Abstract
Background
Antiangiogenesis plays a key role in the treatment of non-small lung cancer (NSCLC). We observed the cavitation of lesions in patients with stage IIIB/IV NSCLC treated with Endostar and vinorelbine-cisplatin (NP) chemotherapy, and evaluated the imaging characteristics and clinical outcome of patients who developed tumor cavitation.
Methods
Our study included 105 untreated NSCLC patients who received Endostar in combination with NP chemotherapy at the Tianjin Lung Cancer Center. Chest computed tomography (CT) was performed to evaluate the efficacy every two cycles. The number of activated circulating endothelial cells (aCECs) was measured by flow cytometry. Rates of tumor cavitation were documented and their clinical CT imaging data were analyzed.
Results
Tumor cavitation occurred in 11 of the 105 (10.5%) patients treated with Endostar and NP. The response rates were 37.2% (35/94) in patients without cavitation, 27.3% (3/11) evaluated by Response Evaluation Criteria in Solid Tumors, and 100.0% (11/11) if evaluated by an alternate method in patients who developed cavitation. Three of the 11 cases with cavitation had a centrally located tumor. No patients had hemoptysis or any other severe side effects. Compared with patients not developing cavitation, cavity formation resulted in a longer median survival time (13.6 vs. 11.8 months, P = 0.011) and an increase in the number of aCECs (244.4/105 vs. 23.3/105, P = 0.000).
Conclusions
Intratumoral cavitation induced by Endostar is common in NSCLC patients, and is not correlated with squamous histology, tumor location or pulmonary hemorrhage. Cavitation might have a significant effect on the number of aCECs and overall prognosis.
Keywords: Activated circulating endothelial cells, angiogenesis, cavitation, Endostar, non-small cell lung cancer
Introduction
Lung cancer is the most frequently diagnosed cancer in developed nations.1 More than 80% of patients are diagnosed with non-small cell lung cancers (NSCLCs), which usually present as incurable locally advanced or metastatic disease.1 For such patients, a platinum-based doublet is the standard first line therapy, which results in median survival times of eight to 10 months.2 Recent investigation to improve treatment for NSCLC has focused on targeted therapies, including angiogenesis inhibitors.3 Bevacizumab, a monoclonal antibody to vascular endothelial growth factor (VEGF), has demonstrated efficacy (improved response rates [RRs] and overall survival [OS]) in phase II and III trials in combination with standard first-line chemotherapy in NSCLC.4,5
In 1997, Folkman et al. first identified endostatin, another antiangiogenesis agent, in the conditioned media of hemangioendothelioma cells as an antiangiogenic molecule.6 Animal studies demonstrated that endostatin strongly inhibited the growth of a variety of murine and xenotransplanted human tumors by suppressing the neovascularization. Thus,it was quickly pushed into clinical trials.7 It was apparently unsuccessful in clinical trials, however, because of problems with the technique for recombinant human endostatin production.8 Reduced solubility of the recombinant endostatin prepared from Escherichia coli led to failure in clinical therapy. A soluble form of endostatin is available from a yeast system that has a relatively low yield and high cost, which has made it difficult to produce endostatin in quantities sufficient for extensive clinical evaluation.9
Endostar is a recombinant humanized endostatin purified in an Escherichia coli system.10 Compared with endostatin, Endostar has an additional nine amino acid sequence (MGGSHHHHH) added to the N-terminal of the protein, which results in the formation of a six-histidine tag and enhancement of affinity with metal ions.11 These changes have simplified the purification and improved the stability of the protein. It is reported that Endostar had a potent effect in animal tumor models, and the half-life of Endostar was longer than endostatin.12 Preclinical data suggest that Endostar inhibits the migration of endothelial cells for angiogenesis, suppresses the formation of new tumor blood vessels, obstructs the nutrition supply for tumor cells, and, thus, inhibits the proliferation or metastasis of tumors.13
A randomly controlled, double blind and multi-center Phase III clinical trial was conducted on the combined administration of Endostar and the vinorelbine-cisplatin (NP) regimen in 493 stage IIIB/IV NSCLC patients.14,15 The results showed that the addition of Endostar to the NP regimen resulted in a significant improvement in the RR (35.4% vs. 19.5%, P = 0.0003), median time to tumor progression (6.3 vs. 3.6 months, P < 0.001), and clinical benefit rate (76.5% vs. 65.0%, P = 0.023) compared with NP alone in advanced NSCLC patients. Based on systemic preclinical and clinical studies, Endostar was approved by the State Food and Drug Administration (SFDA) in China for the treatment of NSCLC in September 2005.16
The current criteria for evaluating antiangiogenic efficacy are insufficient as tumor shrinkage occurs after blood perfusion decreases. Circulating endothelial cells (CECs), which mainly consist of endothelial progenitor cells (EPC) from the bone marrow and endothelial cells shed from the walls of blood vessels, are rarely found in the blood of a healthy body, but increase dramatically during tumor progression where they play an essential role in tumor angiogenesis. Circulating endothelial cells also decrease significantly after effective chemotherapy or tumor resection. Apparently, changes in CEC levels could reflect development and suppression of angiogenesis.
