Abstract
Although general anesthesia still represents the standard when performing thoracic surgery, the interest toward alternative methods is increasing. These have evolved from the employ of just local or regional analgesia techniques in completely alert patients (awake thoracic surgery), to more complex protocols entailing conscious sedation and spontaneous ventilation. The main rationale of these methods is to prevent serious complications related to general anesthesia and selective ventilation, such as tracheobronchial injury, acute lung injury, and cardiovascular events. Trends toward shorter hospitalization and reduced overall costs have also been indicated in preliminary reports. Monitored anesthesia care in thoracic surgery can be successfully employed to manage diverse oncologic conditions, such as malignant pleural effusion, peripheral lung nodules, and mediastinal tumors. Main non-oncologic indications include pneumothorax, emphysema, pleural infections, and interstitial lung disease. Furthermore, as the familiarity with this surgical practice has increased, major operations are now being performed this way. Despite the absence of randomized controlled trials, there is preliminary evidence that monitored anesthesia care protocols in thoracic surgery may be beneficial in high-risk patients, with non-inferior efficacy when compared to standard operations under general anesthesia. Monitored anesthesia care in thoracic surgery should enter the armamentarium of modern thoracic surgeons, and adequate training should be scheduled in accredited residency programs.
Keywords: General anesthesia, lung resection, pleural effusion, VATS
Introduction
Today's thoracic surgeons are expected to minimize the burden of their performance. A means to achieve this goal has been the development of minimally invasive surgical techniques, which are now the standard management in a broad spectrum of thoracic conditions in daily practice. Meanwhile, advances in anesthesiology and postoperative care have led to a remarkable reduction in morbidity rate and hospital stay.
In 2001, upon approval of our Institutional Review Board, we established an investigational program of thoracic operations performed without the employ of general anesthesia (GA) and one-lung ventilation (OLV). This program was supervised by Prof. T.C. Mineo as academic coordinator, and was aimed to refine techniques, indications, and physiopathological knowledge about this kind of operations. In previous papers, we, and other authors as well, adopted the term awake to indicate this surgical practice, as many patients remained fully alert during the operation.1–5 Subsequently, we moved to perform more technically demanding procedures, which required patients being sedated while maintaining spontaneous ventilation. Thus, we now prefer the term “monitored anesthesia care thoracic surgery” (MACTS), as it better fits this operative modality. In this article, we revisit the basics of awake and MACTS, with particular reference to pathophysiologic principles, techniques, presumed advantages (Table 1), and pitfalls. We also focus on the most relevant literature findings in this setting.
Table 1.
Supposed pros and cons of monitored anesthesia care thoracic surgery (MACTS)
| Supposed pros of MACTS |
| Lesser major and minor morbidity |
| Faster recovery |
| Shorter procedural times (extra procedures may be planned in the surgical session) |
| Shorter hospitalization |
| Reduced costs |
| Easier nursing management |
| Non-inferior efficacy assumption versus conventional approaches |
| Preserved antitumor activity as a result of reduced perioperative cortisol release → better NK activity |
| Debated issues |
| TEA-related side effects to be taken into the account when used |
| No availability of large number RCTs |
| Attributable benefits not yet calculated |
| Possible increase of surgical risks |
| Specific training mandatory |
| Patients' acceptance questionable |
NK, natural killer; RCTs, randomized controlled trials; TEA, epidural anesthesia.
Historical perspective
Thoracic surgery performed without GA dates back to World War I, when the need to treat dramatic gunshot wounds led to a better understanding of surgical pneumothorax.6 Thereafter, the familiarity with thoracic operations in non-anesthetized patients noticeably increased. In the 1950s, Vichniewsky7 and Ossipov8 in Russia, as well as Buckingham in the US,9 were able to perform major surgeries with sole locoregional anesthesia techniques in spontaneously ventilating patients.
Soon after the introduction of Carlens' double-lumen, OLV became the gold standard in thoracic surgery, and local or neuraxial analgesia techniques fell into disuse. They were eventually rediscovered in the late 1980s, alongside the widespread explosion of videothoracoscopy.10
Injurious effects of general anesthesia (GA) and one-lung ventilation (OLV)
Ventilator associated lung injury
Ventilator associate lung injury (VALI)11 entails a compartmental inflammatory status which leads, in turn, to interstitial edema, loss of surfactant,12 ventilation-to-perfusion mismatch, and decreased compliance.12 In patients with preexisting lung disease, VALI can evolve into a frank acute lung injury. This occurs in about 4% of major lung resections,13,14 and carries a mortality rate as high as 25%. Subclinical VALI possibly leading to minor respiratory impairment can still occur in healthy lungs, and independently on extent of surgical trauma.15,16
One-lung ventilation related injury
At the dependent lung, OLV induces ventilation-to-perfusion mismatch, and promotes further inflammatory changes.17–24 Hypoxic environment establishing at the non-dependent (non-ventilated) lung causes tissue acidosis, alveolar edema, vascular congestion,17–19 cytokine release,17 and an increased malondialdehyde level. These changes can occur early after minor operations and even in the absence of surgical trauma.24 At re-ventilation, high oxygen fraction triggers oxidative burst,25,26 leading to neutrophil recruitment27–30 and macrophage activation.31
Extrapulmonary side effects of GA and OLV
Extrapulmonary side effects are mediated by the spillover of cytokines toward the systemic circulation, and may include liver injury,32 natural-killer cell impairment33 and arrhytmias.34 The latter also include transient QT-tract prolongation, which has been shown to occur in up to 80% of patients with possible evolution to torsade de pointe in 0.4% of these.35
Hypoxemia
Hypoxemia is defined as SaO2 < 90%, with an inspired oxygen fraction of 0.5. It occurs in 10% of thoracic operations, mostly during right procedures, in the supine position, and in heavy smokers.36 Double-lumen tube dislodgement is also a frequent cause.36
Tracheobronchial rupture
Although its estimated rate is quite low (1/20.000), tracheobronchial rupture may carry a mortality rate as high as 22%.37 Women seem to be at a higher risk, probably as a result of their smaller airway caliber. Deteriorated clinical conditions and radiotherapy also significantly increase the risk; therefore, MACTS seems justified in these kinds of thoracic patients. Injury to the hypopharynx, esophagus, and vocal cords may also rarely occur, although a reliable incidence estimate is not available.
Indications
MACTS is mainly considered when performing minor to intermediate surgery in patients with increased risk using GA, even if it can be offered to healthy patients as well. So far, no definitive recommendations have been established in this regard. We are relying now on the Thoracoscore-based risk stratification38 parameters to select optimal candidates. These include: age >65 years, degree of dyspnea ≥2, American Society of Anesthesiology score ≥3, performance status ≥3, and comorbidity index ≥3. We have set a risk of postoperative death of 2.3% as a reliable threshold to consider patients for awake or MACTS procedures. This figure matches that of an over-65 male patient with composite comorbidity status, a frequent scenario of daily practice. Main contraindications to MACTS are summarized in Table 2.
Table 2.
Contraindications to monitored anesthesia care thoracic surgery (MACTS)
| Technically demanding operation (related to surgeons' skills) |
| Urgent surgery with less than <6 hours fasting (risk of regurgitation). |
| Hemodinamically unstable patients |
| Known allergy to local anesthetic |
| High risk of intraoperative seizure |
| Patient unable to cooperate |
| Past medical history of pleurisy |
| Previous ipsilateral thoracic surgery |
| Medium to severe obesity (BMI > 35) |
| Central hypoventilation syndrome |
| Validated non-surgical options available |
| Spine deformities (if TEA to be used) |
| INR > 1.5 or current antiplatelet therapy |
| Brain edema (if TEA to be used) |
BMI, body mass index; INR, international normalized ratio; TEA, epidural anesthesia.
Basic surgical principles
In spontaneously ventilating subjects, a surgical opening of the chest wall will immediately result in the entry of air within the pleural cavity. As a consequence, the lung will collapse at a volume below its functional residual capacity, and the chest wall will expand at 60% of total lung capacity. The space established between the deflated lung and the expanded chest allows a quite satisfactory surgical exposure, so that additional CO2 insufflation is unnecessary.
