Abstract
Thymic carcinoma is an uncommon neoplasm. The efficacy of second-line treatment with docetaxel in advanced thymic carcinoma has not been well studied. Therefore, we conducted a review of the efficacy of docetaxel-based chemotherapy as a second-line regimen for advanced thymic carcinoma. Fifteen patients with advanced thymic carcinoma who received second-line chemotherapy with docetaxel singlet or docetaxel/platinum combination chemotherapy regimens were retrospectively reviewed. There were 11 males and four females, with a median age of 53 years. Squamous cell carcinoma was most common (n = 10), followed by undifferentiated carcinoma (n = 4), and small cell carcinoma (n = 1). Eight patients received docetaxel/platinum combination chemotherapy and seven docetaxel mono-therapy. Four patients showed partial responses, representing a response rate of 26.7%. The median progression-free survival and overall survival in the 15 patients were 4.0 (2.8–5.2) and 22.0 (14.6–29.4) months, respectively. There was no difference in progression-free survival between the docetaxel singlet or docetaxel/platinum combination chemotherapy (3.5 months vs. 4.0 months, P = 0.889). A docetaxel-based regimen could be a potential therapeutic option as a second-line chemotherapy for advanced thymic carcinoma.
Keywords: Chemotherapy, docetaxel, efficacy, second-line, thymic carcinoma
Introduction
Thymic carcinoma is a rare carcinoma of the thymus arising from the thymic epithelium. From 1973 to 1998 in the US, the overall incidence of malignant thymoma was 0.15 per 100 000 person-years.1 Approximately half of the patients with thymic carcinoma have Stage IV disease at initial presentation.2 It has been difficult to conduct clinical trials for patients with thymic carcinoma because of the low incidence of this disease.3 Systemic chemotherapy has proven effective. Platinum-based chemotherapy is the most frequently used systemic therapy for advanced thymic carcinoma. New anticancer agents including irinotecan, paclitaxel, docetaxel, gemcitabine, and vinorelbine, which are useful in the treatment of lung cancer, were tried in a small series of retrospective and prospective clinical trials for the treatment of thymic carcinoma as first-line chemotherapy,4–7 but there are few reports describing second-line chemotherapy for thymic carcinoma. Docetaxel, a semisynthetic taxane targeting the β-sub-unit of tubulin, exhibits broad-spectrum anticancer activity. In clinical situations, this agent is applied in several kinds of solid carcinomas and has proved effective.8 However, the efficacy evaluation of docetaxel for second-line chemotherapy in thymic carcinoma is lacking.
We conducted a retrospective study to evaluate the efficacy of docetaxel as second-line chemotherapy against advanced thymic carcinoma.
Materials and methods
Patient eligibility
The data recorded included demographic information, clinical assessment, chemotherapy cycle, response, and toxicity. Criteria for inclusion in the study were: (i) classification according to Masaoka criteria Stage IVa or IVb, stage IV including pleural or pericardial dissemination, and lymphogenous or hematogenous metastasis; (ii) failure of prior first-line chemotherapy regimens; (iii) no local treatment, such as radiotherapy or interventional therapy was performed during second-line therapy; (iv) the pathological diagnosis of thymic carcinoma was established according to the histopathological criteria proposed by the World Health Organization (WHO) 2004 version 9 and all patients were diagnosed as type C, exhibiting cytological atypia and cytoarchitectural features not specific to the thymus, but analogous to those seen in carcinomas of other organs.
Treatment methods
Docetaxel was administered at a dose of 75 mg/m2 intravenously over one hour, with the treatment cycle repeated every three weeks. The doublet treatment was followed by carboplatin (AUC=5) or cisplatin at a dose of 75 mg/m2 on day 1. Chemotherapy ceased if no progression occurred at the end of four to six cycles.
Responses and toxicity
Tumor responses were assessed every two cycles, or were evaluated early when significant signs of progression appeared. Objective tumor responses were measured according to the Response Evaluation Criteria in Solid Tumors (RECIST 1.1). Objective tumor responses included complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD). The disease control rate (DCR) was defined as the addition of objective response and stabilization rates (CR+PR+SD). Toxicities were checked every cycle throughout the second-line therapy. All toxicities were evaluated according to the National Cancer Institute Common Toxicity Criteria version 3.0 (CTC3.0).