We observed tumor cavitation in a patient treated with Endostar and chemotherapy in a phase III clinical tria1 in 2004. More patients with cavitation of pulmonary lesions after antiangiogenic therapy have been noted in recent years. The purpose of this retrospective study is to evaluate the frequency, imaging characteristics, and clinical data of patients receiving Endostar and NP chemotherapy who developed tumor cavitation, and correlate these findings with the change of activated circulating endothelial cells (aCECs) and overall prognosis.
Patients and methods
Patient selection and treatment
One hundred and five patients with histologically proven NSCLC who were treated with Endostar in combination with NP chemotherapy at the Tianjin Lung Cancer Center between January 2007 and January 2012 were considered for our retrospective study. All patients in our study were proven stage IIIB or IV NSCLC who had never received antiangiogenic therapy or any other treatment including chemotherapy or radiotherapy, with a Karnofsky performance status ≥70% or an Eastern Cooperative Oncology Group performance status of 0 to 1, with stable hepatic, hematologic, and renal functions. Pathologic type and tumor location were not exclusion criteria. Patients with cardiac disease or hemorrhagic disease were excluded. In this study, patients received 25 mg/m2 vinorelbine on days one and eight, 75 mg/m2 cisplatin on day one, and 7.5 mg/m2 Endostar from day one to day 14, every 21 days (generally 4–6 treatment cycles). All of the agents were administered intravenously. All patients were treated at a single center (Tianjin Lung Cancer Center) and had complete clinical data, including chest computed tomography (CT), performed every two cycles of treatment. The number of aCECs was measured by flow cytometry (FCM) at the same time. Our lung cancer center approved our retrospective study, and this study was in compliance with the Health Insurance Portability and Accountability Act regulations.
Clinical assessments
Clinical data were retrieved from the database system at the Tianjin Lung Cancer Center, Tianjin Cancer Institute & Hospital. Information obtained included: patient's age, gender, stage of disease, histology of lung cancer, clinical response, adverse events, total number of treatment cycles, progression-free survival (PFS) and survival time. Information about drug toxicity was based on the original investigator report and was graded according to the National Cancer Institute Common Toxicity Criteria. Evaluation of the oncologic clinical response was based on two methods. One was the conventional method for response assessment, Response Evaluation Criteria in Solid Tumors (RECIST).17 The other was an alternate, modified method introduced by Crabb et al., especially used in tumor cavitation after antiangiogenic therapy.18 They concluded that incorporating an assessment of cavitation when measuring target lesions might more accurately reflect changes in tumor volume. It was used for target lesions in which the longest diameter of any cavitation (zero if no cavity present) was subtracted from the longest total diameter of the lesion, with each measurement taken in the same plane, to provide an alternate measure that was used to calculate the sum of measurements for all target lesions. All other details regarding the assessment of non-target lesions for this alternate method were identical to RECIST.
Radiologic evaluation
All patients included in the study had chest CT 14 days before starting Endostar and NP chemotherapy. The CT section thickness used was 10 mm. Follow-up scans were performed every 42 days. A full retrospective radiology review was performed by one of two radiologists blinded to clinical details and outcome. Response evaluation was performed first by RECIST. Patients with tumor cavitation after treatment also received response assessment according to the alternate method described by Crabb et al. Imaging characteristics of the cavity in the tumor, including tumor location, histology, pre- and post-therapeutic longest diameter of tumor, longest diameter of cavity, wall of cavity, and distribution of the cavity were recorded.
Sample assay for activated circulating endothelial cells (aCECs)
Blood samples were obtained two days before the beginning of each therapeutic cycle and the last blood sample was collected on the eighth day after completion of the last cycle. All blood samples were anticoagulated with ethylenediamine tetraacetic acid (EDTA), stored at 4°C and processed within 36 hours after collection.
FCM was used to determine aCECs (CD45−,CD146+,CD105+). One hundred μl of blood specimens were anti-coagulated with EDTA. All antibodies were purchased from Becton Dickinson (USA) except for CD105, which was purchased from Chemicon (USA). The samples were incubated for 30 minutes in the dark with 10 μL of the following basic combinations of fluorescein isothiocyanate (FITC), phycoerythrin (PE) and PE-Cy5 antibodies: CD45-PE-Cy5, CD146-PE, CD105-FITC and isotype control IgG1 from mice. After incubation, red blood cells were lysed with lysing solution (purchased from Beckman Coulter, USA) for 20 minutes in the dark and then washed three times in phosphate-buffered saline (PBS) by centrifugation. Using the forward-scatter/side-scatter (FS/SS) gating strategy, the acquisition was performed by FCM (Beckman Coulter, EPICS-XL) equipped with a 488 nm argon-ion laser. A minimum of 100 000 events was collected for each sample. The data of each sample was analyzed by Software-System II (Beckman Coulter).
Statistical analysis
Continuous variables were given as mean ± standard deviation (SD) and categorical variables as absolute numbers. Descriptive statistics were used to summarize the baseline and adverse events of patients. Statistical differences between the two groups were assessed with the t test for continuous variables (e.g. comparison of the number of aCECs between the with and without cavitation groups) and the chi-square and Fisher's exact test for categorical variables (e.g. RR between the with and without cavitation groups). A log-rank test was used to compare the PFS and OS in the two groups. All tests were two-sided. A P value < 0.05 was considered statistically significant. The statistical analysis was performed using SPSS 16.0 software package.
