Abstract
Background
Epidermal growth factor-tyrosine kinase inhibitors (EGFR-TKIs) are one of the effective medicines in advanced lung adenocarcinoma leptomeningeal metastasis (LM) treatment in recent years.
Methods
This paper reports two cases of advanced lung adenocarcinoma with exon-EGFR19 mutation. LM occurred during gefitinib treatment with an extracranial condition under control. Later, erlotinib was adopted with a recurrent response.
Results
The progression free survival of both patients was over six months and their LM was under control for six and a half and nine months, respectively. LM may be sensitive on different levels to different EGFR-TKIs.
Conclusion
Erlotinib can be used to replace gefitinib if intracranial tumor progress occurs during gefitinib treatment. However, our conclusion still needs to be proven by further clinical research.
Keywords: EGFR, erlotinib, LM, lung adenocarcinoma, TKIs
Introduction
Leptomeningeal metastasis (LM) is one of the main lethal factors of advanced malignant tumors, with 4∼15% of incidence and poor prognosis. Without medical intervention, median survival time is only four to six weeks, with a rapid onset of symptoms.1–3 Advanced non small cell lung cancer (NSCLC) has a higher risk of LM developing than other tumors. Because of the limit of the blood brain barrier, traditional cytotoxic medicines are not as effective. There is no standard treatment for such patients. Epidermal growth factor receptor-tyrosine kinase inhibitors (EGFR-TKIs) have proven an effective treatment for advanced NSCLC in recent years. This new kind of molecular targeted medicine is especially effective for patients with exon-EGFR19 sensitive mutations. In clinical application, gefitinib and erlotinib are often used. Since 2003, when Villano et al. first reported that EGFR-TKIs were effective in LM treatment,4 clinical research on EGFR-TKI treatment to central nervous system metastasis of advanced NSCLC has gradually been conducted. However, there are limited clinical documents on LM and most are single case reports. Some research mentions that erlotinib might be effective once again if LM occurred during gefitinib treatment.5–7 This paper reports on two cases of patients who suffered LM during gefitinib treatment and were subsequently successfully treated with erlotinib. We also present a review of the literature.
Case report
Case 1
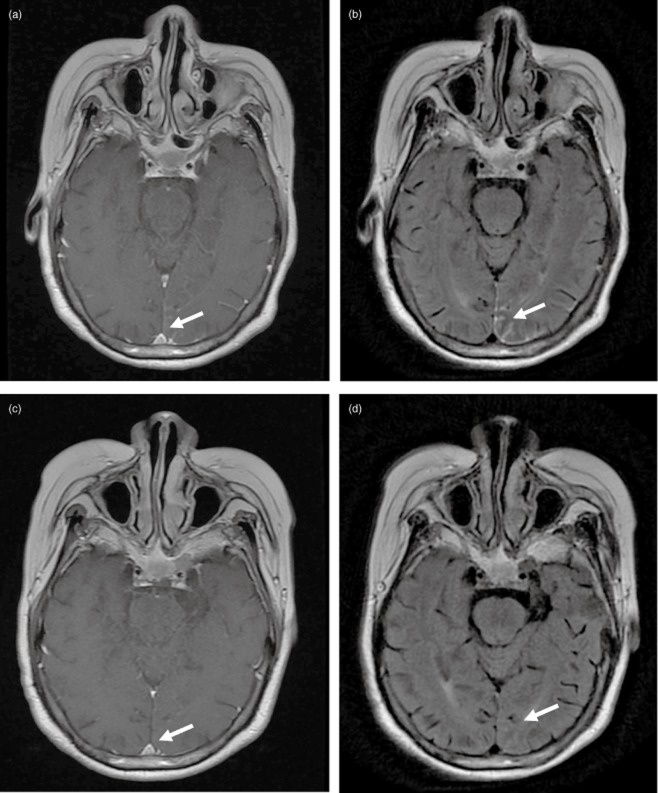
A 63 year-old Asian, non-smoking, female patient was first diagnosed in 2009 at the Cancer Hospital of the Chinese Academy of Medical Sciences with adenocarcinoma of the left lung. She was treated with radical surgery staging IIIA and exon-EGFR19 mutation. Four cycles of chemotherapy with vinorelbine /cisplatin were completed from 21 August 2009. A new metastasis in the right lung was detected during a regular lung computed tomography (CT) examination in August 2010. Subsequently, four further cycles of chemotherapy with docetaxel/cisplatin were given, with stable curative effects. In January 2011, a new metastasis in both lungs was detected during CT examination. The patient was given 250 mg of gefitinib orally once a day. In early November 2011, she started to experience dizziness, headaches, and vomiting. Brain magnetic resonance imaging (MRI) indicated linear high signal intensities on parts of meninges and linear hardening could be seen with enhanced scanning. Thus, LM was diagnosed for the first time (as shown in Fig 1a,b). A CSF (cerebrospinal fluid) laboratory test found adenocarcinoma cells. At that time, her Eastern Cooperative Oncology Group (ECOG) score was 2. Erlotinib (150 mg/day) was used to replace gefitinib. One week later, her symptoms disappeared. CSF tests were subsequently performed several times, but no tumor cells were found. In March 2012, a brain MRI showed that the previous LM was in complete remission (as shown in Fig 1c,d). In August 2012, a brain MRI showed that cerebral metastasis had progressed. Until that time, progression free survival (PFS) of erlotinib re-treatment was nine months with stable extracranial disease.
