Abstract
Background
Hepcidin is a small secreted peptide that plays a key role in iron metabolism. A high level of hepcidin expression may be implicated in colorectal cancer; however, the relationship between hepcidin and lung cancer has not yet been studied.
Methods
Serum hepcidin-25, bone morphogenetic protein (BMP)-2, and interleukin (IL)-6 concentration in 53 patients and 16 non-cancerous individuals was measured by enzyme-linked immune sorbent assay. Reverse transcriptase quantitative polymerase chain reaction (RT-qPCR) was utilized to study the expression of hepcidin mRNA in paired tumor and non-tumor lung tissues in surgical specimens from 65 patients with non small cell lung cancer (NSCLC), as well as in six types of lung cancer cell lines and human bronchial epithelial (HBE) cells. Hepcidin protein expression and cellular localization in NSCLC was determined by immunohistochemistry.
Results
The serum hepcidin-25 concentration was higher in patients with NSCLC than in non-cancerous individuals, and was positively correlated with serum BMP2 concentration, but negatively with serum IL-6 levels. Serum hepcidin was also correlated with lymph node metastasis and clinical stage. Hepcidin mRNA expression was higher in cancerous tissues of NSCLC than in normal pulmonary tissues (P = 0.001). Hepcidin mRNA levels in four lung carcinoma cell lines were higher than in HBE cells. Immunohistochemistry showed that hepcidin protein was increased in cancerous tissues of NSCLC.
Conclusions
The level of hepcidin expression increased in NSCLC tissue and serum. Serum hepcidin-25 level was associated with lymph node metastasis and tumor clinical stage in patients with NSCLC.
Keywords: Clinical stage, hepcidin, non-small cell lung cancer
Introduction
Hepcidin is a small peptide that was first found in the ultrafiltration of human blood by Krause et al.1 Hepcidin is synthesized and secreted by the liver, and is expressed in the heart, brain, lung, and pancreas.1,2 Hepcidin was originally considered to be an antibacterial peptide,3 but was then confirmed to participate in the regulation of iron metabolism.4 Hepcidin-25 can combine with ferroportin on the cell membrane, which leads to its phosphorylation, internalization and degradation.5,6 Ferroportin is primarily responsible for transporting intracellular iron into extracellular fluid. Therefore, because ferroportin degradation is increased after combining with hepcidin-25, the intracellular iron cannot be transported out of the cells, which results in an intracellular accumulation of iron.
Iron is essential for fundamental cellular processes in all living organisms. Iron homeostasis in normal cells is balanced and regulated by the co-ordinated functioning of several systems that are responsible for the uptake, intracellular storage, and removal of iron from cells.7 SLC40A1 (solute carrier family 40, member 1) protein, also named ferroportin, is mainly responsible for transporting iron within the cell to the extracellular fluid;8,9 transferrin protein can bind with serum iron and transport iron throughout the body.10,11 However, this balance is frequently and consistently compromised in cancer cells.12,13 Many animal models and epidemiological investigation have shown that excess iron is one of the important reasons for the promotion of malignant tumors.14–17 Recent studies have shown that the expression of ferroportin in breast cancer is downregulated and related to prognosis. Restoring ferroportin expression in breast cancer could inhibit the growth of tumor cells.18
Much of the current hepcidin research has focused on iron-metabolism-related diseases (such as anemia and hemochromatosis). Hepcidin can regulate iron metabolism, and excess iron can induce malignancies, therefore, the relationship between hepcidin and tumors has attracted more attention recently. It has been reported that hepcidin mRNA expression was suppressed in hepatocellular and renal cell carcinoma (RCC),19,20 but it was increased in 34% of colorectal cancer tissue samples, with low or no expression in the corresponding adjacent non-cancerous tissue.21 However, little is known about its role in lung cancer.
Here, we examined serum hepcidin-25 levels by enzyme-linked immune sorbent assay (ELISA) and compared hepcidin mRNA expression between non small cell lung cancer (NSCLC) and non-neoplastic tissues from the same resected specimens by reverse transcription quantitative polymerase chain reaction (RT-qPCR). We also examined the relationship between the serum level of hepcidin-25 and hepcidin mRNA expression and the clinicopathological features of NSCLC patients. Furthermore, we determined the expression and cellular localization of hepcidin protein by immunohistochemistry in NSCLC and normal pulmonary tissues.
Methods
Patients and tissue specimens
We studied 65 consecutive Chinese patients (48 male and 17 female) aged 36–78 years (mean age 58.9 years), who were diagnosed with NSCLC from 2010 to 2011. All patients routinely underwent imaging studies (computed tomography [CT] and/or positron emission tomography [PET]–computed tomography) for preoperative staging before pulmonary lobectomy. Patients underwent surgery before receiving any other treatment.
Peripheral blood was collected from 56 of the 65 patients and 16 healthy volunteers. The healthy volunteers (10 male and 6 female), aged 34–70 years (mean age 53.7 years), were recruited from January to March 2011. All of the was blood centrifuged at 3500 g for five minutes, and the serum was stored at −80°C. In every patient, tumor and lung tissues from various parts of the non-neoplastic pulmonary lobe were harvested. Parts of the resected tissues were stored immediately in liquid nitrogen and the others were used for pathological study after fixation in 4% paraformaldehyde, dehydration in ethanol, and embedding in paraffin. The tumor grade and clinical stage were determined according to the Tumor Node Metastasis (TNM) Classification for Lung Cancer.22 This study was conducted in accordance with the Declaration of Helsinki and Institutional Review Board approval was obtained. Each patient signed a consent form that had been approved by the Committee on Human Rights in Research at our institution.
Cell lines and cell culture
The NSCLC cell lines H460 (large-cell carcinoma), H520 (squamous cell carcinoma) H23, H125, A549, and H1299 (adenosquamous carcinoma) were obtained from the American Tissue Culture Collection (ATCC). Cells were all grown in RPMI 1640 medium (Hyclone; Thermo Fisher Scientific). RPMI 1640 medium was supplemented with 10% fetal bovine serum (Gibco). All cell lines were maintained in culture at 37°C in an atmosphere of 5% CO2.
