Abstract
Background
The aim of the study was to characterize the histological and epidemiological features of lung cancer in Chinese women.
Methods
Demographic and histological information on female lung cancer cases identified during 1 January 2000 through 31 December 2012 from the Cancer Hospital of the Chinese Academy of Medical Sciences were collected. The International Classification of Diseases for Oncology system was used to classify the histological subtypes. Relative frequencies (RF) were estimated for major histological subtypes and compared by the years of diagnosis and birth, and among residential areas. Statistical differences were tested for RFs in the time periods with a trend test and with Pearson Chi square tests for distribution.
Results
Of 7070 female Chinese lung cancer cases, the major histological subtypes were adenocarcinoma (ADC) 65.79%; squamous cell carcinoma (SCC) 10.21%; small cell cancer 8.12%; large cell carcinoma, 2.79%; and adeno-squamous carcinoma (ASC), 2.19%. ADC increased, with RFs from 46.72% in the cases identified in 2000–2002 to 76.49% in 2011–2012 (Z = 16.998, P < 0.0001); SCC decreased from 15.69% to 5.97% (Z = −8.750, P < 0.0001). Compared to the cases identified in 2000–2006, the age-adjusted RFs of ADC in 2007–2012 consistently increased in all study areas.
Conclusion
The significant increase of ADC of the lung in Chinese women suggests that a persistently strong exposure to potential carcinogens in the Chinese population should be further and fully investigated.
Keywords: Chinese, epidemiology, female, histology, lung cancer
Introduction
Lung cancer has been reported as the most common cause of cancer in Chinese women, with an age-standardized mortality rate (ASR, for world standard population) of 17.27 per 100 000 women reported by the Chinese National Cancer Registry System. Lung cancers have accounted for more than one fifth of all causes of female cancer deaths since 2006.1–4 It is estimated that a total of 150 000 Chinese women died from malignant neoplasms of the trachea, bronchus, and lung (International Classification of Diseases [ICD]-10, C33-34), accounting for 23.3% of all cancer deaths in 2010.5 However, in the 1970's, the ASR of lung cancer among Chinese women was only five per 100 000, contributing to 6% of cancer cases; data from the female population in 94% of the residential areas showed mortality rates under eight per 100 000 for lung cancer.3,4 Dramatic increases in lung cancer mortality among women over recent decades highlight the high risk of lung cancer in Chinese women, and the challenges of cancer prevention and control in China.
The histologic types of lung cancer are classified into four main sub-types: squamous cell carcinoma (SCC), adenocarcinoma (ADC), small cell carcinoma (SCLC), and large cell carcinoma (LCC). The rates and distribution of histological types of lung cancer have changed substantially in men and women over the last decades in many western countries.6–9 Rates of SCC and SCLC among women were generally found to increase, especially in the Netherlands and Norway, while SCC rates among men declined 30% or more in North America and some European countries; the SCLC rate decreased less rapidly.6 Among men and women, ADC rates rose in virtually all areas of Europe, with a more rapid increase in women, with rates more than doubling in Norway, Italy, and France. Incidence rates of all lung cancer types among women and ADC among men and women continue to rise, despite declining cigarette use in many western countries and shifts to filtered/low-tar cigarettes. The changes over time in the incidence of lung cancer in men and women in western countries are primarily attributed to changes in smoking prevalence and a new generation of cigarettes.9–11 Increases in ADC are considered the reflection of an increase over time in the use of filter-tripped and low-tar cigarettes.12Understanding the histologic and epidemiological patterns of lung cancer can provide scientific clues to identify the etiological factors for effective cancer control programs.
