Abstract
Background
Cisplatin-based adjuvant chemotherapy provided a significant advantage in the overall survival (OS) of patients with stage II and III non-small cell lung cancer (NSCLC). However, the compliance and toxicity in cisplatin-based treatment were not always satisfactory. Pemetrexed plus carboplatin (PC) had better chemotherapy compliance and efficiency in advanced non-squamous NSCLC patients. The aim of our study was to investigate the feasibility and efficacy of PC as adjuvant chemotherapy in patients with completely resected non-squamous NSCLC.
Methods
Eighty-two eligible non-squamous NSCLC patients operated on with pathological stage II or IIIA were enrolled in this trial. Adjuvant chemotherapy was initiated between one and four weeks after surgery, and consisted of four cycles of pemetrexed (500 mg/m2) plus carboplatin (AUC = 5) every three weeks. The primary endpoint was the compliance of the regimen and the second endpoint was disease-free survival (DFS).
Results
Patient demographics were median age 58 years (range 32 to 78) and gender ratio 68.3% male/31.7% female. Forty-eight (58.5%) of the patients were at stage II, and the other thirty-four (41.5%) patients were at stage IIIA. Seventy patients (85.4%) received four cycles of therapy over a 12-week period. Reasons for discontinuing therapy included: patient's refusal (n = 10); severe adverse events (n = 1); and surgical complications (n = 1). The primary grade 3 to 4 adverse reaction was hematologic toxicity: neutropenia (13.4%); leucopenia (7.3%); anemia (3.7%); and thrombocytopenia (2.4%). Non-hematological adverse events were mild. No treatment related deaths were observed. Median DFS for stage II and IIIA patients were 38.0 months (95% confidence interval (CI): 28.1 to 47.9 months) and 21.0 months (95%CI: 13.7 to 28.3 months), respectively.
Conclusion
Adjuvant PC chemotherapy was an acceptable regimen in resected non-squamous NSCLC patients.
Keywords: Adjuvant chemotherapy, carboplatin, non-small cell lung cancer, pemetrexed
Introduction
Lung cancer is the leading cause of cancer-related mortality worldwide. Globally, there were 1.35 million new cases and 1.18 million deaths in 2002.1 Non-small cell lung cancer (NSCLC) comprises about 85% of cases and is mainly smoking related. The majority of NSCLC patients present with advanced disease, for which surgery, the most potentially curative treatment, is not feasible. Even for early stage NSCLC resected with curative purpose, 30–60% of patients underwent relapse within five years of surgery.2 Five-year lung cancer-specific survival rates vary from 76.6% in stage IA disease to a disappointing 22.9% in stage IIIA disease.3 Therefore, adjuvant chemotherapy seems especially necessary in order to obtain a better rate of survival.
The efficacy of cisplatin-based adjuvant chemotherapy in NSCLC was not clear until recent large randomized trials (Adjuvant Lung Project Italy [ALPI]; International Adjuvant Lung Cancer Trial [IALT]; National Cancer Institute of Canada JBR.10 trial, JBR.10; Adjuvant Navelbine International Trialist Association [ANITA]; Big Lung Trial [BLT]) and the following meta-analysis. Based on the data of the above five large randomized trials, the Lung Adjuvant Cisplatin Evaluation (LACE) meta-analysis revealed a significant advantage in overall survival (OS) (hazard ratio [HR] = 0.89, 95% confidence interval [CI] 0.82 to 0.96; P = 0.005), corresponding to a five-year absolute benefit of 5.4% from chemotherapy.4 In view of those benefits, cisplatin-based chemotherapy was established in the role of adjuvant chemotherapy in NSCLC.