Measurement of serum concentration of hepcidin-25, bone morphogenetic protein (BMP)-2 and interleukin (IL)-6
The serum level of hepcidin-25 was measured in preoperative blood samples using an ELISA kit for human hepcidin-25 (DRG, Marburg, Germany). We compared the serum hepcidin-25 level with hepcidin mRNA expression in 53 corresponding tumors, and also with the serum levels of interleukin (IL)-6 and serum bone morphogenetic protein (BMP)-2 in the same 53 patients. IL-6 was measured by a human IL-6 ELISA kit (R&D Systems, Minneapolis, MN, USA), and serum BMP-2 was measured by a Quantikine BMP-2 ELISA kit (R&D Systems). For specific ELISA methods of hepcidin, IL-6 and BMP-2, see the instructions of each ELISA kit.
Reverse transcription quantitative polymerase chain reaction (RT-qPCR) assay
Total RNA was purified from all 65 pairs of tumor and non-tumor pulmonary tissue samples, as well as cell lines, with TRIzol reagent (Invitrogen, Carlsbad, CA, USA). Reverse transcription reactions were performed using a RevertAidFirst Strand cDNA Synthesis Kit (Fermentas, Burlington, Ont, Canada). Briefly, 12 μL of the mixture contained 2 μg total RNA, 1 μL random hexamer primer, and nuclease-free water, and was incubated at 65°C for five minutes. Then, 4 μL 5 × reaction buffer, 1 μL Ribolock RNase inhibitor, 2 μL 10 mM dNTP Mix and 1 μL RevertAidH Minus M-MuLV reverse transcriptase were added, and the mixture was incubated for five minutes at 25°C followed by 60 minutes at 42°C. The reaction was terminated by heating for five minutes at 70°C.
Real-time quantitative polymerase chain reaction (qPCR) was performed with an ABI Prism StepOne sequence detector (Applied Biosystems, Warrington, UK). The PCR was carried out in a final volume of 1 μL cDNA, 5 μL 2 × SYBR Premix Ex Taq (Takara, Shiga, Japan), 0.2 μL ROX Reference Dye, 0.4 μL 10 nM sense and antisense primer, and water up to 10 μL. The PCR conditions consisted of 40 cycles at 95°C for five seconds, 58°C for 20 seconds and 72°C for 20 seconds. The sequences of the primers were as follows: β-actin: sense primer 5′-GCACCACACCTTCTACAATGAG-3′, antisense primer 5′-GATAGCACAGCCTGGATAGCA-3′; hepcidin: sense primer 5′-CACAACAGACGGGACAACTT-3′, antisense primer 5′-CGCAGCAGAAAATGCAGATG-3′.19
The level of expression of hepcidin mRNA was calculated as a ratio to that of β-actin. Relative expression in the tissues was shown by the value of 2–ΔCT (ΔCT = mean hepcidin CT – mean β-actin CT).23 The mean value obtained from reverse transcription (RT)-qPCR of three tissue samples was used for analysis. The hepcidin relative expression between the cancerous and normal pulmonary tissues in the same patient was shown by the value of 2–ΔΔCT (ΔΔCT = meanΔCT,Cancer – meanΔCT,Lung).23 We normalized the value of hepcidin expression by log10.
Immunohistochemistry
Immunohistochemistry was performed using paraffin sections of human normal pulmonary lobe (n = 65) and lung cancer (n = 57). All sections were incubated at 95–100°C in sodium citrate buffer solution (0.01 mol/L, pH 6.0) for 20 minutes after dewaxing and hydration, washed in phosphate buffered saline (PBS), and then incubated in 3% H2O2 deionized water for 10 minutes. Sections were then incubated with rabbit polyclonal hepcidin antibody (30760, 1:200 dilution; Abcam, Cambridge, UK) for 12 hours at 4°C. The immunoreactivity was detected with the immunohistochemistry assay kit (PV-9001; Zhongshan Goldenbridge Biotechnology, Beijing, China) and diaminobenzidine (DAB) reagent (ZLI-9017; Zhongshan Goldenbridge Biotechnology). All sections were counterstained in hematoxylin and mounted before visualization. Immunohistochemical staining of these sections was scored microscopically (Leica, Germany) at ×200 magnification in all available tumor or epithelial cells meeting the typical morphological criteria, according to two pathologists, using the qualitative scale described in the literature.24 The number of cells stained was scored as: 0 (no staining); 1 + (less than one of three positive cells); 2 + (more than one of three and less than two of three positive cells); and 3 + (more than two of three positive cells). The intensity of staining was scored from 1 + (weak) to 3 + (strong). The immunoreactivity score was categorized in three groups by comprehensive evaluation of the percentage of positive cells and staining intensity. No staining was considered negative (0 score), and weak, moderate, and strong staining were considered positive (1 score, 2 scores, and 3 scores, respectively).
Statistical analysis
The results of serum hepcidin and RT-qPCR were analyzed using the Mann–Whitney U-test to compare two groups and the Kruskal–Wallis H test to compare three groups. Correlations were analyzed using Spearman's correlation coefficient by rank test. Significance was accepted at P < 0.05. The Pearson χ2 test was used to compare pairs of groups. All analyses were performed using SPSS version 16.0 (SPSS Inc., Chicago, IL, USA).