Another common finding is that the deflated lung remains almost completely motionless during MACTS, despite preserved diaphragmatic function. This fact might be explained by some amount of lung tissue hysteresis39,40 established at lower lung volumes. However, if a further force is applied, the lung will inflate promptly. This can occur by asking the patient to breathe deeply and cough, after having inserted an intrapleural chest tube to favor air outflow. All of these maneuvers are useful to achieve complete lung expansion at the end of the operation, and it would be, therefore, desirable to have the patient able to respond purposefully to commands at this stage.
Cough may disturb surgical manipulations and put the patient at risk of unsafe surgery. It is usually triggered by vagus nerve stretching. Preventive methods include atropine premedication, intravenous or aerosolized lidocaine, stellate ganglion blockade41 or vagus nerve blockade. The latter is performed by instillation of 0.2% bupivacaine at the lower trachea or aortopulmonary window during right and left procedures, respectively.
Anesthesiology techniques
Analgesia
Commonly employed analgesia techniques to perform awake or MACTS surgery entails thoracic epidural (TEA), paravertebral (PVB) or intercostal blockade, or just local anesthetic injection.
TEA can provide a thorough analgesia of the chest wall and pleural cavity, and is indicated to perform more complex procedures. We usually insert a TEA catheter at the T3-T5 level, even though a lower or higher position may be considered depending on surgical needs.41 After insertion, it is useful to have the patient lie on the side to be operated on for 15–20 minutes prior to surgery, to allow gravity diffusion of the analgesic drug.42,43 Non-analgesic effects of TEA include improved myocardial blood flow, prevention of arrhythmias, quicker gastrointestinal recovery, and reduced hypercoagulability.3 Supposed adverse effects include impaired compensatory ventilation44,45 and bronchial constriction.46–48 These effects seem to be of poor practical relevance, although they have not been tested during MACTS procedures. Hypotension may occur as well. Although the estimated risk of epidural hematoma is low (<1/150.000), recent surveys have suggested a trend toward an increase.48 Therefore, it should be avoided in patients with impaired clotting mechanisms. We also avoid TEA in patients with intracranial hypertension, because of the risk of cerebrospinal fluid leak in the event of misplacement.
PVB is being increasingly employed in MACTS surgeries.49 As thoracic paravertebral space lacks anatomical separations between adjacent segments, a single anesthetic drug injection is sufficient to provide a broad analgesic coverage throughout the operation. A paravertebral catheter may be also placed whenever a durable analgesia is desired. Ultrasound guidance can reduce the risk of misplacement and prevent complications, such as pneumothorax (0.5% incidence).50
Patient preparation
A careful preanesthetic assessment must be performed in order to evaluate possible pitfalls and to prepare a contingency plan. Hematosis must be monitored by pulse-oxymeter and repeated blood samples taken through an arterial cannula. Non-invasive end-tidal CO2 measurement on exhaled breath may still be reliable for the same purpose.
Drugs
An ideal MACTS protocol should provide adequate analgesia and sedation, while protecting the airway and the patients' responsiveness (“twilight” sleep). In general, short-acting agents with rapid elimination are preferred. Propofol infusion at subhypnotic dosage is frequently employed, even because of the antiemetic properties. Midazolam can be responsible for postoperative psychomotor impairment and increased airway resistance so we limited its use to preoperative anxiolysis.51 Opioids can also be used, but the risk of hypoventilation and intraoperative nausea should be considered. The synthetic opioid Remifentanil at an infusion rate of 0.1 mcg/kg per minute seems to be particularly suitable for MACTS, as it is rapidly metabolized by tissue and plasma esterases. A summary of the most frequently employed sedation protocols in awake and MACTS procedures is provided in Table 3.
Table 3.
Most relevant literature findings
| Author (Ref) | Patients | Case mix | Analgesia | Sedation level | Drugs | Conversion rate† | Perioperative morbidity |
|---|---|---|---|---|---|---|---|
| Katlic (52) | 353 | Various | LA | ++ | Md (P) | 0% | 3% |
| Ke-Fe-Pro (M) | |||||||
| Tseng (53) | 46 | Small nodules resection | TEA | ++ (Ramsay III) | Fe (P) | 4.3% | 8.7% |
| Pro (M) | |||||||
| Wu (54) | 36 | VATS lobectomy | TEA | ++ (Ramsay III) | Fe (P) | 2.8% | 25%‡ |
| Pro (M) | |||||||
| Al-Abdullatief (41) | 79 | Major surgeries | TEA | + | Md (P) | 9% | 11% |
| >Fe (P) | |||||||
| Smit (55) | 45 | Pleural diseases | LA | −/+ | Dro/Fe (P) | 0% | 0% (2 failures reported) |
| Md (M) | |||||||
| Tschopp (56) | 89 | Primary PNX (talc insufflation) | LA | + | Md/Phe (M) | 0% | 0% (9 redo procedures reported) |
| Our group (57) | 17 | Stage 2 pleural empyema | TEA | −/+ | Md (P) | 0% | 9% (minor; 1 failure reported). |
| PVB | |||||||
| Our group (58) | 11 | VATS LVRS | TEA | – | Md (P) | 9% | 3 patients with prolonged air-leaks |
| Chen (59) | 285 | Lung resections | TEA | ++ (Ramsay III) | Fe (P) | 4.9% | 8.1% |
| Fe/Pro (M) | |||||||
| Sakuraba (60) | 32 | Tubercular effusions | LA | – | None | 0% | 0% |
| Macchiarini (61) | 20 | Tracheal resections | CEA | + | Md (P) | 0% | 20% (minor) |
| Re (M) | |||||||
| Noda (62) | 15 | Secondary PNX (bleb resection + abrasion) | LA | Not reported | Not reported | 0% | 4 patients with prolonged air-leaks§ |
| TEA | |||||||
| Nezu (63) | 36 | Recurrent primary PNX (bleb resection) | LA | + | But (M) | 0% | 10% (minor) |
| Dia (M) | 1 failure reported |
Slash bar indicates combined drug administration. −/+: fully alert patient, light sedation given as required. +: light sedation preferred, ++: mild sedation preferred. †Conversion to general anesthesia/orotracheal intubation. ‡No difference when compared to video-assisted thoracic surgery (VATS) lobectomy under general anesthesia. §0% morbidity rate if surgical complication is excluded (P = 0.02 vs. general anesthesia at propensity score comparison). But, butorphanole; CEA, cervical epidural; Dia, diazepam; Dro, droperidole; Fe, fentanyl; ICB, intercostals block; Ke, ketamine; LA, local anesthesia; LVRS, lung volume reduction surgery; (M), maintenance; Md, midazolam; (P), premedication; Phe, phetidine; PNX, pneumothorax; Pr, propofol; PVB, paravertebral block; Re, remifentanil; Su, sufentanyl; TEA, thoracic epidural.
Pitfalls and complications
In referral publications, the overall conversion rate to general anesthesia ranged between <1% and 9% (mean: 2.4%), with intermediate and major surgeries at increased risk (Tables 3, 4). Conversions are mostly because of surgical reasons, adhesions being the most frequent. Non-surgical causes included respiratory impairment, suboptimal pain control, and cough (Table 4).
Table 4.
Cumulative analysis of causes of conversion to general anesthesia
| Event | N/Relative incidence (%) | Absolute incidence (%) |
|---|---|---|
| Unmanageable adhesions | 10 (27.7%) | 0.69% |
| Mediastinal movements | 5 (13.8%) | 0.34% |
| Bleeding/organ laceration | 5 (13.8%) | 0.34% |
| Changes in surgical plan | 5 (13.8%) | 0.34% |
| Hypoxemia | 4 (11.1%) | 0.27% |
| Suboptimal analgesia | 3 (8.3%) | 0.21% |
| Intractable cough | 1 (2.7%) | <0.1% |
| Hypercarbia | 1 (2.7%) | <0.1% |
| Tachypnea | 1 (2.7%) | <0.1% |
| Cardiac arrest | 1 (2.7%) | <0.1% |
| Total | 36 | 2.4% |
| Total (Intermediate/major operations) | 35 (97.2%) | 5.1% |
Data of 1441 patients from selected references with at least 10 consecutive procedures and our 15 institutional 2002–2013 years experience.