In spite of the survival benefit, there are factors that cannot be ignored, such as compliance and toxicity. According to the LACE meta-analysis, at least 33% of the patients in the chemotherapy arm did not complete the planned three or four cycles of treatment.4 Basic information about the recent large trials is displayed in Table 1.2,5–9 Besides discouraging compliance, the toxicity of cisplatin-based chemotherapy is unsatisfactory, with the rate of overall grade 3 or 4 toxicity reaching up to 66% in four of the five trials registered in the LACE study.4 In advanced NSCLC, two recent meta-analyses10,11comparing cisplatin to carboplatin reported that cisplatin-based chemotherapy frequently led to grade 3 or higher nausea and vomiting, while grade 3 or greater thrombocytopenia was significantly more frequent with carboplatin-based chemotherapy. No significant difference in treatment-related mortality or survival advantage was observed, although cisplatin treatment was associated with a higher objective response rate.10,11 Similarly, because of the favorable therapeutic effect and lower toxicity,12 pemetrexed was approved for second-line treatment of NSCLC. Further research has demonstrated that pemetrexed plus carboplatin (PC) provides better efficacy and tolerability than gemcitabine and carboplatin (GC) in the first-line treatment of advanced non-squamous NSCLC.13 We hypothesized that the decreased toxicity of adjuvant PC could result in increased chemotherapy compliance and, consequently, an improvement in survival. Nevertheless, until now, data reported in detail about the feasibility of PC as adjuvant chemotherapy in patients with curative resected non-squamous NSCLC was rare. We, therefore, designed this phase II study to investigate the compliance and efficacy of this regimen in adjuvant treatment.
Table 1.
Recent large randomize adjuvant chemotherapy trials
| Trial | ALPI | IALT | BLT | JBR.10 | ANITA | CALGB 9633 |
|---|---|---|---|---|---|---|
| Year of publication | 2003 | 2004 | 2004 | 2005 | 2006 | 2008 |
| Total number of patients | 1209 | 1867 | 381 | 482 | 840 | 344 |
| Cancer stages | I, II, IIIA | I, II, III | I, II, III | IB, II | IB, II, IIIA | IB |
| Chemotherapy regimen | Cisplatin, vindesine and mitomycin | Cisplatin-based | Cisplatin-based | Cisplatin and vinorelbine | Cisplatin and vinorelbine | Carboplatin and paclitaxel |
| Designed cycles | 3 | 3 or 4 | 3 | 4 | 4 | 4 |
| Compliance | 69% | 73.8% | 64% | 45% | About 50% | 57% |
| Grade 3 or 4 neutropenia | 28% | 17.5%(grade 4 only) | 40% | 73% | 85% | 35% |
| Overall survival | HR 0.96, P = 0.589 | HR 0.86, P < 0.03 | HR 1.02, P = 0.90 | HR 0.69, P = 0.04 | HR 080, P = 0.017 | HR 0.83, P = 0.12 |
ALPI, Adjuvant Lung Project Italy; ANITA, Adjuvant Navelbine International Trialist Association; BLT, Big Lung Trial; CALGB 9633, Cancer and Leukemia Group B 9633; HR, hazard ratio; IALT, International Adjuvant Lung Cancer Trial; JBR.10, National Cancer Institute of Canada JBR.10 Trial.
Materials and methods
Eligibility criteria
Eighty-two patients with completely resected and pathologically documented stage II or IIIA NSCLC were included in the study. All patients had radiography, computed tomography (CT) scans of chest and upper abdomen, bone scintigraphy, and brain magnetic resonance imaging (MRI) to confirm that there was no distant metastasis before surgery was performed. We did not perform mediastinoscopy for patients prior to surgery and patients classified as N2 by CT scans in pre-operative imaging assessments were not eligible for this trial. All patients underwent lobectomy or pneumonectomy with complete dissection of mediastinal lymph nodes. The revised seventh edition guideline of lung cancer staging criteria was used to stage patients.14 The postsurgical status of all of the patients (age ≥18 years) reflected Eastern Cooperative Oncology Group (ECOG) 1 or 0, no significant weight loss (<5%), adequate renal function (a calculated creatinine clearance of ≥45 mL/minute), adequate hepatic function (aspartate aminotransferase [AST] and alanine aminotransferase [ALT] were within the upper limit of normal), and adequate bone marrow function (absolute neutrophil count of 1500/mm3, hemoglobin ≥8 g/dL, and platelets ≥100 000/mm3). Furthermore, surgeries were completed within one to four weeks prior to enrollment. Patients who received neoadjuvant chemotherapy or radiotherapy were not eligible for this trial. Patients were excluded from the study if they had a history of other neoplasms (except in-situ carcinoma of the cervix or basal cell carcinoma of the skin), cardiac disease, peripheral neuropathy grade 3 or 4, psychiatric disorders, or serious active infection. Potentially pregnant or lactating women were excluded. Patients signed informed consents before any study related procedures were performed. The protocol review committee of the Cancer Center of Sun Yat-sen University and the Investigational Review Board of the institution approved all patients who met the eligibility criteria. The study was conducted in accordance with good clinical practice (GCP) guidelines and the principles that have their origin in the Declaration of Helsinki.
