Abstract
Background
In this study, positron emission tomography-computed tomography (PET-CT) was used to monitor the maximal standard uptake value (SUVmax) in advanced lung adenocarcinoma patients with epithermal growth factor receptor (EGFR) mutation to prove its role in predicting the prognosis of targeted therapy.
Methods
A total of 46 patients with advanced lung adenocarcinoma (IIIb-IV stage) were enrolled in the current study. They were positive for EGFR mutation. All patients received gefitinib (250 mg per day, administered orally). PET-CT was conducted prior to (at baseline) and six months after gefitinib administration for the lesion size and SUVmax. The recommendations of the European Organization for Research and Treatment of Cancer criteria were chosen for PET assessment. Metabolic response (SUV decline < −25%) was compared with morphologic response evaluated by CT scan and overall survival.
Result
Compared to patients with △SUV% ≥ 25% (progressive metabolic disease), the survival time was significantly prolonged in △SUV% < −25% (including complete metabolic response and progressive metabolic disease) (10.6/18.4, P = 0.000), but was not in −25% ≤ △SUV% < 25% (stable metabolic disease) (10.6/10.7, P = 0.088). Patients who achieved △SUV% < −25% after treatment were associated with a longer median survival, higher control rate, and better prognosis. There was a strong correlation between SUV changes (△SUV%) and CT size change (△lesion size%) (R2 = 0.891, P = 0.000).
Conclusion
Changes in the SUV could be used to predict the prognosis of targeted therapy in advanced lung adenocarcinoma.
Keywords: △SUV%, efficacy, gefitinib, lung adenocarcinoma, PET-CT
Introduction
Lung cancer is currently the leading cause of cancer death worldwide. The World Health Organization (WHO) estimates that there will be over one million new cases of lung cancer annually by 2025 in China. The proportion of lung adenocarcinoma increases year by year. Most patients with adenocarcinoma are diagnosed at advanced stage, with typically poor prognoses and a one-year survival rate of just 20%–50%.1,2
Positron emission tomography-computed tomography (PET-CT) is a novel diagnostic device based on PET, combined with the merits of anatomical and functional imaging. It can diagnose lung cancer on the molecular level via standard uptake value (SUV) analysis. A decrease in fludeoxyglucose (FDG) uptake in tumor cells can be detected earlier than structural changes occur.3 Some researchers have deemed that changes in the SUV can serve as a potential predictor of survival in cancer patients.4–6 Nowadays, the platinum-based doublets are still regarded as the first-line chemotherapy choice. However, only one third of patients respond to chemotherapy.7 Second-line chemotherapy regimens are primarily docetaxel-based monotherapy. Those patients poorly sensitive to the first- and second-line chemotherapy may have a poorer prognosis. Gefitinib, an epithermal growth factor receptor-tyrosine kinase inhibitor (EGFR-TKI), has been extensively applied in advanced lung cancer cases in recent years. Numerous studies have shown that Asian female patients with lung adenocarcinoma and no smoking history were more sensitive to gefitinib.8–10 The correlation between EGFR mutations and EGFR TKI sensitivity has been subsequently validated in several studies.11–13Indeed, several clinical trials demonstrate that median progression free survival (PFS) of lung adenocarcinoma patients with gefitinib is at least 5.7 months.14–16 It inhibits the proliferation of tumor cells by blocking EGFR phosphorylation, allowing for targeted therapy. Therefore, we asked whether a change in the SUV could be a predictor for survival in lung cancer patients with gefitinib therapy.
Patients and methods
Inclusion criteria
Patients were eligible for inclusion in our study if they had pathologically confirmed advanced lung adenocarcinoma (IIIb-IV), measurable local or metastatic lesions, EGFR mutations in exon 19 or 21, failed previous first- or second-line chemotherapy, and had cancer history. A total of 46 patients hospitalized in the Nanfang Hospital were enrolled from 1 January to 30 December 30 2008. Patients who died of non-tumor diseases were excluded. All patients were followed up.
