Abstract
Background
This study was conducted to evaluate the efficacy and safety of paclitaxel-carboplatin combined with intercalated gefitinib in patients with advanced, untreated, nonsquamous non-small cell lung cancer.
Methods
A total of 29 patients were enrolled in the study. All patients were Chinese, with a histology type of adenocarcinoma, without a smoking history, and as a result of the limited tissue sample, an epidermal growth factor receptor (EGFR) mutation test could not be performed. All patients received chemotherapy of paclitaxel-carboplatin every 21 days for four cycles, and gefitinib (250 mg/day) was administered on days eight to 17 of the chemotherapy cycle. If the patient responded to chemotherapy, maintenance therapy of 250mg of gefitinib could be administered daily.
Results
All of the 29 patients received at least one cycle of chemotherapy and gefitinib, and 25 patients received four cycles of therapy. Eighteen patients selected maintenance therapy with gefitinib. The objective response rate was 74.1% (95% confidence interval, 53.7% to 88.9%). No complete response was achieved. The median progression-free survival was 16 months, however, the median overall survival was not available by the conclusion of the study. The major adverse event was hematologic toxicity.
Conclusions
The regimen of paclitaxel-carboplatin combined with intercalated gefitinib showed a high response rate and a favorable safety profile. Gefitinib maintenance therapy was proven to be beneficial. This study proposes a good pattern of chemotherapy combined with EGFR tyrosine kinase inhibitors.
Keywords: Carboplatin, EGFR, gefitinib, non-small-cell lung cancer, paclitaxel
Introduction
Small-molecule epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs), such as gefitinib and erlotinib, are important target drugs for the treatment of advanced non-small cell lung cancer (NSCLC). The EGFR gene mutation status determines the efficacy of these drugs, which has been confirmed by the results of the IPASS,1 NEJGSG002,2 and WJTOG 34053 studies. Gefitinib has been approved as the first line treatment for NSCLC patients with sensitive EGFR gene mutation status. However, in clinical practice, because of limited tissue samples or technique, EGFR gene mutation can only be detected in a portion of patients. Chemotherapy remains the standard first-line treatment for patients whose EGFR gene mutation status is unknown.
Earlier clinical studies indicate that patients with some clinical characteristics (oriental, female, non-smokers and adenocarcinoma histology) are more likely to benefit from EGFR-TKIs.4–6 Further molecular biology studies7–9 indicate that these clinical features are generally consistent with patients with EGFR gene mutations. The IPASS study showed the response rate of the gefitinib monotherapy was 43.0% in this population, similar to the efficacy of the standard first-line chemotherapy. Therefore, if the EGFR gene mutation test is not possible, a rational substitute may be to select appropriate patients by their clinical characteristics. These patients also have the right and the opportunity to receive EGFR-TKI treatment.
Chemotherapy combined with TKIs represents a tempting option because patients may benefit from both treatments. Additive or synergistic activities of EGFR-TKIs and several chemotherapeutic drugs have been seen in preclinical models.10–12 Selecting patients sensitive to EGFR-TKIs and using EGFR-TKIs between chemotherapy cycles may be a reasonable strategy. In fact, a phase II and a phase III study,13,14 which used erlotinib between gemcitabine-cisplatin cycles, showed improved survival.
In this prospective study, we evaluated the efficacy and safety of intercalated gefitinib with paclitaxel-carboplatin in advanced untreated NSCLC patients. These patients were selected by clinical characters (oriental, non-smokers, and non-squamous cell carcinoma), as their EGFR gene mutation status was unknown as a result of the limited tissue samples.
Patients and methods
This prospective study was an open-labeled, non-randomized and single-arm phase II trial. All patients were from China with advanced NSCLC and meet the following criteria: histopathologically or cytologically confirmed NSCLC, with the exclusion of squamous cell carcinoma; the EGFR mutation test could not be performed because of a limited tissue sample; no smoking history; untreated patients with stage IIIb or IV who were not suitable for surgery or radiotherapy, or patients with postoperative recurrence who had never been treated by chemotherapy; age ≥18 years; Eastern Cooperative Oncology Group (ECOG) score 0–1 points; absolute neutrophil count (ANC) ≥ 2 × 109/L; platelet count ≥100 × 109/L; alanine aminotransferase (ALT) and aspartate aminotransferase (AST) ≤ 1.5 times the upper limit of normal levels; total bilirubin (TP) ≤ 1.5 times the upper limit of normal levels; serum creatinine level is within the normal range; for female patients, the serum β-hCG pregnancy test should be negative at screening; and subjects must sign prior informed consent documents. The exclusion criteria included: a history of treatments for other malignant tumors within one year prior to enrolment; patients who received other drugs under study or medical devices within one month prior to the present study; and symptomatic patients with brain metastases.
