Abstract
Background
The 7th edition American Joint Committee on Cancer tumor-node-metastasis (AJCC TNM) staging system was published in 2010. Here we evaluate its predictive ability and compare the 6th and 7th editions of the AJCC TNM staging systems in esophageal squamous cell cancer (ESCC) with preoperative radiotherapy.
Methods
A total of 296 esophageal squamous cell carcinoma patients receiving preoperative radiotherapy between 1980 and 2007 were included. Patients were staged using the 6th and 7th edition staging systems. Survival analyses were performed using Cox regression models. The homogeneity, discriminatory ability, and monotonicity of gradients of the two staging systems were compared using linear trend χ2, likelihood ratio statistics, and Akaike information criterion calculation.
Results
The overall five-year survival rate for the entire cohort was 27.1%. Female gender, length, “T,” and “N,” classifications according to the 7th edition staging system were the prognostic factors in univariate analyses. However, tumor histological grade and cancer location did not significantly influence patient survival. The 7th edition staging system has the highest linear trend χ2and likelihood ratio χ2scores. Compared to the 6th edition, the 7th edition staging system also has a smaller Akaike information criterion value, which represents the optimum prognostic stratification.
Conclusions
The strength of the 7th edition AJCC TNM staging system lies in the new descriptors for “T” and “N” classifications. However, we did not find cancer location to be a significant prognostic factor in our cohort. Overall, the 7th edition AJCC TNM staging system performed better than the previous edition.
Keywords: AJCC cancer stage system, esophageal squamous cell carcinoma, preoperative radiotherapy, prognosis
Introduction
Esophageal cancer has a poor prognosis of gastrointestinal tumors, with a five-year survival rate of approximately 25%.1Surgery remains the primary treatment for patients with limited stage esophageal cancer. Postoperative pathologic stage is the main criterion used for prognosis evaluations. Currently, the 6th edition American Joint Committee on Cancer (AJCC) stage system is widely applied in clinical practice. However, for locally advanced esophageal cancer, multimodality treatment is the primary treatment, including preoperative, adjuvant, or concurrent chemoradiation therapy. Preoperative therapy results in postoperative pathological changes. A complete surgical resection with radical lymphadenectomy also provides information for accurate stage determination, which is very important in prognosis prediction and further decisions regarding therapy. In the 6th edition AJCC, the authors suggested evaluating the prognosis of patients who underwent preoperative therapy.2,3However, these results were controversial. Many modifications have been proposed for the 6th edition AJCC TNM system. For example, subdivision of the “M” classification into M1A and M1B according to the presence of non-regional lymph node involvement is considered inappropriate because it provides no advantage in survival prediction.4–6Furthermore, subdivision of the “N” classification, based on the absolute number of involved lymph nodes instead of regional lymph node involvement, has been suggested for better survival stratification.4–6
The 7th edition AJCC Tumor Node Metastasis (TNM) staging system was released in 2010.4,7Currently, no study that evaluates the predictive ability of the 7th edition of the AJCC TNM staging system in esophageal cancer with preoperative or postoperative treatment has been reported. In this study, we evaluated the predictive ability of the 7th edition AJCC TNM staging system and compared the 6th and 7th editions in a cohort of patients who underwent preoperative radiotherapy for esophageal squamous cell cancer (ESCC).
Materials and methods
A total of 296 patients with ESCC who underwent preoperative radiotherapy in our hospital between January 1980 and November 2007 were retrospectively analyzed. Patients without survival information were excluded from these analyses. We also excluded any patient who did not undergo radiotherapy, who underwent intended definitive nonsurgical therapy that was eventually resected (primary chemotherapy), or underwent other preoperative and postoperative concurrent chemoradiotherapy. Patients underwent preoperative planning radiotherapy, with a preoperative radiation dose of 40–50 Gy. Patients underwent surgery eight weeks after the completion of radiotherapy. After surgery, patients did not receive any adjuvant radiotherapy, chemotherapy plus radiation, or chemotherapy. Subjects included 232 male (78.4%) and 64 female (21.6%) patients whose ages ranged from 27 to 78 years (median age 55 years). The clinical characteristics of the 296 patients are shown in Table 1. The Academic Committee of the Chinese Academy of Medical Sciences Cancer Hospital approved this study.