Methods
All patients received 250 mg gefitinib per day, administered orally. PET/CT was conducted prior to (at baseline) and six months after gefitinib administration. Treatment was discontinued if the disease worsened or patients were unable to tolerate the regimen. △SUV% and △lesion size% were calculated using the following formulas.
 |
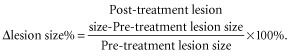 |
Efficacy evaluation
Prior to initiation of therapy, PET-CT was conducted for the site, size, number and SUVmax of lesions. The time point metabolic response was defined according to the recommendations of the European Organization for Research and Treatment of Cancer (EORTC) criteria for PET.17,18 Complete metabolic response (CMR) was achieved when SUVs of all lesions were decreased to an uptake value equivalent to the surrounding tissues. Partial metabolic response (PMR) was defined as a percentage change of the sum of SUVs (△SUV%) < −25%; stable metabolic disease (SMD) was −25% ≤ △SUV% < 25%; and progressive metabolic disease (PMD) was defined as △SUV% ≥ 25%, when the extent of [18F]FDG increased greater than 20% in the longest dimension, or when new [18F]FDG uptake appeared in metastatic lesions. During therapy, CT was conducted every three months to observe lesion changes. Based on the changes, efficacy was assessed using Response Evaluation Criteria In Solid Tumors (RECIST) 1.1: complete remission (CR), partial remission (PR), stable disease (SD) and progressive disease (PD). The total effective cases consisted of CR, PR and SD. Progression-free survival (PFS) was assessed from the data of randomization to the earliest sign of disease progression, as determined by means of RECIST 1.1, or death from any cause. Overall survival (OS) was assessed from the data of randomization until death from any cause.
Statistical analysis
SPSS13.0 was used for statistical analysis. The Wilcoxon test was used to compare the differences between subgroups. Survival curves were calculated by the Kaplan-Meier method and analyzed by log-rank test. To determine independent factors that were significantly related to the prognosis, multivariate analysis was performed using the Cox proportional hazards regression model. The correlation between △SUV% and △lesion size% was analyzed using the Spearman correlation method. Agreement between metabolic response on EORTC recommendations and morphologic overall response on RECIST 1.1 on the sixth month was evaluated using kappa statistics. P < 0.05 was considered statistically different.
Results
△ Standard uptake value (SUV)% and clinical factors
The median age was 62 years (range from 34 to 81), and 63% of patients were female. Clinical and pathological characteristics are shown in Table 1. According to △SUV% and the recommendations of EORTC, patients were divided into three groups (CMR+PMR, SMD, PMD) with only one patient in the CMR group. The correlation between △SUV% and clinicopathological features is shown in Table 2. Subgroup analyses were performed to compare △SUV% between groups defined according to gender, age (< 65 years or ≥65 years), PS (≤1 or ≥2), smoking status, stage (IIIb or IV), and previous chemotherapy cycles (≤1 or ≥2). Significant association between △SUV% and low PS (0–1) (P = 0.015), and non-smoking history (P = 0.016) were detected.
Table 1.
Patients' characteristics
| Characteristics | Number of patients | % |
|---|---|---|
| Gender | ||
| Male | 17 | 37.0 |
| Female | 29 | 63.0 |
| Median age (range) | 62 (34–81) | |
| Stage (UICC) | ||
| IIIb | 13 | 28.3 |
| IV | 33 | 71.7 |
| PS | ||
| 0 | 10 | 21.7 |
| 1 | 15 | 32.6 |
| 2 | 10 | 21.7 |
| 3–4 | 11 | 24.0 |
| Smoking history | ||
| Yes | 16 | 34.8 |
| No | 30 | 65.2 |
| Chemotherapy cycle | ||
| 1 | 10 | 21.7 |
| 2 | 10 | 21.7 |
| ≥ 3 | 26 | 56.6 |
PS, performance status; UICC Union for International Cancer Control.
Table 2.