Treatments
All patients were treated with paclitaxel-carboplatin and gefitinib. The program was as follows: paclitaxel 175 mg/m2 on day one, carboplatin AUC = 5 on day two, and gefitinib 250 mg daily on days eight to 17 of a 21 day cycle for four cycles. The patients who achieved complete remission (CR), partial remission (PR), or stable disease (SD) after four cycles could continue gefitinib maintenance therapy, depending on their intentions. Maintenance therapy of gefitinib 250 mg/day was discontinued when disease progression or intolerable toxicity occurred. In cases where a grade 3/4 adverse event occurred, two paclitaxel or carboplatin dose reductions (from 100% to 75% or from 75% to 50%) were allowed. If the adverse events did not cease, chemotherapy and gefitinib administration were postponed for a maximum of 14 days.
Assessments
The primary endpoint of the study was objective response rate (ORR), and the secondary endpoint was progression-free survival (PFS) and overall survival (OS). Tumor assessments were performed every two cycles, and every two months during maintenance therapy. Tumor response was determined by Response Evaluation Criteria in Solid Tumors (RECIST1.1). PFS and OS were assessed from day one of the first cycle to the date of patient death or the date of objective disease progression. Death was considered a progression event in patients who died before disease progression occurred. Patients without documented death or objective progression at the time of the final analysis were censored at the date when they were last known to be alive or of their last objective tumor assessment, respectively.
Statistical analysis
At the time of the study, the response rate of first-line chemotherapy regimens was about 30%.1,15–18 The IPASS study1 showed that 43% of the patients who were selected on the basis of clinical characteristics responded to EGFR-TKIs. According to this data, our study assumed chemotherapy of paclitaxel-carboplatin and the gefitinib monotherapy in this population could produce a response rate of 35% and 40.0%, respectively. Therefore, the response rate of a combination of chemotherapy and gefitinib should be more than 60% if additive or synergistic effects existed. This study assumed the two kinds of therapy were synergic, and the response rate of 60% was set as the cut-off value, assuming the unilateral statistically significant level was α = 0.05, in 80% power of the test (1-β). Using the Simon Mini-max design of the two-stage method, a total of 26 eligible cases were required on the basis of the study design assumption. If more than 13 patients responded, the desired hypothesis was considered to have been achieved, meaning that paclitaxel-carboplatin with intercalated gefitinib improved the efficacy, and further expansion of the research was required.
The objective response rate and 95% confidence interval (CI) were calculated by an approximate normal distribution method. The median PFS and OS were calculated, and survival curves were mapped out by the Kaplan-Meier method using SPSS 13.5. Adverse events were summarized for all patients who received at least one dose of the drugs.
Results
Patients
A total of 29 patients were enrolled between May 2009 and March 2010. The last follow up date was 1 January 2012. The median follow-up duration was 22 months. All patients were Chinese, and their demographic data is shown in Table 1.
Table 1.
Patient characteristics (n = 29)
| No. | % | |
|---|---|---|
| Gender | ||
| Female | 26 | 89.7 |
| Male | 3 | 10.3 |
| Age, years | ||
| Median | 52 | |
| Range | 23 to 73 | |
| Cancer stage | ||
| IV | 22 | 75.9 |
| IIIB | 5 | 17.2 |
| Relapse | 2 | 6.9 |
| ECOG performance status | ||
| 0 | 21 | 72.4 |
| 1 | 8 | 27.6 |
| Histology | ||
| Adenocarcinoma | 28 | 96.6 |
| Bronchioloalveolar carcinoma | 1 | 3.4 |
| Prior smoking history | ||
| Never | 27 | 93.1 |
| Light passive smoking | 2 | 6.9 |
| Diagnostic method | ||
| Fiber optic bronchoscope biopsy | 14 | 48.2 |
| Hydrothorax cytology | 4 | 13.8 |
| Sputum cytology | 3 | 10.3 |
| Per cutem pneumocentesis | 6 | 15.4 |
| Relapse postoperative | 2 | 6.9 |
ECOG, Eastern Cooperative Oncology Group.