Results
Serum hepcidin-25 concentration is increased in patients with non small cell lung cancer (NSCLC)
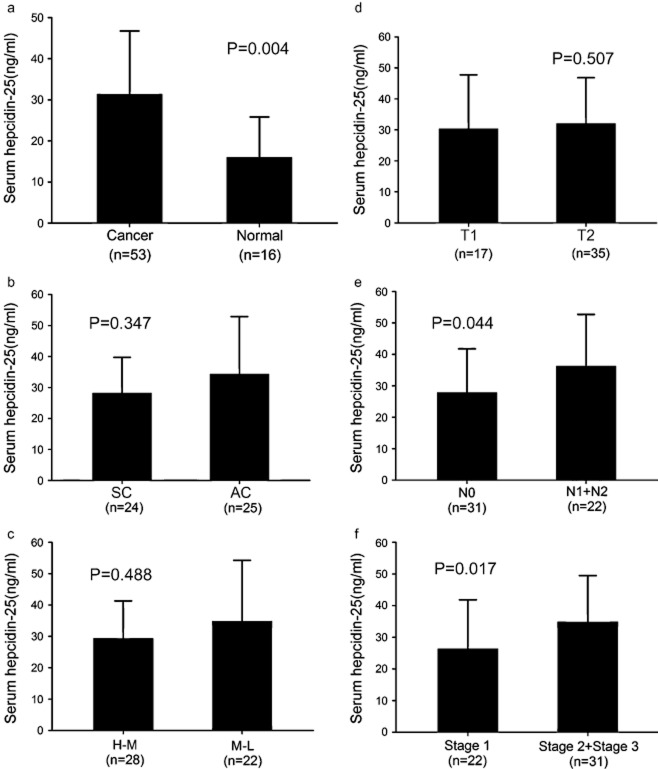
To explore whether hepcidin-25 levels differed between patients with NSCLC and non-cancerous individuals, we measured the serum concentration of hepcidin-25 in patients with NSCLC (n = 53) and healthy volunteers (n = 16) by ELISA. Serum hepcidin-25 levels were higher in patients with NSCLC (31.34 ± 15.41 ng/mL) than in healthy individuals (16.03 ± 9.78 ng/mL) (Fig. 1A, P = 0.004). There was no relationship between the serum hepcidin-25 level and pathological type of NSCLC (mean ± SD, squamous carcinoma, 28.25 ± 11.44 ng/mL; adenocarcinoma, 34.27 ± 18.57 ng/mL, P = 0.347, Fig. 1B), or the grade of differentiation (mean ± SD, high to middle [H-M], 29.30 ± 11.99 ng/mL; middle to low [M-L], 34.83 ± 19.38 ng/mL, P = 0.488, Fig. 1C). The serum hepcidin-25 level was not correlated with tumor stage (mean ± SD, T1, 30.32 ± 17.41 ng/mL; T2, 32.04 ± 14.77 ng/mL, P = 0.507, Fig. 1D). The level of serum hepcidin-25 was significantly different between N0 and lymph node metastasis (N1 + N2) (mean ± SD, N0, 27.86 ± 13.90 ng/mL; N1 + N2, 36.25 ± 16.41ng/ml, P = 0.044, Fig. 1E). Meanwhile, we also determined, by chi-square test (Table 1, P < 0.05), that there was a greater increase in the serum hepcidin level in patients with lymph node metastasis compared with those with non-metastatic lymph nodes. The serum hepcidin-25 level among clinical stage 1 and higher stage (stage 2 + stage 3) tumors was significantly different (mean ± SD, stage 1, 26.39 ± 15.47 ng/mL; stage 2 + stage 3, 34.85 ± 14.62 ng/mL, P = 0.017, Fig. 1F). These results indicate that the elevated serum hepicidin-25 level was associated with lymph node metastasis and the clinical stage of tumors in patients with NSCLC.
Figure 1.
Serum hepcidin-25 concentration in healthy individuals and patients with non small cell lung cancer (NSCLC). (a) The hepcidin-25 level was significantly higher in patients with NSCLC than in non-cancerous individuals (b) Hepcidin-25 levels in patients with squamous carcinoma (SC) and adenocarcinoma (AC). (c) Hepcidin-25 level according to grade of differentiation: high to middle (H-M) and middle to low (M-L). (d) Hepcidin-25 level according to tumor stage T1 and T2. (e) Hepcidin-25 level according to lymph node metastasis N0 and N1 + N2. (F) Hepcidin-25 level according to clinical stage 1 and higher stages (stage 2 + stage 3).
Table 1.
Serum hepcidin level in lymph node metastasis
| Increase (%) | Not increase (%) | |
|---|---|---|
| N0 | 18 (58.1) | 13 (41.9) |
| N1 + N2 | 19 (86.4)* | 3 (13.6) |
P < 0.05. The χ2 test revealed a greater increase in serum hepcidin levels in lymph node metastasis N1 and N2, compared with N0. We measured the serum hepcidin concentration in 16 healthy individuals, and normalized the 95% confidence interval (CI). The 95% CI was from 9.25 to 22.80 ng/mL; the sensitivity.and specificity was 44.4% and 90.5%, respectively. Concentrations that were higher than this value were considered to have increased.
Serum levels of BMP-2 and interleukin (IL)-6 in patients with NSCLC
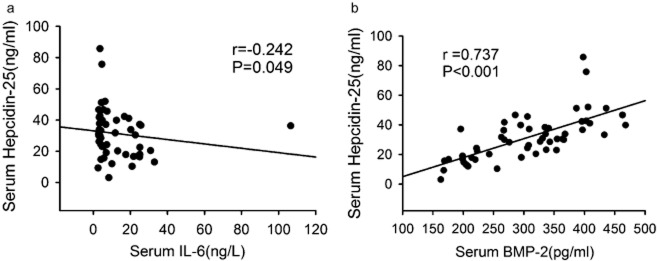
Hepcidin can regulate iron metabolism through binding ferroportin. Some cytokines such as IL-6 and BMP-2 can regulate hepcidin transcription.25 To further explain the reason for the increase in serum hepcidin-25 in NSCLC, we measured serum IL-6 and BMP-2 and analyzed the relationship between serum IL-6, BMP-2 and hepcidin-25. Serum IL-6 concentration in patients with NSCLC and healthy volunteers was 12.45 ± 15.87 and 10.61 ± 9.07 ng/L, respectively (P = 0.917), and the level of serum BMP-2 in patients with NSCLC and healthy volunteers was 305.40 ± 83.42 and 107.5 ± 24.85 pg/mL, respectively (P < 0.001). The serum BMP2 level was significantly increased in patients with NSCLC. Meanwhile, the serum hepcidin-25 level was negatively correlated with serum concentration of IL-6 (r = −0.272, P = 0.049, Fig. 2A), but positively correlated with the serum level of BMP-2 (r = 0.737, P < 0.001, Fig. 2B). Collectively, these results suggest that the increased serum BMP-2, but not IL-6, contributes to the increase in serum hepcidin-25 in patients with NSCLC.
Figure 2.
Spearman rank correlation coefficient relationship between serum concentration of interleukin (IL)-6, bone morphogenetic protein (BMP)-2 and hepcidin-25. (a) The serum hepcidin-25 level was negatively correlated with the serum concentration of IL-6. (b) The serum hepcidin-25 level was positively correlated with the serum level of BMP-2.