Treatment
Chemotherapy consisted of pemetrexed (500 mg/m2) and carboplatin (AUC = 5) every 21 days for four cycles. Both chemotherapy drugs were administered on day one. All patients were supplemented with 350–1000 μg of oral folic acid daily at least one to two weeks before cycle 1 until three weeks after the last administration of pemetrexed, and 1000 μg of vitamin B12 intramuscularly every 3 cycles starting within one week before the first cycle and then at the same day prior to pemetrexed. Patients received 10 mg of dexamethasone and 0.25 mg of palonosetron intravenously before the start of therapy. All patients were required to have an absolute neutrophil count ≥1500/mm3, platelet count of ≥100 000/mm3, and creatinine clearance ≥45 mL/minute, before initiating chemotherapy. Chemotherapy doses were either reduced or withheld following adverse events, such as severe leukopenia or thrombocytopenia. Dose reductions were maintained for subsequent cycles.
Evaluation of toxicities and statistical analysis
The primary endpoint of this single-arm phase II trial was to evaluate the compliance of PC as adjuvant treatment, and the second endpoint was disease-free survival (DFS). Toxicities were graded according to the National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events (CTCAE) version 3.0.15 DFS was measured from the date of enrolment until the first evidence of disease recurrence or death; OS was calculated from the date of enrolment until death. Both DFS and OS were plotted by the standard Kaplan method. All statistical analyses were generated using SPSS for Windows Version 19.0.
Results
Patient characteristics
Eight-two patients were enrolled on the trial between May 2008 and July 2011, comprising 56 (68.3%) males and 26 (31.7%) females with a median age of 58 years, ranging from 32 to 78. Forty-eight (58.5%) of the patients were at stage II, and the other thirty-four (41.5%) of the patients were at stage IIIA. Seventy-seven patients (93.9%) underwent lobectomy, and the remaining five patients (6.1%) underwent pneumonectomy. The predominant histological type was adenocarcinoma (93.9%), with a small representation of large cell carcinoma (6.1%) (Table2).
Table 2.
Clinical characteristics of the 82 patients
| Characteristic | Number of patients (%) |
|---|---|
| Age (year) range | 32–78 |
| Median | 58 |
| Gender | |
| Male | 56 (68.3) |
| Female | 26 (31.7) |
| Smoking status | |
| Yes | 40 (48.8) |
| No | 42 (51.2) |
| Performance status | |
| 0 | 49 (60.0) |
| 1 | 33 (40.0) |
| Histology | |
| Adenocarcinoma | 77 (93.9) |
| Large cell carcinoma | 5 (6.1) |
| Pathological stage | |
| IIA | 12 (14.6) |
| IIB | 36 (43.9) |
| IIIA | 34 (41.5) |
| Type of surgery | |
| Lobectomy | 77 (93.9) |
| Pneumonectomy | 5 (6.1) |
Treatment administration
Seventy (85.4%) of the 82 patients enrolled in the trial received the full-dose of four cycles of therapy over a 12-week timeframe. Twelve patients (14.6%) failed to accomplish the whole treatment: three patients completed three cycles, five patients completed two cycles, and four patients completed one cycle. Reasons for discontinuing therapy included: patient's refusal (n = 10), severe adverse events (n = 1), and surgical complication (n = 1). Seventy-one patients (86.6%) began to receive adjuvant chemotherapy within 14 days of surgery. Therapy was discontinued in one case after two cycles of treatment as a result of an elevated AST, which, even after almost three months of treatment, still exceeded 2.5 times the normal upper limit. Another patient was excluded because of severe surgical complications. He was diagnosed with bronchopleural fistula after one cycle of chemotherapy and had to be treated surgically. No treatment delay or dose reduction occurred on this trial (Table3).
Table 3.
