Abstract
Background
β-elemene (β-ELE) injection is a new anticancer drug extracted from Curcuma zedoaria Roscoe that has been widely used to treat malignant tumors. Recent studies show that β-ELE reverses the drug resistance of tumor cells. To explore the possible mechanisms of β-ELE, we investigated its effects on cisplatin (DDP)-resistant human lung adenocarcinoma A549/DDP cells.
Methods
The effects of β-ELE on the growth of A549/DDP cells in vitro were determined by MTT assay. Apoptosis was assessed by fluorescence microscopy with Hoechst 33258 staining, flow cytometry with Annexin V-FITC/propium iodide double staining; mitochondrial membrane potential using JC-1 fluorescence probe and laser confocal scanning microscopy, and intracellular reactive oxygen species levels were measured by 2’,7'-dichlorfluorescein-diacetate staining and flow cytometry; and contents of cytosolic glutathione were determined by glutathione assay kits. Intracellular Rhodamine-123 fluorescence intensity was detected by flow cytometry, and the expression of P-glycoprotein (P-gp) was detected by Western blotting.
Results
β-ELE inhibited the proliferation of A549/DDP cells in a time- and dose-dependent manner. Furthermore, β-ELE enhanced the sensitivity of A549/DDP cells to cisplatin and reversed the drug resistance of A549/DDP cells. Consistent with a role in activating apoptosis, β-ELE decreased mitochondrial membrane potential, increased intracellular reactive oxygen species concentration and intracellular accumulation of Rhodamine-123, decreased the cytoplasmic glutathione levels and the expression of P-gp in a time- and dose-dependent manner.
Conclusions
These results define a pathway of β-ELE function that involves decreased mitochondrial membrane potential and P-gp expression activated intracellular redox system, and induced apoptosis leading to reverse drug resistance.
Keywords: A549/DDP cell, apoptosis, drug resistance, elemene, lung neoplasms
Introduction
Lung cancer is generally diagnosed too late to be operable, and, consequently, chemotherapy provides a major treatment for most lung cancer patients.1–3 Furthermore, drug resistance of lung cancer to chemotherapeutic drugs is one of the important causes of the failure of chemotherapy.4,5 β-elemene (β-ELE) is a new anticancer drug extracted from Curcuma zedoaria Roscoe, know as zedoary, that includes α, β, γ and δ forms. β-ELE comprises the main anti-tumor effect.6,7 β-ELE injection has been widely used to treat a variety of malignancies including lung cancer,8 liver cancer,9 malignant tumors of the digestive tract,10 and bladder cancer.11Recently studies show that β-ELE could reverse the drug resistance of tumor cells.7,12,13 To explore the mechanisms for β-ELE, we examined the effects of β-ELE in the cisplatin (DDP)-resistant human lung adenocarcinoma cell line A549/DDP. Our results define a pathway of β-ELE function involving the regulation of the mitochondrial membrane and apoptosis signaling proteins, leading to a reversal of drug resistance.
Materials and methods
Reagents and equipment
The cisplatin-sensitive human lung adenocarcinoma cell line, A549 and its cisplatin-resistant derivate, A549/DDP were purchased from the China Military Medical Science Academy of the People's Liberation Army (Beijing, China). Cisplatin (DDP) (Yunnan old Biological Pharmaceutical Co Ltd, batch number: 090202); β-elemene injection (Dalian Jingang Pharmaceutical Co., Ltd, batch number: 081152); mouse anti-human monoclonal antibodies against P-gp, β-actin and horseradish peroxidase (HRP) labeled rabbit anti-mouse immunoglobulin (IgG) were obtained from Santa Cruz Biotechnology (USA). The 2’, 7'-dichlorine fluorescein diacetate (DCFH-DA) fluorescence probe was purchased from Invitrogen (USA); propidium iodide (PI), Annexin V-FITC/PI double dye kits, enhanced chemiluminescence (ECL) reagent kits, Hoechst 33342 staining reagent, MTT cell proliferation assay kits, dimethyl sulfoxide (DMSO), Roswell Park Memorial Institute (RPMI)-1640 culture medium, and JC-1 mitochondrial membrane potential kits were purchased from Nanjing Keygen Development Co. Ltd. (China); and the glutathione (GSH)/GSSG detection kits were purchased from Jiangsu Pik Wan Biotechnology Research Institute (China). Equipment included Coulter Epics XL flow cytometry (Beckman Coulter Inc., Brea, CA, USA), TCS SP2 laser scanning confocal microscope (Leica Microsystems, Wetzlar, Germany), spectrophotometer (Eppendorf, Hamburg, Germany), electrophoresis and transfer film equipment (Bio-Rad, Hercules, CA, USA), OLYMPUS IX71 fluorescence microscope (OLYMPUS Corporation, Shinjuku, Tokyo, Japan), AE31/CCIS inverted microscope (Moltic Co. Ltd, Japan), and 5804R low speed centrifuge (Eppendorf, Hamburg, Germany). β-ELE and cisplatin were diluted with both RPMI 1640 and 10% fetal bovine serum (FBS) medium to various working concentrations when used.
Cell culture
Human lung adenocarcinoma A549, A549/DDP cells (final concentration of 2 μg/mL DDP to maintain drug resistance) were cultured in RPMI-1640 medium supplemented with 10% FBS, 100 U/mL penicillin and 100 mg/L streptomycin in an atmosphere of 5% CO2 at 37°C. A549/DDP cells were cultured for one week in the medium without DDP prior to experimentation. Exponentially growing cells were used in all experiments.