Table 1.
Patients characteristics and univariate analyses of overall survival
| Characteristic | Number | % | Overall survival | ||
|---|---|---|---|---|---|
| HR | 95%CI | P-value* | |||
| Gender | |||||
| Male (ref) | 232 | 78.4 | 1 | ||
| Female | 64 | 21.6 | 0.678 | 0.506–0.908 | 0.009 |
| Age (years) | 55 (27–78) | ||||
| Length (cm) | |||||
| ≤6 (ref) | 107 | 35.8 | 1 | ||
| >6 | 189 | 64.2 | 1.406 | 1.097–1.803 | 0.007 |
| Location | 0.862 | ||||
| Upper | 61 | 20.6 | 1 | ||
| Middle | 209 | 70.6 | 1.118 | 0.821–1.524 | 0.479 |
| Lower | 26 | 8.8 | 1.082 | 0.844–1.388 | 0.533 |
| Anastomosis | |||||
| Neck | 102 | 26.3 | |||
| Thorax | 194 | 73.7 | 1.014 | 0.892–1.153 | 0.833 |
| Histological grade | 0.323 | ||||
| Well | 39 | 13.2 | 1 | ||
| Moderate | 191 | 64.5 | 0.085 | 0.664–1.353 | 0.085 |
| Poor | 66 | 22.3 | 1.104 | 0.891–1.368 | 0.836 |
| yT stage | <0.0001 | ||||
| T0 (ref) | 100 | 33.9 | 1 | ||
| T1 | 10 | 3.4 | 1.381 | 0.715–2.666 | 0.334 |
| T2 | 58 | 20.0 | 0.968 | 0.816–1.149 | 0.713 |
| T3 | 80 | 27.0 | 1.218 | 1.094–1.356 | 0.000 |
| T4a | 15 | 5.1 | 1.284 | 1.146–1.439 | 0.000 |
| T4b | 33 | 11.1 | 1.183 | 1.102–1.269 | 0.000 |
| yN stage | <0.0001 | ||||
| N0 (ref) | 207 | 70.0 | 1 | ||
| LN1-2 | 56 | 18.9 | 1.559 | 1.149–2.114 | 0.004 |
| LN3-6 | 21 | 7.1 | 1.580 | 1.245–2.007 | 0.000 |
| LN ≥7 | 12 | 4.0 | 1.498 | 1.227–1.829 | 0.000 |
| Resection | <0.0001 | ||||
| R0 (ref) | 240 | 81.1 | 1 | ||
| R2 | 56 | 18.9 | 1.405 | 1.210–1.632 | |
| ypStage 7th UICC | |||||
| 0 (T0N0M0 ref) | 78 | 26.3 | 1 | <0.0001 | |
| ypStage I | 46 | 15.5 | 0.994 | 0.641–1.391 | 0.771 |
| ypStage IIA | 52 | 17.6 | 1.367 | 1.126–1.660 | 0.001 |
| ypStage IIB | 25 | 8.4 | 1.080 | 0.960–1.215 | 0.198 |
| ypStage IIIA | 38 | 12.8 | 1.223 | 1.122–1.333 | 0.000 |
| ypStage IIIB | 7 | 2.4 | 1.211 | 1.045–1.403 | 0.007 |
| ypStage IIIC | 50 | 16.9 | 1.198 | 1.131–1.269 | 0.000 |
P-values calculated using Kaplan-Meier methods. 95% CI, 95% confidence interval; HR, hazard ratio; UICC, Union for International Cancer Control.