All 29 patients received at least one cycle of chemotherapy and gefitinib. Of these, one patient withdrew his informed consent after one cycle without providing a reason, and another patient died of hemoptysis, which was considered unrelated to the chemotherapy or gefitinib. Finally, a total of 27 patients received combined therapy for at least two cycles. Of the 27 patients, 25 patients received four cycles of combined therapy and 18 patients selected subsequent maintenance therapy with gefitinib.
Efficacy
Of the 29 patients, 27 patients were eligible for tumor response assessment. The objective response rate was 74.1% (95% CI, 53.7% to 88.9%), indicating that the hypothesis of synergistic activities was probably true. The response rates were 48.1% after two cycles of therapy, and 74.1% after four cycles. The disease control rate (DCR) was 88.9% (95% CI, 70.8% to 97.7%) after four cycles. During the gefitinib maintenance therapy period, the tumor size continued to reduce in 10/18 patients. The changes in tumor size after two and four cycles of therapy are shown in Figures 1 and 2.
Figure 1.
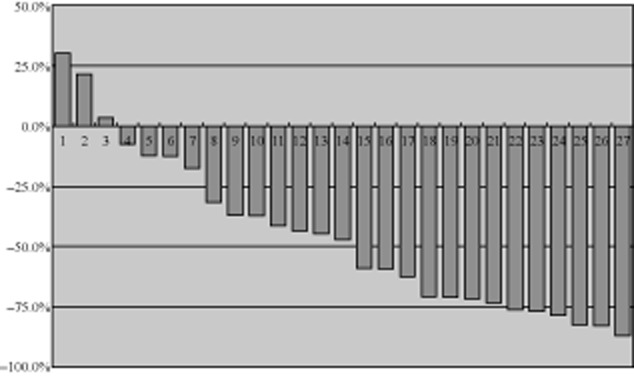
Tumor size (% change against the baseline after two cycles of treatment, n = 27).
Figure 2.
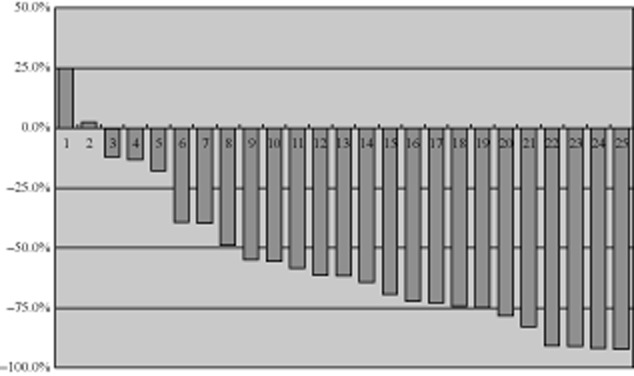
Tumor size (% change against the baseline after four cycles, n = 25).
The median follow-up period was 22 (1.5–32) months for all patients. The median PFS was 16 (1.5–32) months, however, the median OS was not available by the conclusion of the study. One-year PFS was 53.1% ± 9.5% and OS was 82.1% ± 7.2%, which were better results than those of past studies, even in the EGFR mutation positive population. Two-year PFS was 43.2% ± 10.2% and OS was 63.5% ± 9.2%. The PFS and OS curve are shown in Figure 3.
Figure 3.
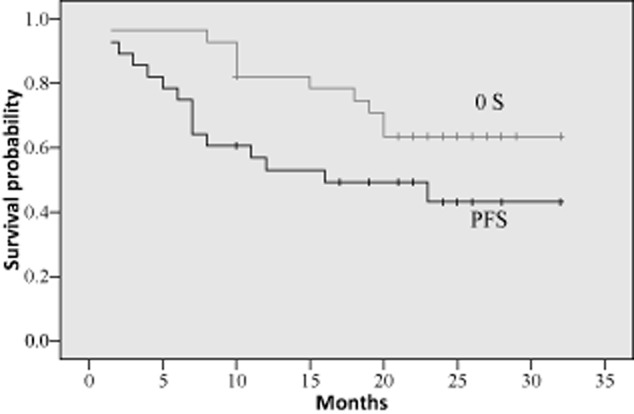
Progression-free survival (PFS) and overall survival (OS) curves (n = 29).
The median duration of gefitinib maintenance therapy was 19 (2–29) months in the 18 patients. The median PFS and OS of these patients were not available by the conclusion of the study. One year PFS was 72.2% ± 10.6% and OS was 94.4% ± 5.4%. Two year PFS was 58.3% ± 12.5% and OS was 77.8% ± 9.8%. The survival curves of the patients with gefitinib maintenance therapy are shown in Figure 4.
