Abstract
Primary extranodal natural killer/T- cell lymphoma, nasal type (NK/TCL) in the lung is extremely rare and associated with Epstein-Barr virus (EBV) infection. An 80-year-old male presented with hemoptysis, which had lasted three days. Physical examination revealed inspiratory crackles at the left lung base and massive splenomegaly. Chest radiograph shows a mass-like lesion in the left lower lung but no active lesion six months earlier. Computed tomography demonstrated a soft tissue mass (size: 6.6 × 5.1 cm) with increased ground-glass opacities in the left lower lobe, several pulmonary nodules, and mediastinal lymphadenopathy. Transthoracic needle biopsy of the left-lower-lobe lung mass was performed. The pathology revealed atypical lymphoid cell infiltration, which is immunoreactive for cytoplasmic CD3, CD30 and CD56, but not reactive for CK and CD20. EBV-encoded RNA (EBER) was also detected in these atypical lymphoid cells. The serum EBV DNA level was 7.03 × 106 copies/mL and subtype 1 EBV was identified. No evidence of lymphoma involvement was found in the extrathoracic site. Primary pulmonary lymphoma showing nasal-type NK/T-cell subtype was diagnosed. Chemotherapy with cyclophosphamide and prednisolone was initiated immediately but the patient deteriorated and died three weeks later. In conclusion, patients presenting with rapidly growing lung mass and massive splenomegaly raise the possibility of aggressive pulmonary lymphoma. Extranodal NK/T-cell lymphoma with high baseline plasma EBV DNA levels signifies poor prognosis. Identifying young high-risk patients may have benefits for early aggressive and successful treatment.
Keywords: Epstein-Barr virus, extranodal NK/T cell lymphoma, lung neoplasm, lymphoma
Introduction
Primary pulmonary lymphoma is a rare disorder, and represents only 0.5-1% of all primary pulmonary malignancies, less than 1% of all cases of non-Hodgkin's lymphoma and 3–4% of all extranodal manifestations of this disease.1 The most frequent form of primary pulmonary lymphoma is low-grade B-cell type that originates from mucosa-associated lymphoid tissue.2 Extranodal natural killer/T- cell lymphoma, nasal type (NK/TCL) in the lung is extremely rare and strongly associated with Epstein-Barr virus (EBV). We present a case of primary extranodal NK/TCL presenting with rapidly growing lung mass and massive splenomegaly mimicking bronchogenic carcinoma.
Case report
An 80-year-old man who was an ex-smoker presented with a cough and blood-streaked sputum lasting for three days. No fever, weight loss or night sweats were identified. Physical examination revealed inspiratory crackles at the left lung base, massive splenomegaly (spleen palpable 8 cm below the costal margin), and no other peripheral lymphadenopathy. Laboratory results were as follows: white blood cell count, 6100/uL; neutrophil/lymphocyte ratio, 88.4/7.8; hemoglobin, 12.4 g/dL; platelet count, 161000/ul; and LDH, 271 U/L. Chest radiography revealed a mass-like lesion in the left lower lung zone and no active lesion six months earlier (Fig 1a,b). A chest computed tomography (CT) scan revealed a soft tissue mass (6.6 × 5.1 cm) with ground-glass attenuation in the left lower lobe, several pulmonary nodules, and mediastinal lymphadenopathy (Fig 1c,d). Percutaneous transthoracic needle biopsy of the left-lower-lobe mass was performed and the pathology revealed monotonous, medium-sized cells infiltrating the lung tissue. These cells have high nuclear cytoplasmic ratio and inconspicuous nucleoli (Fig 2a). Coagulative necrosis and mitotic figures were easily found. Immunohistochemical stains revealed reactivity for cytoplasmic CD3, CD56 and CD30, but no reactivity for CK and CD20 (Fig 2b,c). In situ hybridization for EBV-encoded RNA (EBER) showed a positive reaction in these lymphoid cells (Fig 2d). The serum EBV DNA level was 7.03 × 106 copies/mL and subtype 1 EBV was identified. After serial studies including nasopharyngoscopy, abdominal sonography, and bone marrow biopsy, no evidence of lymphoma involvement was found in the extrapulmonary site. Extranodal NK/TCL primary in the lung was diagnosed. The patient only received induction chemotherapy with cyclophosphamide and prednisolone, not a standard cyclophosphamide, hydroxydaunorubicin, oncovin, prednisolone (CHOP) regimen, in consideration of his advanced age and Eastern Cooperative Oncology Group Performance (ECOG) Status 2. Nevertheless, he died three weeks after chemotherapy as a result of disease progression with increasing tumor size following respiratory failure.
Figure 1.

(a) Posteroanterior chest radiograph shows no active lesion six months earlier. (b) Posteroanterior chest radiograph shows a mass-like lesion in the left lower lung. (c) Axial non-enhanced computed tomography (CT) scan shows a CT halo sign (peritumoral ground-glass attenuation). (d) Mediastinal lymphadenopathy in the pretracheal retrocaval region.
Figure 2.