Treatment
The preoperative radiotherapy dosage was 40 to 50 Gy, with a median dose of 40 Gy (270 cases [91.5%] received 40 Gy and 25 cases [8.5%] received 42–50 Gy) and was given five times per week, 2 Gy each time. Anterior-posterior-opposed radiation fields were used in 284 patients (95.5%), and 3D-conformal radiotherapy (CRT) or intensity-modulated radiation therapy (IMRT) radiotherapy was used in 12 patients (4.5%). The radiation field included the primary lesion and the corresponding lymphatic drainage region. Based on their tumor sites, all patients had undergone thoracic esophagectomy. The adjacent esophagus, carcinoma, adjacent mediastinal lymph node, and lymph nodes in the periphery of the gastric cardia were radically dissected. A total of 3577 lymph nodes were removed, with an average cleaning of 10.5 (0–53) pieces. A total of 89 (30.6%) patients had 263 positive lymph nodes, with an average of one positive lymph node (0–16) piece. Thirty-nine patients had one positive lymph node, 17 patients had two positive lymph nodes, 12 patients had three positive lymph nodes, and 21 had ≥4 positive lymph nodes. Moreover, 191 subjects (64.5%) underwent supra-arch anastomosis, three subjects (1.0%) intra-arch anastomosis, 102 patients (34.5%) cervical anastomosis, 240 patients (81.1%) R0 resection, and 56 patients (18.9%) underwent R2 resection.
Assessment of residual carcinoma and pathologic stage
Tumors in their primary locations were divided into T-pCR (T0) and tumor residual (no T-pCR), as well as into pathological T staging (T1-T4) groups. Each specimen was evaluated for invasion depth and lymph node metastasis, and was staged according to the 7th AJCC criteria for esophageal carcinoma. T-pCR refers to tumors identified by macroscopic evaluation with ulcerated or scarred areas indicating that the therapy fields were submitted completely for histological examination. No T-pCR refers to cases that had remnant tumors in primary locations. For all patients, N staging procedures were performed using postoperative pathology lymph node statuses according to the 7th edition AJCC TNM staging system.4
Follow-up
The final follow-up was in January 2009. Conventional examinations were performed in the three to six months after surgery, whereas patients in other locations had physical examinations at their local hospital or participated in a telephone follow-up interview. Conventional examinations, including esophageal barium meals, chest computed tomography (CT) examinations, abdominal B-ultrasounds, and corresponding examinations, such as magnetic resonance imaging (MRI) brain scans and whole-body bone imaging, were performed based on the patients' symptoms.
Statistical analyses
Survival analyses were performed using Cox regression models and survival curves were plotted using the Kaplan-Meier method. Discrimination can be verified by observing whether there are any overlaps in the Kaplan-Meier curves and the numerical estimates of the hazard ratios. In accordance with Ueno et al.,8criteria for evaluating the performance of the staging systems were: (i) homogeneity within subgroups (small differences in survival among patients within same stage); (ii) discriminatory ability between different groups (greater differences in survival among patients in different stages); and (iii) monotonicity of gradients shown in the association between stages and survival rates (patients with earlier stages have longer survival than those in later stages within the same system). Likelihood ratio χ2tests, related to the Cox regression model, were used to measure homogeneity. The discriminatory ability and monotonicity of gradient assessments were measured using the linear trend χ2test. For potential bias in comparing prognostic systems with different numbers of stages, the Akaike information criterion (AIC) within the Cox proportional hazard regression model was used.9,10A smaller AIC value indicated a better model for predicting outcome. All calculations were performed using SPSS 20.0 software (SPSS Inc., Chicago, IL) and P-values less than 0.05 were considered significant.
Patient characteristics
Patients were followed for a median of 25 months. There were 232 men and 64 women whose ages ranged from 27 to 78 years (median age 55 years). The clinical characteristics of the 296 patients are shown in Table 1. Preoperative esophagrams indicated tumor lengths of 2–12 cm (median length 6.0 cm).
The 6th and 7th American Joint Committee on Cancer (AJCC) staging systems on overall survival
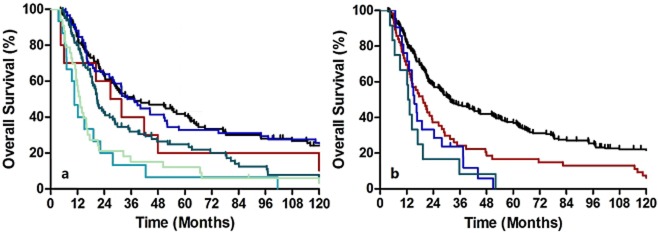
According to the 7th edition staging system, all subclassifications predicted survival accurately (Fig 1a, b). However, further subclassification of seven or greater positive lymph nodes as N3 is unnecessary because patient survival was similar to N2 patients (P= 0.516). Additionally, subclassification of T4 is unnecessary because T4a survival was similar to that of T4b patients (P= 0.576). Another prognostic factor was tumor length (P= 0.007). However, histological grade and cancer location were not significant prognostic factors in our analyses (P= 0.323 and P= 0.839, respectively).
