Abstract
Background
To investigate how the four-dimensional computed tomography (4DCT) technique spares normal tissues in non-small cell lung cancer (NSCLC) radiotherapy by defining individualized internal target volume (ITV).
Materials and Methods
Gross tumor volume (GTV) and clinical target volume (CTV) were contoured on all 10 respiratory phases of 4DCT scans in 10 patients with peripheral NSCLC. Both 3D and 4D treatment plans were performed for each patient using planning target volume (PTV)3D (derived from a single CTV plus conventional margins) and PTV4D (derived from 4D internal target volume, which included all 10 CTVs plus setup margins). Dose volume histogram and normal tissue complication probability (NTCP) values were compared for the lung, heart, and spinal cord between 3D and 4D treatment plans.
Results
The average PTV of the 4D (127.56 ± 70.79) was less than the 3D plans (147.65 ± 76.89). The 4D spared more surrounding normal tissues than the 3D plans, especially in the lung. Compared with 3D plans, V5, V10, V20 and V30 of the total lung decreased from 41.25%, 37.75%, 24.25%, 17.00% to 38.13%, 33.00%, 21.25%, 15.13%, respectively. Without increasing the NTCP of the lung significantly, the 4D plans allowed us to increase the average prescription dose from 60 Gy to 66.00 ± 4.62 Gy.
Conclusions
4DCT based plans can reduce the target volumes, spare more normal tissues, and allow dose escalation compared with 3D plans in NSCLC radiotherapy.
Keywords: 4DCT, dosimetry, internal target volume, non-small cell lung cancer, normal tissue complication probability
Introduction
Radiotherapy has important roles in both curative and palliative treatment of non-small cell lung cancer (NSCLC).1 An estimated 75% of patients with NSCLC might benefit from radiotherapy.2 With improvement in radiation techniques, especially the introduction of intensity-modulated radiotherapy (IMRT), the role of radiotherapy for patients with NSCLC is being reconsidered, however, accurately defining target volume is difficult because of respiratory motion.3,4 To ensure sufficient dose coverage throughout the treatment course, margins, including the internal margin (IM) and setup margin (SM), should be added to the clinical target volume (CTV) to make up the planning target volume (PTV).5 Geometric margins to account for respiratory motion are usually derived from fluoroscopy, clinical experience, or using values reported in the literature.6 Such margins are neither accurate nor patient-specific, allowing for the possibility of geometric miss or over coverage.7
Four-dimensional radiotherapy (4DRT) is the explicit inclusion of the temporal changes in anatomy during the imaging, planning, and delivery of radiotherapy.8 Theoretically, 4D computed tomography (CT) scans can capture intra-fractional tumor mobility for radiotherapy planning and generate accurate internal target volume (ITV), which covers the movement range of CTV. It was recently reported that using 4DCT to determine ITV for lung cancer could substantially reduce the PTV while safely covering the target.9,10 However, few have used this new approach to determine ITV with 10 respiratory phases to quantitatively evaluate the benefit of sparing more normal tissues in Chinese NSCLC patients. This study was designed to investigate how 4DCT spares normal tissues in NSCLC radiotherapy by defining ITV.
Materials and methods
Patient characteristics
The study included 10 patients with primarily peripheral NSCLC who had received IMRT in the Radiation Department of the Shandong Tumor Hospital and Institute between September 2009 and September 2010. The T stage according to the tumor node metastasis (TNM) classification of the 2009 National Comprehensive Cancer Network was stage T1 in all 10 patients. The tumor locations were: four left lobe cases; six right lobe; two upper lobe; four middle lobe; and four lower lobe cases. The data were retrospectively collected and analyzed.
This study was discussed and approved by the Research Ethics Board of Shandong Tumor Hospital and Institute and written informed consent was obtained from each patient.