Wilcoxon test between △standard uptake value (SUV)% (according to the European Organization for Research and Treatment of Cancer) and clinical factors
| Clinical factors | △SUV% | P | ||
|---|---|---|---|---|
| △SUV% < −25% (N = 13) | −25% ≤ △SUV% <25% (N= 22) | △SUV% ≥ 25% (N = 11) | ||
| Age | ||||
| <65 | 8 | 12 | 6 | 0.719 |
| ≥65 | 5 | 10 | 5 | |
| Gender | ||||
| Male | 3 | 13 | 5 | 0.224 |
| Female | 10 | 9 | 6 | |
| Stage (UICC) | ||||
| IIIb | 7 | 18 | 2 | 0.132 |
| IV | 6 | 4 | 9 | |
| ECOG PS scores | ||||
| ≤1 | 11 | 10 | 4 | 0.015 |
| ≥2 | 2 | 12 | 7 | |
| Smoking history | ||||
| No | 11 | 15 | 4 | 0.016 |
| Yes | 2 | 7 | 7 | |
| Chemotherapy cycle | ||||
| ≤1 | 4 | 4 | 2 | 0.486 |
| ≥2 | 9 | 18 | 9 | |
| Response to gefitinib | ||||
| Effective | 13 | 18 | 3 | 0.000 |
| Ineffective | 0 | 4 | 8 | |
ECOG, Eastern Cooperative Oncology Group; PS, performance status; UICC, Union for International Cancer Control.
The assessment of efficacy
Efficacy was assessed at the third and six months, and PET/CT was processed whenever symptoms worsened or new symptoms appeared. After six months of therapy, two (4.3%), 16 (34.8%), 16 (34.8%) and 12 (26.1%) patients achieved CR, PR, SD, and PD, respectively. The total effective rate (CR+PR+SD) was 73.9%. The effective rate was 100.0%, 81.8% and 27.3% in △SUV%<-25% (CMR+PMR), −25% ≤ △SUV% < 25% (SMD), and △SUV% ≥ 25% (PMD), respectively (P = 0.000) (Table 2 ). The effective rate significantly increased when △SUV% was less than 25%.
Comparative analysis of metabolic and morphologic responses
There was a strong correlation between SUV changes (△SUV%) and CT size change (△lesion size%) (R2 = 0.891, P = 0.000) (Fig. 1). There was also a moderate agreement (κ = 0.540) between metabolic response based on EORTC recommendations and morphologic overall response according to RECIST 1.1 at the sixth month of treatment (Table 3). In most cases, shrinking lesions were accompanied by drops of SUV (Fig. 2). In other words, PET/CT could replace CT to assess the therapeutic efficacy and prognosis at the molecular level.
Figure 1.
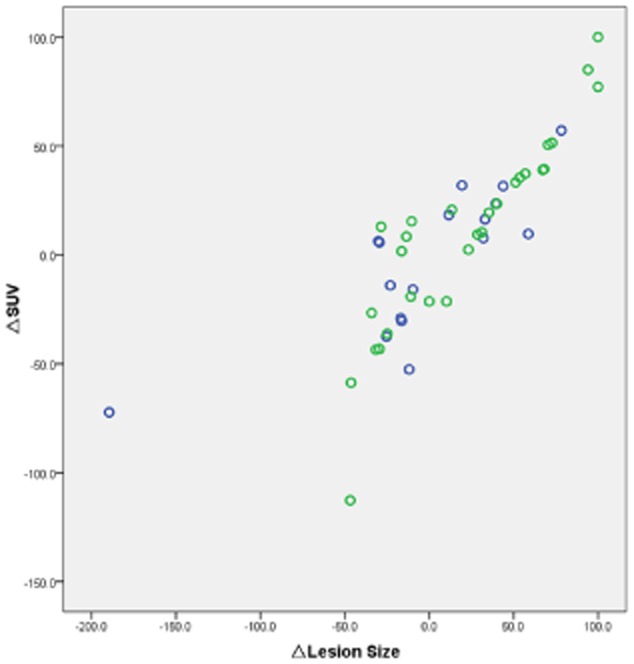
Correlation coefficient, 0.891; P = 0.000. Gender:  , male;
, male;  , female.
, female.
Table 3.
Agreement between metabolic response on European Organization for Research and Treatment of Cancer recommendations and morphologic overall response on Response Evaluation Criteria In Solid Tumors 1.1 at six months (κ = 0.540)
| RECIST1.0 | EORTC | |||
|---|---|---|---|---|
| CMR | PMR | SMD | PMD | |
| CR | 1 | 1 | ||
| PR | 12 | 6 | ||
| SD | 1 | 12 | 3 | |
| PD | 4 | 8 | ||
CMR, complete metabolic response; CR, complete remission; EORTC, European Organization for Research and Treatment of Cancer; PMD, progressive metabolic disease; PD, progressive disease; PMR, partial metabolic response; PR, partial remission; RECIST, Response Evaluation Criteria In Solid Tumors; SMD, stable metabolic disease; SD stable disease.