Hepcidin mRNA expression is increased in NSCLC tissues and cells
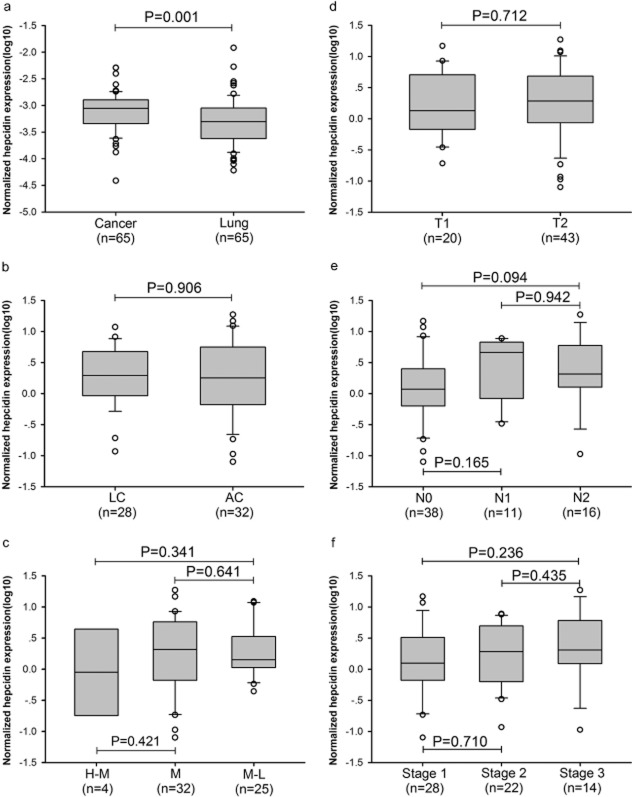
We observed that hepcidin expression was increased in the serum of patients with NSCLC, so we were interested in whether hepcidin was expressed in lung cancer tissues. We examined hepcidin mRNA expression in 65 cancer specimens, as well as their corresponding adjacent normal uninvolved pulmonary tissues. The hepcidin mRNA expression in cancerous tissues was obviously higher than that in the normal pulmonary tissues (Fig. 3A, P = 0.001). The data of 2–ΔΔCT showed that hepcidin mRNA expression in 67.7% of cancerous tissue specimens (44/65) was higher than that in normal pulmonary tissue, whereas in 30.8% of cancerous tissue specimens (20/65) it was lower. Expression in only 1.5% of cancerous tissue specimens (1/65) was no different from that in normal pulmonary tissues (see Fig. S1). Hepcidin mRNA expression was significantly increased in NSCLC tissues.
Figure 3.
Hepcidin mRNA expression in patients with non small cell lung cancer (NSCLC). (a) Hepcidin mRNA in cancerous and normal pulmonary tissues. (b) Hepcidin mRNA in squamous carcinoma (SC) and adenocarcinoma (AC). (c) Hepcidin mRNA level according to grade of differentiation: high to middle (H-M), middle (M), and middle to low (M-L). (d) Hepcidin mRNA level according to tumor stage T1 and T2. (e) Hepcidin mRNA level according to lymph node metastasis N0 to N2. (f) Hepcidin mRNA level according to clinical stage I to III.
The correlation between the expression of hepcidin mRNA and pathological characteristics was also studied. The expression of hepcidin mRNA was not related to the pathological type of NSCLC (Fig. 3B). There was no relationship between hepcidin mRNA expression and the grade of differentiation (Fig. 3C). Expression of hepcidin mRNA was neither correlated with tumor stage (Fig. 3D) nor lymph node metastasis (Fig. 3E). The level of hepcidin mRNA expression was not correlated with the clinical stage of NSCLC (Fig. 3F). However, the concentration of serum hepcidin-25 was not significantly correlated with the expression of hepcidin mRNA in the corresponding cancerous tissues (r = −0.214, P = 0.124, Fig. S2) from 53 patients.
To test further whether hepcidin was also expressed in NSCLC cell lines, and whether the hepcidin mRNA level was higher in NSCLC cell lines than in bronchial epithelial cells, we further detected hepcidin mRNA expression in human bronchial epithelial (HBE) cells and six cell lines from human lung carcinoma, H460, H520, H23, H125, A549, and H1299. The relative expression of hepcidin mRNA in four cell lines (H23, H1299, H125 and H520) of lung carcinoma was significantly higher than that in HBE cells, and it was lower in two cell lines (H460 and A549) (Fig. 4). These results suggested that hepcidin increased expression in most of NSCLC cell lines, however it was lower in large cell carcinoma (H460) and adenosquamous carcinoma (A549).
Figure 4.
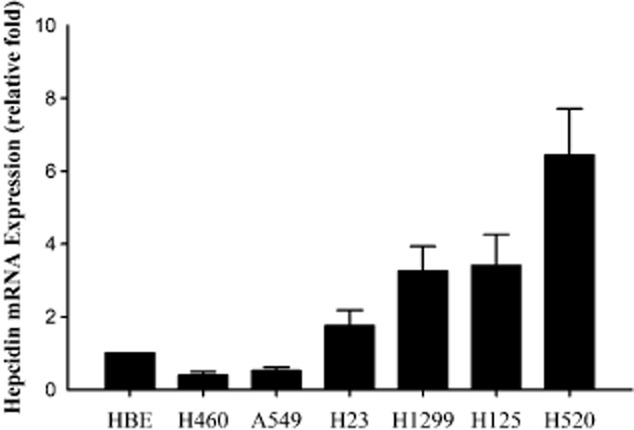
Hepcidin mRNA expression in human bronchial epithelial (HBE) cell lines and six cell lines of lung carcinoma. The expression level of hepcidin mRNA in four lung carcinoma cell lines (H23, H1299, H125 and H520) was higher than that in HBE cells, and lower in only two cell lines (H460 and A549).