Figure 1.
(a) Brain magnetic resonance imaging (MRI) T1+C before treatment; (b) Brain MRI T2 flair image before treatment; (c) Brain MRI T1+C Image after 12 weeks; (d) Brain MRI T2 flair image after 12 weeks of treatment.
Case 2
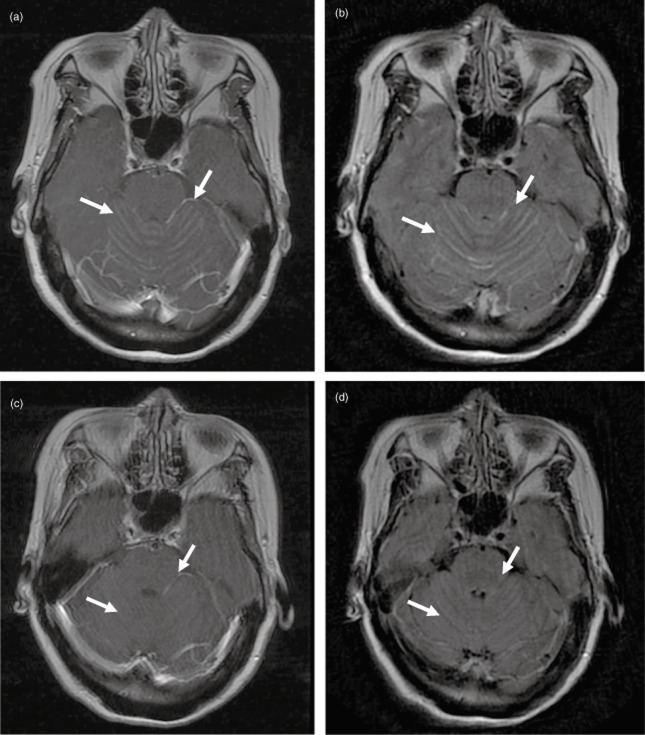
A 57 year-old, Asian, non-smoking, female patient was diagnosed at the Cancer Hospital of the Chinese Academy of Medical Sciences with adenocarcinoma and was treated with resection of the lower lobe of the left lung with staging IIIA and exon-EGFR19 mutation. Four cycles of docetaxel/cisplatin were completed. In September 2011, she was diagnosed by emission computed tomography (ECT) and local enhanced CT scan, with bone metastasis. She commenced regular gefitinib treatment. On 15 May 2012, she started to experience dizziness and headaches. A brain MRI indicated diffuse linear high signal intensities on the temporal lobe and cerebellum, and linear hardening could be seen with enhanced scanning. Thus, LM was diagnosed (as shown in Fig 2a,b) with stable extracranial disease. Erlotinib (150 mg/day) was used to replace gefitinib. After one week, her symptoms were gradually relieved and then disappeared. In July 2012, a regular brain MRI showed that the metastasis was greatly relieved (as shown in Fig 2c,d). The LM was evaluated as stable disease (SD). Subsequently, an overall tumor evaluation was done every eight weeks and the patient was still found with SD. On 3 December 2012, an abdomen CT showed the disease had progressed with multiple metastases to the spleen. The LM focus of the infection was still stable. Erlotinib replaced gefitinib and the patient's PFS was six and a half months, at which time the LM maintained a steady state.
Figure 2.
(a) Brain magnetic resonance imaging (MRI) T1+C before treatment; (b) Brain MRI T2 flair image before treatment; (c) Brain MRI T1+C image after treatment; (d) Brain MRI T2 flair image after 12 weeks of treatment.
Discussion
Patients with advanced NSCLC with LM have a short median survival time. Their conditions worsen quickly as a result of the lack of standard and effective treatment. Radiotherapy of LM has a large radiation field with severe negative reactions and, generally, a negative prognosis. The two cases described in this paper are both female advanced lung adenocarcinoma patients. Neither patient had a smoking history. Their primary lesions had exon-EGFR19 mutations. LM occurred during their gefitinib treatment with stable peripheral conditions. Both patients were sensitive to EGFR-TKI treatment and their extracranial conditions were under control. Performance status showed that chemotherapy was not a suitable treatment. Therefore, erlotinib was used to replace gefitinib in EGFR-TKI treatment and another intrathecal therapy was not utilized. Radiotherapy was postponed in consideration of the extensive LM and long-term low quality of life, which would be caused as a result of radiation damage. The LM was relieved in both cases after using erlotinib for about one week. Without full brain radiotherapy, the PFS rate of both central nervous system tumors and peripheral tumors is over six months.