In Chinese women, both the rates of current smoking and ever smoking have been historically low, all less than 3%.13 However, epidemiological research has shown that cigarette smoking increases the mortality of lung cancer by two to three times in female smokers, similar risks as those found in Chinese male smokers compared to non-smokers.14,15 Among non-smoking women, risk factors for lung cancer, including secondhand smoking (SHS) or environmental smoking (ETS), occupational exposures, fried cooking or cooking fume exposures,16–22were reported. High ASRs of lung cancer were observed among women exposed to high levels of air pollution by burning “smoky” coal indoors.23 While rapid increases of lung cancer incidence and mortality rates have been observed, there is limited information on the distribution of histological subtypes of lung cancers among Chinese women in scientific literature.1–3 There was no adequate histological data from the cancer registration system available to be used to explore the causal links between exposure and development of lung cancer in Chinese women.
In this study, we report our analysis on the data of Chinese female lung cancers identified at the Cancer Hospital of the Chinese Academy of Medical Sciences – Peking Union Medical College (CHCAMS-PUMC) from 2000 to 2012.
Materials and methods
CHCAMS-PUMC in Beijing is one of largest and oldest “class-A” cancer hospitals in China. It has approximately 1200 beds, and services patients from Beijing and all areas of mainland China. It offered comprehensive surgical treatment, radiotherapy, chemotherapy, therapeutic and pathologic services, and consultation to more than 600 000 patients in 2012.
Data sources and selection of cases
Data on female lung cancer cases (ICD-10, C34), confirmed by histology, were collected from the health information system of the CHCAMS-PUMC during the period of 1 January 2000 through 31 December 2012. The cases with complete and valid International Classification of Diseases for Oncology (ICD-O) (sub-site, grade, histological diagnosis) and demographic information (age, year of birth, date of diagnosis, residential area) were selected as study subjects. The area and time variations in more than 200 patients with lung cancer from nine geographic areas were analyzed in this study.
Classification of the study cases
The International classification system was used to define histologic subtypes.24 Lung cancers (ICD-10 coded as C34.1 to C34.9) were sub-divided into SCC (M8050, M8070, M8082, M8083), ADC (M8140, M8144, M8200, M8211, M8250, M8251, M8255, M8260, M8263, M8323, M8333, M8490, M8550, or M8574), SCLC (M8041, or M8045), LCC (M8012, M8013, or M8046), adeno-squamous carcinoma (ASC) (M8560), carcinoid tumor (M8240, M8244, or M8249), sarcomatoid tumor (M8033, M8430, or M8480) or other tumor (M8010, M8020, or M8022). Analyses were limited to histologic sub-groups with at least 100 cases diagnosed during the study period.
Statistical analyses
SAS 9.1 (SAS Institute, Inc., Cary, NC) statistical software was used for data management and analysis. Relative frequencies (RF) of the most frequent histological subtypes/groups of lung cancer were calculated by year of diagnosis, year of birth, and age group. A Pearson Chi-square test was used for statistical testing by histologic subtype, and Z values in trend tests for continuing time periods and age groups. Estimations were made for the area-specific RFs of main histological subtypes, adjusted by the year of diagnosis (1-year interval) and the year of birth (5-year intervals). Area-specific RFs for SCC and ADC were adjusted by age (5-year intervals) and stratified by year of diagnosis (before or after 2007), using the factors derived from all study cases. All P-values quoted are two-sided and values less than 0.05 were considered statistically significant.
Results
A total of 7125 female lung cancer cases were identified from the CHCAMS-PUMC health information system and the data were collected without including names or personal identifying information. The cases with valid and complete information on histology and demography were selected after deleting the duplicates, missing values in histology, date of birth, date of diagnosis, and residential area. Finally, a total of 7070 cases of lung cancer in Chinese women were selected as study subjects.
Overall, the most frequent histological sub-types of lung cancer in women in the study cases were ADC (4651 cases, 65.79%); SCLC (722 cases, 10.21%); SCC (574 cases, 8.12%); LCC (197 cases, 2.79%); and ASC (155 cases, 2.19%). In the cases of ADC, 35.04% were poorly, 50.55 moderately, and 14.41% well differentiated by grade, while in the cases of SCC, 38.47% were poorly, 50.12% moderately, and 13.87% well differentiated. All lobes of the lung were involved, however, the upper (42.55%) and lower (32.23%) lobes were the main sub-sites of disease occurrence.