Four dimensional computed tomography (4DCT) scan
Vacuum bags were used to improve reproducibility during daily treatments. Three millimeter thick CT scan slices were obtained from the lower end of the cricoid cartilage to the lower edge of the liver. The simulation was performed during quiet breathing on the Philips Brilliance Big Bore CT simulator (Philips Medical Systems, Highland Heights, OH, USA). Breath training was conducted before the simulation. A plastic box with a pair of reflective markers was placed on the patient's anterior abdominal surface, approximately midway between the xiphoid and the umbilicus. The 4DCT scanning was performed and the respiration (marker) motion of patients was recorded in synchronization with the Real-time Positioning Management (RPM) system (Varian Medical Systems, Palo Alto, CA, USA) in precise temporal correlation to CT data acquisition. The scanner was operated in axial cine mode, with an interval equal to 1–1.5 seconds plus the patient's average respiratory period. Data acquisition was repeated at each couch position until full longitudinal coverage of the region of interest was obtained. The 4DCT images were sorted into 10 phases according to the respiratory cycle, labeled as CT0, CT10 …CT90 (CT0 for the end inspiration phase, CT20 for the respiratory intermediate state, CT50 for the end expiration phase). The maximum intensity projection (MIP) images (CTMIP) of 4DCT were also reconstructed. Thereafter, the CT images were transmitted to the Varian Eclipse V8.6 (Varian Medical Systems, Palo Alto, CA, USA) treatment planning system (TPS).
Delineation of gross tumor volumes (GTVs) and clinical target volumes (CTVs)
Gross tumor volumes (GTVs) and CTVs were manually contoured on all 10 phases on the 4DCT images. Target volumes were defined as follows: GTV encompassed all of the primary lesions visualized on the CT images. A 7 mm expansion of GTV was generated as CTV on each phase, including all of the subclinical lesions and the possible areas of infiltration.11 For each patient, all of the 10 phases of CTV were combined and fused together on 20% phase CT by image registration on Varian Eclipse TPS. The combined volume of the CTVs in the multiple CT phases was defined as ITV4D. PTV3D was derived from a single CTV of the 20% phase CT scan plus conventional margins. PTV4D was the ITV4D plus the setup margin (SM). For both treatment plans, SM was defined as an isotropic margin of 5 mm, which is typically used for thoracic malignancies.12 To account for mobility and the SM, patients were set on the couch of the Varian Acuity simulator after 4DCT scanning. The motion of the diaphragm, bilateral lungs, and especially the gross tumor, were observed at 0° and 90°/270° (depending on the position of primary lesion) during free breath. Therefore, considering the deviation difference between patients, the conventional margins were defined as 8–10 mm in the laterolateral (LL) and anteroposterior (AP) directions; but 10–25 cm in the craniocaudal (CC) direction.
Organs at risks (OARs) were contoured only on the 20% phase CT scan (CT20). All contours were projected onto CT20. OARs included the total lung, heart, and spinal cord. The total lung was defined as the volume of both the left and right lung minus GTV. To minimize inter-observer variations, all volumes were outlined by a single clinician at the same window width (1600Hu) and window level (600Hu).
Treatment planning and evaluation
The 3D and 4D treatment plans were performed for each patient using the two different PTVs: PTV3D (Plan3D) and PTV4D (Plan4D). IMRT was carried out using a 6MV photon linear accelerator. The prescription dose and design of irradiating fields were identical between the two plans: coplanar 7–9 beams, traditional fractionation (2.0Gy/fraction, 30 fractions), dose-volume objective functions, average target volume normalization. The prescription dose should cover at least 95% of the PTV volume.13
The dose volume histograms (DVHs) of the OARs were obtained for both Plan3D and Plan4D in each patient. The mean lung dose (MLD), V5 (volume included by 5Gy isodose curve), V10, V20, V30 of the total lung, the heart's mean dose (Dmean), and the spinal cord's maximum dose (Dmax), were recorded. Normal tissue complication probability (NTCP) values were also calculated for each OAR with the Lyman-Kutcher-Burman (LKB) model. The three parameters were derived according to Burman identification:14 lung (TD50 = 24.5Gy, n = 0.87, m = 0.18), heart (TD50 = 48.0Gy, n = 0.35, m = 0.10) and spinal cord (TD50 = 66.5Gy, n = 0.05, m = 0.175).
Data statistics and analysis
Statistical analysis was performed using SPSS software (Statistics Package for Social Science, Version 16.0, Inc., Chicago, IL). The Paired-Samples T test was used to compare the target volume and dosimetric evaluations between Plan3D and Plan4D. Differences were considered to be significant if the 2-tailed P-value was less than 0.05.