Chemotherapy compliance
| Parameter | Number of patients (%) |
|---|---|
| Received 4 cycles of chemotherapy within 12 weeks | 70 (85.4) |
| Did not complete chemotherapy | 12 (14.6) |
| Completed 1 cycle | 4 (4.9) |
| Completed 2 cycles | 5 (6.1) |
| Completed 3 cycles | 3 (3.7) |
| Reasons for treatment discontinuation | |
| Patient's refusal | 10 (12.2) |
| Severe adverse events | 1 (1.2) |
| Surgical complication | 1 (1.2) |
Toxicity
The most common hematologic toxicity was neutropenia (63.4%; n = 52), with 11 patients (13.4%) developing grade 3 or 4 neutropenia. Severe anemia or thrombocytopenia was comparatively infrequent. The rate of grade 3 to 4 anemia or thrombocytopenia was 3.7% and 2.4%, respectively. Frequent non-hematologic adverse events included fatigue (43.9%; n = 36), nausea/vomiting (57.3%; n = 47), and elevated AST/ALT (22.0%; n = 18). However, most of the side effects were transient, mild, and manageable. No treatment related deaths occurred in this trial (Table4).
Table 4.
The main toxicities of chemotherapy
| Toxicity | Grade of toxicity | |||||
|---|---|---|---|---|---|---|
| 0 (%) | 1 (%) | 2 (%) | 3 (%) | 4 (%) | 3–4 (%) | |
| Hematologic toxicity | ||||||
| Leukopenia | 34 (41.5) | 29 (35.4) | 13 (15.9) | 5 (6.1) | 1 (1.2) | 6 (7.3) |
| Neutropenia | 30 (36.6) | 23 (28.0) | 18 (22.0) | 9 (11.0) | 2 (2.4) | 11 (13.4) |
| Anemia | 65 (79.3) | 11 (13.4) | 3 (3.7) | 3 (3.7) | 0 (0.0) | 3 (3.7) |
| Thrombocytopenia | 40 (48.8) | 24 (29.3) | 16 (19.5) | 2 (2.4) | 0 (0.0) | 2 (2.4) |
| Non-hematologic adverse events | ||||||
| Constitutional symptoms | ||||||
| Fatigue | 46 (56.1) | 25 (30.5) | 9 (11.0) | 2 (2.4) | 0 (0.0) | 2 (2.4) |
| Fever | 73 (89.0) | 6 (7.3) | 3 (3.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Gastrointestinal | ||||||
| Nausea/Vomiting | 35 (42.7) | 32 (39.0) | 13 (15.9) | 2 (2.4) | 0 (0.0) | 2 (2.4) |
| Mucositis | 72 (87.8) | 7 (8.5) | 3 (3.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Diarrhea | 72 (87.8) | 6 (7.3) | 2 (2.4) | 2 (2.4) | 0 (0.0) | 2 (2.4) |
| Constipation | 76 (92.7) | 6 (7.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Hepatorenal function | ||||||
| AST/ALT | 64 (78.0) | 13 (15.9) | 5 (6.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Creatinine/GFR | 74 (90.2) | 5 (6.1) | 3 (3.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Dermatology | ||||||
| Rash | 76 (92.7) | 5 (6.1) | 1 (1.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Alopecia | 69 (84.1) | 10 (12.2) | 3 (3.7) | — | — | — |
| Petechiae/purpura | 71 (86.6) | 6 (7.3) | 5 (6.1) | 0 (0.0) | — | 0 (0.0) |
| Dyspnea | 78 (95.1) | 4 (4.9) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
Outcomes
The median follow-up was 33 months in this trial, ranging from 9 to 53 months. Median DFS for patients with stage II and IIIA were 38.0 months (95% CI: 28.1 to 47.9 months) and 21.0 months (95% CI: 13.7 to 28.3 months), respectively. The two-year DFS rates were 70.5% and 45.9%, respectively, for patients with stage II and stage IIIA (Fig 1). Median OS was 36 months (95% CI: 25.9 to 46.1 months) in patients with stage IIIA, and not reached in patients with stage II (Fig 2).
Figure 1.