Figure 1.
Kaplan-Meier survival curves for patients stratified by tumor “T” (a) and node “N” (b) classifications. Survival differences were analyzed using Cox regression models. (a)  , T0;
, T0;  , T1;
, T1;  , T2;
, T2;  , T3;
, T3;  , T4a;
, T4a;  , T4b. (b)
, T4b. (b)  , N0;
, N0;  , N1;
, N1;  , N2;
, N2;  , N3.
, N3.
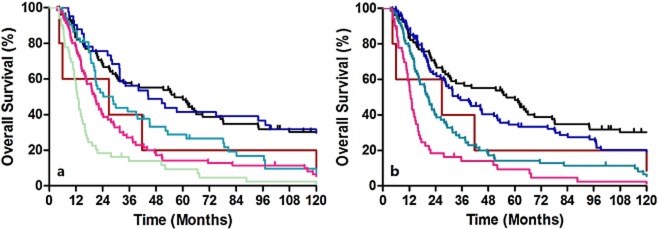
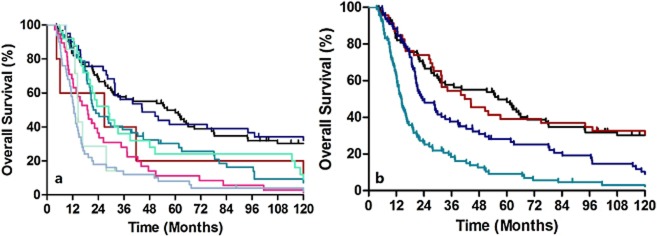
Overall survival based on grade or location did not show any discriminatory ability. Table 2lists the patient distribution and stage specific survival rates. In the 6th edition staging system, the Kaplan-Meier plot showed overlapped survival curves among stages 0 and IIA, stages IIA and IIB, and stages III and IV (Fig 2a). When classified as five major stages (0, I, II, III, and IV), the survival curves of stages III and IV remained similar (Fig 2b). According to the 6th edition staging system, most stage IV patients had non-regional lymph node metastases, whereas stage IIB (T1-2N1M0) and stage III (T3N1M0 and T4N0-1M0) patients had regional lymph node metastases. Because these stages had similar survival rates, our results indicate that identifying non-regional lymph node metastases and labeling these as M1A or M1B is unnecessary. In the 7th edition staging system, when classified as all eight substages, there were similar survival curves between ypStages 0 and IA, ypStages IA and IB, ypStages IIA and IIB, ypStages IIIA and IIIB, and ypStages IIIB and IIIC (Fig 3a). However, when classified as four major stages, the Kaplan-Meier plot showed good discriminatory ability among stages I through III (Fig 3b).
Table 2.
Cross table of the 6th edition by the 7th edition American Joint Committee on Cancer (AJCC) Tumor Node Metastasis (TNM) staging system with patient distribution and stage-specific survival
| 6th Edition | OS according to 7th edition | ||||||
|---|---|---|---|---|---|---|---|
| 0 | I | IIA | IIB | III | IVa | ||
| 7th edition | |||||||
| 0 | 78 | 0 | 0 | 0 | 0 | 0 | 56.2 (33.2–79.2) |
| IA | 0 | 5 | 0 | 0 | 0 | 0 | 26.8 (13.0–71.5) |
| IB | 0 | 0 | 41 | 0 | 0 | 0 | 44.5 (22.3–66.7) |
| IIA | 0 | 0 | 52 | 0 | 0 | 0 | 21.7 (12.5–31.0) |
| IIB | 0 | 0 | 17 | 0 | 0 | 0 | 28.8 (17.5–40.1) |
| IIIA | 0 | 0 | 0 | 4 | 9 | 15 | 18.8 (12.8–25.0) |
| IIIB | 0 | 0 | 0 | 0 | 3 | 4 | 14.5 (14.1–15.0) |
| IIIC | 0 | 0 | 0 | 3 | 33 | 14 | 12.6 (11.2–13.9) |
| OS according to 6th edition | 56.2 (33.2–79.2) | 26.8 (13–71.5) | 31.7 (21.6–41.9) | 23.4 (11.4–35.4) | 14.3 (11.5–17.2) | 14.5 (11.5–17.5) | |
OS, overall survival.