Statistical analysis
Analyses were conducted using the computer software SPSS version 16.0 (SPSS Inc., Chicago, IL, USA). The survival curves were calculated according to the Kaplan-Meier method. Values of P < 0.05 were considered significant. Overall survival was defined as the time from the first day of diagnosis to death or last follow-up. Progression-free survival (PFS) encompassed the time from the first cycle of second-line therapy to documented progression or death from any cause.
Follow-up
All of the patients that were evaluated for second-line tumor response had a PFS. The follow-up rate was 100%. The median follow-up period was 30.1 months (6.5–72) and the last follow-up recorded was 31 December 2011.
Results
Treatment characteristics
Between January 2005 and March 2010, only 37 of the 70 patients with advanced thymic carcinoma diagnosed at the Zhejiang Cancer Hospital received second-line treatment. Twenty-two patients had chemotherapy other than docetaxel; therefore, only the remaining 15 patients treated with docetaxel were included in this study. The clinical and pathological characteristics of the 15 patients are summarized in Tables 1 and 2. Squamous cell carcinoma was the most common histological type. Twelve (80%) patients had a performance status of 0–1 (Table 1). The median (range) number of chemotherapy cycles was four (one to six), and four (26.7%) patients received six cycles of chemotherapy (Table 2).
Table 1.
Characteristics of 15 patients
| Gender | Number (%) |
|---|---|
| Male | 11 (73.3) |
| Female | 4 (26.7) |
| Age | |
| Range | 34–70 |
| Median | 53 |
| <60 | 11 (73.3) |
| ≥60 | 4 (26.7) |
| Smoking history | |
| Current or ever | 8 (53.3) |
| Never | 7 (46.7) |
| Histology | |
| Squamous cell carcinoma | 10 (66.7) |
| Undifferentiated carcinoma | 4 (26.7) |
| Small cell carcinoma | 1 (6.6) |
| Clinical stage | |
| IVa | 5 (33.3) |
| IVb | 10 (66.7) |
| Performance status | |
| 0–1 | 12 (80.0) |
| 2 | 3 (20.0) |
| History of surgery | |
| Yes | 6 (40.0) |
| No | 9 (60.0) |
Table 2.
Clinical profiles and outcomes of 15 patients with advanced thymic carcinoma
| Patient | Gender | Age | Metastasis site | Histology | First-line regimen | Response to first-line | Second-line regimen | Response to second-line | PFS (month) | OS (month) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Male | 53 | Lung | Undifferentiated | TC | SD | DP | SD | 4.5 | 19.5 |
| 2 | Female | 49 | Liver | SCC | TC | PR | DP | PR | 5.6 | 25.0 |
| 3 | Male | 42 | Lung | SCC | CAP | SD | DP | PD | 1.0 | 10.7 |
| 4 | Female | 53 | Lung | SCC | EP | PD | DC | SD | 4.0 | 12.0 |
| 5 | Female | 62 | Bone | SCC | VIP | PR | D | PR | 4.5 | 36.5 |
| 6 | Male | 50 | Pleural | Undifferentiated | TC | PR | DP | PD | 1.5 | 16.0 |
| 7 | Male | 34 | Supraclavicular LN | Undifferentiated | VIP | SD | D | PD | 1.0 | 11.0 |
| 8 | Male | 55 | Supraclavicular LN | SCC | TC | SD | D | SD | 3.5 | 25.0 |
| 9 | Male | 56 | Pleural | SCC | CAP | SD | D | SD | 4.0 | 22.0 |
| 10 | Female | 51 | Pleural | SCC | TC | PR | D | PR | 11.0 | 25.0 |
| 11 | Male | 47 | Lung | Undifferentiated | CAP | PD | DC | PD | 2.0 | 8.5 |
| 12 | Male | 65 | Pleural | SCC | CAP | PR | D | SD | 3.0 | 22.0 |
| 13 | Male | 70 | Lung | SCC | ADOC | SD | D | PD | 1.5 | 12.0 |
| 14 | Male | 61 | Pleural | Small cell | EP | PR | DP | PR | 5.0 | 48.0+ |
| 15 | Male | 59 | Lung | SCC | TC | SD | DP | SD | 6.0 | 26.0 |
ADOC, Cyclophosphamide+doxorubicin+cisplatin+vincristine; CAP, Cyclophosphamide+doxorubicin+cisplatin; CR, complete response; DC, docetaxel+ Carboplatin; DP, docetaxel++cisplatin; OS, overall survival; PD, progressive disease; PFS, progression-free survival; PR, partial response; SCC, Squamous cell carcinoma; SD, stable disease; TC, Carboplatin+paclitaxel; VIP, Ifosfamide+cisplatin+etoposide.