Figure 4.
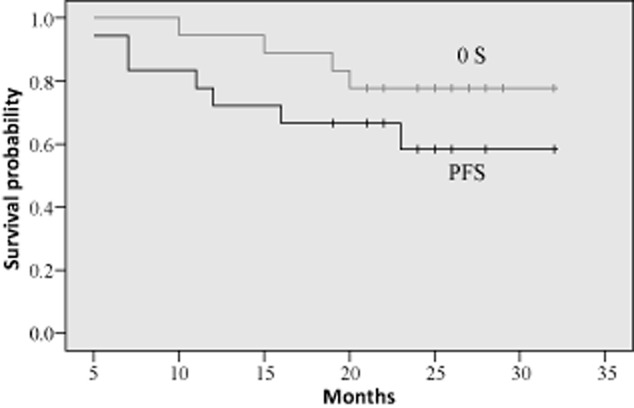
Survival curves of the patients with gefitinib maintenance therapy (n = 18).
Toxicity
Most of the adverse events experienced by the patients were consistent with the known toxicities of the chemotherapy agents. The main adverse events were hematologic. Grade III/IV leukopenia and neutropenia accounted for 57.1% and 78.6%, respectively, but no neutropenic infection occurred. Gefitinib maintenance therapy is a safe treatment. Gefitinib-associated skin events were mild. Drug-related adverse events are shown in Table 2.
Table 2.
Drug-related toxicities
| Grade I/II | Grade III/IV | |
|---|---|---|
| Leukopenia | 96.4% | 57.1% |
| Neutropenia | 92.9% | 78.6% |
| Anemia | 46.4% | 0 |
| Thrombopenia | 10.7% | 0 |
| Nausea and vomiting | 48.3% | 3% |
| Neuropathy-sensory | 37.9% | 0 |
| Acne-like rash | 31% | 0 |
| ALT or AST | 25% | 3.6% |
| Alopecia | 25% | 0 |
| Diarrhea | 3.6% | 0 |
| Thrombotic | 0 | 3.6% |
ALT, alanine aminotransferase; AST, aspartate aminotransferase.
Three serious adverse events (SAE) occurred during the combination therapy period. One patient died from serious hemoptysis after two cycles of therapy, which was considered unrelated to the therapy. Another patient underwent a serious hyponatremia after two cycles of therapy; the reason for this development could not be ascertained. A third patient developed a vein thrombosis of the lower extremities and was hospitalized, but finally completed four cycles of treatment. Two (7.2%) patients dropped out of the study because of adverse events.
Discussion
The IPASS study and several other trials1–3 have demonstrated that EGFR-mutation status is the most important predictor in determining whether to use EGFR TKIs in first line treatment of NSCLC. However, because of sample and technical availability, it is impossible to detect EGFR gene-mutation for all lung adenocarcinoma patients. Although several very sensitive techniques can be used for detecting EGFR gene mutation,19–21 they are not universally available in clinical practice. Earlier trials4,7–9 found that some clinicopathologic features could help identify patients who have a higher likelihood of EGFR gene mutation. At present, for those patients whose EGFR gene-mutation status could not be detected, clinical characteristics (including oriental origin, no smoking or light smoking, and non-squamous carcinoma histology) may help to determine which patients could benefit from EGFR-TKIs, and 40%-50% of them would respond to EGFR-TKI treatment.1,22,23
Of course, a better solution is patients could benefit from both conventional chemotherapy and EGFR-TKIs. The results of earlier trials15–18 suggest that we need to find an appropriate way of combining chemotherapy and EGFR-TKIs. To avoid probable drug interference or antagonistic effects because of concomitant use of EGFR-TKIs and chemotherapy, it would be a good idea to use EGFR-TKIs between chemotherapy cycles, or as a maintenance treatment following chemotherapy. In this study, we used paclitaxel-carboplatin instead of gemcitabine-cisplatin as the chemotherapy regimen, because it was also the standard first-line regimen, and paclitaxel showed synergistic effects with gefitinib in preclinical research.10–12 Therefore, we decided that paclitaxel-carboplatin was a more suitable combination for gefitinib than gemcitabine-cisplatin. Patients received paclitaxel-carboplatin every 21 days and gefitinib on days eight to 17 for 10 days. There was a four to seven day interval between cytotoxic drugs and EGFR-TKIs. This should be long enough to avoid reducing the sensitivity of chemotherapy as a result of EGFR-TKIs blocking tumor cell proliferation.