Results
Patient characteristics
From January 2007 to January 2012, 105 patients with advanced NSCLC received Endostar combined with NP chemotherapy as first line therapy at the Tianjin Lung Cancer Center. Our analysis included 57 men and 48 women, with a mean age of 59.4 ± 8.7 years (range, 31–74 years), who received at least two cycles of Endostar and NP chemotherapy. All patients were followed up from starting treatment for seven to 24 months with a median of 14 months. The majority of patients had stage IV disease (n = 91, 86.7%). There were 45 adenocarcinoma (42.9%), 40 squamous carcinoma (38.1%), seven large-cell carcinoma (6.7%), eight sarcomatoid carcinoma (7.6%) and five adenosquamous carcinoma (4.7%). Grade I/IV neutropenia occurred in 54 patients (51.4%), and grade I/IV thrombocytopenia occurred in 15 patients (14.3%). Mild hypertension occurred in 19 patients (18.1%), and grade I/II adverse cardiologic reactions, including myocardial ischemia and atrial premature beat, occurred in 14 patients (13.3%). Forty-seven patients (44.7%) had grade I/II nausea. No patients had hemoptysis or any other severe side effects. The gender, tumor stage, histology, and adverse events of the patients' with/without tumor cavitation in this study are listed in detail in Table 1.
Table 1.
Baseline and adverse events of non-small cell lung cancer patients receiving Endostar and vinorelbine-cisplatin
| Characteristics | No. Cavitation n (%) | Cavitation n (%) | Cavitation/ Total (%) |
|---|---|---|---|
| Gender | |||
| Male | 55 (58.5) | 6 (54.5) | 9.8 |
| Female | 39 (41.5) | 5 (45.5) | 11.4 |
| Tumor histology | |||
| Adenocarcinoma | 40 (42.6) | 5 (45.5) | 11.1 |
| Squamous carcinoma | 37 (39.4) | 3 (27.3) | 7.5 |
| Other | 17 (18.1) | 3 (27.3) | 15.0 |
| Tumor stage | |||
| IIIB | 12 (12.8) | 2 (18.2) | 14.2 |
| IV | 82 (87.2) | 9 (81.8) | 9.9 |
| Adverse events | |||
| Hypertension | 16 (17.0) | 3 (27.3) | 15.8 |
| Cardiac disease | 11 (11.7) | 3 (27.3) | 21.4 |
| Pulmonary bleedings | 0 | 0 | 0 |
| Nausea/vomiting | 41 (43.6) | 6 (54.5) | 12.8 |
| Gastrointestinal bleedings | 0 | 0 | 0 |
| Deep vein thrombosis | 5 (5.3) | 1 (9.1) | 16.7 |
| Fatigue | 42 (44.7) | 6 (54.5) | 12.5 |
| Total number | 94 | 11 | 10.5 |
Tumor cavitation and clinical, radiologic data
No cavity was detected in any of the patients at the start of treatment. Pulmonary cavitation occurred in 11 cases of 105 in Endostar plus NP chemotherapy (10.5%). The median time from the beginning of therapy to the development of cavitation was 7.6 ± 2.8 weeks: eight cases (72.7%) after six weeks and three cases (27.3%) after 12 weeks. Among 94 patients who did not develop cavitation, 35 (37.2%) experienced partial response (PR) (no complete response [CR]). Among 11 patients who developed cavitation, only three (27.3%) experienced PR if evaluated by RECIST. However, all of them achieved PR (100%) if evaluated by the alternate method described by Crabb et al. (Table 2). There was also a significant difference in RR (evaluated by alternate method) and median survival time (Table 3). There was no significant difference in PFS (P = 0.068, Table 3).
Table 2.
Response assessment and change of activated circulating endothelial cells of 11 cases of tumor cavitation
| No. | Response by RECIST | Response by Alternate Method | Change of aCECs (/105) | ||
|---|---|---|---|---|---|
| Pre-therapy (X) | Post-therapy (Y) | Difference (X-Y) | |||
| 1 | SD | PR | 261 | 16 | 245 |
| 2 | SD | PR | 781 | 74 | 707 |
| 3 | SD | PR | 150 | 0 | 150 |
| 4 | PR | PR | 133 | 11 | 122 |
| 5 | SD | PR | 291 | 64 | 227 |
| 6 | SD | PR | 361 | 125 | 236 |
| 7 | SD | PR | 485 | 106 | 379 |
| 8 | PR | PR | 653 | 89 | 564 |
| 9 | PR | PR | 315 | 121 | 194 |
| 10 | SD | PR | 295 | 88 | 207 |
| 11 | SD | PR | 264 | 106 | 158 |
aCECs, activated circulating endothelial cells; PR, partial response; RECIST, Response Evaluation Criteria in Solid Tumors; SD, stable disease.
Table 3.