Small molecular EGFR-TKIs can pass through the blood brain barrier. Particularly when brain metastasis or LM occurs, some factors, such as incomplete new tumor blood vessels and tumor edema, can further destroy the blood brain barrier, which is helpful in improving the transmission rate of EGFR-TKIs. Although it has been proven that EGFR-TKIs treatment on brain metastases is quite effective and can reduce the incidence of NSCLC brain metastasis, 8–11 encephalic metastasis often occurs in clinical practice when TKIs have already effectively controlled advanced NSCLC extracranial lesions. In terms of central nervous system progress during gefitinib treatment, most researchers believe that it is related to a low level of regular dose gefitinib, which passes through the blood brain barrier. Relative domestic and overseas clinical research shows that the passing rate of a regular dose of gefitinib through the blood brain barrier is only 1%.12–14 One Chinese clinical research tested gefitinib concentration in the CSF and blood of 22 advanced NSCLC patients with central nervous system progress under gefitinib treatment. The mean ratio of CSF concentration to serum concentration was found to be 1.30 ± 0.7%. Although erlotinib and gefitinib are both EGFR-TKIs with similar chemical structural formulas, erlotinib performs better in passing through the blood brain barrier and has a higher concentration in blood.15–17 A report by Broniscer et al. in 2007 revealed the highest passing rate of erlotinib through the blood brain barrier was 7%.16 Based on Japanese and American research, the rate is around 4.50% ∼ 6.3%.7,18,19 LM lesions may have different sensitivities to different EGFR-TKIs. Therefore, most researchers suggest that erlotinib can be used to replace gefitinib only in cases where central nervous system progress occurs during gefitinib treatment. A patient's tolerance to erlotinib can be evaluated based on their ECOG score and blood test results. Regular dosing of erlotinib is a choice to be made by doctor and patient. Research shows that larger doses of erlotinib are safe and effective. Previous research has mainly been based on phase I/II clinical experiments and still needs to be proven by clinical research with sufficient samples.
Conclusion
Clinical application of EGFR-TKIs greatly prolongs the life span of advanced lung adenocarcinoma patients, particularly those with EGFR19/21-sensitive mutation. Such patients are often faced with a situation where their extracranial conditions are under control, however, their intracranial tumor continues to progress. In particular, it is more difficult to treat LM, which has a negative prognosis. Although so far there have been many treatments under exploration, as well as relative molecular research, a standard treatment has yet to be found. Sufficient phase III clinical research is needed for reference.
Acknowledgments
This work was supported with grants from Chinese Geriatric Oncology Society (CGOS).
Disclosure
No authors report any conflict of interest.
References
- Grossman SA, Krabak MJ. Leptomeningeal carcinomatosis. Cancer Treat Rev. 1999;25:103–119. doi: 10.1053/ctrv.1999.0119. [DOI] [PubMed] [Google Scholar]
- Ceresoli GL, Reni M, Chiesa G, et al. Brain metastases in locally advanced nonsmall cell lung carcinoma after multimodality treatment: risk factors analysis. Cancer. 2002;95:605–612. doi: 10.1002/cncr.10687. [DOI] [PubMed] [Google Scholar]
- Taillibert S, Laigle-Donadey F, Chodkiewicz C, Sanson M, Hoang-Xuan K, Delattre JY. Leptomeningeal metastases from solid malignancy: a review. J Neurooncol. 2005;75:85–99. doi: 10.1007/s11060-004-8101-x. [DOI] [PubMed] [Google Scholar]
- Villano JL, Ryan CW. Patients presenting with CNS lesions. Case 2. Subdural presentation of recurrent breast cancer. J Clin Oncol. 2003;21:4060–4062. doi: 10.1200/JCO.2003.09.013. [DOI] [PubMed] [Google Scholar]
- Katayama T, Shimizu J, Suda K, et al. Efficacy of erlotinib for brain and leptomeningeal metastases in patients with lung adenocarcinoma who showed initial good response to gefitinib. J Thorac Oncol. 2009;4:1415–1419. doi: 10.1097/JTO.0b013e3181b62572. [DOI] [PubMed] [Google Scholar]