Response data and survival analysis in second-line treatment
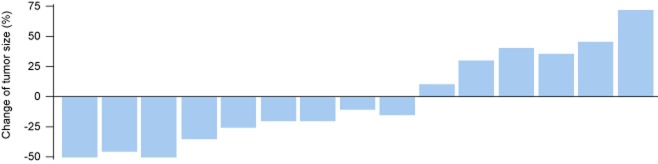
The tumor response to chemotherapy was evaluated in all patients: no patients achieved CR, four had PR, six had SD, and five had PD, which represents a response rate of 26.7% and a DCR of 66.7% (Fig 1). The overall median survival time (MST) was 22.0 months (95% confidence interval [CI], 14.6–29.4, Fig 2). The median PFS was 4.0 months (95% CI, 2.8–5.2). There was no significant association among the PFS and the gender (P = 0.08), age (P = 0.67), stage (P = 0.46), pathological subtype (P = 0.28), or smoking status (P = 0.54). No difference was found in PFS between docetaxel-based monotherapy (single) and doublet chemotherapy (doublet) (3.5 months vs. 4.0 months, P = 0.89, Fig 3). The DCR was 71.4% and 62.5% for mono-therapy and doublet chemotherapy, respectively (P = 0.69). Those patients who reached a PR during the first-line treatment had a PFS of 4.5 months in second-line treatment, which was longer than the PFS of the patients with SD (median 3.5 months) and PD (2.0 months) in the first-line treatment (P = 0.37) (Fig 4).
Figure 1.
Change in tumor size in 15 patients.
Figure 2.
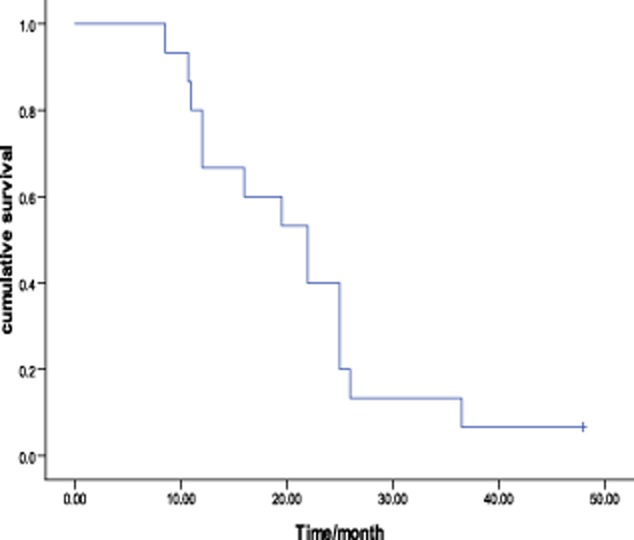
Survival curve of 15 patients using the Kaplan-Meier method.
Figure 3.
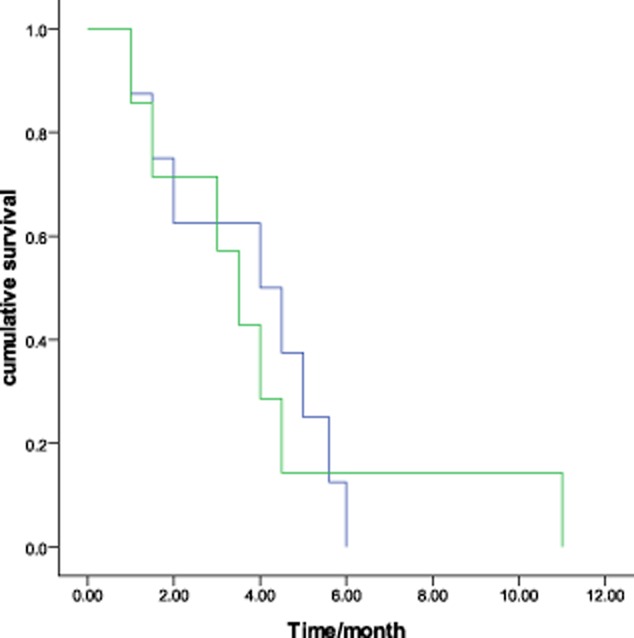
Kaplan-Meier curves comparing progression-free survival of patients with docetaxel and docetaxel-based doublet chemotherapy. (P = 0.89).  , doublet;
, doublet;  , single.