Drug sensitivity assay
The sensitivity of cells to drugs was determined using MTT assay. Briefly, cells were plated in triplicate in 96-well plates at a density of 5 × 103 cells/well for drug-sensitivity assays. Cells were treated with 0.25, 0.5, 1, 2, 4, 8, 16 or 32 μg/mL of DDP for 24 hours, 20 μL MTT dye (5 mg/mL) was added at 37°C for four hours, then culture medium was removed and 150 μL of dimethyl sulfoxide (DMSO) per well was added with oscillation for 10 minutes. Spectrometric absorbance at 570 nm was measured using a microplate reader (reference wavelength 630 nm). The experiment was repeated three times to generate a growth curve. The proliferation rate (%) was determined by calculating the value of the experimental group/the value of the control group × 100%.
MTT cytotoxicity assay
A549/DDP cells were plated in triplicate in 96-well plates at a density of 5 × 103 cells/well. After cells adhered to the plates, a final concentration of 10, 20, 40 or 80 μg/mL β-ELE was added to the experimental groups, and the same amount of drug dissolution medium was added to the control group. MTT assay was used to determine β-ELE cytotoxicity as described previously. The cell proliferation inhibition rate was calculated as one − the proliferation rate (%). The 50% inhibitory concentration (IC50) was calculated by linear regression, and the fold drug resistance was calculated as IC50 of resistant cells/IC50 of sensitive cells.
For assessing β-ELE reversal of A549/DDP cell drug resistance, the control group was treated with varying final concentrations of DDP (0.25∼32 μg/mL), and the experimental group had an additional 20 μg/mL β-ELE. After 24 hours, MTT solution was added and absorbance was measured as described above. The reversal fold was calculated as the IC50 value in absence of β-ELE to that in the presence of β-ELE.
Apoptosis assay
A549/DDP cells were cultured in 2 μg/mL DDP to maintain their drug resistance, and β-ELE was added at 20 or 40 μg/mL to the experimental group. After 24 hours, the cells were collected by centrifugation for Hoechst and Annexin V-FITC staining. For Hoechst staining, nuclei were stained with DNA fluorescent dye and observed under a fluorescence microscope. For Annexin V-FITC staining, the cell pellet was re-suspended in 100 μL of binding buffer containing 10 mM HEPES/NaOH, 140 mM NaCl, 5 mM CaCl2 (pH 7.4), supplemented with 5 μL Annexin V-FITC and 5 μL PI. After the incubation period (30 minutes at 37°C in the dark), an additional 400 μL of binding buffer was added and Annexin V-FITC/PI staining was analyzed within one hour by flow-cytometry. The fluorescence intensity (green Annexin V-FITC and red PI) was measured on Coulter Epics XL flow cytometry (Beckman Coulter). CellQuest Pro software was used for acquisition and analysis of data.
Assessment of the mitochondrial membrane potential (ΔΨm) by JC-1 assay and laser confocal fluorescence microscopy
A549/DDP cells were plated in triplicate in 96-well plates at a density of 1 × 105 cells/well. The control group was plated in 2 μg/mL DDP, and an additional 40 μg/mL β-ELE was added to the experimental group. Cells were cultured for zero, six, 12, or 24 hours in serum-free culture medium, and then 10 mg/L JC-1 dye was added and cells were incubated at 37°C for 15 minutes. Cells were centrifuged, and the excess waste dye was aspirated. The cells were then photographed under a laser confocal microscope, and JC-1 monomer (green fluorescence) was detected at excitation wavelength 488 nm (emission wavelength 530 nm), while JC-1 polymer (red fluorescence) was detected at excitation wavelength 535 nm (emission wavelength 590 nm). Ten fields were randomly selected for calculation of the average fluorescence intensity (Leica, LCS Universal Imaging software). The red fluorescence/green fluorescence optical density ratio indicated the mitochondrial ΔΨm levels, while a decrease in the optical density ratio represented mitochondrial ΔΨm decrease.
Assessment of intracellular reactive oxygen species (ROS) and glutathione (GSH) level
To assess intracellular reactive oxygen species (ROS) levels, A549/DDP cells were plated in triplicate in 96-well plates at a density of 5 × 103 cells/well with 2 μg/mL DDP and 0, 20 or 40 μg/mL ELE for 24 hours. Cells were collected, incubated with 5 uM DCFH-DA probe at 37°C for 20 minutes in serum-free medium, and washed three times. The fluorescence intensity at 488 nm excitation wavelength and 525 nm emission wavelength was detected by flow cytometry. DCFH-DA itself has no fluorescence and can freely pass through cell membrane. After entering the cell, 2’, 7'-DCFH is oxidation by superoxide anion and hydrogen peroxide to fluorescent 2’, 7'-dichlorine fluorescein (DCF). The level of DCF reflects the level of intracellular ROS expression.
At the same time, we measured GSH according to the manufacturer’ s instructions. We measured the total GSSG+GSH content, and then subtracted the amount of GSH in the sample to calculate the GSSG content. The ratio of GSH/(GSSG+GSH) was used as a measure of GSH. The principle of this assay is as follows: GSH reacts with 5'-dinitrobenzene acid (DTNB) to form GSSG and stable 5'-mercapto-2'-nitrobenzene acid (TNB); GSSG is reduced by GSSG reductase and nicotinamide adenine dinucleotide phosphate (NADPH), releasing additional TNB (yellow color), which can be detected by spectrophotometry (maximum absorbance wavelength 412 nm). The amount of TNB is proportional to the GSH released in the samples. All experiments were repeated three times.