Response assessment change of activated circulating endothelial cells in non-small cell lung cancer patients receiving Endostar and vinorelbine-cisplatin
| Response and aCECs | No Cavitation n (%) | Cavitation n (%) | P |
|---|---|---|---|
| Response assessment† | |||
| Complete response | 0 | 0 | |
| Partial response | 35 (37.2) | 11 (100) | 0.008 |
| Stable | 39 (41.5) | 0 | |
| Progression | 20 (21.3) | 0 | |
| Progression-free survival† (median, months) | 4.2 | 4.5 | 0.068 |
| Overall survival time (median, months) | 11.8 ± 0.2 | 13.6 ± 0.8 | 0.011 |
| Change of aCECs | |||
| Pre-therapy (X,/105) | 367.6 ± 170.2 | 362.6 ± 201.1 | 0.757 |
| Post-therapy (Y,/105) | 344.3 ± 149.3 | 118.2 ± 96.3 | 0.000 |
| Difference (X-Y,/105) | 23.3 ± 127.9 | 244.4 ± 122.7 | 0.000 |
Objective response and progression-free survival was assessed by alternate method. aCECs, activated circulating endothelial cells.
CT showed that eight of these 11 cases had a peripherally located tumor (involvement in segmental and subsegmental airways); only three of them had a centrally located tumor (involvement in main bronchus and lobar bronchi). There was only one cavity in each case and most of the pulmonary cavities of the tumor were roundish with an average diameter of 3.0 ± 1.1 cm. There were six cavities with thin and five with thick walls. Eight cavities were situated in the center of the tumor and three were eccentric. Of the 11 patients, there were six men and five women; five with adenocarcinoma, three with squamous carcinoma, one with large-cell carcinoma, one with adenosquamous carcinoma, and one with sarcomatoid carcinoma. Imaging characteristics are listed in Table 4 and shown in Figure 1.
Table 4.
Imaging characteristics of 11 cases of tumor cavitation induced by Endostar and vinorelbine-cisplatin
| Case No. | Tumor location | Histology | Longest diameter of tumor | Longest diameter of cavity (Y, cm) | Longest diameter of active tumor (Z = X-Y, cm) | Percentage of tumor loss (A –Z/A, %) | Wall of cavity† | Distribution of cavity | |
|---|---|---|---|---|---|---|---|---|---|
| Pre- therapy (A, cm) | Post-therapy (X, cm) | ||||||||
| 1 | Superior lobe of left lung (Peripheral) | Adenocarcinoma | 6.6 | 6.4 | 4.1 | 2.3 | 65.2 | Thick | Decentered |
| 2 | Superior lobe of right lung (Peripheral) | Adenosquamous carcinoma | 1.9 | 1.9 | 1.7 | 0.2 | 89.5 | Thin | Centered |
| 3 | Inferior lobe of right lung (Peripheral) | Adenocarcinoma | 2.2 | 2.0 | 1.9 | 0.1 | 95.5 | Thin | Centered |
| 4 | Superior lobe of right lung (Peripheral) | Sarcomatoid carcinoma | 4.2 | 2.8 | 1.5 | 1.3 | 69.0 | Thick | Centered |
| 5 | Inferior lobe of left lung (Peripheral) | Adenocarcinoma | 4.8 | 4.5 | 4.3 | 0.2 | 95.8 | Thin | Centered |
| 6 | Right lung (Central) | Squamous carcinoma | 5.1 | 4.8 | 3.9 | 0.7 | 86.3 | Thick | Centered |
| 7 | Left lung (Central) | Squamous carcinoma | 6.0 | 5.2 | 4.1 | 1.1 | 81.7 | Thick | Decentered |
| 8 | Inferior lobe of left lung (Peripheral) | Large-cell carcinoma | 3.9 | 2.6 | 2.3 | 0.3 | 92.3 | Thin | Centered |
| 9 | Superior lobe of right lung (Peripheral) | Adenocarcinoma | 4.5 | 2.9 | 2.5 | 0.4 | 91.1 | Thin | Centered |
| 10 | Inferior lobe of Left lung (Central) | Squamous carcinoma | 6.0 | 5.6 | 3.5 | 2.1 | 65.0 | Thick | Decentered |
| 11 | Inferior lobe of right lung (Peripheral) | Adenocarcinoma | 4.0 | 3.4 | 3.1 | 0.3 | 92.5 | Thin | Centered |
Thick wall is defined as ≥5 mm in the diameter of wall of cavity, with thin wall defined as <5 mm.
Figure 1.