Increased hepcidin expression in the tumor microenvironment
We not only confirmed an increase in the serum hepcidin level in patients with NSCLC, but also increased hepcidin expression in NSCLC tumor tissues. This shows that lung carcinoma cells may synthesize and secrete hepcidin. As we know, cancer cells gather together and form carcinoma nests. These cells live in the interstitial fluid, through which substances are exchanged between intracellular fluid and blood. On the one hand, serum hepcidin can spread to the interstitial fluid between the tumor cells and act on the tumor cells. On the other hand, the tumor cells can synthesize and secrete hepcidin into the interstitial fluid, which then acts on the cells. Therefore, the level of hepcidin expression in the tumor interstitial fluid (tumor microenvironment) is important. There are two aspects of hepcidin expression in the tumor microenvironment. One is the serum hepcidin level, which is synthesized and secreted by the liver, and the other is hepcidin that is synthesized and secreted by tumor cells. The level of serum hepcidin was low in some of the tumor samples, but increased hepcidin mRNA expression indicated increased hepcidin synthesis and secretion by tumor cells, which maintained a high level of hepcidin in the tumor microenvironment. In other tumor samples, although hepcidin mRNA expression in the tumor was low, there was a high serum level of hepcidin, which maintained a high level of hepcidin in the tumor microenvironment and iron overload in the tumor cells. There was no effective method to detect the hepcidin level in the tumor microenvironment, thus, we combined a measurement of serum hepcidin and hepcidin expression in the tumor to indicate the level of hepcidin in the tumor microenvironment. An increase in the serum hepcidin level or tumor hepcidin mRNA was considered to be indicative of an increase in tumor microenvironment hepcidin. Using this method, the increased rate of tumor microenvironment hepcidin was 88.7% in 53 patients with NSCLC (Table S1). We compared measuring serum hepcidin alone and with the combined measurement of serum hepcidin and expression in tumor tissues. The total increase rate of serum hepcidin and expression in tumors was significantly higher than that with the measurement of serum hepcidin alone (Table 2, P < 0.05).
Table 2.
Comparison of measuring serum hepcidin-25 and combined measuring of serum hepcidin-25 and hepcidin mRNA expression
| Increase (%) | No increase (%) | |
|---|---|---|
| Serum | 37 (69.8) | 16 (30.2) |
| Serum and tumor | 47 (88.7)* | 6 (11.3) |
P < 0.05. The χ2 test revealed a higher total rate of increased hepcidin expression in both serum and tumor compared with serum hepcidin-25 level. We measured serum hepcidin concentration in 16 healthy individuals, and normalized the 95% CI. The 95% CI was from 9.25 to 22.80 ng/mL; the sensitivity.and specificity was 44.4% and 90.5%, respectively. Concentrations that were higher than this value were considered to have increased. Hepcidin mRNA expression was considered to have increased when 2–ΔΔCT was >1. Serum, hepcidin-25 level in serum, serum and tumor, hepcidin-25 level in serum and hepcidin mRNA expression in tumor tissues.
Determination of hepcidin localization in NSCLC tissues
To test whether hepcidin concentrations were also altered in NSCLC tissue, we performed immunohistochemical analysis. The cellular localization of hepcidin was determined by immunohistochemistry. In normal pulmonary tissues, hepcidin expressed strong positive immunoreactivity in lung macrophages (Fig. 5H–G) and weak positive immunoreactivity in HBE cells (Fig. 5E,F). In NSCLC tissue, it showed positive immunoreactivity of hepcidin in the cytoplasm of lung carcinoma cells (Fig. 5A–D). The positive rate of hepcidin immunoreactivity in the HBE cells was only 22.8% (13/57). However, 87.7% (57/65) of NSCLC specimens showed abundant positive immunoreactivity in the cytoplasm of these cancer cells, and the immunoreactivity was stronger than in normal bronchiolar epithelium (Table 3, P < 0.05). This indicated that hepcidin expressed high immunoreactivity in NSCLC tissues and low activity in normal bronchiolar epithelium. It showed that hepcidin protein expression was increased in cancerous tissues.
Figure 5.
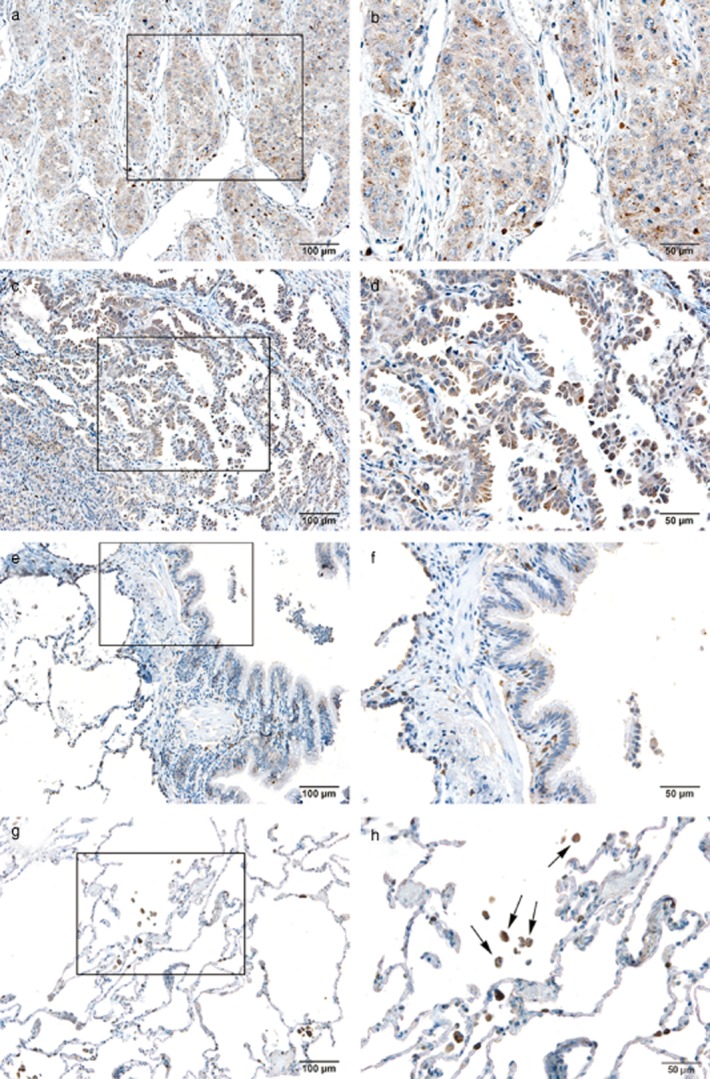
Cellular localization of hepcidin in non small cell lung cancer (NSCLC) and normal pulmonary tissues. (a) Squamous carcinoma (×10). (b) Squamous carcinoma (×20). (c) Adenocarcinoma (×10). (d) Adenocarcinoma (×20). (e) Normal bronchiolar epithelium (×10). (f) Normal bronchiolar epithelium (×20). (g) Normal alveoli (×10). (h) Normal alveoli (×20); the cells marked by arrows are alveolar macrophages.
Table 3.