As shown in Table 1, the number of female lung cancer cases of all histologic subtypes increased through the study period. The subtotal of all cases diagnosed in 2007–2012 (65.79%) was nearly double the number in 2000–2006 (34.21%). There were 83.54% of cases aged 45 to 74 years, and most frequent cases were found in ages 55–64 years. Almost 80% (79.83%) of cases came from six provincial areas including Beijing (36.73%), Hebei (14.00%), Neimenggu (8.15%), Heilongjiang (7.30%), Liaoning (6.89%), and Shandong (6.01%).
Table 1.
General characteristics of the study subjects (N = 7070)
| Class | N (%) |
|---|---|
| Year of diagnosis | |
| 2000–2002 | 854 (12.08) |
| 2003–2004 | 578 (8.18) |
| 2005–2006 | 986 (13.95) |
| 2007–2008 | 1168 (16.52) |
| 2009–2010 | 1608 (22.74) |
| 2011–2012 | 1876 (26.53) |
| Age (year) | |
| <35 | 174 (2.46) |
| 35–44 | 739 (10.45) |
| 45–54 | 1801 (25.47) |
| 55–64 | 2396 (33.89) |
| 65–74 | 1710 (24.18) |
| 75+ | 250 (3.54) |
| Residential area | |
| Beijing | 2597 (36.73) |
| Hebei | 990 (14.00) |
| Neimenggu | 576 (8.15) |
| Heilongjiang | 516 (7.30) |
| Liaoning | 487 (6.89) |
| Shandong | 425 (6.01) |
| Shanxi | 298 (4.21) |
| Henan | 265 (3.75) |
| Jilin | 226 (3.20) |
| Other | 690 (9.76) |
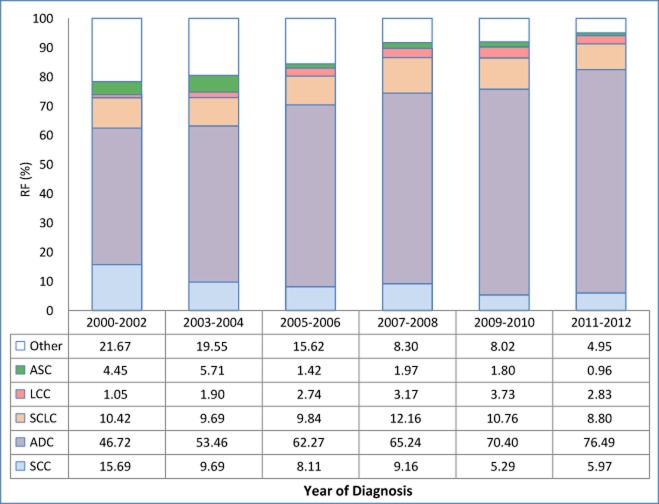
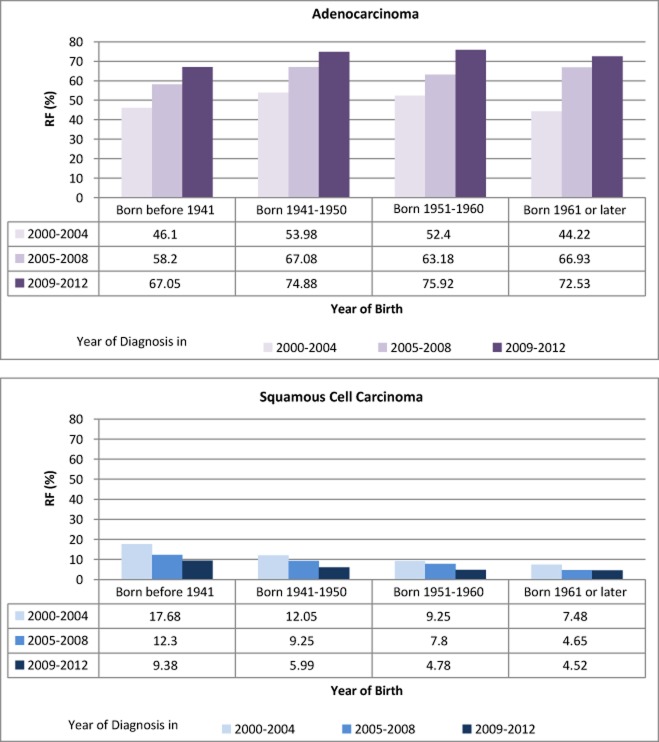
Figure 1 shows the RFs of the five histological sub-types of lung cancer diagnosed in 2000 to 2012 in women in this study. Significant increases of RFs in ADC (from 46.72% to 76.49%, Z = 16.998, P < 0.0001), LCC (from 1.05% to 2.83%, Z = 3.079, P = 0.0021), and decreases in SCC (from 15.69% to 5.97%, Z = −8.750, P < 0.0001), and ASC (4.45% to 0.96%, Z = −6.918, P < 0.0001) were observed. When examined by year of birth (born before 1941, 1941–1950, 1951–1960, or 1961 or later), RFs were found to increase for ADC (from 43.91% to 67.82%, Z = 7.558, P < 0.0001) and LCC (from 0.87% to 3.31%, Z = 2.735, P = 0.0062), and decreased in SCC (from 18.26% to 5.05%, Z = −9.537, P < 0.0001), as shown in Figure 2.
Figure 1.