Figure 2.
Kaplan-Meier survival curves for patients stratified using the 6th edition staging system. (a) Classified as all five substages. (b) Classified as the four major stages. (a)  , 0;
, 0;  , I;
, I;  , IIA;
, IIA;  , IIB;
, IIB;  , III;
, III;  , IV. (b)
, IV. (b)  , 0;
, 0;  , I;
, I;  , II;
, II;  , III;
, III;  , IV.
, IV.
Figure 3.
Kaplan-Meier survival curves for patients stratified using the 7th edition staging system. (a) Classified as all eight substages. (b) Classified as the four major stages. (a)  , 0;
, 0;  , IA;
, IA;  , IB;
, IB;  , IIA;
, IIA;  , IIB;
, IIB;  , IIIA;
, IIIA;  , IIIB;
, IIIB;  , IIIC. (b)
, IIIC. (b)  , 0;
, 0;  , I;
, I;  , II;
, II;  , III.
, III.
According to the 7th edition staging system, T2-3N0M0 could be classified as stages IB, IIA, or IIB depending on histological grade and cancer location. However, as mentioned, we did not recognize grade and location as significant prognostic factors in survival analyses. Therefore, the discriminatory ability was expected to be worse among stages IIA and IIB. For a patient with N0, overall survival (OS) of T2 is significantly greater than T3 (P= 0.002). Kaplan-Meier survival analyses show that ypStage IB and ypStage IIA were statistically different (P= 0.004). The majority of ypStage IIIB cases in our database were N2 lesions (seven of seven, 100%). Because our database demonstrated similar survival rates between N2 and N3 patients (P= 0.516, Fig 1b), it was not surprising that there were no significant survival differences between ypStage IIIB (T3N2M0) and IIIC patients.
The performance of the 6th and 7th edition staging systems, assessed by the linear trend χ2, likelihood ratio χ2, and the Akaike information criterion (AIC) test, are described in Table 3. The 7th edition staging system had better homogeneity (highest likelihood ratio χ2score), discriminatory ability, and monotonicity of gradients (highest linear trend χ2score). Compared to the 6th edition, the 7th edition staging system had a smaller AIC value, which represented the optimum prognostic stratification.
Table 3.
Comparison of the performance of the 6th and 7th editions of the American Joint Committee on Cancer (AJCC)
| TNM staging system | Figure | Model | Linear trend χ2 | Likelihood ratio χ2 | AIC |
|---|---|---|---|---|---|
| 6th Edition | A | 0, I, IIA, IIB, III, IV | 37.41 | 47.120 | 2590.759 |
| B | 0, I, II, III, IV | 34.46 | 46.747 | 2591.183 | |
| 7th Edition | A | 0, IA, IB, IIA, IIB, IIIA, IIIB, IIIC | 49.59 | 64.275 | 2574.686 |
| B | 0, I, II, III | 44.47 | 59.124 | 2578.623 |
AIC, Akaike information criterion; TNM, tumor-node-metastasis.
Discussion
Clinical scientists and patients have increasingly accepted preoperative therapy in recent years. However, according to the results of multiple randomized controlled studies, preoperative treatment for local advanced ESCC has become a preferred choice.11,12Randomized controlled studies have revealed that patients who receive pathological complete response (pCR) after surgery obtain better prognoses than those with residual carcinoma.11For patients who underwent preoperative treatment, good prognostic stratification is very important in prognosis predictions and further therapy decisions. The authors evaluated the prognosis of patients that underwent preoperative therapy in the 6th edition of the AJCC.2,3However, these results were controversial. Until now, no study evaluating the predictive ability of the 7th edition AJCC TNM staging system in esophageal cancer patients that underwent preoperative treatment has been reported.