Several authors have studied the changes in hematosis occurring during awake and MACTS, and the results appear to be univocal. Dong et al. reported no intraoperative impairment in oxygenation indexes in patients undergoing minor MACTS operations.64 In the same paper, arterial PaCO2 rose to an average permissive level of 65 mmHg, but it was never responsible for conversion to general anesthesia. Hypercarbia was likely to be related to a reduced ventilatory drive, rather than an effect of surgical pneumothorax, as suggested by the parallel drop in respiratory rate. We have reported similar changes to occur in empyema operations57 and during lung volume reduction for advanced emphysema.58
Should conversion to GA become necessary, the anesthesiology staff must be equipped to proceed with a rapid orotracheal intubation. If the surgeon doesn't deem OLV to be strictly necessary to complete the operation, a single-lumen tracheal tube, as well as supraglottic devices or just a facial mask can be used to assure mechanical ventilation. A chest drainage connected to waterseal must be ready for use on the scrub nurse table and inserted to avoid lung collapse during anesthesiology maneuvers, while the surgical openings are temporarily closed by means of adhesive dressing.
Monitored anesthesia care thoracic surgery (MACTS) in thoracic oncology
Resection of pulmonary nodules
MACTS may be considered to perform videothoracoscopic or open wedge resection of peripheral lung nodules of any origin42,65–67 (Table 3). However, it is not indicated for deeply located nodules, and those >3 cm in size, as intraoperative detection and resection may be quite demanding in spontaneously ventilating patients. We employ a standard three-port videothoracoscopic approach, under either local or regional analgesia. It is essential to carefully plan the sites for trocars insertion, as a suboptimal surgical exposure may be responsible for a time-consuming and stressful procedure in conscious patients. A wise strategy is to insert first the 30-degree camera at the 4th intercostal space, on the anterior axillary line, with the surgeon standing frontally. At this level, the intercostal space is wider than it is posteriorly, so that preliminary exploration of the pleural cavity will be less painful should the analgesia level be suboptimal. The other accesses may be then performed under visual control, taking into the account the location of the nodule. Tseng et al.,53 have employed needlescopic instrumentation through 3-mm skin incisions. Lesser employed a two-ports method with 2- and 11-mm openings and a laser device for nodule excision.68 Rocco et al. employed a single-port technique in an ambulatory setting.65 Interestingly, these authors obtained lung exclusion by means of a Fogarty balloon inserted via flexible bronchoscopy.
We comparatively analyzed a series of 60 patients undergoing lung nodule excision by standard videothoracoscopy under either GA or MACTS.42 The latter procedure resulted in a significantly shorter hospital stay (two vs. three days) and less nursing care required (2.5 vs. four calls per day). The patients' median satisfaction score and perioperative arterial oxygenation results were better as well. These results have led us to elect this strategy as the elective one in high-risk patients, whenever it is deemed feasible and oncologically adequate.
Non-small cell lung cancer
MACTS may be used to wedge out peripheral, stage I non-small cell lung cancer (NSCLC) in patients unfit for general anesthesia.66,69 We have operated on a small series of such patients, achieving a three-year overall survival rate of 72%. This figure compares well with non-surgical ablative methods, including radiofrequency ablation and stereotaxis.
Wedge resection is not intended to be a substitute of lobar resection, which remains the gold standard in stage I NSCLC. In this regard, however, it should be highlighted that large retrospective studies have found no substantial difference between the two approaches when restricting the analysis to peripheral tumors less than 1 cm in diameter.70 This presentation is likely to become more and more frequent because of the establishment of lung cancer screening programs. When putting these considerations together, we feel that MACTS could be included in the armamentarium of NSCLC management in functionally deteriorated patients, who could actually benefit from an atraumatic tumor excision.
MACTS metastasectomy
MACTS can be considered in selected patients scheduled for videothoracoscopic metastasectomy.67 Its limit, however, is the difficulty to palpate the lung in search of radiologically occult lesions, which can be found in more than 10% of patients. Thus, we reserved this method for patients with a presumably single and easy to remove lung lesion, or whenever resection is indicated for diagnostic purpose. A possible advantage of MACTS in this setting could be a better preservation of perioperative natural-killer cell count,71 which is supposed to protect against further metastatic spread in the early perioperative period. Other fascinating hypotheses regarding the possible link between avoidance of GA and improved oncologic outcome are preserved perioperative immunosurveillance as a result of lesser cortisol release, and the direct anticancer effects of local anesthetic.72 Also, reduced opioid usage has been considered, as they have been found to promote cancer cell growth in vitro.
MACTS for malignant pleural effusion
Patients with recurrent pleural effusion often present with deteriorated conditions as a result of concomitant organ failure and/or underlying malignancy. Therefore, they are expected to benefit from the avoidance of GA. MACTS management of pleural effusion is derivative of the so-called “medical thoracoscopy,” but differs as it implies the surgeon must be skilled in performing more complex maneuvers, such as adhesiolysis and hemostasis.73 During the procedure, the patient can be fully awake, although twilight sedation may be needed in some instances. In this regard, Griffo et al. found no substantial advantage when using midazolam + sufentanyl or continuous remifentanyl administration as the sedation protocol.74 Sole local injection of anesthetic agents at the site of surgical opening(s) can be sufficient to attain a satisfactory analgesic level. We prefer a mixture of 2% lidocaine and 7.5% ropivacaine, to provide a rapid analgesic onset alongside a long-lasting effect. Additional injection can be performed at the site(s) of pleural biopsy, which can be painful in non-anesthetized patients. We usually perform MACTS for pleural effusion by inserting one 20 mm flexible trocar at the 6th–7th intercostal space, on the midaxillary line. The surgical opening resulting from previous chest drainage placement may also be used, unless overwhelming infection is suspected. A 30-degree thoracoscope is then coaxially inserted with the other operative instruments. Alternatively, a videomediastinoscope can be used, as described by Rusch and Mountain in 1987.10 Evacuation of pleural fluid collection should be performed gradually to avoid the risk of re-expansion pulmonary edema. The ability of the lung to expand is checked by asking the patient to breathe deeply, after having inserted chest drainage in the pleural cavity coaxially with the thoracoscope. We prefer to use talc as the elective pleurodesic agent, because of its efficacy75 and possible inhibition of tumor growth via an anti-angiogenic effect.76 The potential risk of severe adverse reactions, such as acute lung injury, can be obviated with the use of a large-particle (5–70 μm) talc powder. Spraying of talc may trigger intraoperative pain, therefore, short-acting local anesthetics should be administered intrapleurally before proceeding with pleurodesis. Occasionally, it can be useful to increase the sedation level at this stage of the operation.
Success rates for videothoracoscopic talc pleurodesis range from 84% to 95%.77–79 These figures are higher than those observed after bedside “slurry” instillation of talc80 or of other agents.77,81 It also seems to achieve better results in terms of quality of life.82 Controversial results, however, have been reported by Debeljiac et al., who found a higher complication rate of MACTS when compared with talc slurry (73% vs. 41%),83 and no superior efficacy. The indication to MACTS should also be balanced against the insertion of an indwelling intrapleural catheter (PleurX).84 The latter has the advantage of very short hospitalization, and is quite a simple technique. However, because of the necessity for continuous home nursing assistance and outpatient visits, the cost-efficacy of PleurX is limited to patients with a very short life-expectancy.85
MACTS in management of anterior mediastinal masses
In patients with huge mediastinal masses, GA can trigger serious cardiac and respiratory complications.86 Therefore, a MACTS procedure could be considered whenever a surgical biopsy is required. An anterior mediastinal mass may be approached by either mediastinotomy87,88 or videothoracoscopy.89 The latter should be preferred whenever concomitant conditions, which also may benefit from videothoracoscopic management, exist, such as pleural effusion, pericardial effusion, chylothorax, and lung nodules. The technique does not differ substantially from the same procedure performed under general anesthesia. A 45-degree flank position allows an ergonomic approach to the anterior mediastinum, and facilitates a switch to the supine position should emergent intubation become necessary. One 20-mm trocar insertion at the 4th–5th on the anterior axillary line for coaxial instrument placement is usually sufficient. Biopsies are taken after instillation of an extra local anesthetic dose on the site to be sampled. In our experience, the diagnostic accuracy of MACTS in this setting was 100%. One of the main concerns, however, is the potential for difficult airway control should urgent intubation become mandatory. This risk is higher in patients with tracheal compression and lumen reduction of >50%. In this instance, the surgical theater should be equipped to perform urgent rigid bronchoscopy. Some authors even recommend being prepared to perform emergent extracorporeal circulation.90
If a clinical patient's presentation indicates a foreseeable risk in this regard, a compromise solution could be to proceed with awake orotracheal intubation, then administer deep sedation without myorelaxants in order to maintain spontaneous ventilation. The alpha-2 agonist dexmedetomidine has been proven to be of great effectiveness in managing cases such as this, as it can provide light to deep sedation without affecting respiratory drive.91
MACTS in non-malignant diseases of the lung and pleural cavity
Primary spontaneous pneumothorax
MACTS videothoracoscopic bullectomy, with or without pleurodesis, can be offered to patients with recurring primary spontaneous pneumothorax.43,63 Supposed pros of this method include a fast recovery, shorter hospitalization, and reduced overall costs,43 although these results have not yet been demonstrated in a large-number, randomized controlled trial. Surgery may be carried out by means of LA or TEA, even if the latter should be preferred when pleural abrasion is planned.43 Usually, young patients with primary spontaneous pneumothorax have limited or no adhesions at all, therefore, surgery is highly feasible in practically all instances. Talc pleurodesis may still be a valid alternative in instances where bullectomy is impractical or unfeasible.56,92
MACTS management of secondary pneumothorax
Noda et al. reported on a propensity-score matched comparison of patients with secondary pneumothorax treated by videothoracoscopic bullectomy under either spontaneous ventilation or GA-OLV.62 Interestingly, they found no respiratory complication in the former group, while adult respiratory distress syndrome and pneumonia occurred in half of patients undergoing GA (P = 0.02). A trend toward increased in-hospital mortality was also found in the latter group, although statistical significance was not reached (P = 0.06). Yet another presumed advantage of MACTS in this setting could be a reduced risk of intraoperative contralateral pneumothorax, although no evidence is currently available to support this hypothesis.