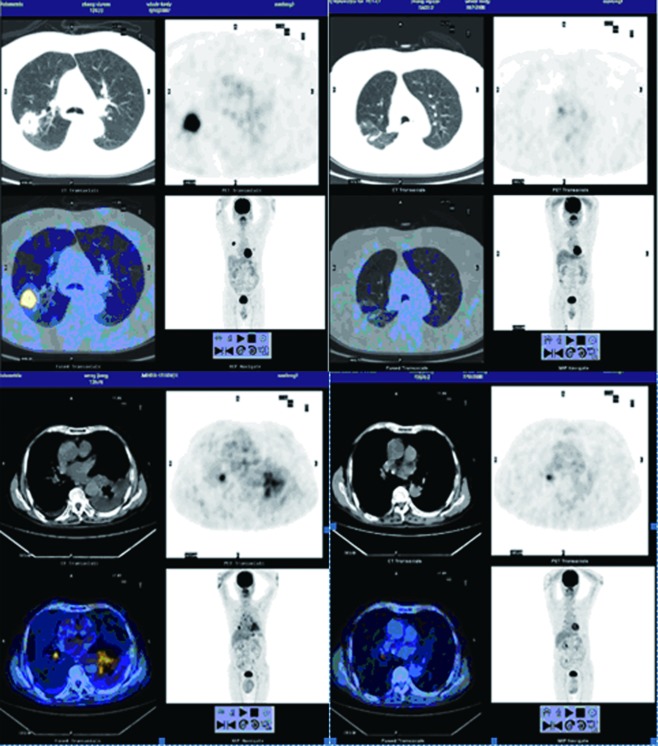
Figure 2.
Positron emission tomography-computed tomography (PET-CT): standard uptake value (SUV) dramatically drops after six months of treatment with gefitinib.
Association between SUV change and patient survival
The one- and two-year survival rates were 54.3% and 8.7%, respectively. Kaplan-Meier analysis showed that the survival time was longer in females (the median survival 12.5 vs. 10.7; P = 0.011), patients in early stage (IIIB) (15.4 vs. 10.7; P = 0.001), with a low PS (≤1) (14.3 vs. 9.9; P = 0.003), and no smoking history (13.1 vs. 7.9; P = 0.000) (Table 4). Compared to △SUV% ≥ 25%, the survival time was significantly prolonged in △SUV% < −25% (10.6/18.4, P = 0.000), but was not in −25% ≤ △SUV% < 25% (10.6/10.7, P = 0.088) (Fig. 3). The Cox multiple regression analysis suggested that stage (hazard ratio [HR], 0.340;95% confidence interval [CI], 0.150∼0.772; P = 0.010), PS scores (HR, 2.033; 95% CI, 1.060∼3.903; P = 0.033), smoking history (HR, 0.215; 95% CI, 0.095∼0.487; P = 0.000), and △SUV% < −25% (HR, 0.170; 95% CI, 0.057∼0.511; P = 0.002) were independent prognostic factors in our study (Table 5). We also found that, compared to △SUV% ≥ 25%, △SUV% < −25% had a lower risk.
Table 4.
Univariate survival analysis
| Variable | N | Median survival (months) | χ2 | P-value |
|---|---|---|---|---|
| Age | ||||
| <65 | 27 | 11.4 | 0.040 | 0.842 |
| ≥65 | 19 | 12.4 | ||
| Gender | ||||
| Male | 17 | 10.7 | 6.454 | 0.011 |
| Female | 29 | 12.5 | ||
| Stage (UICC) | ||||
| IIIb | 13 | 15.4 | 11.949 | 0.001 |
| IV | 33 | 10.7 | ||
| PS | ||||
| ≤1 | 25 | 14.3 | 8.986 | 0.003 |
| ≥2 | 21 | 9.9 | ||
| Smoking history | ||||
| Yes | 16 | 7.9 | 22.665 | 0.000 |
| No | 30 | 13.1 | ||
| Chemotherapy cycle | ||||
| ≤1 | 10 | 12.3 | 1.046 | 0.306 |
| ≥2 | 36 | 12.2 | ||
| △SUV% | ||||
| △SUV% < −25% | 13 | 18.4 | 23.856 | 0.000† |
| −25% ≤ △SUV% < 25% | 22 | 10.7 | 2.902 | 0.088† |
| △SUV% ≥ 25% | 11 | 10.6 | 22.302 | 0.000‡ |
Compared with △SUV% ≥ 25% (PMD);
The whole Log-rank inspection, the comparison Bonferroni (× 3) correction. PS, performance status; SUV, standardized uptake value; UICC, Union for International Cancer Control.