In this study, we report that the survival rate of ypT0 patients is significantly superior to ypT1-4 patients (P= 0.001). For patients with residual carcinoma, the prognosis of ypT3 subjects is significantly worse than ypT1-2 subjects (P= 0.002), and ypT4 is also inferior to ypT3 (P= 0.002). However, ypT4a patients are similar to ypT4b patients. Stratified analyses have shown that the depth of tumor invasion remains an important factor in determining prognosis even for patients who undergo preoperative treatment. However, T4 should not be subdivided into T4a and T4b.
The 7th edition staging system strengthens the role of positive lymph nodes, and the N classification is subdivided into N0 to N3. Research has focused on whether local lymph node involvement or the number of positive lymph nodes affect postoperative long-term survival and local recurrence in patients that undergo surgery alone. Therefore, in the 7th AJCC staging system, the N staging criteria (N0-3) is that four groups are divided based on numbers of lymph node metastases. It must then be determined whether the number of involved local lymph nodes affects the postoperative long-term survival of patients who receive preoperative therapy. A study that included 47 esophageal adenocarcinoma cases with preoperative therapy found that the involvement of local lymph nodes is the only relevant factor related to prognosis.13Retrospective analyses by Gu et al. included 187 patients with adenocarcinoma in the lower esophagus or esophagogastric junction, all of whom received preoperative radio-chemotherapy. In cases with more than two positive lymph nodes, the median survival time and overall survival are significantly lower than those with only one positive lymph node (47.1 months vs. 21.2 months, 34% vs. 6%, P= 0.02).14This is the primary reason why the 7th edition of AJCC is a better prognostic model than the 6th edition.
After preoperative therapy, Stage T and Stage N presented different levels of descent stage. In this study, the number of involved local lymph nodes included in the staging system was based on the difference in the numbers of positive lymph nodes. Rice et al. reported that 4628 patients with esophageal cancer who underwent surgery alone were included according to the 7th AJCC TNM stage, 2032 of which (44%) had lymph node metastases.6In this study, only 83 cases in 311 patients (26.7%) had lymph node metastases, which is significantly lower than the group undergoing surgery alone. In addition, a report by Wang Mei et al. in China randomly divided 418 patients with esophageal cancer into two groups. One group received preoperative radiotherapy (40 Gy) plus surgery (195 cases), and the other group received surgery alone (223 cases).15Postoperative pathology showed that the lymph node metastasis rate of the former was 22.3%, significantly lower than that of the latter (40.8%).15In this study, the total number of patients in the combined N2/N3 stage was 33 (21 in N2 and 12 in N3). Therefore, N0-3 may be more complicated for patients with preoperative radiotherapy.
Although our sample size was relatively small compared with the worldwide esophageal cancer collaboration database, we report a single institutional experience where most patients underwent preoperative radiotherapy. The surgical procedures, pathologic examinations, and patient follow-up were uniform throughout the entire study period. In contrast, previous published worldwide esophageal cancer collaboration data were assembled from 13 centers, with an era spanning nearly 30 years. Thus, bias is inevitable.6Furthermore, the databases do not represent patients that underwent preoperative radiotherapy. Therefore, our experience is very important for the formation of a prognostic system for adjuvant treatment.
Conclusion
Cancer staging is a dynamic process. With improvements in the understanding of cancer biology, the staging system will need to be revised. In conclusion, this study showed better prognostic stratifications of the 7th edition compared to the 6th edition TNM staging system. Moreover, according to different treatment schedules, the modified 7th edition demonstrated better prognostic prediction than the other two systems.
Acknowledgments
We thank Professor Xiaohui Lin for editing the manuscript. This work was supported by: Capital Foundation for Medical Research and Development (2007-2012); National Natural Science Foundation (81021061); National Natural Science Foundation (39925020).
Disclosure
No authors report any conflict of interest.