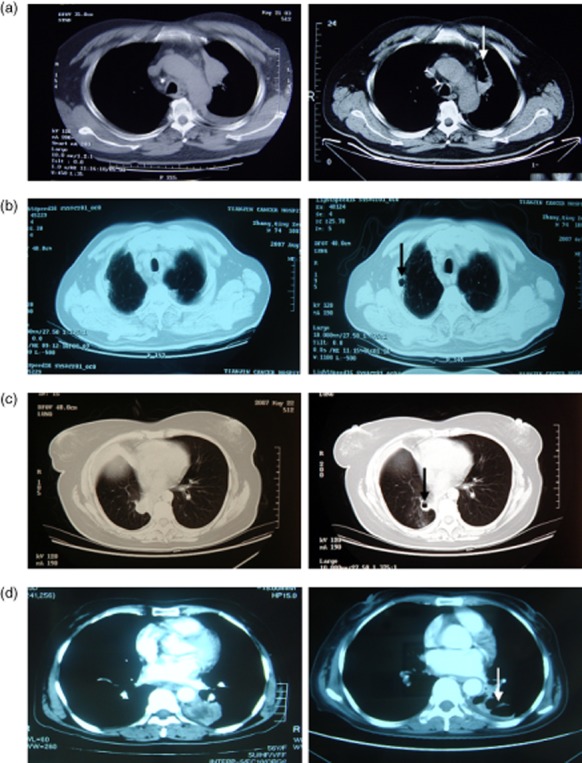
Tumor cavitation of four patients. (a) Case 1 – a 54-year old man with lung adenocarcinoma. Pulmonary cavitation in the left superior lobe mass near the aortic arch occurred after two cycles of Endostar and vinorelbine-cisplatin chemotherapy. The patient died of progressive disease (PD) 13.6 months after initiation of treatment. (b) Case 2 – a 74-year old man with lung adenosquamous carcinoma. Pulmonary cavitation in the right superior lobe mass near the chest wall occurred after two cycles of therapy. The patient died of PD 14.5 months after initiation of treatment. (c) Case 3 – a 44-year old woman with lung adenocarcinoma. Pulmonary cavitation in the right inferior lobe mass occurred after two cycles of therapy. The patient survived for 38 months after the initiation of treatment. (d) Case 4 – a 56-year old woman with lung adenocarcinoma. Pulmonary cavitation in the left inferior lobe mass near the aorta descendens occurred after two cycles of therapy. The patient died of PD 13.5 months after initiation of treatment.
Tumor cavitation and change of aCECs
In general, the number of aCECs had a tendency to decrease from 366.1/105 to 307.2/105 (P = 0.059) after treatment with Endostar and NP in this study. It decreased especially in patients achieving PR or stable disease (SD) (from 389.2/105 to 294.5/105, P = 0.010). In patients with progressive disease (PD), no significant difference in the number of aCECs was found (from 305.7/105 to 371.1/105, P = 0.257). The number of aCECs decreased remarkably in 11 patients with tumor cavitation after treatment (P = 0.001). There was no difference in pre-therapy aCECs between the groups with and without cavitation (P = 0.757). On the contrary, a significant difference was found in post-therapy aCECs (P = 0.000), and in the number of aCECs (pre-therapeutic amount minus post-therapeutic amount) (P = 0.000) between them. The numbers of aCECs in detail are shown in Figure 2 and Table 3.
Figure 2.
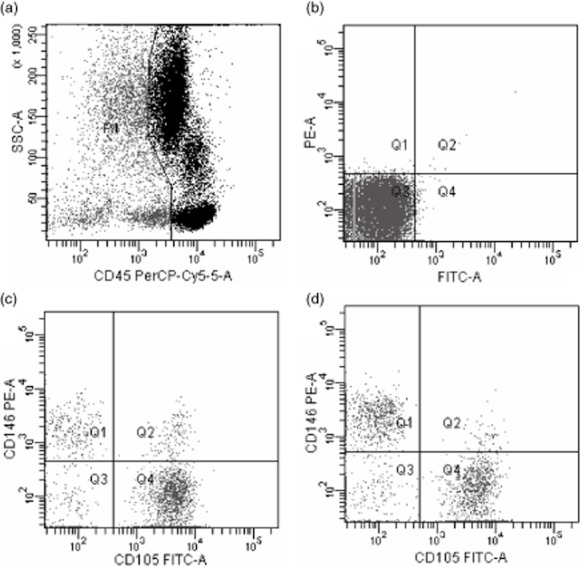
Circulating endothelial cell enumeration by flow cytometry. (a) Panels show the gate used to exclude CD45-positive cells; (b) Negative control; (c) Panels show the gate used to count activated circulating endothelial cells (aCECs) (defined as CD45− CD146+ CD105+) pre-therapeutically; (d) Panels show the gate used to count aCECs (defined as CD45- CD146+ CD105+) post-therapeutically.
Discussion
The presence of an abnormal hollow space within the lung parenchyma, either partially filled with fluid or vacant, is defined as a lung cavity. Cavitation in the pulmonary parenchyma can be caused by a wide variety of pathologic conditions, such as pneumonia, tuberculosis, and cancer.19 As patients with advanced NSCLC have a median survival of approximately 10 months when treated with traditional platinum-based therapy, inhibition of tumor-related angiogenesis has become an attractive target for anticancer therapy. Marom et al. first elaborated in their study on the development of tumor cavitation after treatment of antiangiogenesis agents in 10 different clinical trials.20 In our study, we analyzed the clinical and imaging data of 11 patients who developed tumor cavitation caused by Endostar and NP chemotherapy. Endostar, a kind of recombined humanized endostatin and an endothelial cell growth inhibitor with independent intellectual property rights in China, was categorized to novel antiangiogenesis drugs.21,22 Multi-center clinical trials have proved that combinative use of Endostar and chemotherapy can raise the RR and TTP (time to progression) of advanced NSCLC.14,15 To our knowledge, no previous reports on pulmonary cavitation caused by Endostar have been published. Our analysis of 105 advanced NSCLC patients treated with Endostar and NP chemotherapy showed that 11 patients developed pulmonary cavitation (10.5%). Both their objective RR and median survival time appeared to be superior to those of patients who did not develop cavitation, which did not match Marom et al's results.20
Bevacizumab, a monoclonal antibody with a high affinity for VEGF, is the most studied antiangiogenesis agent in patients with NSCLC.23,24 In a randomized phase II trial, a higher incidence of bleeding was noted in the bevacizumab-treated patients.4 Severe pulmonary hemorrhage, which was observed in six patients (9.1%) and led to four fatalities, was associated with squamous cell histology, tumor cavitation, central tumors, and disease location close to major blood vessels. In a phase III trial (E4599), there was also a significantly higher incidence of hematologic toxicities, febrile neutropenia, hemorrhage, hypertension, and proteinuria for bevacizumab-treated patients.5 Thus, advanced NSCLC patients with squamous histology are not eligible for bevacizumab treatment.