Rhodamine-123 (Rh-123) retention assay test A549/DDP cells P-glycoprotein (P-gp) function
Intracellular rhodamine-123 (Rh-123) retention was determined using flow cytometry as a functional index of P-glycoprotein (P-gp) activity. A549/DDP cells were plated in triplicate in 96-well plates at a density of 5 × 103 cells/well with 2 μg/mL DDP and 0, 20 or 40 μg/mL ELE for 24 hours prior to the addition of 5 μg/mL Rh-123. After incubation at 37°C for one hour, cells were harvested and centrifuged at 300 g for 10 minutes. Cell pellets were resuspended with 500 μL of ice-cold phosphate buffered saline (PBS) and immediately used for flow cytometry at 488 nm analysis of Rh-123 retention.
Western analysis of P-gp expression
A549/DDP cells were plated in triplicate in 96-well plates at a density of 5 × 103 cells/well with 2 μg/mL DDP plus 0, 20 or 40 μg/mL ELE, cells were collected in lysis buffer, and incubated on ice for 15 minutes. The lysates were centrifuged at 4°C for 10 minutes, and 50 μg of protein were electrophoresed by 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinyl difluoride ethylene membranes. The membranes were then blocked with 5% skim milk for two hours, washed in tris-buffered saline plus tween 20 (TBST), and incubated with mouse anti-human monoclonal antibody against P-gp (at a dilution of 1:200, Santa Cruz, Inc., USA) or β-actin (1:2000 dilution). HRP-labeled secondary antibodies (1:2000 dilution) were added for two hours at room temperature, followed by ECL.
Statistical analysis
Statistical analysis was performed using SPSS 11.5 and Origin 8.5 software. Statistical data represent mean ± s and were determined using single factor analysis of variance. Comparisons between the two groups were performed using a student t-test or a Chi-square test. P < 0.05 was considered statistically significant.
Results
Determination of A549 and A549/DDP cell drug sensitivity
To verify the differential sensitivity of A549 and its derivative cell line, A549/DDP, to DDP, cells were exposed to a gradient of DDP concentrations for 24 hours, and cell viability was assessed by MTT assay. Results show that the concentration of DDP required to inhibit the proliferation of A549 cells [IC50 = (5.73 ± 2.11) μg/mL] is lower than the concentration to inhibit the proliferation of A549/DDP cells [C50 = (15.34 ± 1.05) μg/mL] (Fig 1). The difference in IC50 was statistically significant (t = 2.3571, P < 0.01), verifying that A549/DDP cells are DDP resistant.
Figure 1.
The growth inhibitory effects of different concentrations of cisplatin (DDP) on A549 and A549/DDP cells. Cell viability, as assessed by MTT assay, was determined 24 hours after exposure of A549 or A549/DDP cells to increasing amounts of DDP. Results represent the average of triplicate wells and are representative of three independent experiments.  , A549;
, A549;  , A549/DDP.
, A549/DDP.
Effects of β-ELE on A549/DDP cell toxicity
To begin to assess the effects of β-ELE on A549/DDP cells, we performed MTT assays over a range of doses and times. Results show that β-ELE inhibits A549/DDP cell growth in a dose-dependent manner (Fig 2; 20 vs. 40 μg/mL β-ELE: χ2 = 2.6249, P < 0.05 at 24 hours; χ2 = 2.1449, P < 0.05 at 48 hours). This effect was also partially time-dependent, depending on the β-ELE dose (24 vs. 48 hours for 20 μg/mL β-ELE: χ2 = 27.4632, P > 0.05; for 40 μg/mL β-ELE: χ2 = 2.4136, P < 0.05). Based on these results, we selected 20 μg/mL ELE treatment for 24 hours as the optimum concentration and time that ELE reverses drug resistance of A549/DDP cells.
Figure 2.
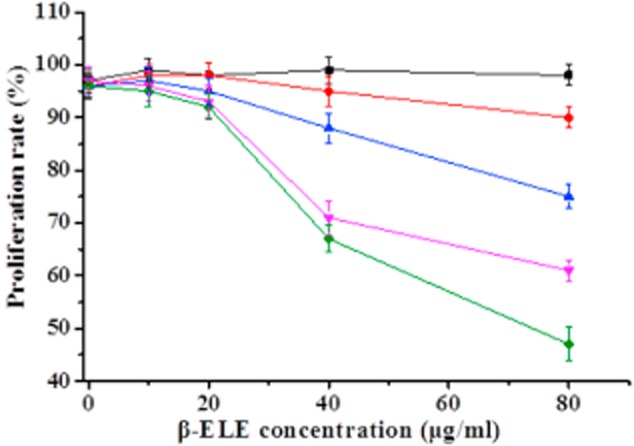
Time- and dose-dependent growth inhibitory effects of β-ELE on A549/cisplatin (DDP) cells. Cell viability, as assessed by MTT assay, was determined at a range of times after of A549/DDP cells to increasing amounts of DDP. Viability is normalized to 100% at time zero. Results represent the average of triplicate wells and are representative of three independent experiments.  , 0 hours;
, 0 hours;  , 12 hours;
, 12 hours;  , 24 hours;
, 24 hours;  , 48 hours;
, 48 hours;  , 72 hours.
, 72 hours.
