Abstract
Background
Platinum doublets are standard first-line treatment for stage IV non-small cell lung cancer (NSCLC) without targetable driver mutations. Oxaliplatin is more potent than cisplatin, requiring fewer DNA adducts to provide equivalent cytotoxicity. The objective of this study was to evaluate the efficacy and safety of oxaliplatin combined with docetaxel as a first-line treatment for stage IV NSCLC.
Methods
This was a prospective, single-center, phase II trial. Patients with chemotherapy-naive NSCLC received 60 mg/m2 docetaxel (day 1) and 70 mg/m2 oxaliplatin (day 2) every three weeks for up to six cycles. The primary endpoint was objective response rate (ORR), and the secondary endpoints were progression-free survival (PFS), overall survival (OS), and safety. Treatment response was evaluated according to Response Evaluation Criteria in Solid Tumors version 1.1.
Results
Thirty-three patients were enrolled and a response evaluation was available in 31 patients. There were 11 patients with a partial response, 15 with stable disease, and five with progressive disease. Two patients ceased further treatment after the first cycle of chemotherapy. Thus, the ORR was 33.3% in the 33 patients of the intention-to-treat population. Median PFS was 3.6 months (95% confidence interval [CI], 2.8–4.5), and median OS was 10.9 months (95% CI, 8.2–13.6). The most common hematologic toxicity was neutropenia. Grade 3–4 neutropenia occurred in 51.5% of patients.
Conclusion
The results suggest that the combination of oxaliplatin and docetaxel is effective in patients with NSCLC with reasonable toxicity.
Keywords: Carcinoma, docetaxel, non-small-cell lung, oxaliplatin
Introduction
Platinum doublets are standard first-line treatment for stage IV non-small cell lung cancer (NSCLC) without targetable driver mutations, such as epidermal growth factor receptor or anaplastic lymphoma kinase.1,2 For patients with NSCLC of non-squamous histology, a pemetrexed (A) plus cisplatin (P) regimen is superior to gemcitabine (G) plus cisplatin in terms of efficacy and toxicity.3 However, GP is superior to AP in patients with squamous cell carcinoma. Thus AP and GP regimens are preferred for non-squamous and squamous NSCLC, respectively.
Docetaxel plus platinum is standard first-line treatment for NSCLC.2,4 The docetaxel plus cisplatin (DP) regimen shows a survival benefit compared with that of vinorelbine plus cisplatin4 or vindesine plus cisplatin5 regimens. However, no prospective phase III trials have directly compared the efficacy of AP with DP in patients with non-squamous NSCLC or GP with DP in patients with squamous NSCLC.
Docetaxel is used at 75 mg/m2 over a three-week interval in most countries. However, 60 mg/m2 is the standard dose in Japan.6 One study conducted a clinical trial comparing DP at two different doses as first-line treatment for advanced NSCLC in a Korean population.7 The objective response rate (ORR) for 60 mg/m2 in three weekly doses of docetaxel was not inferior to that of 75 mg/m2, and the safety profile was better in the 60 mg/m2 arm than in the 75 mg/m2 arm.
Oxaliplatin induces more double-strand breaks in DNA adducts compared with cisplatin, with increased cytotoxicity.8 Oxaliplatin has a survival benefit over cisplatin in patients with gastric and colon cancer and is associated with less toxicity and better tolerability.9 Oxaliplatin is active as a monotherapy10 and in doublets with vinorelbine,11 paclitaxel,12 and gemcitabine13 in patients with NSCLC.
In a phase I dose escalation study of patients with NSCLC and breast cancer, 70 mg/m2 docetaxel on day one and 70 mg/m2 oxaliplatin on day two was recommended for a phase II trial.14 The phase II trial, as second-line treatment for NSCLC, was followed by a Greek oncology group.15 The results suggested that the docetaxel plus oxaliplatin doublet was superior to docetaxel monotherapy in second-line treatment of NSCLC. Another phase II trial as first-line treatment for stage IV NSCLC16 was conducted using 70 mg/m2 docetaxel, 130 mg/m2oxalipaltin, and 6 mg pegfilgrastim every 21 days. The ORR was 37% and median progression-free survival (PFS) and overall survival (OS) rates were 4.6 and 10.9 months, respectively, with a lower incidence of low-grade neutropenia.