Results
Displacement of GTV during respiration
The results of GTV centroid motion in 3D direction seen with 4DCT and fluoroscopy are shown in Table 1. Analysis of the center of the GTV revealed predominant CC movement, with a mean of 0.64 ± 0.53 cm (range 0.1–1.8 cm). Mobility in the LL and AP directions was much more limited. The mobility differences between LL and AP directions were also significant (P = 0.019). From an anatomical point, the mobility of the tumor target gets bigger and bigger from the upper to middle lobe and to the lower lobe.
Table 1.
Tumor displacement at three-dimensional directions
| Patient | Tumor position | X-axis | Y-axis | Z-axis |
|---|---|---|---|---|
| 1 | upper lobe | 0.1† | 0.1 | 0.1 |
| 2 | upper lobe | 0.1 | 0.2 | 0.3 |
| 3 | middle lobe | 0.1 | 0.1 | 0.1 |
| 4 | middle lobe | 0.1 | 0.7 | 0.3 |
| 5 | middle lobe | 0.1 | 0.3 | 0.6 |
| 6 | middle lobe | 0.2 | 0.3 | 0.5 |
| 7 | lower lobe | 0.1 | 0.2 | 0.8 |
| 8 | lower lobe | 0.1 | 0.4 | 1.8 |
| 9 | lower lobe | 0.1 | 0.2 | 1.2 |
| 10 | lower lobe | 0.2 | 0.3 | 0.7 |
| Average | - | 0.12 ± 0.04‡ | 0.28 ± 0.18 | 0.64 ± 0.53 |
Unit: cm. ‡Average ± standard deviation. X-axis: lateral direction; Y-axis: anteroposterior direction; Z-axis: craniocaudal direction.
Comparison of target volumes
The average PTV volume in the 4D plans was 127.56 ± 70.79 cm3, which was 20.09 ± 7.22 cm3 or 15.45% (range 8.51–23.53%) less than the 3D plans (147.65 ± 76.89 cm3) (P = 0.000) (Table 2). The PTV3D was larger than PTV4D in all 10 patients, even though patient specifics differed. The transect area of PTV4D and PTV3D in three orthogonal planes were also analyzed. The PTV4D was smaller than PTV3D in all three orthogonal planes for most of the 10 patients (8/10). This indicates that conventional margins added to CTV in the 3D plan exceeded those actually needed and resulted in unnecessary irradiation of normal tissues, especially for the lung and spinal cord (central-type lung cancer). However, for the other two patients, PTV4D exceeded PTV3D in some slices. For example, in patient nine, although the PTV4D was smaller than PTV3D (236.89 cm3 vs. 205.42 cm3), there was a part of PTV4D not included in PTV3D in LL (0.17 cm) and AP (0.25) directions. This illustrated that the PTV3D not only included excess normal tissues in CC direction, but also resulted in missing the target during certain phases of the breathing cycle.
Table 2.
Geometric comparison between PTV-3D and PTV-4D
| Patients | PTV (cm3) | Decreased by (%) | |
|---|---|---|---|
| PTV-3D | PTV-4D | ||
| 1 | 171.75 | 149.86 | 12.75 |
| 2 | 52.24 | 39.95 | 23.53 |
| 3 | 281.48 | 257.53 | 8.51 |
| 4 | 45.98 | 35.63 | 22.51 |
| 5 | 124.08 | 108.67 | 12.42 |
| 6 | 125.35 | 108.54 | 13.41 |
| 7 | 89.87 | 73.53 | 18.18 |
| 8 | 201.76 | 170.87 | 15.31 |
| 9 | 236.89 | 205.42 | 13.28 |
| 10 | 147.12 | 125.63 | 14.61 |
| Average | 147.65 ± 76.89† | 127.56 ± 70.79 | 15.45 ± 4.68 |
Average ± standard deviation. 3D, three dimensional; 4D, four dimensional; PTV, planning target volume.
Dosimetric evaluation for organs at risk (OARs)
Table 3 shows the dosimetric evaluation result of 3D and 4D plans for OARs. The 4D plans spared more lung, heart, and spinal cord tissue than the 3D plans, especially in the lung (P = 0.025). Compared to 3D plans, the lung's V5 decreased from 41.25% to 38.13% (P < 0.010), V10 decreased from 37.75% to 33.00% (P < 0.010), V20 decreased from 24.25% to 21.25% (P < 0.010), and V30 decreased from 17.00% to 15.13% (P < 0.010); the MLD decreased from 13.04Gy to 12.11Gy (P < 0.010); and the Dmean of the heart and the Dmax of the spinal cord were lower in the 4D plans (P = 0.010 and P = 0.036 respectively).