β-ELE reverses drug resistance of A549/DDP cells
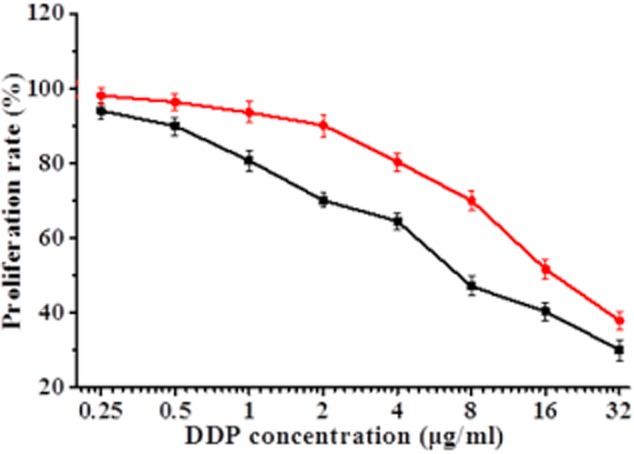
To determine whether β-ELE can reverse drug resistance of A549/DDP cells, we exposed cells for 24 hours to a range of doses of DDP in the absence or presence of 20 μg/mL β-ELE. The β-ELE-treated cells showed increased sensitivity to DDP at all concentrations (Fig 3, P < 0.05). Furthermore, the IC50 value of the experimental group (4.15 ± 0.89) μg/mL was significantly lower than the IC50 value of the control group (15.46 ± 1.23) μg/mL (t = 1.4321, P < 0.01), with the drug resistance ratio reversed (3.73 ± 0.38 times)(Table 1, Fig 3). The results suggest that β-ELE enhances the sensitivity of A549/DDP cells to DDP.
Figure 3.
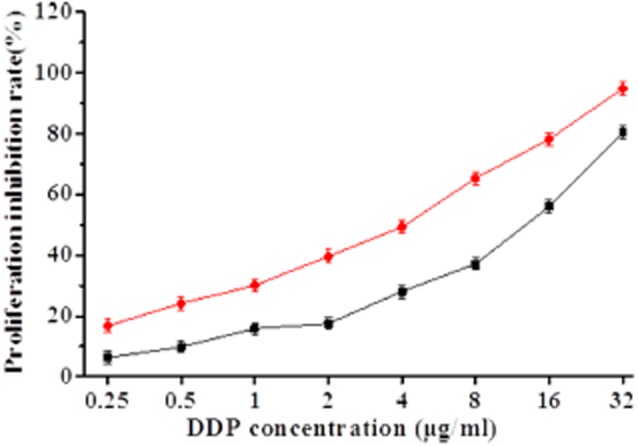
Effect of β-elemene (ELE) on cisplatin (DDP) inhibition of A549/DDP cell proliferation. Proliferation inhibition (cell proliferation inhibition rate was calculated as one – optical density (OD) value of the experimental group/OD value of the control group × 100%) was determined by MTT assays 24 hours after exposure to either a range of concentrations of DDP (control group) or 20 μg/mL β-ELE plus a range of concentrations of DDP (experimental group). The values corresponding to this graph and associated statistical analysis is provided in Table 1. Results represent the average of three experiments performed in triplicate.  , experiment group;
, experiment group;  , control group.
, control group.
Table 1.
The effect of β-elemene (ELE) in reversing the drug resistance of A549/cisplatin (DDP) cells (n = 3,  )
)
| DDP/ (μg/mL) | Cell proliferation inhibition rate (%) | |
|---|---|---|
| Control group | Experimental group | |
| 0.25 | 6.35 ± 1.03 | 16.79 ± 1.85* |
| 0.5 | 9.88 ± 0.99 | 24.20 ± 0.13* |
| 1 | 15.89 ± 0.46 | 30.14 ± 0.47* |
| 2 | 17.55 ± 1.35 | 39.64 ± 0.09* |
| 4 | 28.11 ± 0.65 | 49.34 ± 0.05* |
| 8 | 37.21 ± 1.45 | 65.37 ± 1.05* |
| 16 | 55.96 ± 2.03 | 78.21 ± 0.79* |
| 32 | 80.44 ± 0.77 | 94.85 ± 0.91* |
P < 0.05, versus control group (A549/cisplatin [DDP] cells treated with different concentrations of DDP); Experimental group: β-elemene (ELE) (20 μg/mL) combined with different concentrations of DDP; cell proliferation inhibition rate was calculated as one − cell proliferation rate (%), cell proliferation rate (%) was determined by calculating the OD value of the experimental group/OD value of the control group × 100%. DDP, cisplatin.
β-ELE increases levels of A549/DDP cell apoptosis
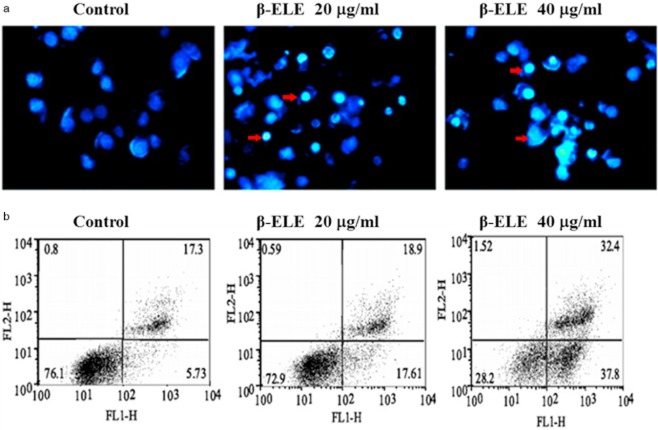
To determine whether the enhanced sensitivity to DDP conferred by β-ELE is related to increased levels of apoptosis, we performed Hoechst 33342 fluorescent staining following β-ELE treatment. Results showed that upon treatment with 20 and 40 μg/mL β-ELE for 24 hours, A549/DDP cell nuclei became progressively smaller with more dense granular chromatin staining, which suggests typical morphological changes of apoptosis (Fig 4a). We verified these findings by flow cytometry following Annexin V-FITC/PI staining. Our results demonstrate that the apoptosis rate is dose-dependent for cells treated with 20 and 40 μg/mL β-ELE as compared to the control, which was apparent for cells in early apoptosis (17.61 ± 0.10% and 37.80 ± 0.12% vs. 5.73 ± 0.09%) and cells in middle-late apoptosis (18.9 ± 0.11% and 32.4 ± 0.13% vs. 17.3 ± 0.11%). The differences between these values were statistical (P < 0.05) (Fig 4b). However, there were no differences between early and middle-late apoptosis in the 20 and 40 μg/mL β-ELE groups (P > 0.05). Collectively, these results suggest that β-ELE increases the levels of apoptosis (early and middle-late apoptosis) in A549/DDP cells.