The objective of this study was to evaluate the efficacy and safety of 60 mg/m2 docetaxel plus 70 mg/m2 oxaliplatin every three weeks for up to six cycles, as first-line treatment for stage IV NSCLC, without using prophylactic myeloid growth factors.
Patients and methods
Chemotherapy-naive patients with histologically or cytologically diagnosed stage IIIB/IV or relapsed NSCLC were eligible to participate. Other criteria included ≥18 years of age, Eastern Cooperative Oncology Group performance status 0–2, presence of unidimensionally measurable lesion(s) by Response Evaluation Criteria in Solid Tumors (RECIST) criteria, no prior chemotherapy for NSCLC, adequate bone marrow function, adequate liver and renal function, no prior malignancy, and written informed consent.
Exclusion criteria were: a history of another malignancy within the last five years, except cured basal cell carcinoma of the skin; any other morbidity or contraindication for chemotherapy (e.g. active infection, myocardial infarction in the preceding six months; symptomatic heart disease, including unstable angina, congestive heart failure or uncontrolled arrhythmias; immunosuppressive treatment); pregnant or lactating women; women and men of child bearing potential who were willing to use adequate contraception.
The chemotherapy consisted of 60 mg/m2 docetaxel on day one followed by 70 mg/m2 oxaliplatin on day two, repeated every three weeks for up to six cycles. To proceed to the next cycle of chemotherapy, neutrophil had to be ≥1500 /μL, and platelets ≥100 000/μL. A 20% dosage reduction was allowed for febrile neutropenia, grade 4 neutropenia, or thrombocytopenia, and grade 3 or higher non-hematologic toxicities. In cases with prolonged recovery from toxicity, treatment could be delayed for up to four weeks. The delivered dose intensity (mg/m2/week) was calculated as the total dose delivered over the total time to complete chemotherapy. Relative dose intensity was calculated as the ratio of delivered dose intensity to standard dose intensity and was expressed as a percentage.17
This trial was a prospective, single-center, phase II trial, approved by the institutional review board of the author's institution and the Korean Food and Drug Administration. Before enrolling the first patient, registration to a clinical trial database was completed (ClinicalTrials.gov). The primary endpoint was ORR and the secondary endpoints were PFS, OS, and safety of the intention-to-treat (ITT) population. Treatment response was evaluated after two cycles of chemotherapy according to RECIST version 1.1.18 PFS and OS were defined as the interval between the date of registration and the date of an event or last follow up. Toxicities were graded according to Common Terminology Criteria for Adverse Events version 4.0.
Simon's two-stage design19 was used in this trial. A success probability of 10% was considered unacceptable and, if true, would imply that the treatment regimen does not warrant further investigation. The targeted success probability of 30%, if true, would imply that the treatment regimen does warrant further investigation. The null hypothesis is that the true response rate is 10%, and it will be tested against a one-sided alternative of 30%. Ten patients would be accrued in the first stage. If there was only one responder, the study would be stopped. Otherwise, more patients would be accrued for a second stage. Considering a 10% drop out rate, 5% type I error rate, and 80% power, 33 patients were accrued.
Results
Thirty-three patients were enrolled from December 2010 to May 2012, and a response evaluation was feasible in 31 cases (Table1). Among the 10 patients accrued in the first stage, three patients showed partial response, thus, more patients was accrued for a second stage. The mean number of chemotherapy cycles administered was 3.6 (standard deviation: 1.5 cycles). Among 33 patients, two ceased further treatment after the first cycle of chemotherapy, and 11 patients ceased treatment after cycles 2 or 3. Fourteen and six patients completed four and six cycles of chemotherapy, respectively.
Table 1.
Patient characteristics
| Intention to Treat | N = 33 |
|---|---|
| Age (years, mean ± standard deviation) | 64.0 ± 9.7 |
| Male/ Female | 30/ 3 |
| Adenocarcinoma/ Squamous/ NSCLC | 8/ 24/ 1 |
| Stage IIIB / Stage IV or Relapsed | 1/ 32 |
| Never-smoker/ Ex-Smoker/ Current Smoker | 3/ 23/ 7 |
| Diabetes Mellitus | 8 |
NSCLC, non-small cell lung cancer.