Table 3.
Comparison between Plan3D and Plan4D on dosimetric factors of OARs
| Item | Plan3D | Plan4D | p value |
|---|---|---|---|
| Total lung | |||
| V5 (%) | 41.25 ± 15.01† | 38.13 ± 15.05 | <0.010 |
| V10 (%) | 37.75 ± 11.39 | 33.00 ± 10.61 | <0.010 |
| V20 (%) | 24.25 ± 8.22 | 21.25 ± 7.36 | <0.010 |
| V30 (%) | 17.00 ± 6.57 | 15.13 ± 5.28 | <0.010 |
| MLD (Gy) | 13.04 ± 4.44 | 12.11 ± 3.68 | <0.010 |
| Dmean of heart (Gy) | 4.50 ± 3.81 | 3.72 ± 3.28 | 0.010 |
| Dmax of spinal-cord (Gy) | 31.63 ± 16.19 | 29.68 ± 15.38 | 0.036 |
| NTCP of total lung (%) | 2.80 ± 3.82 | 1.10 ± 1.85 | 0.028 |
| NTCP of heart (%) | 1.20 ± 1.69 | 0.50 ± 0.71 | 0.081 |
| NTCP of spinal-cord (%) | 1.60 ± 1.07 | 1.30 ± 1.06 | 0.066 |
Average ± standard deviation. 3D, three-dimensional; 4D, four-dimensional; Dmean, mean dose; Dmax, maximum dose; OARs, organs at risk; MLD, mean lung dose; NTCP, normal tissue complication probability.
Comparison of normal tissue complication probability (NTCP) and dose escalation
Table 3 also shows the NTCP evaluation result of 3D and 4D plans for OARs. The NTCP of the total lung decreased from 2.80% to 1.10% (P = 0.028), which was the only significant group of comparison. There were no significant differences in the NTCP of the heart and spinal cord in either plan. As the NTCP of the lung was not increased, the 4D plans of seven patients allowed for an increase in the prescription dose by 6.00 ± 4.62Gy (from 60Gy to 66.00 ± 4.62Gy) (Fig 1). Figure 2 is an average DVH of the total lung for all 10 patients comparing the two plans at the prescribed dose of 60Gy and the plan after dose escalation. It was interesting to note that a prescription dose increase from 60Gy to 66Gy in the 4D plan elevated the V20 of the total lung from 21.25% to 23.34%; however, it was still less than the V20 under the 3D plan at 60Gy (24.25%).
Figure 1.
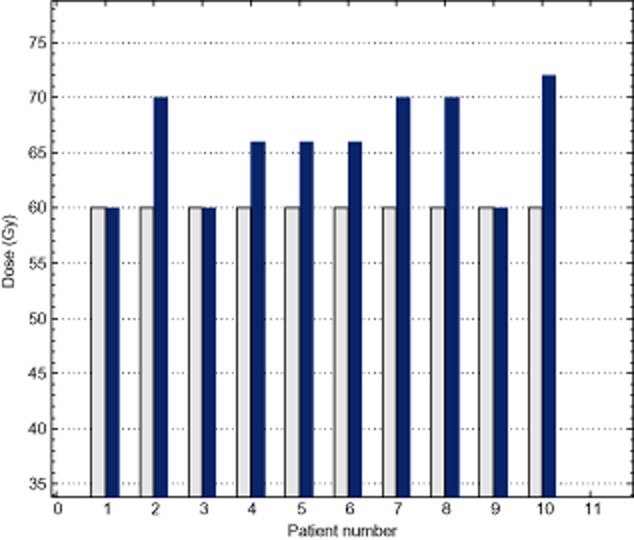
Dose escalation for four-dimensional plans.  , Prescription dose of escalated 4D plans;
, Prescription dose of escalated 4D plans;  , Prescription dose of 3D plans.
, Prescription dose of 3D plans.
Figure 2.