, single.
Figure 4.
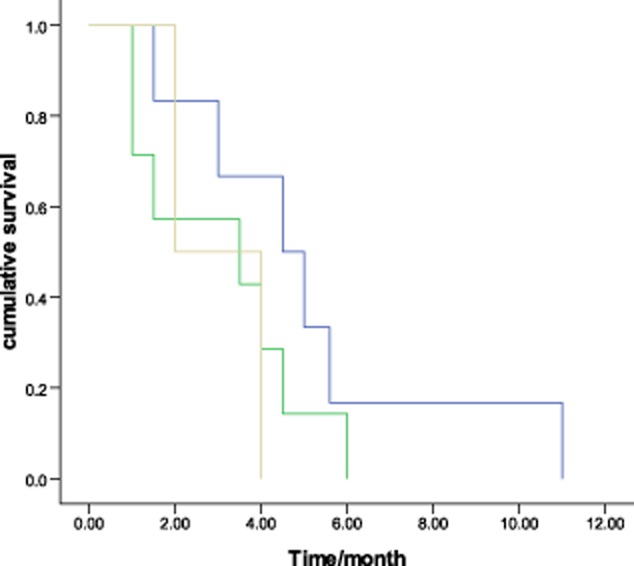
Progression-free survival of second-line treatment according to first-line chemotherapy efficacy (P = 0.37).  , partial response (PR);
, partial response (PR);  , stable disease (SD);
, stable disease (SD);  , progressive disease (PD).
, progressive disease (PD).
Toxicity of second-line therapy
All patients treated with second-line therapy were assessed for toxicity. One patient in the doublet chemotherapy group refused further therapy because of severe toxicities (grade 4 neutropenia) and one changed from doublet to single docetaxel treatment. The overall rate of grade 3/4 toxicity was 60%. The grade 3/4 toxicity was lower in the single agent arm than in the doublet therapy group (3/7 and 6/8, P = 0.46, respectively, Table 3).
Table 3.
The main grade 3–4 toxicity of docetaxel-based chemotherapy
| Docetaxel (n = 7) | Docetaxel/platinum combination (n = 8) | P | |
|---|---|---|---|
| Non-hematological toxicities | |||
| Nausea /Vomiting | 0 (0.0%) | 1 (12.5%) | 0.95 |
| Neurotoxicity | 0 (0.0%) | 1 (12.5%) | 0.95 |
| Hematological toxicities | |||
| Neutropenia | 2 (28.6%) | 3 (37.5%) | 0.85 |
| Thrombocytopenia | 1 (14.3%) | 1 (12.5%) | 0.51 |
| Total | 3 (42.9%) | 6 (75.0%) | 0.46 |
Discussion
This analysis involved 15 cases of advanced thymic carcinoma treated with docetaxel mono-therapy or doublet chemotherapy and demonstrated that docetaxel appears to be active for advanced thymic carcinoma in terms of efficacy and tolerability.