The delay in chemotherapy was up to 25 days (mean ± standard deviation 7.3 ± 7.3 days). Compared to standard dose intensity of docetaxel (20.0 mg/m2/week) and oxaliplatin (23.3 mg/m2/week), the relative dose intensity of each drug was 88.7 ± 11.0% and 88.8 ± 10.5%, respectively.
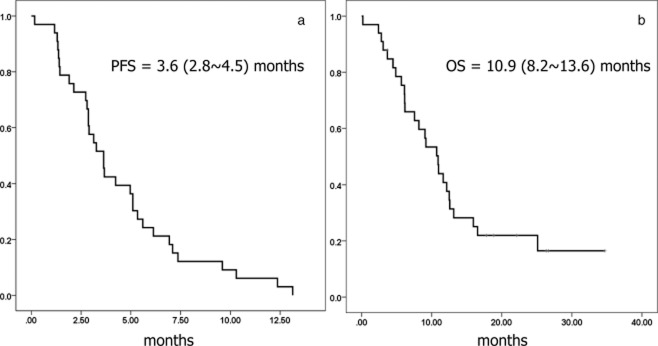
Among the 33 patients in the ITT population, there were 11 partial responses, 15 with stable disease, five with progressive disease, and two patients were not evaluable; thus, the ORR was 33.3% in the ITT population (Fig 1). The median follow-up time was 26.3 months (95% confidence interval, [CI], 17.7–35.0). At the time of analysis, three patients were alive, four patients were lost to follow up, and 26 patients had died. The median PFS was 3.6 months (95% CI, 2.8–4.5; Fig 2a), and median OS was 10.9 months (95% CI, 8.2–13.6; Fig 2b).
Figure 1.
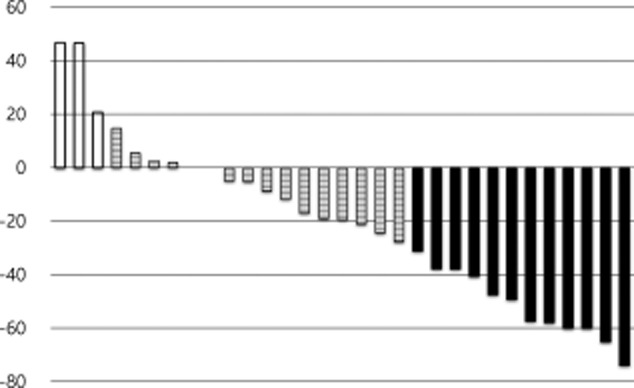
Waterfall plot of response after two cycles of chemotherapy in 31 response-evaluable patients.  , progressive disease (PD);
, progressive disease (PD);  , stable disease (SD);
, stable disease (SD);  , partial response (PR).
, partial response (PR).
Figure 2.
(a) Progression-free and (b) overall survival of the 33 patients in the intention-to-treat population.
The most common hematologic toxicity was neutropenia. Grade 3–4 neutropenia occurred in 51.5% of patients (Table 2). The most common non-hematologic grade 3–4 toxicities were infection (21.2%), diarrhea (15.2%), and hyperglycemia (15.2%). Peripheral neuropathy occurred in 9.1% of cases. No grade 5 toxicity was observed (Table 3).
Table 2.
Hematologic toxicities observed among the 33 patients in the intention-to-treat population
| Grade | 1 | 2 | 3 | 4 | 5 | 3 or 4 (%) | Any (%) |
|---|---|---|---|---|---|---|---|
| Neutropenia | 3 | 8 | 5 | 12 | 0 | 51.5 | 84.8 |
| Febrile neutropenia | 0 | 0 | 1 | 1 | 0 | 6.1 | 6.1 |
| Anemia | 20 | 7 | 1 | 0 | 0 | 3.0 | 84.8 |
| Thrombocytopenia | 3 | 0 | 1 | 0 | 0 | 3.0 | 12.1 |
Table 3.