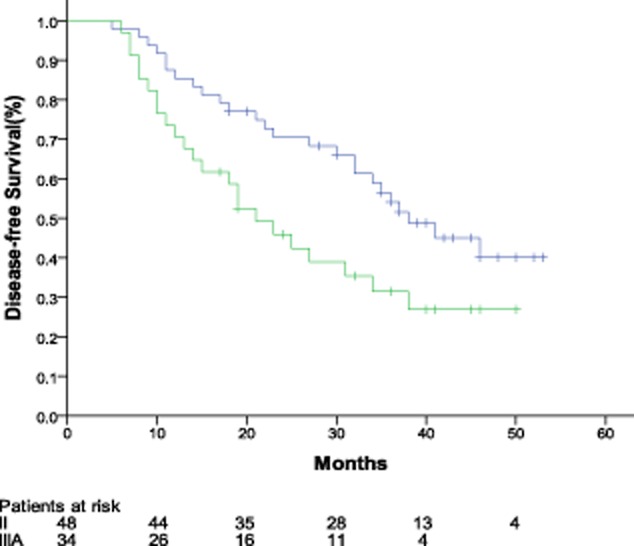
Kaplan-Meier disease-free survival curve.  , stage II;
, stage II;  , stage IIIA;
, stage IIIA;  , stage II-censored;
, stage II-censored;  , stage IIIA-censored.
, stage IIIA-censored.
Figure 2.
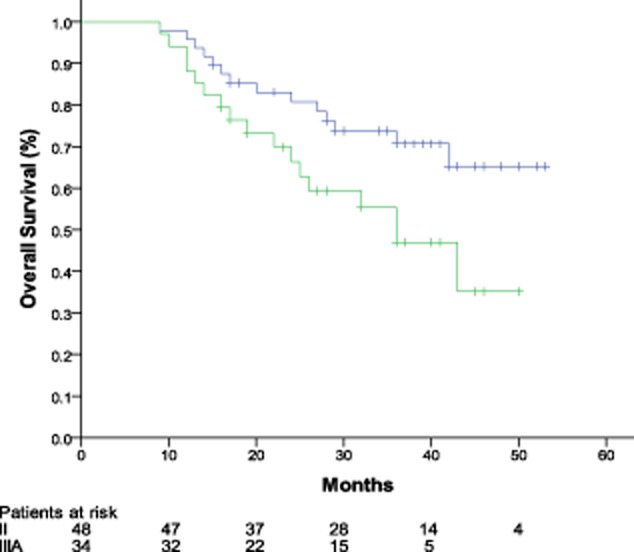
Kaplan-Meier overall survival curve.  , stage II;
, stage II;  , stage IIIA;
, stage IIIA;  , stage II-censored;
, stage II-censored;  , stage IIIA-censored.
, stage IIIA-censored.
Of the 82 patients who had been followed for survival information, 48 (58.5%) experienced relapse and 31 (37.8%) died. Four people died without documented relapse. Of the patients who experienced a relapse, 23 patients (47.9%) were confined to intrathoracic relapse, and the other 25 patients (52.1%) appeared to have a distant relapse. Brain metastases may play an important role in the cause of the patients' death. Of the 31 patients who died, 13 had a documented brain relapse before death.
Discussion
According to the LACE meta-analysis,4 the pooled analysis of the recent large trials of adjuvant cisplatin-based chemotherapy in NSCLC confirmed the effect of chemotherapy on both OS (5.4% absolute benefit at five years) and DFS (5.8% benefit), with unsatisfying compliance and toxicity. The observed compliance among these trials ranged from 45% to 74%, and grade 3 or 4 neutropenia appeared in 18% to 85% patients (Table 1). However, the chemotherapy compliance seen on our trial was more favorable than that of cisplain-based adjuvant chemotherapy. About 85% of patients completed four cycles of PC chemotherapy within 12 weeks. Neutropenia was the most frequent hematological toxicity, occurring in 63.4% of the patients, and grade 3 or 4 neutropenia was experienced by 13.4% of the patients.
The JBR.10 trial carried out a multivariate analysis of the chemotherapy compliance of patients, which showed that there were many factors associated with chemotherapy compliance, such as extent of surgery, gender, age, and race.16 In their report, 19 of the 54 patients who underwent pneumonectomies completed four cycles of therapy. Patients who underwent pneumonectomies were more likely to fail to complete chemotherapy than patients with lesser resections (P < 0.05). Data in our trial showed that four out of five patients who experienced pneumonectomies completed four cycles of chemotherapy. The JBR.10 trial also reported that female patients were less likely than males (P = 0.02) to complete chemotherapy. However, results from Stinchcombe et al.17 revealed that 79.2% (19 of 24) of female patients completed four cycles of therapy, and, thus, no different to male patients (79.2%, 38 of 48). Similarly, there were 85.7% female patients and 84.6% male patients (P = 1) who finished four cycles of therapy in our trial.