- Yi HG, Kim HJ, Kim YJ, et al. Epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) are effective for leptomeningeal metastasis from non-small cell lung cancer patients with sensitive EGFR mutation or other predictive factors of good response for EGFR TKI. Lung Cancer. 2009;65:80–84. doi: 10.1016/j.lungcan.2008.10.016. [DOI] [PubMed] [Google Scholar]
- Masuda T, Hattori N, Hamada A, et al. Erlotinib efficacy and cerebrospinal fluid concentration in patients with lung adenocarcinoma developing leptomeningeal metastases during gefitinib therapy. Cancer Chemother Pharmacol. 2011;67:1465–1469. doi: 10.1007/s00280-011-1555-6. [DOI] [PubMed] [Google Scholar]
- Kim JE, Lee DH, Choi Y, et al. Epidermal growth factor receptor tyrosine kinase inhibitors as a first-line therapy for never-smokers with adenocarcinoma of the lung having asymptomatic synchronous brain metastasis. Lung Cancer. 2009;65:351–354. doi: 10.1016/j.lungcan.2008.12.011. [DOI] [PubMed] [Google Scholar]
- Wu C, Li YL, Wang ZM, Li Z, Zhang TX, Wei Z. Gefitinib as palliative therapy for lung adenocarcinoma metastatic to the brain. Lung Cancer. 2007;57:359–364. doi: 10.1016/j.lungcan.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Wu YL, Zhou C, Cheng Y, et al. A phase II study (CTONG0803) of erlotinib as second-line treatment in advanced non-small cell lung cancer patients (NSCLC) with asymptomatic brain metastases (BM) after first-line chemotherapy (CT) J Clin Oncol. 2011;29(Suppl):Abstract 7605. . 2011 ASCO Annual Meeting Proceedings. [Google Scholar]
- Heon S, Yeap BY, Britt GJ, et al. Development of central nervous system metastases in patients with advanced non-small cell lung cancer and somatic EGFR mutations treated with gefitinib or erlotinib. Clin Cancer Res. 2010;16:5873–5882. doi: 10.1158/1078-0432.CCR-10-1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackman DM, Holmes AJ, Lindeman N, et al. Response and resistance in a non-small-cell lung cancer patient with an epidermal growth factor receptor mutation and leptomeningeal metastases treated with high-dose gefitinib. J Clin Oncol. 2006;24:4517–4520. doi: 10.1200/JCO.2006.06.6126. [DOI] [PubMed] [Google Scholar]
- Fukuhara T, Saijo Y, Sakakibara T, et al. Successful treatment of carcinomatous meningitis with gefitinib in a patient with lung adenocarcinoma harboring a mutated EGF receptor gene. Tohoku J Exp Med. 2008;214:359–363. doi: 10.1620/tjem.214.359. [DOI] [PubMed] [Google Scholar]
- Wang M, Jing Z, Minjiang C. Cerebral penetration of gefitinib in patients with lung adenocarcinoma. J Clin Oncol. 2011;29(Suppl):Abstract 7608. . 2011 ASCO Annual Meeting Proceedings. [Google Scholar]
- Baselqa J, Rischin D, Ranson M, et al. Phase I safety, pharmacokinetic, and pharmacodynamic trial of ZD1839, a selective oral epidermal growth factor receptor tyrosine kinase inhibitor, in patients with five selected solid tumor types. J Clin Oncol. 2002;20:4292–4302. doi: 10.1200/JCO.2002.03.100. [DOI] [PubMed] [Google Scholar]
- Broniscer A, Panetta JC, O'Shaughnessy M, et al. Plasma and cerebrospinal fluid pharmacokinetics of erlotinib and its active metabolite 0SI-420. Clin Cancer Res. 2007;13:1511–1515. doi: 10.1158/1078-0432.CCR-06-2372. [DOI] [PubMed] [Google Scholar]
- Hidalgo M, Siu LL, Nemunaitis J, et al. Phase I and pharmacologic study of OSI-774, an epidermal growth factor receptor tyrosine kinase inhibitor, in patients with advanced solid malignancies. J Clin Oncol. 2001;19:3267–3279. doi: 10.1200/JCO.2001.19.13.3267. [DOI] [PubMed] [Google Scholar]
- Togashi Y, Masago K, Fukudo M, et al. Efficacy of increased-dose erlotinib for central nervous system metastases in non-small cell lung cancer patients with epidermal growth factor receptor mutation. Cancer Chemother Pharmacol. 2011;68:1089–1092. doi: 10.1007/s00280-011-1691-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Togashi Y, Masago K, Fukudo M, et al. Cerebrospinal fluid concentration of erlotinib and its active metabolite OSI-420 in patients with central nervous system metastases of non-small cell lung cancer. J Thorac Oncol. 2010;5:950–955. doi: 10.1097/JTO.0b013e3181e2138b. [DOI] [PubMed] [Google Scholar]