Figure 4.
β-elemine (ELE) induces morphological features of apoptosis. (a) A549/cisplatin (DDP) cells were exposed to 0, 20, 40 μg/mL β-ELE for 24 hours, and then were stained with Hoechst. The small nuclei and condensed granular blue chromatin staining in the β-ELE-treated cells represents typical apoptosis morphology (see the red arrow; fluorescence staining × 400). (b) The levels of apoptosis in A549/DDP cells which were exposed to 0, 20, 40 μg/mL β-ELE were determined by Annexin-V-FITC/propium iodide staining. Cells positive for Annexin-V-FITC (FL1-H) alone represent early apoptotic cells, while cells positive for staining with both chromagens represent middle-late apoptotic cells. Compared with the control group, the experimental groups (20 and 40 μg/mL-ELE group) differences were statistically significant (P < 0.05). However, there were no differences between early and middle-late apoptosis in the 20 and 40 μg/mL β-ELE groups (P > 0.05). Results are representative of three independent experiments.
β-ELE decreases the mitochondrial membrane potential (ΔΨm) of A549/DDP cells
During apoptosis, the mitochondrial membrane potential decreases.14,15 As an additional verification of the effects of β-ELE, we assessed the mitochondrial membrane potential of A549/DDP cells before and after six, 12, and 24 hours of drug treatment. Most of the control cells were stained red by JC-1 assay, indicating an intact cell membrane, with clearly visible nuclei. In contrast, cells treated with 20 or 40 μg/mL β-ELE showed increasing amounts of green fluorescence, cell rupture, and cell content outflow, suggesting a decline in the mitochondrial ΔΨm. The effect was more obvious for the 40 μg/mL β-ELE group, which showed clear pyknosis (Fig 5a). Analysis of the red/green fluorescent light density ratio showed that the decrease was time-dependent and was statistically significant for the 20 and 40 μg/mL ELE groups (six hours: χ2 = 2.2447, P < 0.05, χ2 = 2.0256, P < 0.05; 12 h: χ2 = 2.0143, P < 0.05, χ2 = 1.3121, P < 0.01; 24 hours: χ2 = 1.3084, P < 0.01, χ2 = 1.0034, P < 0.01) (Fig 5b).
Figure 5.
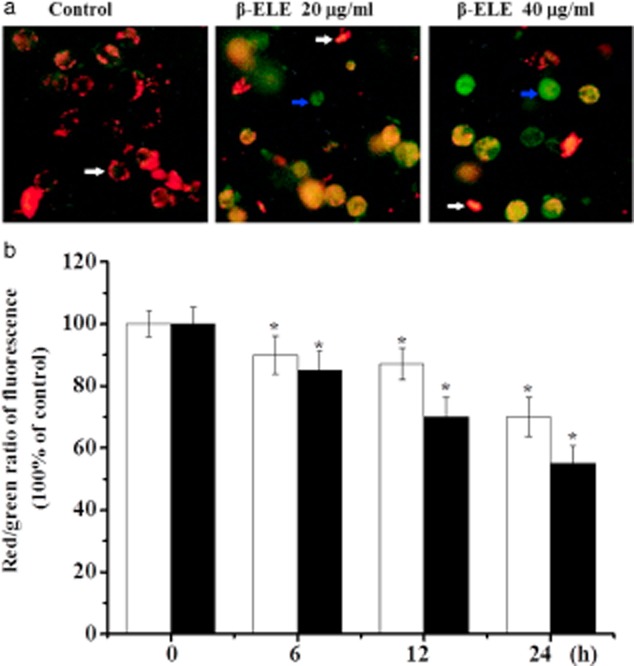
β-elemene (ELE) induces an increase in the mitochondrial ΔΨm of A549/cisplatin (DDP) cells. (a) Mitochondrial ΔΨm was measured by laser confocal scanning microscopy for cells exposed to 0, 20 or 40 μg/mL β-ELE (×400). Red represents resting cells with clearly visible nuclei (see white arrow). In contrast, green represents cells with decreased mitochondrial ΔΨm, cell rupture and cell content outflow, and pyknotic nuclei (see the blue arrow). (b) The ratio of red/green was compared at different times of exposure to β-ELE. Values are standardized to 100% in untreated cells and represent averages ± standard deviation (SD) of triplicate wells. Results are representative of three independent experiments. *P < 0.05 versus control group.  , 20 μg/mL;
, 20 μg/mL;  , 40 μg/mL.
, 40 μg/mL.