Relative frequency (RF) of major subtypes of female lung cancer cases by year of diagnosis. Trend tests were analyzed for squamous cell carcinoma, SCC (Z = −8.750, P < 0.0001); adenocarcinoma, ADC (Z = 16.998, P < 0.0001); small cell carcinoma, SCLC (Z = −0.933, P = 0.3508); large cell carcinoma, LCC (Z = 3.079, P = 0.0021); and adeno-squamous carcinoma ASC (Z = −6.918, P < 0.0001).
Figure 2.
Relative frequency (RF) of female lung cancer cases by histological sub-type, stratified by year of diagnosis within categories of year of birth, Cancer Hospital of the Chinese Academy of Medical Sciences – Peking Union Medical College, 2000–2012.
Table 2 shows the RFs of the major histological sub-types of female lung cancer cases in six study areas, after adjusting the year identified and the year of birth. The RFs were 7.05% (Beijing) to 13.48% (Liaoning) in SCC, 57.33% (Neimenggu) to 70.91% (Beijing) in ADC, 6.88% (Beijing) to 17.44% (Hebei) in SCLC, 1.10% (Liaoning) to 3.58% (Neimenggu) in LCC, and from 1.31% (Shadong) to 2.61% (Hebei) in ASC, respectively. The distributions of the major sub-types of lung cancer were significantly different in the six study areas in SCC (X2 = 21.916, P = 0.0050), ADC (X2 = 34.475, P < 0.0001), and SCLC (X2 = 106.485, P < 0.0001).
Table 2.
Distribution of major histological groups of lung cancer in women
| SCC | ADC | SCLC | LCC | ASC | Other | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (N) | RF† | (N) | RF† | (N) | RF† | (N) | RF† | (N) | RF† | (N) | RF† | |
| All areas | (574) | 8.12 | (4651) | 65.79 | (722) | 10.21 | (197) | 2.79 | (155) | 2.19 | (771) | 10.99 |
| Beijing | (213) | 7.05 | (1757) | 70.91 | (189) | 6.88 | (66) | 2.76 | (51) | 1.76 | (321) | 10.64 |
| Hebei | (71) | 7.49 | (591) | 59.07 | (178) | 17.44 | (25) | 2.44 | (24) | 2.61 | (101) | 10.95 |
| Neimenggu | (45) | 8.09 | (353) | 57.33 | (89) | 15.38 | (24) | 3.58 | (11) | 1.99 | (54) | 13.63 |
| Liaoning | (55) | 13.48 | (314) | 59.95 | (46) | 8.33 | (7) | 1.10 | (10) | 2.34 | (55) | 14.80 |
| Heilongjiang | (61) | 12.46 | (337) | 64.78 | (46) | 8.49 | (11) | 1.81 | (12) | 2.28 | (49) | 10.18 |
| Shandong | (28) | 8.40 | (302) | 65.24 | (41) | 9.17 | (12) | 3.09 | (7) | 1.31 | (35) | 12.79 |
Percentages adjusted with year of diagnosis by one-year and year of birth by five-year intervals.