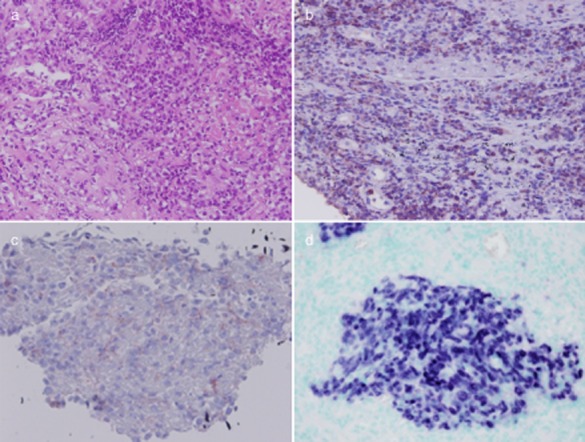
(a) Percutaneous transthoracic needle biopsy specimen revealed monotonous, medium-sized cells infiltrating the lung tissue. Tumor cells have high nuclear cytoplasmic ratio and inconspicuous nucleoli (Hematoxylin & Eosin [H&E] staining, magnification, 200×). (b,c) Immunohistochemical stains revealed reactivity for cytoplasmic CD3, CD56 (magnification, 200×). (d) In situ hybridization for Epstein-Barr virus-encoded RNA (EBER) showed positive reaction in tumor cells (magnification, 200×).
Discussion
Extranodal NK/TCL represents a distinct clinicopathological entity characterized by the presence of lymphoma cell involvement, ulcerative mucosa, and angiodestructive growth pattern. The tumor cells mostly expressed CD56 and EBV in the great majority of cases.3 This disorder is rare in the United States and Europe, but common in Asia, South and Central America, and Mexico.3 Sinonasal disease is a usual finding, although skin and aerodigestive mucosae are commonly involved, but rare in the lung.4
Only a few cases have been reported for primary extranodal NK/TCL of the lung. Most of the cases presented with fever, cough, and dyspnea.5 Radiographic findings may present multiple nodular lesions,5,6 mass or mass-like consolidation,5,6 consolidation with cavitation,5 consolidation with airbronchogram,5,6 halo sign (ground-glass attenuation surrounding a pulmonary nodule),6 pleural effusion,5,6 and absence of hilar or mediastinal lymphadenopathy.5,6 Most cases were also associated with EBV, including positive EBER in the tumor cells or EBV viremia.5,6 Extranodal NK/TCL, nasal type is associated with EBV. Circulating EBV DNA levels are related to a tumor burden and can serve as an invaluable tumor marker for assessing treatment response, monitoring disease progress, and as strongly predicting outcomes.7 Patients with high baseline plasma EBV DNA levels (≥600 copies/mL) had a significantly poorer overall survival expectancy than those with low levels (<600 copies/mL).7
The present case was an ex-smoker with a rapidly growing lung mass, pulmonary nodules, and mediastinal lymphadenopathy, giving the first impression of bronchogenic carcinoma. Nevertheless, splenomegaly is not a usual presentation in lung cancer and might be a diagnostic clue for malignant lymphoma. The differential diagnostic possibilities are much fewer when the spleen is massively enlarged; that is, it is palpable more than 8 cm below the left costal margin or its weight exceeds 1000 g. The vast majority of such patients will have non-Hodgkin's lymphoma, chronic lymphocytic leukemia, hairy cell leukemia, chronic myelogenous leukemia, myelofibrosis with myeloid metaplasia, or polycythemia vera.8 In addition, a very rapidly growing lung tumor suggests infection, infarction, small cell lung cancer, aggressive lymphoma9 or a fast-growing metastasis from tumors, such as germ cell tumors and certain sarcomas.10 Therefore, rapid growth of a lung tumor in six months with massive splenomegaly, as in our patient, indicates aggressive pulmonary lymphoma.
The diagnosis of extranodal NK/TCL is based on histopathology, immunophenotype and EBV status in conjunction with the anatomic site of presentation. Histopathological examination usually reveals: lymphoma cells with a broad cytologic spectrum; small, large or anaplastic in appearance; nucleoli are generally inconspicuous or small; and an angiodestructive growth pattern is frequently present.11 The immunophenotypic profile of the tumor cells bear CD2, and CD56 cytoplasmic CD3, while surface CD3 is usually absent.12 In addition, clonal Epstein-Barr virus (EBV) genomes are virtually always present. In situ hybridization for EBER is the preferred method of demonstrating the presence of EBV.13
There is no recommended treatment at present because of the extreme rarity of these cases. CHOP-based chemotherapy and surgical resection have been reported in the literature.14 Other treatment strategies, including combined field radiotherapy and chemotherapy, autologous bone marrow transplantation, and L-asparaginase treatment in relapsed cases,15,16 have been used in extranodal NK/TCL, but it remains difficult to cure.17 The five-year survival rate in extranodal NK/TCL is less than 50% and the median survival for advanced stage disease is only six to 12 months.17
In our case, positive EBER in tumor cells confirmed the positive correlation between lymphoma and EBV. Subtype 1 EBV identified from serum was compatible with its predominant geographic distribution in Asia. The patient didn't receive chemotherapy with the standard CHOP regimen because of advanced age and performance status. In conclusion, patients presenting with rapidly growing lung mass and massive splenomegaly may have aggressive pulmonary lymphoma. High circulating EBV DNA in extranodal natural killer/T-cell lymphoma would mean a poor prognosis. Identifying young high-risk patients may have benefits for early aggressive and successful treatment.