Chemotherapy treatment in the elderly is complicated by a number of age-related issues, such as decreased hepatic, renal, and bone marrow functions that increase toxicity, particularly cisplatin-related toxicity.18,19 The issue of cisplatin-based or carboplatin-based therapy for elderly patients with advanced NSCLC has recently been addressed in some retrospective analyses of large randomized trials.20–23 Treatment outcomes of patients younger and older than 70 years of age enrolled on these trials were compared. Globally, these analyses found no differences in survival between elderly and younger patients, with a small increase in toxicity in the elderly, and suggest that advanced age alone should not preclude platinum-based chemotherapy for NSCLC. In fact, those aforementioned analyses could suffer from selection bias. Elderly patients enrolled in this sort of trial do not represent the whole elderly population, but, instead, a small subgroup believed by investigators to be eligible for aggressive treatments.24 A retrospective analysis25 of elderly patients (age >65 years) on the JBR.10 trial revealed that adjuvant therapy provided a survival benefit for elderly patients (HR = 0.61, 95% CI 0.38 to 0.98; P = 0.04). None of the elderly patients received the full 16 doses of vinorelbine and only 32% received the intended eight doses of cisplatin. Besides, fewer elderly patients completed treatment and more refused treatment (P = 0.03). This data suggested that there was a survival benefit to treatment, even with a reduced dose delivery of adjuvant chemotherapy, and that chemotherapy compliance in the elderly may be a significant challenge. A similar outcome was observed in one adjuvant chemotherapy (vinorelbine-cisplatin or paclitaxel-carboplatin) trial of early stage NSCLC in Canada.26 Patients aged 70 years and older were almost twice as likely not to receive adjuvant chemotherapy as younger patients, even after adjusting for co-morbidities. The median age of patients on our trial was 58 years, 19 patients (23.2%) exceeded 65 years, and six patients (7.2%) were aged >70 years. Sixteen (84.2%) of the 19 patients who were aged >65 years completed four cycles of therapy, while fifty-three (85.7%) of 63 patients who were aged ≤65 years completed four cycles of therapy. According to the outcomes, we may predict that, with the application of lower toxic chemotherapy, age alone should not be a factor to affect acceptance of chemotherapy.
Recently, Karapanagiotou et al.27 reported a phase II study on PC as adjuvant chemotherapy in resected NSCLC. In the trial, 45 eligible NSCLC patients with a pathological diagnosis of stage IB, II, or IIIA after surgery received postoperative adjuvant chemotherapy with carboplatin (AUC = 5) and pemetrexed (500 mg/m2) administered on days 1 and 14 on a 28-day cycle, for a total of three cycles. Notably, 17 patients (37.8%) with squamous cell lung cancer were included in that trial. The observed side effects were only grade I and II, and grade II toxicities included neutropenia (2.2%), anemia (6.7%), and elevated ALT (13.3%). It is noteworthy that during this study recombinant human granulocyte colony-stimulating factors (rhG-CSF) were used prophylactically (150 mg/m2/d subcutaneously) on days 8 to 11, but in our trial rhG-CSF was used only at the appearance of severe neutropenia or leukopenia. The rates of grade 3 to 4 neutropenia or leukopenia occurring in our trial were only 13.4% and 7.3%, respectively. Additionally, the overall median time to disease recurrence of patients in that research was 26 months. In our study, median DFS was 38 months (95% CI: 28.1 to 47.9 months) for patients with stage II and 21.0 months (95% CI: 13.7 to 28.3 months) for patients with stage IIIA. PC adjuvant chemotherapy revealed a trend to improve DFS compared to cisplatin-based chemotherapy regimens.7 The median OS in our trial reached 36 months (95% CI: 25.9 to 46.1 months) for patients with stage IIIA.
Conclusion
In conclusion, this study addressed whether the administration of PC is feasible in the adjuvant setting of completely resected non-squamous NSCLC. Our findings confirm that this regimen is safe and well-tolerated. Most patients completed the four cycles of therapy within 12 weeks. We demonstrate here that adjuvant therapy with PC is an attractive alternative for those patients with non-squamous NSCLC who cannot tolerate the toxicity of cisplatin-based therapy.