Distributions of cases in the study areas were statistically significant for squamous cell carcinoma (SCC) (X2 = 21.916, P = 0.005), adenocarcinoma (ADC) (X2 = 34.475, P < 0.0001), and small cell carcinoma (SCLC) (X2 = 106.785, P < 0.0001), and not significant for large cell carcinoma (LCC) (X2 = 9.511, P = 0.301) and adeno-squamous carcinoma (ASC) (X2 = 2.564, P = 0.959). Rf, relative frequency.
When stratified, analysis conducted by time of diagnosis into 2000–2006 and 2007–2012, less frequent SCC (−3.49 to −14.22), and more frequent ADC cases (+15.04% to +24.20%) were found consistently in each study area in recent years, as shown in Table 3.
Table 3.
Relative frequencies of squamous cell carcinoma and adenocarcinoma in women in study areas in 2000–2006 and 2007–2012
| All cases | SCC | Changes of SCC | ADC | Changes of ADC | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2000–2006 | 2007–2012 | 2000–2006 | 2007–2012 | 2000–2006 | 2007–2012 | |||||||
| (N) | (N) | (N) | RFa | (N) | RFb | (RFb-RFa) | (N) | RFa | (N) | RFb | (RFb-RFa) | |
| All areas | (2418) | (4652) | (270) | 11.16 | (304) | 6.53 | −4.63 | (1322) | 54.83 | (3329) | 71.49 | +16.66 |
| Beijing | (1104) | (1493) | (125) | 10.24 | (88) | 5.72 | −4.52 | (617) | 56.91 | (1140) | 77.30 | +20.39 |
| Hebei | (295) | (695) | (31) | 10.72 | (40) | 5.83 | −4.89 | (142) | 47.66 | (449) | 64.71 | +17.05 |
| Neimenggu | (165) | (411) | (17) | 11.40 | (28) | 6.77 | −4.63 | (87) | 49.93 | (266) | 64.97 | +15.04 |
| Liaoning | (137) | (350) | (27) | 22.40 | (28) | 8.18 | −14.22 | (66) | 46.23 | (248) | 70.43 | +24.20 |
| Heilongjiang | (171) | (345) | (24) | 14.30 | (37) | 10.81 | −3.49 | (86) | 51.23 | (251) | 71.36 | +20.13 |
| Shandong | (123) | (302) | (12) | 9.91 | (16) | 5.38 | −4.53 | (71) | 57.67 | (231) | 77.16 | +19.49 |
1: Percentages adjusted with age, by five-year intervals. N: The number of cases.
ADC, adenocarcinoma; SCC, squamous cell carcinoma.
Discussion
In the present study, the number of female lung cancer cases diagnosed at CHCAMS-PUMC increased dramatically from 2000 to 2012, and the increase was predominantly in ADC (Fig 1). Cases identified as ADC in 2011–2012 accounted for 76.49% of female lung cancer cases, an increase of 30% from 46.72% in 2000–2002. While the RFs of SCC cases declined gradually (15.69% SCC in 2000–2002), RFs of ADC consistently increased among the cases born before 1941, in 1941–1950, 1951–1960, and after 1961. Even though the overall RFs of ADC varied by geographic region, such as 57.33% from Neimenggu and 70.91% from Beijing, consistent increases in age-adjusted RFs of ADC, from 15.04% to 20.39%, were observed among cases in all geographic areas, including Beijing, Hebei, Neimenggu, Liaoning, Heilongjiang, and Shandong in 2007–2012 compared to 2000–2006.