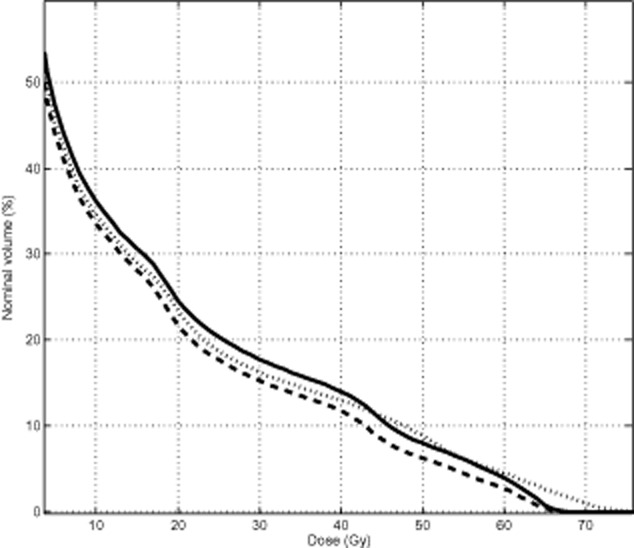
Average dose-volume histogram comparison among the three-dimensional plans (solid lines at 60Gy prescription dose), four-dimensional plans (dashed lines at 60Gy prescription dose), and the plans after dose escalation (dotted lines) for the total lung in ten patients.  , Plan3D-60Gy;
, Plan3D-60Gy;  , Plan4D-60Gy;
, Plan4D-60Gy;  , Plan4D-after dose esclation.
, Plan4D-after dose esclation.
Discussion
4DCT is a new CT scanning protocol that supplements the “time” factor on traditional 3D CT images. This technology is also the basis to develop 4D-radiotherapy.15,16 4DCT not only has the high spatial resolution of traditional 3D-CT, but also contains movement information. It is a powerful tool to track the motion status of a tumor and normal tissues during the radiotherapy process. With 4DCT technology, Weiss et al.17 studied the volume and position changing regulation of a thoracic tumor and normal tissues during the respiration process and proposed that, during respiration, the magnitude of contoured volumes varied up to 62.5% for GTVs, 25.5% for lungs, and 12.6% for the heart. The range of maximum 3D centroid movement for individual patients was 1.3–24.0 mm for GTV, 2.4–7.9 mm for the heart, 5.2–12.0 mm for the lungs, 0.3–5.5 mm for skin markers, 2.9–10.0 mm for the trachea, and 6.6–21.7 mm for the diaphragm. With 4DCT, volumetric changes and positional alterations, as well as changes in the position of contoured structures relative to the GTV, were observed with large variations between individual patients. Although the interpretation of 4DCT data can be uncertain because of 4DCT artifacts, observer variations, and limited acquisition time, the findings might have a significant impact on treatment planning. In the traditional 3D treatment planning process, the work of delineation, beam setting, and dose calculation are based on 3D-CT images, which is just a snapshot for one occasion during immobilization and CT scanning. However, with the 4DCT technique, all of the motion locus of the tumor and normal tissues can be recorded, and the MIP images present the organs at different phases. In lung cancer, the moving tumor and other normal tissues have a higher density than the lung. These occupy a certain volume of the lung in MIP images. Therefore, MIP images underestimate the volume of lung. They will also overestimate the dose distributed on the normal lung tissue if MIP is used to make a treatment plan and evaluate dose distribution. Sun et al. studied the variation of lung volume and its respiratory movement characteristics based on 4DCT. They concluded that the lung volume at series CT images scan on 20%, 30%, and 80% respiratory phases are most similar to the average lung volume during free respiration, and it is reasonable to take these phases for radiation dose calculation. Compared with inspiratory, the motion during the expiration process is more relaxed and smooth, so the 20% phase was chosen as the reference image to calculate dose distribution in this study.
The anatomy of the tumor in relation to nearby organs is important in predicting the tumor motion. It is well known that there is not much motion in an apical tumor, but substantial CC motion in a tumor close to the diaphragm. When the tumor is close to the heart, the motion can also be affected by the heartbeat. From the results of tumor motion on 3D directions, the largest displacement is on the Z-axis with smaller displacement on the X- and Y-axes, which forms an irregular spindle shape movement. From the observation, we also found that there is a significant relationship between the range of displacement and tumor location (upper, middle or lower lobe). A tumor on a lower lobe has the largest displacement on the Z-axis, while a tumor on the middle or upper lobe has a decreasing motion range.