This new regimen showed a high response rate and DCR, suggesting that paclitaxel-carboplatin combined with gefitinib could enhance the response rate. According the statistical hypothesis of this study, if the response rate was more than 60%, the combination of chemotherapy and gefitinib had a synergistic effect. Further, the majority of patients who received gefitinib maintenance therapy had an optimistic PFS and OS. All adverse events were predictable from the safety profiles of paclitaxel-carboplatin and gefitinib. There was no significant increase in adverse events of chemotherapy combining gefitinib than for chemotherapy alone. The results confirmed that this regimen had a favorable safety profile.
This study proposed a good pattern of chemotherapy combined with EGFR-TKIs. The patients with an unknown EGFR gene status would also have the opportunity to use EGFR-TKIs. But the sample of this study was small, and no contrast group of chemotherapy or gefitinib alone was designed. Further studies are needed to confirm the value of this regimen. In addition, it also can be studied in the following aspects. Because of the high response rate of this regimen, it may become a promising new-adjuvant therapy regimen. Moreover, for the patients who benefitted from disease control by EGFR-TKIs, once the disease progresses slowly or local progression appears, maintaining EGFR-TKIs therapy while adding a new chemotherapy may be a sound and successful treatment option.
Acknowledgments
This study obtained financial support of the Wu Jieping Medical Foundation(2009001); Special Funds for Central Health Authority (B2009B124); National Major Project for New Drug Innovation (2008ZX09312, 2012ZX09303012); Beijing Municipal Science and Technology Commission Major Project for New Drug Innovation (Z121107005112005, Z121102009212055).
Disclosure
No authors report any conflict of interest.
References
- Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 361:947–957. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362:2380–2388. doi: 10.1056/NEJMoa0909530. [DOI] [PubMed] [Google Scholar]
- Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol. 2010;11:121–128. doi: 10.1016/S1470-2045(09)70364-X. [DOI] [PubMed] [Google Scholar]
- Wu YL, Zhong WZ, Li LY, et al. Epidermal growth factor receptor mutations and their correlation with gefitinib therapy in patients with non-small cell lung cancer: a meta-analysis based on updated individual patient data from six medical centers in mainland China. J Thorac Oncol. 2007;2:430–439. doi: 10.1097/01.JTO.0000268677.87496.4c. [DOI] [PubMed] [Google Scholar]
- Koyama N, Jinn Y, Takabe K, et al. The characterization of gefitinib sensitivity and adverse events in patients with non-small cell lung cancer. Anticancer Res. 2006;26:4519–4525. [PubMed] [Google Scholar]
- Kaneda H, Tamura K, Kurata T, Uejima H, Nakagawa K, Fukuoka M. Retrospective analysis of the predictive factors associated with the response and survival benefit of gefitinib in patients with advanced non-small-cell lung cancer. Lung Cancer. 2004;46:247–254. doi: 10.1016/j.lungcan.2004.04.032. [DOI] [PubMed] [Google Scholar]
- Wang F, Fu S, Tang T, et al. Relationship between mutations of epidermal growth factor receptor gene and clinicopathologic features of non-small cell lung cancers. Zhonghua Bing Li Xue Za Zhi. 2011;40:664–666. (In Chinese.) [PubMed] [Google Scholar]
- Sasaki H, Endo K, Takada M, et al. L858R EGFR mutation status correlated with clinico-pathological features of Japanese lung cancer. Lung Cancer. 2006;54:103–108. doi: 10.1016/j.lungcan.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Feng Q, Li XH, Chen Z, et al. Epidermal growth factor receptor gene mutations and clinicopathologic correlation in 309 patients with non-small cell lung cancer. Zhonghua Bing Li Xue Za Zhi. 2011;40:660–663. (In Chinese.) [PubMed] [Google Scholar]
- Solit DB, She Y, Lobo J, et al. Pulsatile administration of the epidermal growth factor receptor inhibitor gefitinib is significantly more effective than continuous dosing for sensitizing tumors to paclitaxel. Clin Cancer Res. 2005;11:1983–1989. doi: 10.1158/1078-0432.CCR-04-1347. [DOI] [PubMed] [Google Scholar]