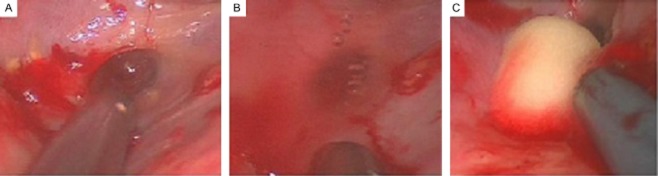
A limitation of MACTS, however, is the difficulty to detect air-leaks without the help of positive pressure ventilation. A reliable tool in this regard could be the intrapleural instillation of a contrast mean trough, a double-lumen chest tube under thoracography control prior to surgery.62 Whenever an air leak is found and stapled resection or suturing is unpractical, air-leak repair can be attempted by the application of biologic or synthetic sealants (Figs 1, 2). Cyanocrylate glue is particularly suitable for use in MACTS, because of its simplicity of use, adhesive strength, and fast sealing.93
Figure 1.
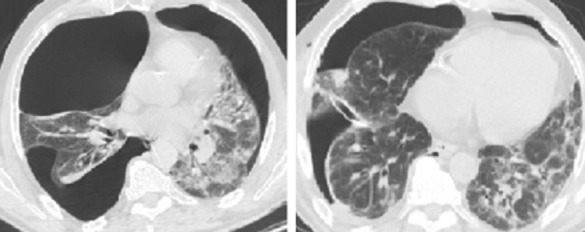
A 53 year old patient with complex bilateral pneumothorax secondary to bronchiectasis and interstitial lung disease (left). An attempt at blind drainage insertion resulted in parenchymal injury because of adhesions (right).
Figure 2.
Same case as Figure 1. (a) A 53 year old patient with complex bilateral pneumothorax secondary to bronchiectasis and interstitial lung disease. A round-shaped wound is found on the lung surface. (b) Bubbling is seen on spontaneous ventilation, indicating an active air-leak. (c) The defect was repaired by means of thrombin foam instillation under local anesthesia and twilight sedation.
MACTS treatment of pulmonary emphysema
In 2005, we presented a videothoracoscopic nonresectional technique to perform lung volume reduction (LVR) for advanced emphysema,58 basically consisting of introflexive stapling of the lung apex. As the technique allows a fast and atraumatic LVR, it is highly suitable to be performed in spontaneously ventilating patients, thus avoiding the detrimental adverse effects of GA and OLV in these delicate patients. As no portion of lung is cut, introflexive stapling also can significantly reduce the rate of postoperative air-leak,94 which is the most frequent complication after LVR.
In our preliminary reports, introflexive stapling under spontaneous ventilation was as effective as standard resectional LVR under GA-OLV in relieving symptoms and improving lung function. However, adequate training is required, as hyperinflated lung and adhesions may render the surgery quite demanding. The results of our technique should also be prospectively balanced against non-surgical LVR options.
MACTS management of bullous emphysema
MACTS may be used to manage bullous emphysema as well.95,96 The bulla can be removed or obliterated with the use of a no-cut endoscopic stapler. Under spontaneous ventilation, a large bulla will remain inflated, thus grasping and excision can be more demanding than under GA-OLV. In these instances, it is useful to make the bulla deflate by performing a small opening on its surface. We have operated on 35 patients in this way, with a median volume of the bulla of 688 mL.96 The median hospital stay was 4.9 days, and no major complication occurred. Only one patient required conversion to open procedure under general anesthesia and OLV because of unmanageable adhesions. A randomized comparison is needed to better understand the real benefits of this technique over conventional surgical approaches.
MACTS lung biopsy for interstitial lung disease
Whenever a lung biopsy is required to diagnose an interstitial lung disease, the MACTS approach should be considered. Indeed, these patients must be deemed at highest risk for GA and OLV, which may carry a mortality rate ranging from 1.5% to 4.7%.97–99
Surgery can be accomplished by either TEA or local anesthesia injection. It should be highlighted, however, that TEA has been experimentally shown to inhibit right ventricular adaption to increased pulmonary vascular resistance.100 This pathophysiologic mechanism, though not yet demonstrated in humans, may be responsible for acute right ventricular failure in the perioperative period. We have also found, in a preliminary comparative evaluation, that TEA in these patients results in higher intraoperative PaCO2 than local injection alone (data unpublished).
The surgical procedure entails one or more wedge lung resections. A three-trocar approach should be preferred. Indeed, it allows the procurement of multiple samples from lung areas presenting with diverse macroscopic change, and is a faster method than a single access approach. The latter may also result in a more demanding and time-consuming procedure, as a result of instrument competition.
We have performed MACTS on 41 patients, with no postoperative complications and a diagnostic yield of 100%. In more than 70%, it resulted in management change, a figure in agreement with that reported for the equivalent procedure under GA.98,99 Thus, we are prone now to consider MACTS as the elective procedure in these instances.
MACTS management of empyema thoracis
MACTS may be considered in high-risk patients needing treatment for fibrinopurulent, stage II empyema thoracis.10,52,57 During surgery, the patient is placed in a full lateral position with slight trunk elevation. Flexible bronchoscopy is mandatory prior to the procedure to rule out bronchial fistula, which may result in inundation of the contralateral lung during surgery. Sites for trocars insertion should be planned based on computed tomography (CT) scan findings, paying attention to avoid any injury to the underlying lung, as focal adhesions are likely to exist. Preoperative ultrasound assessment can also be quite a useful tool in this regard.101
As the lung is entrapped by thickened visceral pleura, respiratory motion is virtually absent; therefore, the feasibility of the procedure equals that of video-assisted thoracic surgery (VATS) under GA. On the other hand, slight lung inflation can improve identification of the visceral pleural layer during removal. Gradual lung re-expansion is checked in real time, while decortication is performed.
We have performed MACTS on a series of 19 patients, obtaining a success rate of 93%. A switch to general anesthesia was not necessary, even in patients requiring conversion to limited muscle-sparing thoracotomy.57
A concern exists that using TEA in these patients may carry a certain risk of epidural abscess, because a pathway between the purulent collection and the epidural space can be created in case of misplacement. The estimated risk of epidural abscess ranges between 1.1/100.000 and 1/1930,102 although it is mostly attributable to the use of indwelling epidural catheter for perioperative analgesia, rather than intraoperative use. However, one should be aware of this severe complication, and confirmatory magnetic resonance imaging should be immediately required in case of clinical suspicion. Also, it is wise to consider alternative analgesia techniques whenever conditions favoring epidural catheter misplacement or bacterial spread do exist.103
Major awake and MACTS procedures
Myasthenia gravis
The Kanazawa University group (Japan) has performed extended thymectomy, through either videothoracoscopic or infrasternal approach, in patients with grade I-II myasthenia gravis.104,105 Anesthesia protocol included TEA and spontaneous ventilation in fully alert patients. Patients with thymoma less than 3 cm in diameter were also included. Feasibility was excellent, and no major intraoperative complications occurred in this series. One patient had mild hypoxemia (SaO2: 92%) as a result of bilateral pneumothorax, which was managed without conversion to GA. The main rationale was avoidance of myorelaxant drugs, which may be responsible for an intensive-care unit stay in myasthenic patients. Overall, their results have been quite satisfactory, and the vast majority patients were able to eat and drink within one hour of surgery.