References
- Mariette C, Piessen G, Triboulet JP. Therapeutic strategies in oesophageal carcinoma: role of surgery and other modalities. Lancet Oncol. 2007;8:545–553. doi: 10.1016/S1470-2045(07)70172-9. [DOI] [PubMed] [Google Scholar]
- Rizk NP, Venkatraman E, Bains MS, et al. American Joint Committee on Cancer staging system does not accurately predict survival in patients receiving multimodality therapy for esophageal adenocarcinoma. J Clin Oncol. 2007;25:507–512. doi: 10.1200/JCO.2006.08.0101. [DOI] [PubMed] [Google Scholar]
- Chirieac LR, Swisher SG, Ajani JA, et al. Posttherapy pathologic stage predicts survival in patients with esophageal carcinoma receiving preoperative chemoradiation. Cancer. 2005;103:1347–1355. doi: 10.1002/cncr.20916. [DOI] [PubMed] [Google Scholar]
- Rice TW, Rusch VW, Blackstone EH. AJCC/UICC staging of esophageal cancer. In: Shields TW, Locicero J, Reed CE, Feins RH, editors. General Thoracic Surgery. Vol. 2. Amsterdam, Netherlands: Wolters Kluwer; 2009. pp. 2013–2015. . In: (eds). Vol. [Google Scholar]
- Rice TW, Blackstone EH, Rybicki LA, et al. Refining esophageal cancer staging. J Thorac Cardiovasc Surg. 2003;125:1103–1113. doi: 10.1067/mtc.2003.170. [DOI] [PubMed] [Google Scholar]
- Rice TW, Rusch VW, Apperson-Hansen C, et al. Worldwide esophageal cancer collaboration. Dis Esophagus. 22:1–8. doi: 10.1111/j.1442-2050.2008.00901.x. [DOI] [PubMed] [Google Scholar]
- Edge S, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A . AJCC Cancer Staging Manual, Seventh Edition. New York: Springer; 2010. pp. 103–111. [Google Scholar]
- Ueno S, Tanabe G, Sako K, et al. Discrimination value of the new western prognostic system (CLIP score) for hepatocellular carcinoma in 662 Japanese patients. Cancer of the Liver Italian Program. Hepatology. 2001;34:529–534. doi: 10.1053/jhep.2001.27219. [DOI] [PubMed] [Google Scholar]
- Cho YK, Chung JW, Kim JK, et al. Comparison of 7 staging systems for patients with hepatocellular carcinoma undergoing transarterial chemoembolization. Cancer. 2008;112:352–361. doi: 10.1002/cncr.23185. [DOI] [PubMed] [Google Scholar]
- Kee KM, Wang JH, Lee CM, et al. Validation of clinical AJCC/UICC TNM staging system for hepatocellular carcinoma: analysis of 5,613 cases from a medical center in southern Taiwan. Int J Cancer. 2007;120:2650–2655. doi: 10.1002/ijc.22616. [DOI] [PubMed] [Google Scholar]
- Fiorica F, Di Bona D, Schepis F, et al. Preoperative chemoradiotherapy for oesophageal cancer: a systematic review and meta-analysis. Gut. 2004;53:925–930. doi: 10.1136/gut.2003.025080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366:2074–2084. doi: 10.1056/NEJMoa1112088. [DOI] [PubMed] [Google Scholar]
- Dunne B, Reynolds J. Mulligan E. Kelly A. Griffin M. A pathological study of tumour regression in oesophageal adenocarcinoma treated with preoperative chemoradiotherapy. J Clin Pathol. 2001;54:841–845. doi: 10.1136/jcp.54.11.841. , and. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y, Swisher SG, Ajani JA, et al. The number of lymph nodes with metastasis predicts survival in patients with esophageal or esophagogastric junction adenocarcinoma who receive preoperative chemoradiation. Cancer. 2006;106:1017–1025. doi: 10.1002/cncr.21693. [DOI] [PubMed] [Google Scholar]
- Wang M, Gu X, Huang G, Yang Z, Wang L, Chen D. Randomized trial on combined pre-operative irradiation and surgery in the treatment of esophageal carcinoma-report on 418 patients. Chin J Radiat Oncol. 2001;10:168–172. . (In Chinese.) [Google Scholar]