Non-hematologic toxicities observed among the 33 patients in the intention-to-treat population
| Grade | 1 | 2 | 3 | 4 | 5 | 3 or 4 (%) | Any (%) |
|---|---|---|---|---|---|---|---|
| Anorexia | 9 | 11 | 1 | 0 | 0 | 3.0 | 63.6 |
| Nausea/ Vomiting | 5 | 4 | 2 | 0 | 0 | 6.1 | 33.3 |
| Diarrhea | 4 | 3 | 5 | 0 | 0 | 15.2 | 36.4 |
| Mucositis | 3 | 4 | 0 | 0 | 0 | 0.0 | 21.2 |
| Rash | 3 | 3 | 0 | 0 | 0 | 0.0 | 18.2 |
| Fatigue | 9 | 8 | 0 | 0 | 0 | 0.0 | 51.5 |
| Dyspnea | 3 | 10 | 1 | 0 | 0 | 3.0 | 42.4 |
| Thromboembolism | 0 | 0 | 1 | 0 | 0 | 3.0 | 3.0 |
| Liver abnormality | 0 | 2 | 1 | 0 | 0 | 3.0 | 9.1 |
| Neuropathy | 1 | 1 | 1 | 0 | 0 | 3.0 | 9.1 |
| Hypo/Hyperkalemia | 1 | 1 | 2 | 0 | 0 | 6.1 | 12.1 |
| Hyperglycemia | 0 | 0 | 2 | 3 | 0 | 15.2 | 15.2 |
| Infection | 1 | 2 | 4 | 3 | 0 | 21.2 | 30.3 |
| Atrial Fibrillation | 0 | 0 | 1 | 1 | 0 | 6.1 | 6.1 |
| Transaminitis† | 3 | 0 | 1 | 0 | 0 | 3.0 | 12.1 |
| Acute Renal Injury | 0 | 1 | 1 | 0 | 0 | 3.0 | 6.1 |
Increased alanine aminotransferase and aspartate aminotransferase.
Discussion
The development of chemotherapy drugs effective against specific histology and the discovery of target treatments have led us to no longer regard NSCLC as a single disease entity. A recent genomic analysis discovered many molecular targets not only in adenocarcinoma,20 but also in squamous cell carcinoma.21 More than half of adenocarcinomas are driven by known targetable mutations. However, few drugs are available for most genomic alterations. However, the majority of patients with NSCLC require cytotoxic chemotherapy during the course of their illness. Thus, cytotoxic chemotherapies are the main therapeutic tools for stage IV NSCLC.
In this trial, we evaluated the efficacy and safety of 60 mg/m2 docetaxel and 70 mg/m2 oxaliplatin as first-line treatment for stage IV NSCLC. The ORR and PFS durations are compared with those of previous trials in Table 4. Similar efficacy in terms of response rate and survival were observed in this trial compared to previous studies. A response rate of 33.3% in the ITT population is worthy of further investigation for this combination regimen.
Table 4.
Comparison of previous trials using docetaxel (D) and cisplatin (P) or oxaliplatin (Ox) with this study (Beloxan)
| ECOG15942 | Tax3264 | JTLCSG5 | ATOM01915 | Raez et al.16 | Beloxan† | |
|---|---|---|---|---|---|---|
| (D75-P75) | (D75-P75) | (D60-P80) | (D70-Ox70) | (D70-Ox130) | (D60-Ox70) | |
| N = 289 | N = 408 | N = 151 | N = 25 | N = 29 | N = 33 | |
| Age (years, mean) | 63 | 61 | 63 | 62 | 57 | 64 |
| Male (%) | 63 | 72 | 64 | 76 | 48 | 91 |
| Squamous cell cancer (%) | N/A‡ | 32.4 | 11.3 | 20.0 | 17.2 | 72.7 |
| Stage IV (%) | 86 | 67 | 100 | 100 | 93 | 94 |
| Overall response (%) | 17 | 32 | 37 | 20 | 37 | 33.3 |
| Median PFS (months) | 3.7 | N/A‡ | N/A‡ | 5.0 | 4.6 | 3.6 |
| Median OS (months) | 7.4 | 11.3 | 11.3 | 11.0 | 10.9 | 10.9 |
| Grade ¾ neutropenia (%) | 69 | 75 | 74 | 56 | 0 | 52 |
This trial. ‡Not available from the cited reference. ECOG, Eastern Cooperative Oncology Group; OS, overall survival; PFS, progression-free survival.