Measuring hepcidin expression in non small cell lung cancer (NSCLC) tissues and normal bronchiolar epithelium by immunohistochemistry
| Positive (%) | Negative (%) | |
|---|---|---|
| NSCLC | 57 (87.7)* | 8 (12.3) |
| NBE | 13 (22.8) | 44 (77.2) |
P < 0.05. The χ2 test revealed the higher positive rate of hepcidin expression in NSCLC tissues compared with normal bronchiolar epithelium. NBE, normal bronchiolar epithelium; NSCLC, non small cell lung cancer.
Discussion
Hepcidin is a small peptide hormone that plays a key role in regulating iron homeostasis, such as dietary iron absorption, plasma iron concentrations, and tissue iron distribution. It is mainly secreted by the liver because it is highly expressed in that organ.3 Recent studies have shown increased mRNA expression of hepcidin in some cancers. Ward et al.21 demonstrated increased hepcidin expression in colorectal cancer. An influential study reported that hepcidin mRNA is detected in normal breast epithelial cells and in all breast cancer cell lines, and that the concentration of prohepcidin protein is higher in all breast cancer cells when compared with non-malignant breast cells.18 Another study confirmed by cDNA microarray analysis that hepcidin expression is upregulated in squamous carcinoma.26 In the present study, we found increased hepcidin mRNA expression in NSCLC tissues and squamous carcinoma cells, which was consistent with previously reported results.
In our study, the serum hepcidin-25 level in patients with NSCLC was significantly higher than in healthy individuals. In addition, we found that serum hepcidin was also correlated with lymph node metastasis and clinical stage. This implied that the synthesis and secretion of hepcidin changed in these patients, and this disorder was probably caused by tumor growth and invasion. There are two main types of cytokines that can increase the level of hepcidin in the body: inflammatory cytokines, such as IL-6, IL-1α and IL-1β; and BMPs, such as BMP2, BMP4 and BMP6. BMPs are cytokines belonging to the transforming growth factor-β superfamily. Experimental evidence has shown that, BMP2, BMP4 and BMP6 interact with hemojuvelin, which is a co-receptor for BMP signaling and BMP type I (R-I) and type II receptors (R-II) to generate an active signaling complex, and upregulate hepatocyte hepcidin expression.27–29 It has been found that IL-6 promotes hepcidin expression through signal transducer and activator of transcription (STAT)3.30,31 This demonstrates that STAT3 is the crucial transcription factor in the upregulation of human hepcidin by the inflammatory cytokine IL-6.32 In the present study, we found that the serum BMP2 level was increased in patients with NSCLC, and it was positively correlated with the level of serum hepcidin. However, the serum IL-6 level was decreased in patients with NSCLC, and it was negatively correlated with the level of serum hepcidin. This shows that increased BMP2 expression is the main reason for promoting increased serum hepcidin in NSCLC, but the expression of IL-6 is inhibited. Although BMPs and IL-6 can promote the expression of hepcidin, they also play a role by activating different transcriptional regulatory elements. BMPs can play a stronger role than IL-6 in promoting the synthesis and transcription of hepcidin.33 Several studies have consistently shown that BMP-2 and BMP-4 enhance the migration and invasion in established cancer cell lines.34,35 It has been confirmed that BMP2 is overexpressed in NSCLC.34 Yong Park et al.36 have reported that serum BMP-2 levels are elevated in patients with gastric cancer and correlate with disease progression. This shows that high levels of BMP promote increased hepcidin expression, while high levels of hepcidin may promote tumor development.
Although serum hepcidin in NSCLC patients was higher than in healthy subjects in our study, the data showed that the distributions of serum hepcidin levels in NSCLC patients and healthy subjects seemed to be overlapped. Because hepcidin is mainly synthesized and secreted by the liver, some studies have indicated that there is a synthesis and secretion of hepcidin in other organs.37,38 NSCLC may influence the serum hepcidin level. In lung tissues, we found that hepcidin was mainly expressed in alveolar macrophages and bronchial epithelial cells, therefore, the autocrine of hepcidin in carcinoma cells has great significance for hepcidin maintenance of the tumor microenvironment. We found that hepcidin expression was increased in the tumor microenvironment when we used combined measure hepcidin mRNA expression and serum levels. This indicated that hepcidin, in circulating blood and expression in tumors, played an important role in maintaining the hepcidin level in the tumor microenvironment.
High levels of hepcidin have great significance for cancer cells. Hepcidin-25 binds to ferroportin, which exports iron from the intracellular to the extracellular space, and leads to internalization and degradation of ferroportin; however, ferroportin is the most important molecule in controlling the iron output of cells. High levels of hepcidin cause reduced expression of ferroportin, which results in an intracellular accumulation of iron. All living cells possess an absolute requirement for iron, and it has been suggested that reactions catalyzed by iron may have constituted an initial step in the origin of life.39,40 The role of iron in cell growth may be important because many iron-containing proteins catalyze key reactions involved in energy metabolism and DNA synthesis. On the one hand, tumor cells increase in demand for iron because of strong growth and division, while on the other hand, intracellular iron overload causes a Fenton reaction (Fe[II] + H2O2 → Fe[III] + ·OH + OH−[Eqn 1]) that produces hydroxyl radicals, which are the most reactive chemical species in biological systems.41 Excess hydroxyl radicals can cause oxidative damage of DNA in cells and promote tumor generation. With the further development of malignant tumors, when hepcidin levels continue to increase, this results in the reduction of iron absorption in intestinal epithelial mucosa, and reduced release of iron from other iron storage cells, such as macrophages, which can cause anemia. This may be an important mechanism of anemia in patients with malignant tumors.
Conclusions
This study showed that hepcidin expression was increased in NSCLC tissues, and the elevated serum hepicidin-25 level was associated with lymph node metastasis and the clinical stage of tumors in patients with NSCLC. In view of the present evidence, further research focusing on the role of hepcidin in tumors and the regulatory mechanism of hepcidin in the tumor microenvironment is needed.
Acknowledgments
We thank Dr. Lei Shi and Dr. Zhongqing Fan for technical assistance with immunohistochemistry. This research was supported by grants from the National Natural Scientific Foundation of China (81101767).
Disclosure
No authors report any conflict of interest.
Supporting Information
Figure S1 Comparison of hepcidin mRNA expression in 65 pairs of non small cell lung cancer (NSCLC) and non-cancerous lung tissues. (A,B) Hepcidin mRNA expression was upregulated in Cases 1–30 of lung cancer. (C) Hepcidin mRNA expression was upregulated in Cases 31–44; only Case 45 showed no difference. (D) Hepcidin mRNA expression was downregulated in Cases 46–55 of lung cancer. (E) Hepcidin mRNA expression was downregulated in Cases 56–65.