The efficacy of combined chemotherapy, as a first-line treatment, has been shown in patients with thymic carcinoma in previous studies.2 Fornasiero et al.9 reported that modified ADOC therapy is effective against thymic carcinoma. Loehr et al.10 reported that a combined etoposide, ifosfamide, and cisplatin regimen exhibits moderate activity against thymic carcinoma. In contrast, there are few reports on second-line chemotherapies for thymic carcinoma. The octreotide activity was confirmed by a phase II study that was conducted in patients with octreotide scan-positive thymic epithelial tumors.11 Out of 38 patients, only six patients had thymic carcinoma or carcinoid and received second-line treatment. Unfortunately, none of six patients with thymic carcinoma had an objective response to therapy. A phase II study by Palmieri et al. involving 15 patients with thymic carcinoma and invasive thymoma showed a promising result with capecitabine and gemcitabine in second-line treatment.12 Kanda et al. described seven cases of unresectable thymic carcinoma treated with irinotecan plus platinum as second-line chemotherapy and reported a response rate of 28.6%.13
Recent studies have focused on the role of targeted therapy in advanced thymic carcinomas. It has been shown that thymic carcinomas generally express c-KIT expression. Based on these data, a few case reports have documented clinical responses to treatment with biologic agents like sunitinib14 and sorafenib.15 Several cases showed effective results. The targeted agents may be a promising treatment in the future.
Docetaxel, a semisynthetic taxane targeting the β-sub-unit of tubulin, exhibits broad-spectrum anticancer activity. In clinical situations, this agent is used as first-line chemotherapy in non-small cell lung cancer, breast, and other solid carcinomas. There is only one case report showing the efficacy of docetaxel as mono-therapy for thymic carcinoma as second-line therapy,16 and we found no reports of its use as second-line treatment in any series. Our analysis showed that chemotherapy using docetaxel was active for advanced thymic carcinoma patients. The response rate was 26.7%, with a median PFS of four months.
Because of the rarity of thymic carcinomas, studies aimed at detecting the effective predictive factor of chemotherapy are lacking. A retrospective study by Okuma, et al.17 including 40 patients suggested that the prognosis for advanced thymic carcinoma could be predicted based on sensitivity to first-line chemotherapy. In our study, the patients who achieved a PR in first-line treatment had a longer PFS of second-line than SD and PD patients, which may indicate that first-line efficacy may influence second-line treatment.
The adverse events of our study occurred with a similar incidence as previous reports in other solid tumor studies.18 Neutropenia is the most frequently reported toxicity, and doublet chemotherapy-related toxicity is much higher than docetaxel single-agent.
Although our study has the caveats of retrospective analyses and is limited by the heterogeneity of docetaxel-based chemotherapy regimen and no control group, it provides relevant insight into the efficacy of docetaxel-based treatment of advanced thymic carcinoma.
Conclusion
Our results suggest that a docetaxel-based regimen could be a potential therapeutic option as a second-line chemotherapy for platinum drug pre-treated thymic carcinoma, but further studies are required to fully quantify the efficacy of this agent.
Acknowledgments
This work was supported by grants from the Natural Science Foundation of Zhejiang (No.Y13H160076), the Medical Scientific Research Foundation of Zhejiang Province (No. 2013KYB049, 2012KYA023) and the Fund of Development Center for Medical Science and Technology, Ministry of Health (W2012FZ134).
Disclosure
No authors report any conflict of interest.
References
- Engels EA, Pfeiffer RM. Malignant thymoma in the United States: demographic patterns in incidence and associations with subsequent malignancies. Int J Cancer. 2003;105:546–551. doi: 10.1002/ijc.11099. [DOI] [PubMed] [Google Scholar]