During phase I-III clinical trials, Endostar was administered to 470 advanced patients.15,25 Frequent adverse reactions (1–10%) mainly occurred in the heart, and rare adverse reactions (0.1–1%) occurred mainly in the digestive system and in skin/annexa allergies. Thirty patients (6.38%) had degree I/II or mild/moderate adverse cardiologic reactions – mainly myocardial ischemia within two to seven days of administration of the Endostar – but these symptoms did not pose a danger to the life of the patients. In patients with previous coronary heart disease and hypertension, Endostar can induce mild ST-T change, atrioventricular conduction blocking, atrial premature beat, and rare ventricular premature beat. Most adverse reactions affecting the digestive system are reversible, and mild cases do not require symptomatic treatment. No death related to adverse reactions was observed in this multi-center trial of 470 Endostar treated patients.
In view of the results above, patients with cardiac or hemorrhagic disease were excluded from treatment with Endostar. However, pathologic type and tumor location were not exclusion criteria. Forty (38.1%) patients with squamous carcinoma were treated in our study. Eight of them had centrally located tumors with infiltration of central blood vessels prior to starting the treatment, but none developed tumor cavitation or pulmonary bleeding. Fortunately, no pulmonary hemorrhage occurred in any of the 11 patients with cavitation in our study. Interestingly, our study showed that only three squamous cell lung cancer patients developed pulmonary cavitation and another eight patients had no squamous carcinoma, central tumor or tumor located near major blood vessels. The difference in the frequency of hemorrhage between bevacizumab and Endostar has remained unclear so far.
We also investigated 98 NSCLC patients treated with NP chemotherapy alone in our lung cancer center from January 2007 to January 2012. As a concurrent control, none of them developed tumor cavitation during treatment. According to our experience, very few patients with lung cancer will develop tumor cavitation after chemotherapy alone. With the introduction of more antiangiogenesis agents into clinical practice in recent years, frequency in the development of tumor cavitation has tended to increase.18,20 However, how to assess tumor response in patients with tumor cavitation is not clear. In our study, three patients attained PR and eight patients attained SD according to RECIST, whereas all of them achieved PR if assessed by the Crabb et al. method. We think that RECIST, widely used in response assessment of conventional cytotoxic agents, has limitations with angiogenesis inhibitors, especially when cavitation occurs. One important reason is that RECIST, which focuses on the change in the sum of the longest diameter of target lesions, does not seem to adequately describe change in tumor tissue volume if cavitation is present. The alternate method introduced by Crabb et al. is more accurate. In this study, cavitation patients had a longer survival time than non-cavitation patients. We believe that the main reason for this is that all cavitation patients (100%) achieved PR, while only 35 non-cavitation patients (36.5%) achieved PR (all evaluated by the alternate method).
CEC plays an important role in angiogenesis.26–29 Mancuso et al. reported that resting and activated endothelial cells are increased in the peripheral blood of cancer patients.30 Beerepoot et al. found that increased levels of viable circulating endothelial cells are an indicator of progressive disease in cancer patients.31 CECs are recognized as executive endothelial cells of capillaries in normal tissue, and endothelial progenitor cells (EPCs) in marrow running into peripheral circulation after activation by cytokines secreted by tumor cells. They are termed as “aCECs” for their ability to migrate, proliferate, and form neo-vessels around tumors. In our study, we chose CD45-CD146+CD105+ to mark aCECs (Fig. 1). This is because CD146 is regarded to characterize most endothelial cells,30 expressing on almost all endothelial cells in circulating blood, including EPCs.32 CD105 is considered to be a good marker of aCECs,30,33 with CD45 used to eliminate the confounding of monocytes (CD45+) in blood. Our study revealed that, in general, the amount of aCECs tended to decrease after treatment with Endostar and NP (P = 0.059). It particularly decreased in patients achieving clinical benefit (P = 0.010). No significant difference was found in the change of aCECs in patients with PD (P = 0.257). A potential link between change of aCECs and tumor cavitation has not been reported. Our data indicated that patients who developed tumor cavitation after antiangiogenesis and chemotherapy experienced a remarkable decrease in the number of aCECs, from 362.6/105 to 118.2/105, which was much higher than that of patients without tumor cavitation after treatment (P = 0.000). Specifically, the amount of aCECs was reduced from 150.0/105 to 0/105 (undetectable level by FCM) in one patient with a significant cavitation whose OS time amounted to 38 months. We hypothesized that tumor cavitation in combination with the decrease in the number of aCECs indicated a good prognosis of NSCLC treated with antiangiogenesis.