Our studies of lung cancer among men and women show that the proportion of ADC lung cancer cases increased among both men and women, but the proportion of ADC among women was consistently more than twice that among men.25 While the proportion of SCC cases among men decreased from 39.11% in 2000–2002 to 32.23% in 2011–2012, among women the decrease was from 15.69% in 2000–2002 to 5.97% in 2011–2012.
The evidence is sufficient to conclude that the risk of developing ADC of the lung from cigarette smoking has increased since the 1960s6–12 and tobacco control strategies and policies must be implemented against the tobacco epidemic.26–28 The ADC epidemic is also, in part, a result of cigarette design changes, including increases in tobacco specific nitrosamines, manipulation of droplet size, and ventilated filters in western countries since the 1990s. Filter ventilation provides low machine measured yields of tar and nicotine and facilitates increased depth of inhalation, which together increase puff frequency. Deep inhalation is also assisted by production of optimal droplet size particles, thus, exposing the periphery of the lung to great volumes of smoke and delivering nicotine and the carcinogenic mix with maximum efficiency.9–12,26 As there is convincing evidence to confirm that despite changes in the cigarette's design, no reduction in the health risk has been made, health advocates in Canada convinced the government that current standards on tobacco, such as low tar yield, are deceptive.28 In the US, an increasing number of states and localities have implemented comprehensive policies prohibiting tobacco smoking in all indoor areas in public places and worksites, and most adults report having voluntary smoke-free homes (81.1%) and vehicles (73.6%) to avoid SHS exposure.29
In mainland China, the average tar delivery per cigarette in the market was about 27 mg in 1983, when no restrictions on tar delivery were applied.30 Since the regulation to restrict tar delivery per cigarette, the rates in China were 17 mg in 2000, 15 mg in 2005, 13 mg in 2008, and 12 mg in 2010.31 The total amount of cigarettes consumed in China have continued to increase since 2000, and the smoking rates in men have not substantially reduced from 2002 (57.4%) to 2010 (54.0%), therefore, exposure to SHS remains a serious problem in China.32–38 Large numbers of people smoke inside workplaces and public places, and many continue to smoke in areas where smoking is banned. More than 90% of families accept smoking in part or in all indoor areas in their households; 72% of non-smokers reported being exposed to SHS, and 38% were exposed on daily basis. While age-adjusted RFs of ADC have increased and surpassed SCC in Chinese male lung cancer cases in 2000–2012,25 a dramatic increase of ADC in female lung cancer cases was found in our study, parallel with histological changes in men. These findings suggest that there could be strong exposure causing both the development of lung cancer and the changes in histology of the disease in Chinese men and women. Individual data on exposure and the disease should be collected and further investigated to confirm the association of active smoking and SHS with the development of lung cancer in the Chinese population. Specific and realistic tobacco control strategies and preventive measures are needed.
This study was based on hospital in-patient records, and was not population based, which is a limitation of the study. We also did not have individual level information on smoking history or history of second-hand smoke exposure. While the increase in RF of ADC was accompanied by a decrease in the proportion of cases classified as “other” histologic subtype, the increase in the proportion of ADC could not be accounted for by the decrease in lung cancers classified as “other” histology.
Conclusions
The increase in the rate of adenocarcinoma of the lung in Chinese women in this study suggests that changes to environmental risk factors should be further investigated for specific and effective preventive measures.
Acknowledgments
This work was supported by the NIH project “Epidemiology and Intervention Research for Tobacco Control in China” (R01RFA-TW-06-006) and the Beijing Hope Run Fund (YF2010-48).