Figure 3.
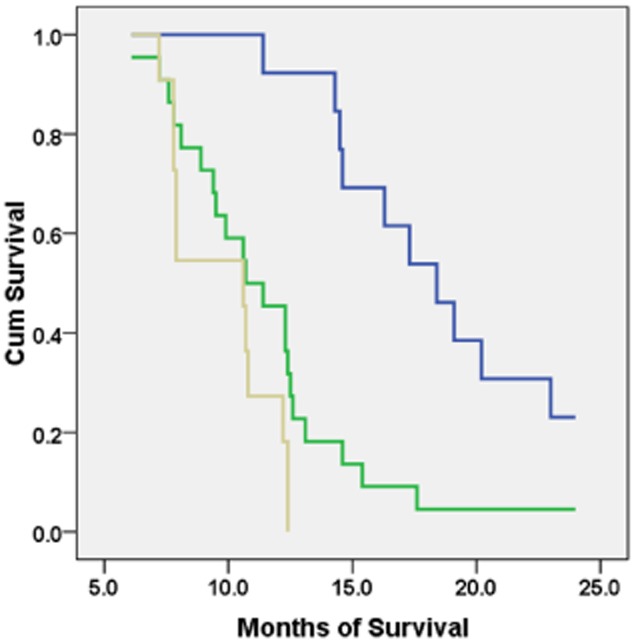
Kaplan-Meier survival analysis among groups with different changes in △ standard uptake value (SUV)%.  , △SUV% < −25%;
, △SUV% < −25%;  , −25% ≤ △SUV% < 25%;
, −25% ≤ △SUV% < 25%;  , △SUV% ≥ 25%.
, △SUV% ≥ 25%.
Table 5.
Multiple regression analysis
| Variable | Standard | HR | 95% CI | Wald χ2 | P-value |
|---|---|---|---|---|---|
| Stage | – | 0.340 | 0.150∼0.772 | 6.657 | 0.010 |
| PS scores | – | 2.033 | 1.060∼3.903 | 4.553 | 0.033 |
| Smoking | – | 0.215 | 0.095∼0.487 | 13.533 | 0.000 |
| △SUV% | Whole | – | – | 11.926 | 0.001 |
| △SUV% ≥ 25% | – | – | – | – | |
| −25% ≤ △SUV% < 25% | 0.649 | 0.279∼1.513 | 1.001 | 0.317 | |
| △SUV% < −25% | 0.170 | 0.057∼0.511 | 9.978 | 0.002 |
95% CI, 95% confidence interval; HR, hazards ratio; PS, performance status; SUV, Standardized Uptake Value.
Discussion
Molecular imaging is an entirely new technique, which can investigate cellular functions and metabolism in the living body for diagnosis, efficacy judgement, and new drug development. This technique utilizes the molecular probe to bind the special molecule within the cell in vivo and then the image in vitro with PET-CT, with the characteristics of high specificity, sensitivity, and resolution. PET-CT plays a vital role in the diagnosis and staging of lung cancer, allowing for non-invasive positioning, qualitative and quantitative analyses.
Much data demonstrates that EGFR TKIs, gefitinib and erlotinib, induce dramatic responses in a subpopulation of patients with adenocarcinoma. Although the presence of somatic mutations in the EGFR gene has been shown to be the best predictor of response to these TKIs,13,19 an alternative approach optimizing the clinical outcome of EGFR TKI therapy is necessary to accurately select patients who will benefit from the therapy.