Disclosure
No authors report any conflict of interest.
References
- Cadranel J, Wislez M, Antoine M. Primary pulmonary lymphoma. Eur Respir J. 2002;20:750–762. doi: 10.1183/09031936.02.00404102. [DOI] [PubMed] [Google Scholar]
- Tamura A, Komatsu H, Yanai N, et al. Primary pulmonary lymphoma: relationship between clinical features and pathologic findings in 24 cases. The Japan National Chest Hospital Study Group for Lung Cancer. Jpn J Clin Oncol. 1995;25:140–152. [PubMed] [Google Scholar]
- Ooi GC, Chim CS, Liang R, Tsang KW, Kwong YL. Nasal T-cell/natural killer cell lymphoma: CT and MR imaging features of a new clinicopathologic entity. AJR Am J Roentgenol. 2000;174:1141–1145. doi: 10.2214/ajr.174.4.1741141. [DOI] [PubMed] [Google Scholar]
- Kinney MC. The role of morphologic features, phenotype, genotype, and anatomic site in defining extranodal T-cell or NK-cell neoplasms. Am J Clin Pathol. 1999;111(Suppl):S104–118. [PubMed] [Google Scholar]
- Cao MS, Cai HR, Yin HL, et al. Primary natural killer/T cell lymphoma of the lung: two cases report and clinical analysis. Zhonghua Jie He He Hu Xi Za Zhi. 2008;31:120–124. (In Chinese.) [PubMed] [Google Scholar]
- Xiao YL, Zhang DP, Wang Y. Primary pulmonary involvement of NK/T-cell lymphoma: report of two cases with literature review. Zhonghua Nei Ke Za Zhi. 2007;46:988–991. (In Chinese.) [PubMed] [Google Scholar]
- Lei KIK, Chan LYS, Chan WY, Johnson PJ, Lo YMD. Diagnostic and prognostic implications of circulating cell-free Epstein-Barr virus DNA in natural killer/T-cell lymphoma. Clin Cancer Res. 2002;8:29–34. [PubMed] [Google Scholar]
- Levy L, Nasereddin A, Rav-Acha M, Kedmi M, Rund D, Gatt ME. Prolonged fever, hepatosplenomegaly, and pancytopenia in a 46-year-old woman. PLoS Med. 2009;6:e1000053. doi: 10.1371/journal.pmed.1000053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunnick NR, Parker BR, Castellino RA. Rapid onset of pulmonary infiltration due to histiocytic lymphoma. Radiology. 1976;118:281–285. doi: 10.1148/118.2.281. [DOI] [PubMed] [Google Scholar]
- Collins VP, Loeffler RK, Tivey H. Observations on growth rates of human tumors. Am J Roentgenol Radium Ther Nucl Med. 1956;76:988–1000. [PubMed] [Google Scholar]
- Chan JK, Sin VC, Wong KF, et al. Nonnasal lymphoma expressing the natural killer cell marker CD56: a clinicopathologic study of 49 cases of an uncommon aggressive neoplasm. Blood. 1997;89:4501–4513. [PubMed] [Google Scholar]
- Jaffe ES. Classification of natural killer (NK) cell and NK-like T-cell malignancies. Blood. 1996;87:1207–1210. [PubMed] [Google Scholar]
- Chan JK, Yip TT, Tsang WY, et al. Detection of Epstein-Barr viral RNA in malignant lymphomas of the upper aerodigestive tract. Am J Surg Pathol. 1994;18:938–946. doi: 10.1097/00000478-199409000-00009. [DOI] [PubMed] [Google Scholar]
- Laohaburanakit P, Hardin KA. NK/T cell lymphoma of the lung: a case report and review of literature. Thorax. 2006;61:267–270. doi: 10.1136/thx.2004.025767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaccard A, Gachard N, Marin B, et al. Efficacy of L-asparaginase with methotrexate and dexamethasone (AspaMetDex regimen) in patients with refractory or relapsing extranodal NK/T-cell lymphoma, a phase 2 study. Blood. 2011;117:1834–1839. doi: 10.1182/blood-2010-09-307454. [DOI] [PubMed] [Google Scholar]
- Reyes VE, Jr, Al-Saleem T, Robu VG, Smith MR. Extranodal NK/T-cell lymphoma nasal type: efficacy of pegaspargase. Report of two patients from the United States and review of literature. Leuk Res. 2010;34:e50–54. doi: 10.1016/j.leukres.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt C, Sako N, Bagot M, Huang Y, Gaulard P, Bensussan A. Extranodal NK/T-cell lymphoma: toward the identification of clinical molecular targets. J Biomed Biotechnol. 2011;2011:790871. doi: 10.1155/2011/790871. [DOI] [PMC free article] [PubMed] [Google Scholar]