After the achievement of 4DCT images, the primary issue is to delineate the tumor target accurately. Rietzel et al.16 argued that, when using non-patient-specific treatment planning margins, respiratory motion may lead to a geometric miss of the target while unnecessarily irradiating normal tissue. However, imaging different respiratory states of a patient allows patient-specific target design. Therefore, with the 4DCT technique, Rietzel et al. found that comparing PTVs with margins of 15 mm on composite 4D target volumes to PTVs with 20 mm on helical CT data, resulted in a decrease in target volume sizes by 23% on average. This result proved that delineation on 4DCT may reduce the irradiation area, which is coincident with our investigation here.
Concomitant with the reduction in PTV volume is a reduction in the amount of uninvolved tissue irradiated. Our data suggest that 4D plans can reduce the target volume to spare more normal tissues than 3D plans. This is especially true and important for the lung in NSCLC irradiation. Compared to 3D plans, 4D plans decreased the irradiation dose to the lung and permitted dose escalation with stable NTCPs. Studies have demonstrated that a dose-response relationship exists in localized radiotherapy for NSCLC and better response rates and prolonged tumor control can be achieved in groups that receive higher RT doses.18 Machtay et al.19 concluded that a 1-Gy biological effective dose (BED) increase in radiotherapy dose intensity was statistically significant and associated with approximately three percent relative improvement in local-regional control. Thus, in theory, dose escalation based on 4D plans may elevate the tumor response rate and prolong overall survival. The irradiated dose of the heart and spinal cord was limited and their NTCP values are smaller than the lung because most patients who undergo 4DCT simulation have peripheral lung cancer. Therefore, the limit of dose escalation in this study is to evaluate the NTCP of lung.
The Lyman NTCP model has been widely used to quantitatively evaluate normal tissue complications. The three parameters for certain tissue are notably different among the literature. So far, there has been no reliable estimation of parameters for the lung with conventional fractions in Chinese patients. This is the reason we ultimately cited the Burman parameters.14 The parameters cited in this study cannot predict actual complication probabilities. The small NTCP values calculated here were used for reference and comparison only.
Conclusion
Compared with 3D technology in NSCLC radiotherapy, the application of 4DCT will reduce the extension from CTV to PTV, and, furthermore, decrease the dose distributed on normal tissues and allow prescription dose escalation. However, contouring all 10 phases will increase the contouring time significantly, creating logistical difficulties and variation of contouring in individual phases. If a contrast medium is used in 4DCT, the timing of the contrast injection is difficult to control, contrast distribution is less satisfactory, and the delineation of mediastinal lymph nodes is more difficult compared to 3D planning.
Acknowledgments
This study was supported by grants from the National Natural Science Foundation of China (Nos. 81272699, 81301298, 61271312, 61173078, 61203105) and from the Shandong Provincial Natural Science Foundation (Nos. ZR2012FQ016).
Disclosure
No authors report any conflict of interest.
References
- Goldstraw P, Ball D, Jett JR, et al. Non-small-cell lung cancer. Lancet. 2011;378:1727–1740. doi: 10.1016/S0140-6736(10)62101-0. [DOI] [PubMed] [Google Scholar]
- Delaney G, Barton M, Jacob S, Jalaludin B. A model for decision making for the use of radiotherapy in lung cancer. Lancet Oncol. 2003;4:120–128. doi: 10.1016/s1470-2045(03)00984-7. [DOI] [PubMed] [Google Scholar]
- Balter JM, Ten Haken RK, Lawrence TS, Lam KL, Robertson JM. Uncertainties in CT-based radiation therapy treatment planning associated with patient breathing. Int J Radiat Oncol Biol Phys. 1996;36:167–174. doi: 10.1016/s0360-3016(96)00275-1. [DOI] [PubMed] [Google Scholar]