Figure S2 Spearman rank correlation coefficient relationship between hepcidin mRNA expression and serum hepcidin level in patients with non small cell lung cancer (NSCLC).The serum hepcidin-25 level was not significantly correlated with the expression of hepcidin mRNA in the corresponding cancerous tissues from 53 patients with NSCLC.
Table S1 Distribution of increased serum hepcidin and hepcidin mRNA expression in 53 patients with non small cell lung cancer (NSCLC). We measured serum hepcidin concentration in 16 healthy individuals, and normalized the 95% confidence interval (CI). Concentrations higher than this value were considered to have increased. Hepcidin mRNA expression was considered to have increased when 2–ΔΔCT was >1. Increased serum hepcidin level or hepcidin mRNA expression was scored as 1, and no increase as 0.
References
- Krause A, Neitz S, Mägert HJ, et al. LEAP-1, a novel highly disulfide-bonded human peptide, exhibits antimicrobial activity. FEBS Lett. 2000;480:147–150. doi: 10.1016/s0014-5793(00)01920-7. [DOI] [PubMed] [Google Scholar]
- Pigeon C, Ilyin G, Courselaud B, et al. A new mouse liver-specific gene, encoding a protein homologous to human antimicrobial peptide hepcidin, is overexpressed during iron overload. J Biol Chem. 2001;276:7811–7819. doi: 10.1074/jbc.M008923200. [DOI] [PubMed] [Google Scholar]
- Park CH, Valore EV, Waring AJ, Ganz T. Hepcidin, a urinary antimicrobial peptide synthesized in the liver. J Biol Chem. 2001;276:7806–7810. doi: 10.1074/jbc.M008922200. [DOI] [PubMed] [Google Scholar]
- Nicolas G, Viatte L, Bennoun M. Beaumont C, Kahn A, Vaulont S. Hepcidin, a new iron regulatory peptide. Blood Cells Mol Dis. 2002;29:327–335. doi: 10.1006/bcmd.2002.0573. [DOI] [PubMed] [Google Scholar]
- De Domenico I, Ward DM, Langelier C, et al. The molecular mechanism of hepcidin-mediated ferroportin down-regulation. Mol Biol Cell. 2007;18:2569–2578. doi: 10.1091/mbc.E07-01-0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeth E, Tuttle MS, Powelson J, et al. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306:2090–2093. doi: 10.1126/science.1104742. [DOI] [PubMed] [Google Scholar]
- Hentze MW, Muckenthaler MU, Galy B, Camaschella C. Two to tango: regulation of Mammalian iron metabolism. Cell. 2010;142:24–38. doi: 10.1016/j.cell.2010.06.028. [DOI] [PubMed] [Google Scholar]
- Donovan A, Brownlie A, Zhou Y, et al. Positional cloning of zebrafish ferroportin1 identifies a conserved vertebrate iron exporter. Nature. 2000;403:776–781. doi: 10.1038/35001596. [DOI] [PubMed] [Google Scholar]
- Donovan A, Lima CA, Pinkus JL, et al. The iron exporter ferroportin/Slc40a1 is essential for iron homeostasis. Cell Metab. 2005;1:191–200. doi: 10.1016/j.cmet.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Kennard ML, Richardson DR, Gabathuler R, Ponka P, Jefferies WA. A novel iron uptake mechanism mediated by GPI-anchored human p97. EMBO J. 1995;14:4178–4186. doi: 10.1002/j.1460-2075.1995.tb00091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson D, Baker E. The uptake of inorganic iron complexes by human melanoma cells. Biochim Biophys Acta. 1991;1093:20–28. doi: 10.1016/0167-4889(91)90133-i. [DOI] [PubMed] [Google Scholar]
- Richardson DR, Ponka P. The molecular mechanisms of the metabolism and transport of iron in normal and neoplastic cells. Biochim Biophys Acta. 1997;1331:1–40. doi: 10.1016/s0304-4157(96)00014-7. [DOI] [PubMed] [Google Scholar]
- Kwok JC, Richardson DR. The iron metabolism of neoplastic cells: alterations that facilitate proliferation? Crit Rev Oncol Hematol. 2002;42:65–78. doi: 10.1016/s1040-8428(01)00213-x. [DOI] [PubMed] [Google Scholar]
- Buss JL, Torti FM, Torti SV. The role of iron chelation in cancer therapy. Curr Med Chem. 2003;10:1021–1034. doi: 10.2174/0929867033457638. [DOI] [PubMed] [Google Scholar]
- Kowdley KV. Iron, hemochromatosis, and hepatocellular carcinoma. Gastroenterology. 2004;127(5 Suppl. 1):S79–S86. doi: 10.1016/j.gastro.2004.09.019. [DOI] [PubMed] [Google Scholar]
- Holmström P, Gåfvels M, Eriksson LC, et al. Expression of iron regulatory genes in a rat model of hepatocellular carcinoma. Liver Int. 2006;26:976–985. doi: 10.1111/j.1478-3231.2006.01316.x. [DOI] [PubMed] [Google Scholar]
- Chen G, Fillebeen C, Wang J, Pantopoulos K. Overexpression of iron regulatory protein 1 suppresses growth of tumor xenografts. Carcinogenesis. 2007;28:785–791. doi: 10.1093/carcin/bgl210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinnix ZK, Miller LD, Wang W, et al. Ferroportin and iron regulation in breast cancer progression and prognosis. Sci Transl Med. 2010;2:43ra56. doi: 10.1126/scisignal.3001127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kijima H, Sawada T, Tomosugi N, Kubota K. Expression of hepcidin mRNA is uniformly suppressed in hepatocellular carcinoma. BMC Cancer. 2008;8:167. doi: 10.1186/1471-2407-8-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamai T, Tomosugi N, Abe H, Arai K, Yoshida K. Increased serum hepcidin-25 level and increased tumor expression of hepcidin mRNA are associated with metastasis of renal cell carcinoma. BMC Cancer. 2009;9:270. doi: 10.1186/1471-2407-9-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward DG, Roberts K, Brookes MJ, et al. Increased hepcidin expression in colorectal carcinogenesis. World J Gastroenterol. 2008;14:1339–1345. doi: 10.3748/wjg.14.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobin LH, Gospodarowicz MK, Wittekind C. TNM Classification of Malignant Tumours. 7th edn. Hoboken, NJ 2010: Blackwell Publishing; [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Brambilla E, Travis WD, Colby TV, Corrin B, Shimosato Y. The new World Health Organization classification of lung tumours. Eur Respir J. 2001;18:1059–1068. doi: 10.1183/09031936.01.00275301. [DOI] [PubMed] [Google Scholar]