MACTS major lung resections
A series of 137 MACTS videothoracoscopic lobectomies for stage I NSCLC patients have been published by Chen et al.69,106 The conversion rate was 4.5%, mostly resulting from refractory hypoxemia, unsatisfactory analgesia, bleeding, and adhesions (Tables 3, 4). The authors found a significantly lower rate of anesthesia-related complications when compared to VATS lobectomies performed with GA, and a non-significant trend toward a reduced global morbidity (P = 0.057) and shorter hospital stay (P = 0.07). Interestingly, the feasibility of mediastinal sampling was the same between groups.106 Wu et al. have reported similar results when restricting the comparative analysis to elderly patients.54 However, data on long term survival is not yet available, therefore, the oncological adequacy of this pioneering technique is still to be evaluated.
MACTS for tracheal resection-anastomosis
Macchiarini has performed tracheal resection-anastomosis under cervical epidural anesthesia in a series of 20 patients.61 The main rationale was to avoid endotracheal tube traumatism at the anastomotic level. The mean length of resected trachea was 4.5 cm, and mobilization maneuvers were necessary in 60% of cases. No major complications were reported, and the postoperative length of stay was three days.
Conclusions
In recent years, awake- and MACTS have become reliable instruments in the armamentarium of an increasing number of thoracic surgeons worldwide, and we are confident that adequate training will be shortly planned in accredited residency programs. It would be highly desirable, however, to look at these methods in a multidisciplinary and patient-centered light, rather than merely considering these as “alternative” surgical options. Anesthesiologists, oncologists, interventional radiologists, chest physicians, and specialized nurses should be involved in the decision-making process, as well as in designing clinical trials. We believe that this policy will help to overcome a certain understandable reluctance to consider this surgical practice as a fully reliable tool. We hope that further speculation will soon contribute to a better definition of the indications and potentialities of awake- and MACTS in the public health care service.
Acknowledgments
We wish to thank Dr. Federico Tacconi, Senior Researcher, for support in the literature review.
Disclosure
No authors report any conflict of interest.
References
- Kao MC, Lan CH, Huang CJ. Anesthesia for awake video-assisted thoracic surgery. Acta Anaesthesiol Taiwan. 2012;50:126–130. doi: 10.1016/j.aat.2012.08.007. [DOI] [PubMed] [Google Scholar]
- Mineo TC, Ambrogi V. Efficacy of awake thoracic surgery. J Thorac Cardiovasc Surg. 2012;143:249–250. doi: 10.1016/j.jtcvs.2011.08.046. [DOI] [PubMed] [Google Scholar]
- Mineo TC. Epidural anesthesia in awake thoracic surgery. Eur J Cardiothorac Surg. 2007;32:13–19. doi: 10.1016/j.ejcts.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Mineo TC, Ambrogi V. Awake thoracic surgery for secondary spontaneous pneumothorax: another advancement. J Thorac Cardiovasc Surg. 2012;144:1533–1534. doi: 10.1016/j.jtcvs.2012.06.061. [DOI] [PubMed] [Google Scholar]
- Guarracino F, Gemignani R, Pratesi G, Melfi F, Ambrosino N. Awake palliative thoracic surgery in a high-risk patient: one-lung, non-invasive ventilation combined with epidural blockade. Anaesthesia. 2008;63:761–763. doi: 10.1111/j.1365-2044.2008.05443.x. [DOI] [PubMed] [Google Scholar]
- Heuer GJ. Empyema of the pleural cavity. Ann Surg. 1923;78:711–724. doi: 10.1097/00000658-192312000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vischnevski AA. Local anesthesia in thoracic surgery: lungs, heart and esophagus. Minerva Anestesiol. 1954;20:432–435. (In Italian.) [PubMed] [Google Scholar]
- Ossipov BK. Local anesthesia in thoracic surgery: 20 years experience with 3265 cases. Anesth Analg. 1960;39:327–332. [PubMed] [Google Scholar]
- Buckingham WW, Beatty AJ, Brasher CA, Ottosen P. The technique of administering epidural anesthesia in thoracic surgery. 1950. Chest. 2009;136:e30. doi: 10.1378/chest.17.5.561. (5 Suppl.): [DOI] [PubMed] [Google Scholar]
- Rusch VW, Mountain C. Thoracoscopy under regional anesthesia for the diagnosis and management of pleural diseases. Am J Surg. 1987;154:274–278. doi: 10.1016/0002-9610(89)90609-0. [DOI] [PubMed] [Google Scholar]
- Belperio JA, Keane MP, Lynch JP, 3rd, Strieter RM. The role of cytokines during the pathogenesis of ventilator-associated and ventilator-induced lung injury. Semin Respir Crit Care Med. 2006;27:350–364. doi: 10.1055/s-2006-948289. [DOI] [PubMed] [Google Scholar]
- Pavone LA, Albert S, Carney D, Gatto LA, Halter JM, Nieman GF. Injurious mechanical ventilation in the normal lung causes a progressive pathologic change in dynamic alveolar mechanics. Crit Care. 2007;11:R64. doi: 10.1186/cc5940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licker M, Fauconnet P, Villiger Y, Tschopp JM. Acute lung injury and outcomes after thoracic surgery. Curr Opin Anaesthesiol. 2009;22:61–67. doi: 10.1097/ACO.0b013e32831b466c. [DOI] [PubMed] [Google Scholar]
- Licker M, de Perrot M, Spiliopoulos A, et al. Risk factors for acute lung injury after thoracic surgery for lung cancer. Anesth Analg. 2003;97:1558–1565. doi: 10.1213/01.ANE.0000087799.85495.8A. [DOI] [PubMed] [Google Scholar]
- Herndon B, Yagan M, Reisz G, Ireland JC. Metabolic and biochemical responses of the healthy human lung to nonthoracic surgery. Lung. 2008;186:63–70. doi: 10.1007/s00408-007-9058-2. [DOI] [PubMed] [Google Scholar]
- Pinheiro de Oliveira R, Hetzel MP, dos Anjos Silva M, Dallegrave D, Friedman G. Mechanical ventilation with high tidal volume induces inflammation in patients without lung disease. Crit Care. 2010;14:R39. doi: 10.1186/cc8919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilling T, Kozian A, Huth C, et al. The pulmonary immune effects of mechanical ventilation in patients undergoing thoracic surgery. Anesth Analg. 2005;101:957–965. doi: 10.1213/01.ane.0000172112.02902.77. [DOI] [PubMed] [Google Scholar]
- Sugasawa Y, Yamaguchi K, Kumakura S, et al. The effect of one-lung ventilation upon pulmonary inflammatory responses during lung resection. J Anesth. 2011;25:170–177. doi: 10.1007/s00540-011-1100-0. [DOI] [PubMed] [Google Scholar]
- Yin K, Gribbin E, Emanuel S, et al. Histochemical alterations in one lung ventilation. J Surg Res. 2007;137:16–20. doi: 10.1016/j.jss.2006.04.038. [DOI] [PubMed] [Google Scholar]
- Funakoshi T, Ishibe Y, Okazaki N, et al. Effect of re-expansion after short–period lung collapse on pulmonary capillary permeability and pro-inflammatory cytokine gene expression in isolated rabbit lungs. Br J Anaesth. 2004;92:558–563. doi: 10.1093/bja/aeh101. [DOI] [PubMed] [Google Scholar]