- Shimizu S, Shirato H, Kagei K, et al. Impact of respiratory movement on the computed tomographic images of small lung tumors in three-dimensional (3D) radiotherapy. Int J Radiat Oncol Biol Phys. 2000;46:1127–1133. doi: 10.1016/s0360-3016(99)00352-1. [DOI] [PubMed] [Google Scholar]
- Xi M, Liu MZ, Deng XW, et al. Defining internal target volume (ITV) for hepatocellular carcinoma using four-dimensional CT. Radiother Oncol. 2007;84:272–278. doi: 10.1016/j.radonc.2007.07.021. [DOI] [PubMed] [Google Scholar]
- Rietzel E, Chen GT, Choi NC, Willet CG. Four-dimensional image-based treatment planning: target volume segmentation and dose calculation in the presence of respiratory motion. Int J Radiat Oncol Biol Phys. 2005;61:1535–1550. doi: 10.1016/j.ijrobp.2004.11.037. [DOI] [PubMed] [Google Scholar]
- Burdett S, Stewart L. Postoperative radiotherapy in non-small-cell lung cancer: update of an individual patient data meta-analysis. Lung Cancer. 2005;47:81–83. doi: 10.1016/j.lungcan.2004.09.010. [DOI] [PubMed] [Google Scholar]
- Keall PJ, Joshi S, Vedam SS, Siebers JV, Kini VR, Mohan R. Four-dimensional radiotherapy planning for DMLC-based respiratory motion tracking. Med Phys. 2005;32:942–951. doi: 10.1118/1.1879152. [DOI] [PubMed] [Google Scholar]
- Underberg RW, Lagerwaard FJ, Slotman BJ, Cuijpers JP, Senan S. Benefit of respiration-gated stereotactic radiotherapy for stage I lung cancer: an analysis of 4DCT datasets. Int J Radiat Oncol Biol Phys. 2005;62:554–560. doi: 10.1016/j.ijrobp.2005.01.032. [DOI] [PubMed] [Google Scholar]
- van der Geld YG, Senan S, van Sörnsen de Koste JR, et al. Evaluating mobility for radiotherapy planning of lung tumors: a comparison of virtual fluoroscopy and 4DCT. Lung Cancer. 2006;53:31–37. doi: 10.1016/j.lungcan.2006.03.013. [DOI] [PubMed] [Google Scholar]
- Li FX, Li JB, Zhang YJ, et al. Comparison of the planning target volume based on three-dimensional CT and four-dimensional CT images of non-small-cell lung cancer. Radiother Oncol. 2011;99:176–180. doi: 10.1016/j.radonc.2011.03.015. [DOI] [PubMed] [Google Scholar]
- Zhu J, Zhang ZC, Li BS, et al. Analysis of acute radiation-induced esophagitis in non-small-cell lung cancer patients using the Lyman NTCP model. Radiother Oncol. 2010;97:449–454. doi: 10.1016/j.radonc.2010.09.025. [DOI] [PubMed] [Google Scholar]
- Hodapp N. The ICRU Report 83: prescribing, recording and reporting photon-beam intensity-modulated radiation therapy (IMRT)] Strahlenther Onkol. 2012;188:97–99. doi: 10.1007/s00066-011-0015-x. (In German.) [DOI] [PubMed] [Google Scholar]
- Burman C, Kutcher GJ, Emami B, Goitein M. Fitting of normal tissue tolerance data to an analytic function. Int J Radiat Oncol Biol Phys. 1991;21:123–135. doi: 10.1016/0360-3016(91)90172-z. [DOI] [PubMed] [Google Scholar]
- Keall P. 4-dimensional computed tomography imaging and treatment planning. Semin Radiat Oncol. 2004;14:81–90. doi: 10.1053/j.semradonc.2003.10.006. [DOI] [PubMed] [Google Scholar]
- Rietzel E, Liu AK, Doppke KP, et al. Design of 4D treatment planning target volumes. Int J Radiat Oncol Biol Phys. 2006;66:287–295. doi: 10.1016/j.ijrobp.2006.05.024. [DOI] [PubMed] [Google Scholar]
- Weiss E, Wijesooriya K, Dill SV, Keall PJ. Tumor and normal tissue motion in the thorax during respiration: analysis of volumetric and positional variations using 4D CT. Int J Radiat Oncol Biol Phys. 2007;67:296–307. doi: 10.1016/j.ijrobp.2006.09.009. [DOI] [PubMed] [Google Scholar]
- Wang L, Correa CR, Zhao L, et al. The effect of radiation dose and chemotherapy on overall survival in 237 patients with Stage III non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2009;73:1383–1390. doi: 10.1016/j.ijrobp.2008.06.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machtay M, Bae K, Movsas B, et al. Higher biologically effective dose of radiotherapy is associated with improved outcomes for locally advanced non-small cell lung carcinoma treated with chemoradiation: an analysis of the Radiation Therapy Oncology Group. Int J Radiat Oncol Biol Phys. 2012;82:425–434. doi: 10.1016/j.ijrobp.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]