Disclosure
No authors report any conflict of interest.
References
- Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- Winton T, Livingston R, Johnson D, et al. Vinorelbine plus cisplatin vs. observation in resected non-small-cell lung cancer. N Engl J Med. 2005;352:2589–2597. doi: 10.1056/NEJMoa043623. [DOI] [PubMed] [Google Scholar]
- Ravdin PM, Davis G. Prognosis of patients with resected non-small cell lung cancer: impact of clinical and pathologic variables. Lung Cancer. 2006;52:207–212. doi: 10.1016/j.lungcan.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Pignon JP, Tribodet H, Scagliotti GV, et al. Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE Collaborative Group. J Clin Oncol. 2008;26:3552–3559. doi: 10.1200/JCO.2007.13.9030. [DOI] [PubMed] [Google Scholar]
- Waller D, Peake MD, Stephens RJ, et al. Chemotherapy for patients with non-small cell lung cancer: the surgical setting of the Big Lung Trial. Eur J Cardiothorac Surg. 2004;26:173–182. doi: 10.1016/j.ejcts.2004.03.041. [DOI] [PubMed] [Google Scholar]
- Strauss GM, Herndon JE, II, Maddaus MA, et al. Adjuvant paclitaxel plus carboplatin compared with observation in stage IB non-small-cell lung cancer: CALGB 9633 with the Cancer and Leukemia Group B, Radiation Therapy Oncology Group, and North Central Cancer Treatment Group Study Groups. J Clin Oncol. 2008;26:5043–5051. doi: 10.1200/JCO.2008.16.4855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scagliotti GV, Fossati R, Torri V, et al. Randomized study of adjuvant chemotherapy for completely resected stage I, II, or IIIA non-small-cell Lung cancer. J Natl Cancer Inst. 2003;95:1453–1461. doi: 10.1093/jnci/djg059. [DOI] [PubMed] [Google Scholar]
- Douillard JY, Rosell R, De Lena M, et al. Adjuvant vinorelbine plus cisplatin versus observation in patients with completely resected stage IB-IIIA non-small-cell lung cancer (Adjuvant Navelbine International Trialist Association [ANITA]): a randomised controlled trial. Lancet Oncol. 2006;7:719–727. doi: 10.1016/S1470-2045(06)70804-X. [DOI] [PubMed] [Google Scholar]
- Arriagada R, Bergman B, Dunant A, Le Chevalier T, Pignon JP, Vansteenkiste J. Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer. N Engl J Med. 2004;350:351–360. doi: 10.1056/NEJMoa031644. [DOI] [PubMed] [Google Scholar]
- Hotta K, Matsuo K, Ueoka H, Kiura K, Tabata M, Tanimoto M. Meta-analysis of randomized clinical trials comparing cisplatin to carboplatin in patients with advanced non-small-cell lung cancer. J Clin Oncol. 2004;22:3852–3859. doi: 10.1200/JCO.2004.02.109. [DOI] [PubMed] [Google Scholar]
- Ardizzoni A, Boni L, Tiseo M, et al. Cisplatin- versus carboplatin-based chemotherapy in first-line treatment of advanced non-small-cell lung cancer: an individual patient data meta-analysis. J Natl Cancer Inst. 2007;99:847–857. doi: 10.1093/jnci/djk196. [DOI] [PubMed] [Google Scholar]
- Hanna N, Shepherd FA, Fossella FV, et al. Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol. 2004;22:1589–1597. doi: 10.1200/JCO.2004.08.163. [DOI] [PubMed] [Google Scholar]
- Gronberg BH, Bremnes RM, Flotten O, et al. Phase III study by the Norwegian lung cancer study group: pemetrexed plus carboplatin compared with gemcitabine plus carboplatin as first-line chemotherapy in advanced non-small-cell lung cancer. J Clin Oncol. 2009;27:3217–3224. doi: 10.1200/JCO.2008.20.9114. [DOI] [PubMed] [Google Scholar]
- Goldstraw P, Crowley J, Chansky K, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol. 2007;2:706–714. doi: 10.1097/JTO.0b013e31812f3c1a. [DOI] [PubMed] [Google Scholar]