- Kozian A, Schilling T, Röcken C, Breitling C, Hachenberg T, Hedenstierna G. Increased alveolar damage after mechanical ventilation in a porcine model of thoracic surgery. J Cardiothorac Vasc Anesth. 2010;24:617–623. doi: 10.1053/j.jvca.2009.09.016. [DOI] [PubMed] [Google Scholar]
- You Z, Feng D, Xu H, et al. Nuclear factor-kappa B mediates one-lung ventilation-induced acute lung injury in rabbits. J Invest Surg. 2012;25:78–85. doi: 10.3109/08941939.2011.603817. [DOI] [PubMed] [Google Scholar]
- Misthos P, Katsaragakis S, Milingos N, et al. Postresectional pulmonary oxidative stress in lung cancer patients. The role of one-lung ventilation. Eur J Cardiothorac Surg. 2005;27:379–382. doi: 10.1016/j.ejcts.2004.12.023. [DOI] [PubMed] [Google Scholar]
- Ahn HJ, Kim JA, Yang M, Shim WS, Park KJ, Lee JJ. Comparison between conventional and protective one-lung ventilation for ventilator-assisted thoracic surgery. Anaesth Intensive Care. 2012;40:780–788. doi: 10.1177/0310057X1204000505. [DOI] [PubMed] [Google Scholar]
- Tekinbas C, Ulusov H, Yulug E, et al. One-lung ventilation: for how long? J Thorac Cardiovasc Surg. 2007;134:405–410. doi: 10.1016/j.jtcvs.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Bastin AJ, Sato H, Davidson SJ, Quinlan GJ, Griffiths MJ. Biomarkers of lung injury after one-lung ventilation for lung resection. Respirology. 2011;16:138–145. doi: 10.1111/j.1440-1843.2010.01870.x. [DOI] [PubMed] [Google Scholar]
- Olivant Fisher A, Husain K, Wolfson MR, et al. Hyperoxia during one lung ventilation: inflammatory and oxidative responses. Pediatr Pulmonol. 2012;47:979–986. doi: 10.1002/ppul.22517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YJ, Chan KC, Chien CT, Sun WZ, Lin CJ. Oxidative stress during 1-lung ventilation. J Thorac Cardiovasc Surg. 2006;132:513–518. doi: 10.1016/j.jtcvs.2006.03.060. [DOI] [PubMed] [Google Scholar]
- Cobelens PM, van Putte BP, Kavelaars A, Heijnen CJ, Kesecioglu J. Inflammatory consequence of lung ischemia-reperfusion injury and low-pressure ventilation. J Surg Res. 2009;153:295–301. doi: 10.1016/j.jss.2008.04.022. [DOI] [PubMed] [Google Scholar]
- Leite CF, Calixto MC, Toro IF, Antunes E, Mussi RK. Characterization of pulmonary and systemic inflammatory responses produced by lung re-expansion after one-lung ventilation. J Cardiothorac Vasc Anesth. 2012;26:427–432. doi: 10.1053/j.jvca.2011.09.028. [DOI] [PubMed] [Google Scholar]
- Wolf PS, Merry HE, Farivar AS, McCourtie AS, Mulligan MS. Stress-activated protein kinase inhibition to ameliorate lung ischemia reperfusion injury. J Thorac Cardiovasc Surg. 2008;135:656–665. doi: 10.1016/j.jtcvs.2007.11.026. [DOI] [PubMed] [Google Scholar]
- Yulug E, Tekinbas C, Ulusoy H, et al. The effects of oxidative stress on the liver and ileum in rats caused by one-lung ventilation. J Surg Res. 2007;139:253–260. doi: 10.1016/j.jss.2006.08.041. [DOI] [PubMed] [Google Scholar]
- Tonnesen E, Höhndorf K, Lerbjerg G, Christensen NJ, Hüttel MS, Andersen K. Immunological and hormonal responses to lung surgery during one-lung ventilation. Eur J Anaesthesiol. 1993;10:189–195. [PubMed] [Google Scholar]
- Misthos P, Katsaragakis S, Theodorou D, Milingos N, Skottis I. The degree of oxidative stress is associated with major adverse effects after lung resection: a prospective study. Eur J Cardiothorac Surg. 2006;29:591–595. doi: 10.1016/j.ejcts.2005.12.027. [DOI] [PubMed] [Google Scholar]
- Nagele P, Pal S, Brown F, Blood J, Miller JP, Johnston J. Postoperative QT interval prolongation in patients undergoing noncardiac surgery under general anesthesia. Anesthesiology. 2012;117:321–328. doi: 10.1097/ALN.0b013e31825e6eb3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guenoun T, Journois D, Silleran-Chassany J, et al. Prediction of arterial oxygen tension during one-lung ventilation: analysis of preoperative and intraoperative variables. J Cardiothorac Vasc Anesth. 2002;16:199–203. doi: 10.1053/jcan.2002.31067. [DOI] [PubMed] [Google Scholar]
- Miñambres E, Burón J, Ballesteros MA. Llorca J, Muñoz P, González-Castro A. Tracheal rupture after endotracheal intubation: a literature systematic review. Eur J Cardiothorac Surg. 2009;35:1056–1062. doi: 10.1016/j.ejcts.2009.01.053. [DOI] [PubMed] [Google Scholar]
- Chamogeorgakis TP, Connery CP, Bhora F, Nabong A, Toumpoulis IK. Thoracoscore predicts midterm mortality in patients undergoing thoracic surgery. J Thorac Cardiovasc Surg. 2007;132:883–887. doi: 10.1016/j.jtcvs.2007.06.020. [DOI] [PubMed] [Google Scholar]
- Rimensberger PC, Cox PN, Frndova H, Bryan AC. The open lung during small tidal volume ventilation: concepts of recruitment and “optimal” positive end-expiratory pressure. Crit Care Med. 1999;27:1946–1952. doi: 10.1097/00003246-199909000-00038. [DOI] [PubMed] [Google Scholar]
- Axe JR, Abbrecht PH. Analysis of the pressure-volume relationship of excised lungs. Ann Biomed Eng. 1985;13:101–117. doi: 10.1007/BF02584233. [DOI] [PubMed] [Google Scholar]
- Al-Abdullatief M, Wahood A, Al-Shirawi N, et al. Awake anaesthesia for major thoracic surgical procedures: an observational study. Eur J Cardiothorac Surg. 2007;32:346–350. doi: 10.1016/j.ejcts.2007.04.029. [DOI] [PubMed] [Google Scholar]
- Pompeo E, Mineo D, Rogliani P, Sabato AF, Mineo TC. Feasibility and results of awake thoracoscopic resection of solitary pulmonary nodules. Ann Thorac Surg. 2004;78:1761–1768. doi: 10.1016/j.athoracsur.2004.05.083. [DOI] [PubMed] [Google Scholar]
- Pompeo E, Tacconi F, Mineo D, Mineo TC. The role of awake video-assisted thoracoscopic surgery in spontaneous pneumothorax. J Thorac Cardiovasc Surg. 2007;133:786–790. doi: 10.1016/j.jtcvs.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Kochi T, Sako S, Nishino T, Mizuguchi T. Effect of high thoracic extradural anaesthesia on ventilatory response to hypercapnia in normal volunteers. Br J Anaesth. 1989;62:362–367. doi: 10.1093/bja/62.4.362. [DOI] [PubMed] [Google Scholar]
- Warner DO, Warner MA, Ritman EL. Human chest wall function during epidural anesthesia. Anesthesiology. 1996;85:761–773. doi: 10.1097/00000542-199610000-00011. [DOI] [PubMed] [Google Scholar]
- Groeben H. Epidural anesthesia and pulmonary function. J Anesth. 2006;20:290–299. doi: 10.1007/s00540-006-0425-6. [DOI] [PubMed] [Google Scholar]
- Groeben H. Effects of high thoracic epidural anesthesia and local anesthetics on bronchial hyperreactivity. J Clin Monit Comput. 2000;16:457–463. doi: 10.1023/a:1011448927817. [DOI] [PubMed] [Google Scholar]
- Horlocker TT. Complications of regional anesthesia and acute pain management. Anesthesiol Clin. 2011;29:257–278. doi: 10.1016/j.anclin.2011.04.006. [DOI] [PubMed] [Google Scholar]