- Lee P, Peng H, Gelbart T, Wang L, Beutler E. Regulation of hepcidin transcription by interleukin-1 and interleukin-6. Proc Natl Acad Sci U S A. 2005;102:1906–1910. doi: 10.1073/pnas.0409808102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachi S, Yoneda K, Wu R. Interactome-transcriptome analysis reveals the high centrality of genes differentially expressed in lung cancer tissues. Bioinformatics. 2005;21:4205–4208. doi: 10.1093/bioinformatics/bti688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babitt JL, Huang FW, Wrighting DM, et al. Bone morphogenetic protein signaling by hemojuvelin regulates hepcidin expression. Nat Genet. 2006;38:531–539. doi: 10.1038/ng1777. [DOI] [PubMed] [Google Scholar]
- Babitt JL, Huang FW, Xia Y, Sidis Y, Andrews NC, Lin HY. Modulation of bone morphogenetic protein signaling in vivo regulates systemic iron balance. J Clin Invest. 2007;117:1933–1939. doi: 10.1172/JCI31342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Y, Babitt JL, Sidis Y, Chung RT, Lin HY. Hemojuvelin regulates hepcidin expression via a selective subset of BMP ligands and receptors independently of neogenin. Blood. 2008;111:5195–5204. doi: 10.1182/blood-2007-09-111567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verga Falzacappa MV, Vujic Spasic M, Kessler R. Stolte J, Hentze MW, Muckenthaler MU. STAT3 mediates hepatic hepcidin expression and its inflammatory stimulation. Blood. 2007;109:353–358. doi: 10.1182/blood-2006-07-033969. [DOI] [PubMed] [Google Scholar]
- Huang YH, Chuang JH, Yang YL. Huang CC, Wu CL, Chen CL. Cholestasis downregulate hepcidin expression through inhibiting IL-6-induced phosphorylation of signal transducer and activator of transcription 3 signaling. Lab Invest. 2009;89:1128–1139. doi: 10.1038/labinvest.2009.82. [DOI] [PubMed] [Google Scholar]
- Wrighting DM, Andrews NC. Interleukin-6 induces hepcidin expression through STAT3. Blood. 2006;108:3204–3209. doi: 10.1182/blood-2006-06-027631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truksa J, Peng H, Lee P, Beutler E. Different regulatory elements are required for response of hepcidin to interleukin-6 and bone morphogenetic proteins 4 and 9. Br J Haematol. 2007;139:138–147. doi: 10.1111/j.1365-2141.2007.06728.x. [DOI] [PubMed] [Google Scholar]
- Langenfeld EM, Bojnowski J, Perone J, Langenfeld J. Expression of bone morphogenetic proteins in human lung carcinomas. Ann Thorac Surg. 2005;80:1028–1032. doi: 10.1016/j.athoracsur.2005.03.094. [DOI] [PubMed] [Google Scholar]
- Deng H, Makizumi R, Ravikumar TS, Dong H, Yang W, Yang WL. Bone morphogenetic protein-4 is overexpressed in colonic adenocarcinomas and promotes migration and invasion of HCT116 cells. Exp Cell Res. 2007;313:1033–1044. doi: 10.1016/j.yexcr.2006.12.020. [DOI] [PubMed] [Google Scholar]
- Park Y, Kang MH, Seo HY, et al. Bone morphogenetic protein-2 levels are elevated in the patients with gastric cancer and correlate with disease progression. Med Oncol. 2010;27:1192–1199. doi: 10.1007/s12032-009-9358-x. [DOI] [PubMed] [Google Scholar]
- Merle U, Fein E, Gehrke SG, Stremmel W, Kulaksiz H. The iron regulatory peptide hepcidin is expressed in the heart and regulated by hypoxia and inflammation. Endocrinology. 2007;148:2663–2668. doi: 10.1210/en.2006-1331. [DOI] [PubMed] [Google Scholar]
- Aigner E, Felder TK, Oberkofler H, et al. Glucose acts as a regulator of serum iron by increasing serum hepcidin concentrations. J Nutr Biochem. 2013;24:112–117. doi: 10.1016/j.jnutbio.2012.02.017. [DOI] [PubMed] [Google Scholar]
- Wächtershäuser G. Evolution of the first metabolic cycles. Proc Natl Acad Sci U S A. 1990;87:200–204. doi: 10.1073/pnas.87.1.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wächtershäuser G. Groundworks for an evolutionary biochemistry: the iron-sulphur world. Prog Biophys Mol Biol. 1992;58:85–201. doi: 10.1016/0079-6107(92)90022-x. [DOI] [PubMed] [Google Scholar]
- Imlay JA, Chin SM, Linn S. Toxic DNA damage by hydrogen peroxide through the Fenton reaction in vivo and in vitro. Science. 1988;240:640–642. doi: 10.1126/science.2834821. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Comparison of hepcidin mRNA expression in 65 pairs of non small cell lung cancer (NSCLC) and non-cancerous lung tissues. (A,B) Hepcidin mRNA expression was upregulated in Cases 1–30 of lung cancer. (C) Hepcidin mRNA expression was upregulated in Cases 31–44; only Case 45 showed no difference. (D) Hepcidin mRNA expression was downregulated in Cases 46–55 of lung cancer. (E) Hepcidin mRNA expression was downregulated in Cases 56–65.
Figure S2 Spearman rank correlation coefficient relationship between hepcidin mRNA expression and serum hepcidin level in patients with non small cell lung cancer (NSCLC).The serum hepcidin-25 level was not significantly correlated with the expression of hepcidin mRNA in the corresponding cancerous tissues from 53 patients with NSCLC.
Table S1 Distribution of increased serum hepcidin and hepcidin mRNA expression in 53 patients with non small cell lung cancer (NSCLC). We measured serum hepcidin concentration in 16 healthy individuals, and normalized the 95% confidence interval (CI). Concentrations higher than this value were considered to have increased. Hepcidin mRNA expression was considered to have increased when 2–ΔΔCT was >1. Increased serum hepcidin level or hepcidin mRNA expression was scored as 1, and no increase as 0.