β-ELE increases levels of ROS generation and GSH release in A549/DDP cells
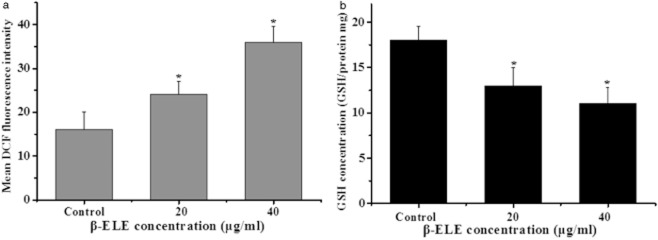
The generation of ROS and the decline of intracellular GSH contents are also associated with apotosis.16,17 When the cellular redox system is destroyed, oxygen free radicals are increased in the cell, thus, oxygen free radicals will cause mitochondrial membrane injury, decrease the mitochondrial membrane potential, activate caspase-3, and then initiate apoptosis. To examine ROS concentration, we performed a DCF assay following treatment with β-ELE for 24 hours. Results showed a statistical increase in DCF fluorescence intensity (χ2 = 3.2443, P < 0.05; χ2 = 2.1254, P < 0.05) for cells treated with 20 or 40 μg/mL β-ELE. These results suggest that the content of ROS is increased by β-ELE treatment (Fig 6a). Further assessment of GSH content showed that the GSH/(GSSG+GSH) ratio decreased, suggesting a decrease in GSH content that was dose-dependent (χ2 = 2.8437, P < 0.05; χ2 = 2.1244, P < 0.05) (Fig 6b). These results suggest that β-ELE activates a pathway of apoptosis that involves both the generation of ROS and the decline of intracellular GSH.
Figure 6.
β-elemene (ELE) promotes an increase in the reactive oxygen species (ROS) concentration and a decrease in glutathione (GSH) content in A549/cisplatin (DDP) cells. (a) The mean dichlorine fluorescein (DCF) fluorescence intensity is shown as a measure of ROS activity 24 hours after exposure of A549/DDP cells to 0, 20 or 40 μg/mL β-ELE. (b) GSH contents are shown 24 hours after exposure of A549/DDP cells to 0, 20 or 40 μg/mL β-ELE. Values represent averages ± standard deviation (SD) of triplicate wells and are representative of three independent experiments. *P < 0.05 versus control group.  , ROS;
, ROS;  , GSH.
, GSH.
β-ELE increases Rh-123 intracellular accumulation in A549/DDP cells
The Rh-123 dye is widely used as an indicator of P-gp activity, and intracellular Rh-123 fluorescence intensity reflects the expression level of the P-gp of the cell membranes.18 By this rationale, we used Rh-123 to assess the function of P-gp. After treatment with various concentrations of β-ELE (10, 20, 40 μg/mL) for 24 hours, the retention of Rh-123 in A549/DDP cells was increased by 4.5%, 21.5%, and 34.7%, respectively, in comparison to the medium control (χ2 = 1.3437, P > 0.05; χ2 = 4.1244; χ2 = 2.6437, P < 0.05; χ2 = 4.1244, P < 0.01) (Fig 7), indicating that β-ELE might affect the pumping function, as well as the expression of P-gp.
Figure 7.
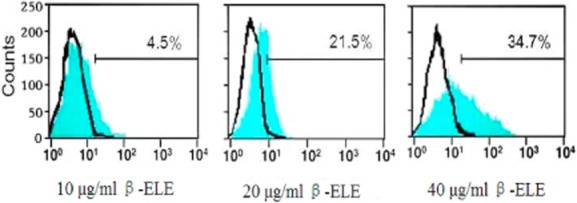
β-elemene (ELE) increases Rhodamine-123 (Rh-123) intracellular accumulation in A549/cisplatin (DDP) cells. The intracellular Rh-123 mean fluorescence intensity is shown as a measure of P-glycoprotein (P-gp) activity after exposure of A549/DDP cells to 10, 20 or 40 μg/mL β-ELE for 24 hours. Values represent averages ± standard deviation (SD) of triplicate wells and are representative of three independent experiments.
β-ELE decreases P-gp expression in A549/DDP cells
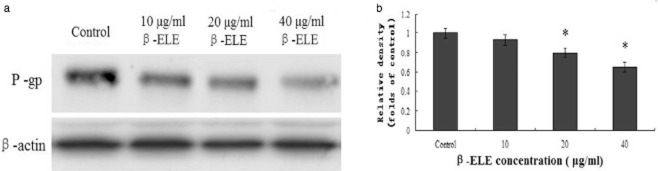
To further demonstrate the effect of β-ELE on the expression of P-gp proteins in A549/DDP cells, Western blotting was performed to detect P-gp protein levels. Results showed that P-gp protein levels were significantly down-regulated in cells incubated with 20 and 40 μg/mL β-ELE compared with the control group (79.47% and 65.28%, P < 0.05) (Fig 8). These results showed that 20 and 40 μg/mL β-ELE could obviously decrease the expression of P-gp relative to reverse drug resistance.
Figure 8.
The levels of P-glycoprotein (P-gp) expression in A549/cisplatin (DDP) cells are modulated by β-elemene (ELE). (a) Representative Western blots are shown for A549/DDP cells exposed to 0, 10, 20 or 40 μg/mL β-ELE for 24 hours, β-actin is shown as a loading control. (b) The mean ± standard deviation SD intensity (grey value) of Western bands were from three independent experiments for the treatments in panel A. Results are standardized to one in the control, untreated cells. *P < 0.05 versus the control group.