The major difference between this trial and previous trials was the proportion of patients with squamous cell carcinoma. More than 70% of patients in this trial had squamous cell carcinoma, compared to between 11-30% in previous trials. Gemcitabine platinum was superior to pemetrexed platinum in squamous cell carcinoma.3 However, there has been no prospective randomized trial comparing gemcitabine platinum versus docetaxel platinum specifically in squamous cell carcinoma. Thus, further trials comparing the efficacy and toxicity of docetaxel and platinum with gemcitabine platinum in patients with squamous cell carcinoma are warranted.
In contrast to a previous study by Raez et al.16 we did not use pegfilgrastim prophylactically. However, hematological toxicities, including the incidence of neutropenia, were numerically lower than in previous trials2,4,5,15 that did not use prophylactic myeloid growth factors. This can be explained by the fact that we used a lower dose of docetaxel compared to other trials,2,4,15,16 and we also used oxaliplatin compared to cisplatin as in a Japanese trial.5
Prophylactic use of myeloid growth factors is recommended by the National Comprehensive Cancer Network for chemotherapy in patients with high (>20%) risk for febrile neutropenia. Myeloid growth factors can be considered for regimens with an intermediate (10–20%) risk of febrile neutropenia, such as taxane platinum doublets. However, the use of myeloid growth factors is a difficult decision and requires careful assessment.
The most common non-hematologic toxicities were infection, diarrhea, and hyperglycemia related to dexamethasone premedication. Peripheral neuropathy, a significant toxicity associated with prolonged use of oxaliplatin was not observed in our cohort. This can be explained by the short-term use of oxaliplatin of no more than six cycles with lower dose intensity (23.3 mg/m2/week) compared to that of colon carcinoma (42.5 mg/m2/week).
Conclusion
In conclusion, our results suggest that a combination of docetaxel and oxaliplatin is effective in patients with NSCLC, with reasonable toxicities. Further investigation of this regimen, particularly for squamous cell lung carcinoma, is warranted.
Acknowledgments
We thank the patients and their families for their support and participation in this trial. We are grateful to Chong Kun Dang Pharm Korea for generous support of this trial.
Disclosure
Dr. Young-Chul Kim received grants for this clinical trial from Chong Kun Dang Pharm (m601NSC10B), and he is an advisory board member of AstraZeneca Korea, Lilly Korea and Hanmi Pharm Korea. He also received grants for other studies from AstraZeneca Korea, Roche Korea, and received honoraria from AstraZeneca Korea, Roche Korea and Lilly Korea. No other authors report any conflict of interest.
References
- National Comprehensive Cancer Network. 2014. NCCN Clinical Practice Guidelines in Oncology, Non-Small Cell Cancer: NCCN [Cited Mar 2014.] Available from URL: http://www.nccn.org.
- Schiller JH, Harrington D, Belani CP, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002;346:92–98. doi: 10.1056/NEJMoa011954. [DOI] [PubMed] [Google Scholar]
- Scagliotti GV, Parikh P, von Pawel J, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol. 2008;26:3543–3551. doi: 10.1200/JCO.2007.15.0375. [DOI] [PubMed] [Google Scholar]
- Fossella F, Pereira JR, von Pawel J, et al. Randomized, multinational, phase III study of docetaxel plus platinum combinations versus vinorelbine plus cisplatin for advanced non-small-cell lung cancer: the TAX 326 study group. J Clin Oncol. 2003;21:3016–3024. doi: 10.1200/JCO.2003.12.046. [DOI] [PubMed] [Google Scholar]
- Kubota K, Watanabe K, Kunitoh H, et al. Phase III randomized trial of docetaxel plus cisplatin versus vindesine plus cisplatin in patients with stage IV non-small-cell lung cancer: the Japanese Taxotere Lung Cancer Study Group. J Clin Oncol. 2004;22:254–261. doi: 10.1200/JCO.2004.06.114. [DOI] [PubMed] [Google Scholar]