- Tomaszek S, Wigle DA, Keshavjee S, Fischer S. Thymomas: review of current clinical practice. Ann Thorac Surg. 2009;87:1973–1980. doi: 10.1016/j.athoracsur.2008.12.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubens MA. Treatment updates in advanced thymoma and thymic carcinoma. Curr Treat Options Oncol. 2012;13:527–534. doi: 10.1007/s11864-012-0211-7. [DOI] [PubMed] [Google Scholar]
- Koizumi T, Takabayashi Y, Yamagishi S, et al. Chemotherapy for advanced thymic carcinoma: clinical response to cisplatin, doxorubicin, vincristine, and cyclophosphamide (ADOC chemotherapy) Am J Clin Oncol. 2002;25:266–268. doi: 10.1097/00000421-200206000-00012. [DOI] [PubMed] [Google Scholar]
- Igawa S, Murakami H, Takahashi T, et al. Efficacy of chemotherapy with carboplatin and paclitaxel for unresectable thymic carcinoma. Lung Cancer. 2010;67:194–197. doi: 10.1016/j.lungcan.2009.03.031. [DOI] [PubMed] [Google Scholar]
- Okuma Y, Shimokawa T, Takagi Y, et al. S-1 is an active anticancer agent for advanced thymic carcinoma. Lung Cancer. 2010;70:357–363. doi: 10.1016/j.lungcan.2010.09.004. [DOI] [PubMed] [Google Scholar]
- Okuma Y, Hosomi Y, Takagi Y, Iguch M, Okamura T, Shibuya M. Cisplatin and irinotecan combination chemotherapy for advanced thymic carcinoma: evaluation of efficacy and toxicity. Lung Cancer. 2011;74:492–496. doi: 10.1016/j.lungcan.2011.05.013. [DOI] [PubMed] [Google Scholar]
- Sun Y, Wu YL, Zhou CC, et al. Second-line pemetrexed versus docetaxel in Chinese patients with locally advanced or metastatic non-small cell lung cancer: a randomized, open-label study. Lung Cancer. 2013;79:143–150. doi: 10.1016/j.lungcan.2012.10.015. [DOI] [PubMed] [Google Scholar]
- Fornasiero A, Daniele O, Ghiotto C, et al. Chemotherapy for invasive thymoma. A13-year experience. Cancer. 1991;68:30–33. doi: 10.1002/1097-0142(19910701)68:1<30::aid-cncr2820680106>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Loehrer PJ, Sr, Jiroutek M, Aisner S, et al. Combined etoposide, ifosfamide, and cisplatin in the treatment of patients with advanced thymoma and thymic carcinoma: an intergroup trial. Cancer. 2001;91:2010–2015. [PubMed] [Google Scholar]
- Loehrer PJ, Sr, Wang W, Johnson DH, Aisner SC, Ettinger DS. Octreotide alone or with prednisone in patients with advanced thymoma and thymic carcinoma: an Eastern Cooperative Oncology Group Phase II Trial. J Clin Oncol. 2004;22:293–299. doi: 10.1200/JCO.2004.02.047. [DOI] [PubMed] [Google Scholar]
- Palmieri G, Merola G, Federico P, et al. Preliminary results of phase II study of capecitabine and gemcitabine (CAP-GEM) in patients with metastatic pretreated thymic epithelial tumors (TETs) Ann Oncol. 2010;21:1168–1172. doi: 10.1093/annonc/mdp483. [DOI] [PubMed] [Google Scholar]
- Kanda S, Koizumi T, Komatsu Y, et al. Second-line chemotherapy of platinum compound plus CPT-11 following ADOC chemotherapy in advanced thymic carcinoma: analysis of seven cases. Anticancer Res. 2007;27:3005–3008. [PubMed] [Google Scholar]
- Ströbel P, Bargou R, Wolff A, et al. Sunitinib in metastatic thymic carcinomas: laboratory findings and initial clinical experience. Br J Cancer. 2010;103:196–200. doi: 10.1038/sj.bjc.6605740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XF, Chen Q, Huang WX, Ye YB. Response to sorafenib in cisplatin-resistant thymic carcinoma: a case report. Med Oncol. 2009;26:157–160. doi: 10.1007/s12032-008-9100-0. [DOI] [PubMed] [Google Scholar]
- Oguri T, Achiwa H, Kato D, et al. Efficacy of docetaxel as a second-line chemotherapy for thymic carcinoma. Chemotherapy. 2004;50:279–282. doi: 10.1159/000082626. [DOI] [PubMed] [Google Scholar]
- Okuma Y, Hosomi Y, Takagi Y, et al. Clinical outcomes with chemotherapy for advanced thymic carcinoma. Lung Cancer. 2013;80:75–80. doi: 10.1016/j.lungcan.2012.12.012. [DOI] [PubMed] [Google Scholar]
- Fossella F, Pereira JR, von Pawel J, et al. Randomized, multinational, phase III study of docetaxel plus platinum combinations versus vinorelbine plus cisplatin for advanced non-small cell lung cancer: the TAX 326 study group. J Clin Oncol. 2003;21:3016–3024. doi: 10.1200/JCO.2003.12.046. [DOI] [PubMed] [Google Scholar]