43.3.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Banerjee A, Kaplan JB, Soherwardy A, Nudell Y, Mackenzie GA, Johnson S, Balashova NV. 2013. Characterization of TEM-1 β-lactamase-producing Kingella kingae clinical isolates. Antimicrob Agents Chemother 57:4300–4306. doi: 10.1128/AAC.00318-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rouli L, Robert C, Raoult D, Yagupsky P. 2014. Kingella kingae KK247, an atypical pulsed-field gel electrophoresis clone A strain. Genome Announc 2:e01228-14. doi: 10.1128/genomeA.01228-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carraro N, Poulin D, Burrus V. 2015. Replication and active partition of integrative and conjugative elements (ICEs) of the SXT/R391 family: the line between ICEs and conjugative plasmids is getting thinner. PLoS Genet 11:e1005298. doi: 10.1371/journal.pgen.1005298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Basmaci R, Bidet P, Jost C, Yagupsky P, Bonacorsi S. 2015. Penicillinase-encoding gene blaTEM-1 may be plasmid borne or chromosomally located in Kingella kingae species. Antimicrob Agents Chemother 59:1377–1378. doi: 10.1128/AAC.04748-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yagupsky P, Weiss-Salz I, Fluss R, Freedman L, Peled N, Trefler R, Porat N, Dagan R. 2009. Dissemination of Kingella kingae in the community and long-term persistence of invasive clones. Pediatr Infect Dis J 28:707–710. doi: 10.1097/INF.0b013e31819f1f36. [DOI] [PubMed] [Google Scholar]