Discussion
Inherent or acquired drug resistance is a serious barrier to effective chemotherapy for lung cancer. β-ELE is a new natural compound that has a wide spectrum of antitumor activity. In a previous study, Li et al.19 found that β-ELE not only inhibited cell growth and proliferation, but also increased cisplatin cytotoxicity towards human bladder cancer cells and small-cell lung cancer in vitro. β-ELE-enhanced cisplatin cytotoxicity was associated with increased apoptosis and increased activities of caspase-3, -7, -8, -9, and -10 in bladder cancer cell lines. These results suggested that β-ELE augments the antitumor activity of cisplatin by enhancing the induction of cellular apoptosis via a caspase-dependent mechanism. In our study, we demonstrated that β-ELE, a non-cytotoxic agent, exerts potent reverse effects on drug resistance in drug-resistant human non small-cell lung cancer cell line A549/DDP in vitro through down-regulation of P-gp expression, inhibition of P-gp or multidrug resistance protein (MRP)-dependent drug efflux, and, thus, increases the chemosensitivity to anticancer drugs.
P-gp belong to the adenosine 5'-triphosphate (ATP)-binding cassette superfamily of transport protein, efflux anticancer agents out of cells and, therefore, decrease their intracellular accumulation.20 In this study, we used Western blotting analysis to determine the effects of β-ELE on the protein expression of P-gp. Results revealed that β-ELE significantly down-regulated the expression of P-gp in A549/DDP cells. To further determine the effects of β-ELE on the function of P-gp, we investigated the accumulation of the retention of intracellular Rh-123, an indicator of P-gp function. Results showed that β-ELE dose-dependently enhanced the intracellular Rh-123 retention in A549/DDP cells. This suggested β-ELE could effectively inhibit P-gp-mediated Rh-123 efflux, and enhance intracellular anti-cancer drug accumulation in drug-resistant cancer cells.
We know that the mitochondria are the apoptosis control center.21,22 We used the JC-1 fluorescent probe detection method to assess changes in the mitochondrial membrane potential following β-ELE treatment. Our results suggest that β-ELE causes pyknosis, cell rupture, and outflow of contents. Further analysis suggests that the effects are time- and dose-dependent and are accompanied by a decrease in the membrane potential. Decline in the membrane potential is a hallmark of apoptosis initiation and is indicative of membrane damage. Therefore, these results indicate that β-ELE reverses drug resistance in A549/DDP cells by inducing damage to the mitochondrial membrane and decreasing the membrane potential.
Mitochondria are the “energy processing plan” of biology. Failure or inhibition of the respiratory electron transport chain is likely to lead to increased mitochondrial ROS, an activated oxygen free radical that attacks the mitochondrial membrane and decreases mitochondrial potential, resulting in increased permeability.23 We demonstrated that the decreased mitochondrial membrane potential in A549/DDP cells was accompanied by increased ROS, suggesting a pathway of apoptosis induced by β-ELE. We also observed a decrease in intracellular GST contents. GST is the scavenger of oxygen free radicals that removes intracellular ROS, which accumulates during cell injury.24Therefore, our results indicate that β-ELE reverses the drug resistance of A549/DDP cells through increased ROS contents in cells, which leads to a reduction of intracellular GSH contents. These intracellular mediators, in turn, cause further aggravation of the damage to mitochondrial membrane, resulting in a further progression towards apoptosis. This cycle is consistent with the results of Yang et al.25 who showed that the application of fucoidan to the hepatocellular carcinoma cell line SMMC-7721 activates an increase in ROS, a decrease in GSH, and mitochondrial membrane depolarization.
Acknowledgments
This research was supported by the Fujian Provincial Natural Science Foundation, China (No. 2010D014).
Conclusion
In summary, our results demonstrate that β-ELE reverses A549/DDP cell drug resistance, possibly involves decreased mitochondrial membrane potential and P-gp expression, activates intracellular redox system and induces cells apoptosis leading to reverse drug resistance.
Disclosure
No authors report any conflict of interest.