- Trotti A, Colevas AD, Setser A, et al. CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol. 2003;13:176–181. doi: 10.1016/S1053-4296(03)00031-6. [DOI] [PubMed] [Google Scholar]
- Alam N, Shepherd FA, Winton T, et al. Compliance with post-operative adjuvant chemotherapy in non-small cell lung cancer. An analysis of National Cancer Institute of Canada and intergroup trial JBR.10 and a review of the literature. Lung Cancer. 2005;47:385–394. doi: 10.1016/j.lungcan.2004.08.016. [DOI] [PubMed] [Google Scholar]
- Stinchcombe TE, Harper HD, Hensing TA, et al. The feasibility of adjuvant carboplatin and docetaxel in patients with curatively resected non-small cell lung cancer. J Thorac Oncol. 2008;3:145–151. doi: 10.1097/JTO.0b013e318160c5f1. [DOI] [PubMed] [Google Scholar]
- Gridelli C, Perrone F, Gallo C, et al. Chemotherapy for elderly patients with advanced non-small-cell lung cancer: the Multicenter Italian Lung Cancer in the Elderly Study (MILES) phase III randomized trial. J Natl Cancer Inst. 2003;95:362–372. doi: 10.1093/jnci/95.5.362. [DOI] [PubMed] [Google Scholar]
- Cheong KA, Chrystal K, Harper PG. Management of the elderly patient with advanced non-small cell lung cancer. Int J Clin Pract. 2006;60:340–343. doi: 10.1111/j.1368-5031.2005.00795.x. [DOI] [PubMed] [Google Scholar]
- Lilenbaum RC, Herndon JE, II, List MA, et al. Single-agent versus combination chemotherapy in advanced non-small-cell lung cancer: the cancer and leukemia group B (study 9730) J Clin Oncol. 2005;23:190–196. doi: 10.1200/JCO.2005.07.172. [DOI] [PubMed] [Google Scholar]
- Langer CJ, Manola J, Bernardo P, et al. Cisplatin-based therapy for elderly patients with advanced non-small-cell lung cancer: implications of Eastern Cooperative Oncology Group 5592, a randomized trial. J Natl Cancer Inst. 2002;94:173–181. doi: 10.1093/jnci/94.3.173. [DOI] [PubMed] [Google Scholar]
- Hensing TA, Peterman AH, Schell MJ, Lee JH, Socinski MA. The impact of age on toxicity, response rate, quality of life, and survival in patients with advanced, Stage IIIB or IV nonsmall cell lung carcinoma treated with carboplatin and paclitaxel. Cancer. 2003;98:779–788. doi: 10.1002/cncr.11548. [DOI] [PubMed] [Google Scholar]
- Blanchard EM, Moon J, Hesketh PJ, et al. Comparison of platinum-based chemotherapy in patients older and younger than 70 years: an analysis of Southwest Oncology Group Trials 9308 and 9509. J Thorac Oncol. 2011;6:115–120. doi: 10.1097/JTO.0b013e3181fbebfd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrone F, Gallo C, Gridelli C. Re: Cisplatin-based therapy for elderly patients with advanced non-small-cell lung cancer: implications of Eastern Cooperative Oncology Group 5592, a randomized trial. J Natl Cancer Inst. 2002;94:1029–1030. doi: 10.1093/jnci/94.13.1029. ; author reply 1030–1021. [DOI] [PubMed] [Google Scholar]
- Pepe C, Hasan B, Winton TL, et al. Adjuvant vinorelbine and cisplatin in elderly patients: National Cancer Institute of Canada and Intergroup Study JBR.10. J Clin Oncol. 2007;25:1553–1561. doi: 10.1200/JCO.2006.09.5570. [DOI] [PubMed] [Google Scholar]
- Winget M, Fleming J, Li X, Gao Z, Butts C. Uptake and tolerance of adjuvant chemotherapy in early stage NSCLC patients in Alberta, Canada. Lung Cancer. 2011;72:52–58. doi: 10.1016/j.lungcan.2010.07.005. [DOI] [PubMed] [Google Scholar]
- Karapanagiotou EM, Boura PG, Papamichalis G, et al. Carboplatin-pemetrexed adjuvant chemotherapy in resected non-small cell lung cancer (NSCLC): a phase II study. Anticancer Res. 2009;29:4297–4301. [PubMed] [Google Scholar]