Our study demonstrates good efficacy of gefitinib in the Asian population. The one- and two-year survival was 54.3% and 8.7%, respectively, consistent with studies in other Asian countries.20,21 The efficacy of gefitinib mainly depends on the mutant rate of EGFR, which is higher in the Asian population.
In our univariate and multivariate analysis, △SUV% at the sixth month could serve as an independent predictor of prolonged OS when a cut-off value of −25% in SUV decline was used. The survival was longer in patients with △SUV < −25% than those with △SUV ≥ 25%. An increase in SUV post-therapy suggests poorer prognosis in these patients. Although our study was single-centered with a small number of patients, we propose that △SUV% at the sixth month could be a superior predictor of post-gefitinib outcome.
Similar findings were observed in previous studies by Weber et al.22 and Nahmias et al.5 Fifty-seven patients with stage IIIB or IV non-small cell lung cancer (NSCLC) scheduled to undergo platinum-based chemotherapy were included in Weber et al.'s study to prospectively elucidate the predicting response of FDG-PET to chemotherapy. A reduction of tumor FDG uptake by more than 20% as assessed by SUV was used as a criterion for a metabolic response. There was a close correlation between metabolic response and best response to therapy according to RECIST (P < 0.0001; sensitivity and specificity for prediction of best response, 95% and 74%, respectively). Median time to progression and OS were significantly longer for metabolic responders than for metabolic non-responders (163 vs. 54 days and 252 days vs. 151 days, respectively). When therapeutic effects were evaluated by PET at one and three weeks after chemotherapy in 16 patients with NSCLC, Nahmias et al. also reported that survival time was more than six months in the group with △SUV < −50%, but less than six months in the group with △SUV > −50%. Our study found a similar result in that patients with △SUV < 25% after receiving gefitinib could have a significantly longer median survival time, compared with those patients with △SUV ≥ 25%.
We also found that there was a strong correlation between SUV changes (△SUV%) and CT size change (△lesion size%). There was a moderate agreement between metabolic response based on EORTC recommendations and morphologic overall response according to RECIST 1.1 at the sixth month.
Conclusion
In conclusion, PET-CT can replace CT to predict the efficacy and prognosis of targeted therapy in advanced lung adenocarcinoma at the molecular level.
Acknowledgments
This work is supported by the Province Natural Fund of Guangdong (S2012010009392). We would like to express our warmest gratitude to Rongcheng Luo, our tutor, for his instructive suggestions and valuable comments on the writing of this thesis. We are also grateful to Rong Li for providing us with valuable advice on the thesis. We wish to thank the Department of Medical Statistics, Southern Medical University and PET center, Nanfang Hospital.
Disclosure
No authors report any conflict of interest.
References
- Scagliotti GV, De Marinis F, Rinaldi M, et al. Phase III randomized trial comparing three platinum-based doublets in advanced non-small-cell lung cancer. J Clin Oncol. 2002;20:4285–4291. doi: 10.1200/JCO.2002.02.068. [DOI] [PubMed] [Google Scholar]
- Schiller JH, Harrington D, Belani CP, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002;346:92–98. doi: 10.1056/NEJMoa011954. [DOI] [PubMed] [Google Scholar]
- Vansteenkiste J, Fischer BM, Dooms C, Mortensen J. Positron-emission tomography in prognostic and therapeutic assessment of lung cancer: systematic review. Lancet Oncol. 2004;5:531–540. doi: 10.1016/S1470-2045(04)01564-5. [DOI] [PubMed] [Google Scholar]
- Na, Byun BH, Kang HJ, et al. 18F-fluoro-2-deoxy-glucose uptake predicts clinical outcome in patients with gefitinib-treated non-small cell lung cancer. Clin Cancer Res. 2008;14:2036–2041. doi: 10.1158/1078-0432.CCR-07-4074. [DOI] [PubMed] [Google Scholar]