References
- Cicenas S, Zaliene A, Atkocius V. Treatment outcome of locally advanced stage IIIA/B lung cancer] Medicina (Kaunas) 2009;45:452–459. (In Lithuanian.) [PubMed] [Google Scholar]
- Szkorupa M, Klein J, Bohanes T, Neoral C, Kolek V, Grygárková I. Neoadjuvant chemotherapy and surgical treatment in advanced stages of non-small cell lung cancer] Rozhl Chir. 2011;90:433–439. (In Czech.) [PubMed] [Google Scholar]
- Spásová I. The position of neoadjuvant chemotherapy in the treatment of non-small-cell lung carcinoma] Vnitr Lek. 2007;53:715–723. (In Czech.) [PubMed] [Google Scholar]
- Lee Y, Kim HY, Lee SH, et al. Clinical significance of heterogeneity in response to retreatment with epidermal growth factor receptor tyrosine kinase inhibitors in patients with lung cancer acquiring secondary resistance to the drug. Clin Lung Cancer. 2014;15:145–151. doi: 10.1016/j.cllc.2013.11.008. [DOI] [PubMed] [Google Scholar]
- Goldberg SB, Oxnard GR, Digumarthy S, et al. Chemotherapy with erlotinib or chemotherapy alone in advanced non-small cell lung cancer with acquired resistance to EGFR tyrosine kinase inhibitors. Oncologist. 2013;18:1214–1220. doi: 10.1634/theoncologist.2013-0168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju J, Fu C, Ma L. Modern research and clinical application of β-elemene. Qi Lu Yi Yao. 2008;27:546–548. (In Chinese.) [Google Scholar]
- Guo HQ, Zhang GN, Wang YJ, et al. β-elemene, a compound derived from Rhizoma zedoariae, reverses multidrug resistance mediated by the ABCB1 transporter. Oncol Rep. 2014;31:858–866. doi: 10.3892/or.2013.2870. [DOI] [PubMed] [Google Scholar]
- Hao S, Zhang J, Zhang T. A study of Elemene Emulsion treated advanced non small cell lung cancer on platinum based chemotherapy. Lin Chuan Hui Cui. 2012;27:1529–1531. (In Chinese.) [Google Scholar]
- Zhao C, Zhang Y, Sun Y, Jia Y. A study of elemene injection combined with interventional chemotherapy treated primary liver cancer. Chin J Diffic and Compl Cas. 2012;11:882–883. (In Chinese.) [Google Scholar]
- Cheng H, Yang Z, Zhang M, Cheng Z. Clinical observation of elemene combined with paclitaxel/tegafur in the treatment of advanced esophageal carcinoma. An Hui Yi Yao Xue Za Zhi. 2012;16:1679–1681. (In Chinese.) [Google Scholar]
- Ren W, Du S. Different effect of elemene and BCG on preventing the recurrence of postoperative superficial bladder. Shan Xi Yi Xue Za Zhi. 2012;41:1151–1152. (In Chinese.) [Google Scholar]
- Li QQ, Lee RX, Liang HS, Zhong Y, Reed E. Enhancement of cisplatin-induced apoptosis by β-elemene resistant human ovarian cancer cells. Med Oncol. 2013;30(1):424–444. doi: 10.1007/s12032-012-0424-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Mu X-D, Li E-Z, et al. The role of E3 ubiquitin ligase Cbl proteins in β -elemene reversing multi-drug resistance of human gastric adenocarcinoma cells. Int J Mol Sci. 2013;14:10075–10089. doi: 10.3390/ijms140510075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miedlich SU, Zalutskaya A, Zhu ED, Demay MB. Phosphate-induced apoptosis of hypertrophic chondrocytes is associated with a decrease in mitochondrial membrane potential and is dependent upon Erk1/2 phosphorylation. J Biol Chem. 2010;285:18270–18275. doi: 10.1074/jbc.M109.098616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong J, Samudio I, Chintharlapalli S, Safe S. 1,1-bis(3'-indolyl)-1-(p-substituted phenyl) methanes decrease mitochondrial membrane potential and induce apoptosis in endometrial and other cancer cell lines. Mol Carcinog. 2008;47:492–507. doi: 10.1002/mc.20407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo L, Motherwell MS. The impact of reactive oxygen species and genetic mitochondrial mutations in Parkinson's disease. Gene. 2013;532:18–23. doi: 10.1016/j.gene.2013.07.085. [DOI] [PubMed] [Google Scholar]
- Circu ML, Aw TY. Glutathione and modulation of cell apoptosis. Biochim Biophys Acta. 2012;1823:1767–1777. doi: 10.1016/j.bbamcr.2012.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv J, Tian Y. Effect of Src tyrosine kinase inhibition on the drug-resistance as well as MDR1 and LRP expression of the human cis-platinum-resistant lung cancer cell line A549/DDP] Zhong Guo Fei Ai Za Zhi. 2012;15:501–506. doi: 10.3779/j.issn.1009-3419.2012.09.01. (In Chinese.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li QQ, Lee RX, Liang H, et al. β-Elemene enhances susceptibility to cisplatin in resistant ovarian carcinoma cells via downregulation of ERCC-1 and XIAP and inactivation of JNK. Int J Oncol. 2013;43:721–728. doi: 10.3892/ijo.2013.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T, Oka M, Aizawa K, et al. Direct interaction between a quinoline derivative, MS-209, and multidrug resistance protein (MRP) in human gastric cancer cells. Biochem Biophys Res Commun. 1999;255:618–624. doi: 10.1006/bbrc.1999.0245. [DOI] [PubMed] [Google Scholar]
- Tait JF. Imaging of apoptosis. J Nucl Med. 2008;49:1573–1576. doi: 10.2967/jnumed.108.052803. [DOI] [PubMed] [Google Scholar]
- Radović N, Cucić S, Altarac S. Molecular aspects of apoptosis] Acta Med Croatica. 2008;62:249–256. (In Croatian.) [PubMed] [Google Scholar]
- Prosperini A, Juan-García A, Font G, Ruiz MJ. Beauvericin-induced cytotoxicity via ROS production and mitochondrial damage in Caco-2 cells [J] Toxicol Lett. 2013;222:204–211. doi: 10.1016/j.toxlet.2013.07.005. [DOI] [PubMed] [Google Scholar]
- Yan F, Yang WK, Li XY, et al. A trifunctional enzyme with glutathione S-transferase, glutathione peroxidase and superoxide dismutase activity. Biochim Biophys Acta. 2008;1780:869–872. doi: 10.1016/j.bbagen.2008.03.003. [DOI] [PubMed] [Google Scholar]
- Yang L, Wang P, Wang H, et al. Fucoidan derived from Undaria pinnatifida induces apoptosis in human hepatocellular carcinoma SMMC-7721 cells via the ROS-mediated mitochondrial pathway. Mar Drugs. 2013;11:1961–1976. doi: 10.3390/md11061961. [DOI] [PMC free article] [PubMed] [Google Scholar]