Our study has some limitations. It is based on the retrospective analysis of patients from a single-institution. In addition, Endostar, a novel recombinant human endostatin, has not been widely used outside China. However, Endostar might exert antiangiogenic effects via a similar mechanism with endostatin, and moreover, Folkman et al. found that Endostar is at least twice as potent as endostatin in animal tumor models.12 There are homogeneous standards in patient baseline conditions, therapeutic agents, and post study therapy.
Acknowledgments
We thank Xin Cheng, Zhaoxiang Ye for their assistance in conducting this study and Xi-Yin Wei for assistance with the measurement of aCECs by flow cytometry. This work was supported by a grant from the National Natural Science Foundation of China (no. 81372467).
Conclusion
In conclusion, our data suggest that pulmonary cavitation of a tumor is one of the particular imaging characteristics found in NSCLC patients after treatment with Endostar and NP chemotherapy, seen in 10.5% of patients. We did not find any clear link between pulmonary hemorrhage and tumor cavitation. Cavitation induced by Endostar is not correlated with squamous histology or tumor location. The alternate method described by Crabb et al. is more reasonable than conventional RECIST in the response assessment of tumors with cavitation after antiangiogenic therapy. Cavitation seems to have a significant effect on the number of aCECs and overall prognosis.
Disclosure
No authors report any conflict of interest.
References
- Reck M, Heigener DF, Mok T, Soria JC, Rabe KF. Management of non-small-cell lung cancer: recent developments. Lancet. 2013;382:709–719. doi: 10.1016/S0140-6736(13)61502-0. [DOI] [PubMed] [Google Scholar]
- Schiller JH, Harrington D, Belani CP, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002;346:92–98. doi: 10.1056/NEJMoa011954. [DOI] [PubMed] [Google Scholar]
- Schmid-Bindert G. Update on antiangiogenic treatment of advanced non-small cell lung cancer (NSCLC) Target Oncol. 2013;8:15–26. doi: 10.1007/s11523-013-0261-1. [DOI] [PubMed] [Google Scholar]
- Johnson DH, Fehrenbacher L, Novotny WF, et al. Randomized phase II trial comparing bevacizumab plus carboplatin and paclitaxel with carboplatin and paclitaxel alone in previously untreated locally advanced or metastatic non-small-cell lung cancer. J Clin Oncol. 2004;22:2184–2191. doi: 10.1200/JCO.2004.11.022. [DOI] [PubMed] [Google Scholar]
- Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small cell lung cancer. N Engl J Med. 2006;355:2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- O'Reilly MS, Boehm T, Shing Y, et al. Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell. 1997;88:277–285. doi: 10.1016/s0092-8674(00)81848-6. [DOI] [PubMed] [Google Scholar]
- Mundhenke C, Thomas JP, Wilding G, et al. Tissue examination to monitor antiangiogenic therapy: a phase I clinical trial with endostatin. Clin Cancer Res. 2001;7:3366–3374. [PubMed] [Google Scholar]
- Herbst RS, Hess KR, Tran HT, et al. Phase I study of recombinant human endostatin in patients with advanced solid tumors. J Clin Oncol. 2002;20:3792–3803. doi: 10.1200/JCO.2002.11.061. [DOI] [PubMed] [Google Scholar]
- Huang X, Wong MK, Zhao Q, et al. Soluble recombinant endostatin purified from Escherichia coli: antiangiogenic activity and antitumor effect. Cancer Res. 2001;61:478–481. [PubMed] [Google Scholar]
- Han Q, Fu Y, Zhou H, He Y, Luo Y. Contributions of Zn (II)-binding to the structural stability of endostatin. FEBS Lett. 2007;581:3027–3032. doi: 10.1016/j.febslet.2007.05.058. [DOI] [PubMed] [Google Scholar]
- Song HF, Liu XW, Zhang HN, et al. Pharmacokinetics of His-tag recombinant human endostatin in Rhesus monkeys. Acta Pharmacol Sin. 2005;26:124–128. doi: 10.1111/j.1745-7254.2005.00009.x. [DOI] [PubMed] [Google Scholar]
- Jia H, Kling J. China offers alternative gateway for experimental drugs. Nat Biotechnol. 2006;24:117–118. doi: 10.1038/nbt0206-117. [DOI] [PubMed] [Google Scholar]
- Rong B, Yang S, Li W, Zhang W, Ming Z. Systematic review and meta-analysis of Endostar (rh-endostatin) combined with chemotherapy versus chemotherapy alone for treating advanced non-small cell lung cancer. World J Surg Oncol. 2012;10:170–181. doi: 10.1186/1477-7819-10-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Wang J, Liu Y, et al. Results of phase III trial of rh-endostatin (YH-16) in advanced non-small cell lung cancer (NSCLC) patients. 2005 Annual ASCO Meeting Proceedings J Clin Oncol. 2005;23(Suppl) .): (Abstract 7134) [Google Scholar]
- Wang JW, Sun Y, Liu YY, et al. Results of randomized, multicenter, double-blind phase III trial of rh-endostatin (YH-16) in treatment of advanced non-small cell lung cancer patients. Chin J Lung Cancer. 2005;8:283–290. doi: 10.3779/j.issn.1009-3419.2005.04.07. (In Chinese.) [DOI] [PubMed] [Google Scholar]
- China Food and Drug Administration. 2005. . [Cited 12 Sep 2008.] Available from URL: http://app1.sfda.gov.cn/datasearch/face3/base.jsp.
- Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors: European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- Crabb SJ, Patsios D, Sauerbrei E, et al. Tumor cavitation: impact on objective response evaluation in trials of angiogenesis inhibitors in non–small-cell lung cancer. J Clin Oncol. 2009;27:404–410. doi: 10.1200/JCO.2008.16.2545. [DOI] [PubMed] [Google Scholar]
- Woodring JH, Fried AM, Chuang VP. Solitary cavities of the lung: diagnostic implications of cavity wall thickness. AJR Am J Roentgenol. 1980;135:1269–1271. doi: 10.2214/ajr.135.6.1269. [DOI] [PubMed] [Google Scholar]
- Marom EM, Martinez CH, Truong MT, et al. Tumor cavitation during therapy with antiangiogenesis agents in patients with lung cancer. J Thorac Oncol. 2008;3:351–357. doi: 10.1097/JTO.0b013e318168c7e9. [DOI] [PubMed] [Google Scholar]
- Ma X, Yao Y, Yuan D, et al. Recombinant human endostatin endostar suppresses angiogenesis and lymphangiogenesis of malignant pleural effusion in mice. PLoS ONE. 2012;7:e53449. doi: 10.1371/journal.pone.0053449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao B, Jia J, Ma L, et al. Recombinant human endostatin could eliminate the pro-angiogenesis priority of SP cells sorted from non-small cell lung cancer cells. Clin Transl Oncol. 2012;14:575–585. doi: 10.1007/s12094-012-0844-9. [DOI] [PubMed] [Google Scholar]
- Sandler AB, Schiller JH, Gray R, et al. Retrospective evaluation of the clinical and radiographic risk factors associated with severe pulmonary hemorrhage in first-line advanced, unresectable non-small-cell lung cancer treated with Carboplatin and Paclitaxel plus bevacizumab. J Clin Oncol. 2009;27:1405–1412. doi: 10.1200/JCO.2008.16.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortimer J, Zonder HB, Pal SK. Lessons learned from the bevacizumab experience. Cancer Control. 2012;19:309–316. doi: 10.1177/107327481201900407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Wang JW, Sun Y, et al. Randomized phase II trial on escalated doses of Rh-endostatin (YH-16) for advanced non-small cell lung cancer. Chin J Oncol. 2006;28:138–141. (In Chinese.) [PubMed] [Google Scholar]
- Mancuso P, Calleri A, Cassi C, et al. Circulating endothelial cells as a novel marker of angiogenesis. Adv Exp Med Biol. 2003;522:83–97. doi: 10.1007/978-1-4615-0169-5_9. [DOI] [PubMed] [Google Scholar]
- Goon PK, Lip GY, Stonelake PS, Blann AD. Circulating endothelial cells and circulating progenitor cells in breast cancer: relationship to endothelial damage/dysfunction/apoptosis, clinicopathologic factors, and the Nottingham Prognostic Index. Neoplasia. 2009;11:771–779. doi: 10.1593/neo.09490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancuso P, Bertolini F. Circulating endothelial cells as biomarkers in clinical oncology. Microvasc Res. 2010;79:224–228. doi: 10.1016/j.mvr.2010.02.007. [DOI] [PubMed] [Google Scholar]
- Yu HK, Lee HJ, Choi HN, et al. Characterization of CD45-/CD31+/CD105+ circulating cells in the peripheral blood of patients with gynecologic malignancies. Clin Cancer Res. 2013;19:5340–5350. doi: 10.1158/1078-0432.CCR-12-3685. [DOI] [PubMed] [Google Scholar]
- Mancuso P, Burlini A, Pruneri G, Goldhirsch A, Martinelli G, Bertolini F. Resting and activated endothelial cells are increased in the peripheral blood of cancer patients. Blood. 2001;97:3658–3661. doi: 10.1182/blood.v97.11.3658. [DOI] [PubMed] [Google Scholar]
- Beerepoot LV, Mehra N, Vermaat JS, Zonnenberg BA, Gebbink MF, Voest EE. Increased levels of viable circulating endothelial cells are an indicator of progressive disease in cancer patients. Ann Oncol. 2004;15:139–145. doi: 10.1093/annonc/mdh017. [DOI] [PubMed] [Google Scholar]
- Delorme B, Basire A, Gentile C, et al. Presence of endothelial progenitor cells, distinct from mature endothelial cells, within human CD146+ blood cells. Thromb Haemost. 2005;94:1270–1279. doi: 10.1160/TH05-07-0499. [DOI] [PubMed] [Google Scholar]
- Allanore Y, Batteux F, Avouac J, Assous N, Weill B, Kahan A. Levels of circulating endothelial progenitor cells in systemic sclerosis. Clin Exp Rheumatol. 2007;25:60–66. [PubMed] [Google Scholar]