- Sumitomo M, Asano T, Asakuma J, Asano T, Horiguchi A, Hayakawa M. ZD1839 modulates paclitaxel response in renal cancer by blocking paclitaxel-induced activation of the epidermal growth factor receptor-extracellular signal-regulated kinase pathway. Clin Cancer Res. 2004;10:794–801. doi: 10.1158/1078-0432.ccr-0948-03. [DOI] [PubMed] [Google Scholar]
- Takabatake D, Fujita T, Shien T, et al. Tumor inhibitory effect of gefitinib (ZD1839, Iressa) and taxane combination therapy in EGFR-overexpressing breast cancer cell lines (MCF7/ADR, MDA-MB-231) Int J Cancer. 2007;120:181–188. doi: 10.1002/ijc.22187. [DOI] [PubMed] [Google Scholar]
- Mok TS, Wu YL, Yu CJ, et al. Randomized, placebo-controlled, phase II study of sequential erlotinib and chemotherapy as first-line treatment for advanced non-small-cell lung cancer. J Clin Oncol. 2009;27:5080–5087. doi: 10.1200/JCO.2008.21.5541. [DOI] [PubMed] [Google Scholar]
- Mok TSK, Lee JS, Zhang L, et al. Biomarker analyses and overall survival (OS) from the randomized, placebo-controlled, phase 3, FASTACT-2 study of intercalated erlotinib with first-line chemotherapy in advanced non-small-cell lung cancer (NSCLC) 37th European Society for Medical Oncology (ESMO) Congress 28 September-2 October, 2012, Vienna, Austria: Abstract 1023.
- Giaccone G, Herbst RS, Manegold C, et al. Gefitinib in combination with gemcitabine and cisplatin in advanced non-small-cell lung cancer: a phase III trial – INTACT 1. J Clin Oncol. 2004;22:777–784. doi: 10.1200/JCO.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Herbst RS, Giaccone G, Schiller JH, et al. Gefitinib in combination with paclitaxel and carboplatin in advanced non-small-cell lung cancer: a phase III trial–INTACT 2. J Clin Oncol. 2004;22:785–794. doi: 10.1200/JCO.2004.07.215. [DOI] [PubMed] [Google Scholar]
- Gatzemeier U, Pluzanska A, Szczesna A, et al. Phase III study of erlotinib in combination with cisplatin and gemcitabine in advanced non-small-cell lung cancer: the Tarceva Lung Cancer Investigation Trial. J Clin Oncol. 2007;25:1545–1552. doi: 10.1200/JCO.2005.05.1474. [DOI] [PubMed] [Google Scholar]
- Herbst RS, Prager D, Hermann R, et al. TRIBUTE: a phase III trial of erlotinib hydrochloride (OSI-774) combined with carboplatin and paclitaxel chemotherapy in advanced non-small-cell lung cancer. J Clin Oncol. 2005;23:5892–5899. doi: 10.1200/JCO.2005.02.840. [DOI] [PubMed] [Google Scholar]
- Liu Y, Liu B, Li XY, et al. A comparison of ARMS and direct sequencing for EGFR mutation analysis and tyrosine kinase inhibitors treatment prediction in body fluid samples of non-small-cell lung cancer patients. J Exp Clin Cancer Res. 2011;30:111. doi: 10.1186/1756-9966-30-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, Lee KY, Kim YC, et al. Detection and comparison of peptide nucleic acid-mediated real-time polymerase chain reaction clamping and direct gene sequencing for epidermal growth factor receptor mutations in patients with non-small cell lung cancer. Lung Cancer. 2012;75:321–325. doi: 10.1016/j.lungcan.2011.08.005. [DOI] [PubMed] [Google Scholar]
- Lopez-Rios F, Angulo B, Gomez B, et al. Comparison of molecular testing methods for the detection of EGFR mutations in formalin-fixed paraffin-embedded tissue specimens of non-small cell lung cancer. J Clin Pathol. 2013;66:381–385. doi: 10.1136/jclinpath-2012-201240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi M, Matsui K, Katakami N, et al. Phase II study of gefitinib as a first-line therapy in elderly patients with pulmonary adenocarcinoma: West Japan Thoracic Oncology Group Study 0402. Jpn J Clin Oncol. 2011;41:948–952. doi: 10.1093/jjco/hyr087. [DOI] [PubMed] [Google Scholar]
- Lin WC, Chiu CH, Liou JL, Chen YM, Perng RP, Tsai CM. Gefitinib as front-line treatment in Chinese patients with advanced non-small-cell lung cancer. Lung Cancer. 2006;54:193–199. doi: 10.1016/j.lungcan.2006.07.013. [DOI] [PubMed] [Google Scholar]