- Piccioni F, Langer M, Fumagalli L, Haeusler E, Conti B, Previtali P. Thoracic paravertebral anaesthesia for awake video-assisted thoracoscopic surgery daily. Anaesthesia. 2010;65:1221–1224. doi: 10.1111/j.1365-2044.2010.06420.x. [DOI] [PubMed] [Google Scholar]
- Bondár A, Szucs S, Iohom G. Thoracic paravertebral blockade. Med Ultrason. 2010;12:223–227. [PubMed] [Google Scholar]
- Molliex X, Dureuil B, Montravers P, Desmonts JM. Effects of midazolam in respiratory drive in healthy volunteers. Ann Fr Anesth Reanim. 1995;14:271–275. doi: 10.1016/s0750-7658(95)80006-9. (In French.) [DOI] [PubMed] [Google Scholar]
- Katlic MR, Facktor MA. Video-assisted thoracic surgery utilizing local anesthesia and sedation: 384 consecutive cases. Ann Thorac Surg. 2010;90:240–245. doi: 10.1016/j.athoracsur.2010.02.113. [DOI] [PubMed] [Google Scholar]
- Tseng YD, Cheng YJ, Hung MH, Chen KC, Chen JS. Nonintubated needlescopic video-assisted thoracic surgery for management of peripheral lung nodules. Ann Thorac Surg. 2012;93:1049–1054. doi: 10.1016/j.athoracsur.2012.01.062. [DOI] [PubMed] [Google Scholar]
- Wu CY, Chen JS, Lin YS, et al. Feasibility and safety of nonintubated thoracoscopic lobectomy for geriatric lung cancer patients. Ann Thorac Surg. 2013;95:405–411. doi: 10.1016/j.athoracsur.2012.10.082. [DOI] [PubMed] [Google Scholar]
- Smit HJ, Schramel FM, Sutedja TG, Ter Laak-Uytenhaak LS, Nannes-Pols MH, Postmus PE. Video-assisted thoracoscopy is feasible under local anesthesia. Diagn Ther Endosc. 1998;4:177–182. doi: 10.1155/DTE.4.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschopp JM, Brutsche M, Frey JG. Treatment of complicated spontaneous pneumothorax by simple talc pleurodesis under thoracoscopy and local anaesthesia. Thorax. 1997;52:329–332. doi: 10.1136/thx.52.4.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tacconi F, Pompeo E, Fabbi E, Mineo TC. Awake video-assisted pleural decortication for empyema thoracis. Eur J Cardiothorac Surg. 2010;37:594–601. doi: 10.1016/j.ejcts.2009.08.003. [DOI] [PubMed] [Google Scholar]
- Mineo TC, Pompeo E, Mineo D, Tacconi F, Marino M, Sabato AF. Awake nonresectional lung volume reduction surgery. Ann Surg. 2006;243:131–136. doi: 10.1097/01.sla.0000182917.39534.2c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CK, Cheng YJ, Hung MH, Tseng YD, Chen JS. Nonintubated thoracoscopic lung resection: a 3-year experience with 285 cases in a single institution. J Thorac Dis. 2012;4:347–351. doi: 10.3978/j.issn.2072-1439.2012.08.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakuraba M, Masuda K, Hebisawa A, Sagara Y, Komatsu H. Thoracoscopic pleural biopsy for tuberculous pleurisy under local anesthesia. Ann Thorac Cardiovasc Surg. 2006;12:245–248. [PubMed] [Google Scholar]
- Macchiarini P, Rovira I, Ferrarello S. Awake upper airway surgery. Ann Thorac Surg. 2010;89:387–390. doi: 10.1016/j.athoracsur.2009.10.044. [DOI] [PubMed] [Google Scholar]
- Noda M, Okada Y, Maeda S, et al. Is there a benefit of awake thoracoscopic surgery in patients with secondary spontaneous pneumothorax? J Thorac Cardiovasc Surg. 2012;143:613–616. doi: 10.1016/j.jtcvs.2011.07.067. [DOI] [PubMed] [Google Scholar]
- Nezu K, Kushibe K, Tojo T, Takahama M, Kitamura S. Thoracoscopic wedge resection of blebs under local anesthesia with sedation for treatment of a spontaneous pneumothorax. Chest. 1997;111:230–235. doi: 10.1378/chest.111.1.230. [DOI] [PubMed] [Google Scholar]
- Dong Q, Liang L, Liu J, et al. Anesthesia with nontracheal intubation in thoracic surgery. J Thorac Dis. 2012;1:126–130. doi: 10.3978/j.issn.2072-1439.2012.03.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocco G, Romano V, Accardo R, et al. Awake single-access (uniportal) video-assisted thoracoscopic surgery for peripheral pulmonary nodules in a complete ambulatory setting. Ann Thorac Surg. 2010;89:1625–1627. doi: 10.1016/j.athoracsur.2010.01.087. [DOI] [PubMed] [Google Scholar]
- Pompeo E, Mineo TC. Awake operative videothoracoscopic pulmonary resections. Thorac Surg Clin. 2008;18:311–320. doi: 10.1016/j.thorsurg.2008.04.006. [DOI] [PubMed] [Google Scholar]
- Mineo TC. Thoracoscopic approach to lung metastases. Minerva Chir. 2008;63:511–516. [PubMed] [Google Scholar]
- Lesser TG. Laser application enables awake thoracoscopic resection of pulmonary nodules with minimal access. Surg Endosc. 2012;26:1181–1186. doi: 10.1007/s00464-011-2000-y. [DOI] [PubMed] [Google Scholar]
- Chen KC, Cheng YJ, Hung MH, Tseng YD, Chen JS. Nonintubated thoracoscopic lung resection: a 3-year experience with 285 cases in a single institution. J Thorac Dis. 2012;4:347–351. doi: 10.3978/j.issn.2072-1439.2012.08.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamogeorgakis T, Ieromonachos C, Georgiannakis E, Mallios D. Does lobectomy achieve better survival and recurrence rates than limited pulmonary resection for T1N0M0 non-small cell lung cancer patients? Interact Cardiovasc Thorac Surg. 2009;8:364–372. doi: 10.1510/icvts.2008.178947. [DOI] [PubMed] [Google Scholar]
- Vanni G, Tacconi F, Sellitri F, Ambrogi V, Mineo TC, Pompeo E. Impact of awake videothoracoscopic surgery on postoperative lymphocyte responses. Ann Thorac Surg. 2010;90:973–978. doi: 10.1016/j.athoracsur.2010.04.070. [DOI] [PubMed] [Google Scholar]
- Niwa H, Rowbotham DJ, Lambert DG, Buggy DJ. Can anesthetic techniques or drugs affect cancer recurrence in patients undergoing cancer surgery? J Anesth doi: 10.1007/s00540-013-1615-7. . doi: 10.1007/s00540-013-1615-7. [DOI] [PubMed] [Google Scholar]
- Rahman NM, Ali NJ, Brown G, et al. Local anaesthetic thoracoscopy: British Thoracic Society pleural disease guideline 2010. Thorax. 2013;65:ii54–60. doi: 10.1136/thx.2010.137018. (Suppl. 2) [DOI] [PubMed] [Google Scholar]
- Griffo S, Gravino E, Luciano A, Ferrante G. The treatment by VATS and MAC of secondary neoplastic pleural effusion in the old patient (>70 years) Acta Biomed. 2005;76:S72–75. (Suppl. 1) [PubMed] [Google Scholar]
- Roberts ME, Neville E, Berrisford RG, Antunes G, Ali NJ. Management of a malignant pleural effusion: British Thoracic Society Pleural Disease Guideline 2010. Thorax. 2010;65:ii32–40. doi: 10.1136/thx.2010.136994. (Suppl. 2) [DOI] [PubMed] [Google Scholar]
- Nasreen N, Mohammed KA, Brown S, et al. Talc mediates angiostatis in malignant pleural effusions via endostatin induction. Eur Respiro J. 2007;29:761–769. doi: 10.1183/09031936.00061606. (Published erratum in 2007;: 1286.) [DOI] [PubMed] [Google Scholar]
- Hartman DL, Gaither JM, Kesler KA, Mylet DM, Brown JW, Mathur PN. Comparison of insufflated talc under thoracoscopic guidance with standard tetracycline and bleomycin pleurodesis for control of malignant pleural effusion. J Thorac Cardiovasc Surg. 1993;105:743–747. [PubMed] [Google Scholar]