- Nahmias C, Hanna WT, Wahl LM, Long MJ, Hubner KF, Townsend DW. Time course of early response to chemotherapy in non-small cell lung cancer patients with 18F-FDG PET/CT. J Nucl Med. 2007;48:744–751. doi: 10.2967/jnumed.106.038513. [DOI] [PubMed] [Google Scholar]
- Riely GJ, Kris MG, Zhao B, et al. Prospective assessment of discontinuation and reinitiation of erlotinib or gefitinib in patients with acquired resistance to erlotinib or gefitinib followed by the addition of everolimus. Clin Cancer Res. 2007;13:5150–5155. doi: 10.1158/1078-0432.CCR-07-0560. [DOI] [PubMed] [Google Scholar]
- Breathnach OS, Freidlin B, Conley B, et al. Twenty-two years of phase III trials for patients with advanced non-small-cell lung cancer: sobering results. J Clin Oncol. 2001;19:1734–1742. doi: 10.1200/JCO.2001.19.6.1734. [DOI] [PubMed] [Google Scholar]
- Jiang H. Overview of gefitinib in non-small cell lung cancer: an Asian perspective. Jpn J Clin Oncol. 2009;39:137–150. doi: 10.1093/jjco/hyn139. [DOI] [PubMed] [Google Scholar]
- Park K, Goto K. A review of the benefit-risk profile of gefitinib in Asian patients with advanced non-small-cell lung cancer. Curr Med Res Opin. 2006;22:561–573. doi: 10.1185/030079906X89847. [DOI] [PubMed] [Google Scholar]
- Pfister DG, Johnson DH, Azzoli CG, et al. American Society of Clinical Oncology treatment of unresectable non-small-cell lung cancer guideline: update 2003. J Clin Oncol. 2004;22:330–353. doi: 10.1200/JCO.2004.09.053. [DOI] [PubMed] [Google Scholar]
- Bai H, Mao L, Wang HS, et al. Epidermal growth factor receptor mutations in plasma DNA samples predict tumor response in Chinese patients with stages IIIB to IV non-small-cell lung cancer. J Clin Oncol. 2009;27:2653–2659. doi: 10.1200/JCO.2008.17.3930. [DOI] [PubMed] [Google Scholar]
- Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–2139. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- Paez JG, Jänne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–1500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- Mok TS, Wu YL, Thongprasert FS, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947–957. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- Kim ES, Hirsh V, Mok T, et al. Gefitinib versus docetaxel in previously treated non-small-cell lung cancer (INTEREST) a randomised phase III trial. Lancet. 2008;372:1809–1818. doi: 10.1016/S0140-6736(08)61758-4. [DOI] [PubMed] [Google Scholar]
- Han JY, Park K, Kim SW, et al. First-SIGNAL: first-line single-agent iressa versus gemcitabine and cisplatin trial in never-smokers with adenocarcinoma of the lung. J Clin Oncol. 2012;30:1122–1128. doi: 10.1200/JCO.2011.36.8456. [DOI] [PubMed] [Google Scholar]
- Young H, Baum R, Cremerius U, et al. Measurement of clinical and subclinical tumor response using [18F]-fluorodeoxyglucose and positron emission tomography: review and 1999 EORTC recommendations. Eur J Cancer. 1999;35:1773–1782. doi: 10.1016/s0959-8049(99)00229-4. [DOI] [PubMed] [Google Scholar]
- Takahashi R, Hirata H, Tachibana I, et al. Early [18F]fluorodeoxyglucose positron emission tomography at two days of gefitinib treatment predicts clinical outcome in patients with adenocarcinoma of the lung. Clin Cancer Res. 2012;18:220–228. doi: 10.1158/1078-0432.CCR-11-0868. [DOI] [PubMed] [Google Scholar]
- Lynch TL, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–2139. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- Chang A, Parikh P, Thongprasert S, et al. Gefitinib (IRESSA) in patients of Asian origin with refractory advanced non-small cell lung cancer: subset analysis from the ISEL study. J Thorac Oncol. 2006;1:847–855. [PubMed] [Google Scholar]
- Kim KS, Jeong JY, Kim YC, et al. Predictors of the response to gefitinib in refractory non-small cell lung cancer. Clin Cancer Res. 2005;11:2244–2251. doi: 10.1158/1078-0432.CCR-04-2081. [DOI] [PubMed] [Google Scholar]
- Weber WA, Petersen V, Schmidt B, et al. Positron emission tomography in non-small-cell lung cancer: prediction of response to chemotherapy by quantitative assessment of glucose use. J Clin Onco. 2003;21:2651–2657. doi: 10.1200/JCO.2003.12.004. [DOI] [PubMed] [Google Scholar]