- Okamoto H, Watanabe K, Segawa Y, et al. Phase II study of docetaxel and cisplatin in patients with previously untreated metastatic non-small-cell lung cancer. Int J Clin Oncol. 2000;5:316–322. [Google Scholar]
- Kim KS, Oh IJ, Ban HJ, et al. Comparison of docetaxel/cisplatin dosages of 75/60 and 60/60 mg/m2 for the treatment of non-small cell lung cancer. Exp Ther Med. 2012;4:317–322. doi: 10.3892/etm.2012.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaney SG, Campbell SL, Bassett E, Wu Y. Recognition and processing of cisplatin- and oxaliplatin-DNA adducts. Crit Rev Oncol Hematol. 2005;53:3–11. doi: 10.1016/j.critrevonc.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Montagnani F, Turrisi G, Marinozzi C, Aliberti C, Fiorentini G. Effectiveness and safety of oxaliplatin compared to cisplatin for advanced, unresectable gastric cancer: a systematic review and meta-analysis. Gastric Cancer. 2011;14:50–55. doi: 10.1007/s10120-011-0007-7. [DOI] [PubMed] [Google Scholar]
- Monnet I, Brienza S, Hugret F, et al. Phase II study of oxaliplatin in poor-prognosis non-small cell lung cancer (NSCLC). ATTIT. Association pour le Traitement des Tumeurs Intra Thoraciques. Eur J Cancer. 1998;34:1124–1127. doi: 10.1016/s0959-8049(98)00007-0. [DOI] [PubMed] [Google Scholar]
- Monnet I, Soulié P, de Cremoux H, et al. Phase I/II study of escalating doses of vinorelbine in combination with oxaliplatin in patients with advanced non-small-cell lung cancer. J Clin Oncol. 2001;19:458–463. doi: 10.1200/JCO.2001.19.2.458. [DOI] [PubMed] [Google Scholar]
- Winegarden JD, Mauer AM, Otterson GA, et al. A phase II study of oxaliplatin and paclitaxel in patients with advanced non-small-cell lung cancer. Ann Oncol. 2004;15:915–920. doi: 10.1093/annonc/mdh215. [DOI] [PubMed] [Google Scholar]
- Cappuzzo F, Novello S, De Marinis F, et al. Phase II study of gemcitabine plus oxaliplatin as first-line chemotherapy for advanced non-small-cell lung cancer. Br J Cancer. 2005;93:29–34. doi: 10.1038/sj.bjc.6602667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouroussis C, Agelaki S, Mavroudis D, et al. A dose escalation study of docetaxel and oxaliplatin combination in patients with metastatic breast and non-small cell lung cancer. Anticancer Res. 2003;23(1B):785–791. [PubMed] [Google Scholar]
- Belvedere O, Follador A, Rossetto C, et al. A randomised phase II study of docetaxel/oxaliplatin and docetaxel in patients with previously treated non-small cell lung cancer: an Alpe-Adria Thoracic Oncology Multidisciplinary group trial (ATOM 019) Eur J Cancer. 2011;47:1653–1659. doi: 10.1016/j.ejca.2011.03.020. [DOI] [PubMed] [Google Scholar]
- Raez LE, Santos ES, Lopes G, et al. Efficacy and safety of oxaliplatin and docetaxel in patients with locally advanced and metastatic non-small-cell lung cancer (NSCLC) Lung Cancer. 2006;53:347–353. doi: 10.1016/j.lungcan.2006.05.023. [DOI] [PubMed] [Google Scholar]
- Lenhart C. Relative dose intensity: improving cancer treatment and outcomes. Oncol Nurs Forum. 2005;32:757–764. doi: 10.1188/05.ONF.757-764. [DOI] [PubMed] [Google Scholar]
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- Simon R. Optimal two-stage designs for phase II clinical trials. Control Clin Trials. 1989;10:1–10. doi: 10.1016/0197-2456(89)90015-9. [DOI] [PubMed] [Google Scholar]
- Imielinski M, Berger AH, Hammerman PS, et al. Mapping the hallmarks of lung adenocarcinoma with massively parallel sequencing. Cell. 2012;150:1107–1120. doi: 10.1016/j.cell.2012.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancer Genome Atlas Research Network. Comprehensive genomic characterization of squamous cell lung cancers. Nature. 2012;489:519–525. doi: 10.1038/nature11404. . (Published erratum appears in Nature 2012; 491: 288) [DOI] [PMC free article] [PubMed] [Google Scholar]