- Danby CA, Adebonojo SA, Moritz DM. Video-assisted talc pleurodesis for malignant pleural effusions utilizing local anesthesia and IV sedation. Chest. 1998;113:739–742. doi: 10.1378/chest.113.3.739. [DOI] [PubMed] [Google Scholar]
- Kolschmann S, Ballin A, Gillisen A. Clinical efficacy and safety of thoracoscopic talc pleurodesis in malignant pleural effusions. Chest. 2005;128:1431–1435. doi: 10.1378/chest.128.3.1431. [DOI] [PubMed] [Google Scholar]
- Stefani A, Natali P, Casali C, Morandi U. Talc poudrage versus talc slurry in the treatment of malignant pleural effusion. A prospective comparative study. Eur J Cardiothorac Surg. 2006;30:827–832. doi: 10.1016/j.ejcts.2006.10.002. [DOI] [PubMed] [Google Scholar]
- Diacon AH, Wyser C, Bolliger CT, et al. Prospective randomized comparison of thoracoscopic talc poudrage under local anesthesia versus bleomycin instillation for pleurodesis in malignant pleural effusion. Am J Respir Crit Care Med. 2000;162:1445–1449. doi: 10.1164/ajrccm.162.4.2002030. [DOI] [PubMed] [Google Scholar]
- Dresler CM, Olak J, Herndon JE, 2nd, et al. Phase III intergroup study of talc poudrage vs talc slurry sclerosis for malignant pleural effusion. Chest. 2005;127:909–915. doi: 10.1378/chest.127.3.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debeljak A, Kecelj P, Triller N, et al. Talc pleurodesis: comparison of talc slurry instillation with thoracoscopic talc insufflation for malignant pleural effusion. J BUON. 2006;11:463–467. [PubMed] [Google Scholar]
- Sioris T, Sihvo E, Salo J, Räsänen J, Knuuttila A. Long-term indwelling pleural catheter (PleurX) for malignant pleural effusion unsuitable for talc pleurodesis. Eur J Surg Oncol. 2009;35:546–551. doi: 10.1016/j.ejso.2008.06.009. [DOI] [PubMed] [Google Scholar]
- Olden AM, Holloway R. Treatment of malignant pleural effusion: PleurX catheter or talc pleurodesis? A cost-effectiveness analysis. J Palliat Med. 2010;13:59–65. doi: 10.1089/jpm.2009.0220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gothard JW. Anesthetic considerations for patients with anterior mediastinal masses. Anesthesiol Clin. 2008;26:305–314. doi: 10.1016/j.anclin.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Mahmodlou R, Mohtazeri V, Rahimi-Rad MH, Sekoti M. Mini-mediastinotomy under local anesthesia for biopsy of anterior mediastinal masses with airway compression. Pneumologia. 2011;60:143–146. [PubMed] [Google Scholar]
- Rendina EA, Venuta F, De Giacomo T, et al. Biopsy of anterior mediastinal masses under local anesthesia. Ann Thorac Surg. 2002;74:1720–1722. doi: 10.1016/s0003-4975(02)03821-3. [DOI] [PubMed] [Google Scholar]
- Pompeo E, Tacconi F, Mineo TC. Awake video-assisted thoracoscopic biopsy in complex anterior mediastinal masses. Thorac Surg Clin. 2010;20:225–233. doi: 10.1016/j.thorsurg.2010.01.003. [DOI] [PubMed] [Google Scholar]
- Goh MH, Liu XY, Goh YS. Anterior mediastinal masses: an anaesthetic challenge. Anaesthesia. 1999;54:670–674. doi: 10.1046/j.1365-2044.1999.00961.x. [DOI] [PubMed] [Google Scholar]
- Abdelmalak B, Marcanthony N, Abdelmalak J, Machuzak MS, Gildea TR, Doyle DJ. Dexmedetomidine for anesthetic management of anterior mediastinal mass. J Anesth. 2010;24:607–610. doi: 10.1007/s00540-010-0946-x. [DOI] [PubMed] [Google Scholar]
- Ramoz-Izquierdo R, Moya J, Macia I, et al. Treatment of primary spontaneous pneumothorax by videothoracoscopic talc pleurodesis under local anesthesia: a review of 133 procedures. Surg Endosc. 2010;24:984–987. doi: 10.1007/s00464-009-0707-9. [DOI] [PubMed] [Google Scholar]
- Tacconi F, Pompeo E, Mineo TC. Late-onset occult pneumothorax after lung-volume reduction surgery. Ann Thorac Surg. 2005;80:2008–2012. doi: 10.1016/j.athoracsur.2005.06.013. [DOI] [PubMed] [Google Scholar]
- Tacconi F, Pompeo E, Mineo TC. Duration of air leak is reduced after awake nonresectional lung volume reduction surgery. Eur J Cardiothorac Surg. 2009;35:822–828. doi: 10.1016/j.ejcts.2009.01.010. [DOI] [PubMed] [Google Scholar]
- Inoue K, Moriyama K, Takeda J. Remifentanil for awake thoracoscopic bullectomy. J Cardiothorac Vasc Anesth. 2010;24:386–387. doi: 10.1053/j.jvca.2009.05.012. [DOI] [PubMed] [Google Scholar]
- Pompeo E, Tacconi F, Frasca L, Mineo TC. Awake thoracoscopic bullaplasty. Eur J Cardiothorac Surg. 2011;39:1012–1017. doi: 10.1016/j.ejcts.2010.09.029. [DOI] [PubMed] [Google Scholar]
- Kreider ME, Hansen-Flaschen J, Ahmad NN, et al. Complications of video-assisted thoracoscopic lung biopsy in patients with interstitial lung disease. Ann Thorac Surg. 2007;83:1140–1144. doi: 10.1016/j.athoracsur.2006.10.002. [DOI] [PubMed] [Google Scholar]
- Sigurdsson MI, Isaksson HJ, Gudmundsson G, Gudbjartsson T. Diagnostic surgical lung biopsies for suspected interstitial lung diseases: a retrospective study. Ann Thorac Surg. 2009;88:227–232. doi: 10.1016/j.athoracsur.2009.04.002. [DOI] [PubMed] [Google Scholar]
- Ooi A, Iyenger S, Ferguson J, Ritchie AJ. VATS lung biopsy in suspected, diffuse interstitial lung disease provides diagnosis, and alters management strategies. Heart Lung Circ. 2005;14:90–92. doi: 10.1016/j.hlc.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Rex S, Missant C, Segers P, Wouters PF. Thoracic epidural anesthesia impairs the hemodynamic response to acute pulmonary hypertension by deteriorating right ventricular-pulmonary arterial coupling. Crit Care Med. 2007;35:222–229. doi: 10.1097/01.CCM.0000250357.35250.A2. [DOI] [PubMed] [Google Scholar]
- Medford AR, Agrawal S, Bennet JA, Free CM, Entwisle JJ. Thoracic ultrasound prior to medical thoracoscopy improves pleural access and predicts fibrous septation. Respirology. 2010;15:804–808. doi: 10.1111/j.1440-1843.2010.01768.x. [DOI] [PubMed] [Google Scholar]
- Wang LP, Hauerberg J, Schmidt JF. Epidural abscess after epidural catheterization. Frequency and case reports. Ugeskr Laeger. 2000;162:5460–5461. (In Danish.) [PubMed] [Google Scholar]
- Tacconi F, Pompeo E, Fabbi E, Mineo TC. Reply to Hung et al. Eur J Cardiothorac Surg. 2011;39:146. [Google Scholar]
- Matsumoto I, Oda M, Watanabe G. Awake endoscopic thymectomy via an infrasternal approach using sternal lifting. Thorac Cardiovasc Surg. 2008;56:311–313. doi: 10.1055/s-2008-1038632. [DOI] [PubMed] [Google Scholar]
- Tsunezuka Y, Oda M, Matsumoto I, Tamura M, Watanabe G. Extended thymectomy in patients with myasthenia gravis with high thoracic epidural anesthesia alone. World J Surg. 2004;28:962–965. doi: 10.1007/s00268-004-7480-7. [DOI] [PubMed] [Google Scholar]
- Chen JS, Cheng YJ, Hung MH, Tseng YD, Chen KC, Lee YC. Nonintubated thoracoscopic lobectomy for lung cancer. Ann Surg. 2011;254:1038–1043. doi: 10.1097/SLA.0b013e31822ed19b. [DOI] [PubMed] [Google Scholar]