Disclosure
No authors report any conflict of interest.
References
- National Office for Cancer Prevention and Control & National Central Cancer Registry, Disease Prevention and Control Bureau of Ministry of Health. 2009 Chinese Cancer Registry Annual Report: Cancer Incidence and Mortality in Chinese Cancer Registration Areas in 2009. Beijing: Military Medical Science Press; 2010. pp. 26–35. , 54–55. (In Chinese.) [Google Scholar]
- The Ministry of Health of the People's Republic of China. Report on Third National Retrospective Sampling Death Survey. Beijing: Peking Union Medical College Press; 2008. p. 22. . (In Chinese.) [Google Scholar]
- Zhou YS. Lung Cancer. Investigation on Cancer Mortality in China. Beijing: People's Health Press; 1980. pp. 148–174. [Google Scholar]
- Zou XN. Epidemiology of lung cancer in China. Chin J Cancer Prev Treat. 2007;14:881–883. [Google Scholar]
- Chen WQ, Zhang SW, Zeng HM, et al. Report of Cancer incidence and mortality in China, 2010. China Cancer. 2014;23:1–10. [Google Scholar]
- Devesa SS, Bray F, Vizcaino AP, Parkin DM. International lung cancer trends by histological type: male:female differences diminishing and adenocarcinoma rates rising. Int J Cancer. 2005;117:294–299. doi: 10.1002/ijc.21183. [DOI] [PubMed] [Google Scholar]
- Wahbah M, Boroumand N, Castro C, El-Zeky F, Eltorky M. Changing trends in the distribution of the histologic types of lung cancer: a review of 4439 cases. Ann Diagn Pathol. 2007;11:89–96. doi: 10.1016/j.anndiagpath.2006.04.006. [DOI] [PubMed] [Google Scholar]
- Muscat JE, Wynder EL. Lung cancer pathology in smokers, ex-smokers and never smokers. Cancer Lett. 1995;88:1–5. doi: 10.1016/0304-3835(94)03608-l. [DOI] [PubMed] [Google Scholar]
- Thun MJ, Lally CA, Flannery JT, Calle EE, Flanders WD, Heath CW., Jr Cigarette smoking and changes in the histopathology of lung cancer. J Natl Cancer Inst. 1997;89:1580–1586. doi: 10.1093/jnci/89.21.1580. [DOI] [PubMed] [Google Scholar]
- Khuder SA, Dayal HH, Mutgi AB, Willey JC, Dayal G. Effect of cigarette smoking on major histological types of lung cancer in men. Lung Cancer. 1998;22:15–21. doi: 10.1016/s0169-5002(98)00068-3. [DOI] [PubMed] [Google Scholar]
- Gray N. The consequences of the unregulated cigarette. Tob Control. 2006;15:405–408. doi: 10.1136/tc.2006.017277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns DM, Anderson CM, Gray N. Do changes in cigarette design influence the rise in adenocarcinoma of the lung? Cancer Causes Control. 2011;22:13–22. doi: 10.1007/s10552-010-9660-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang GH, Li Q, Wang CX, et al. Findings from 2010 Global Adult Tobacco Survey: implementation of MPOWER policy in China. Biomed Environ Sci. 2010;23:422–429. doi: 10.1016/S0895-3988(11)60002-0. [DOI] [PubMed] [Google Scholar]
- Liu BQ, Peto R, Chen ZM, et al. Emerging tobacco hazards in China: 1. retrospective proportional mortality study of one million deaths. BMJ. 1998;317:1411–1422. doi: 10.1136/bmj.317.7170.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang W, Kang Y, Shi GY, et al. Comparisons of multiple characteristics between young and old lung cancer patients. Chin Med J (Engl) 2012;125:72–80. [PubMed] [Google Scholar]
- Gan DK, Han JX, Zheng SH, et al. Adverse effect of passive smoking on women health. J Environ Health. 2006;23:106–108. [Google Scholar]
- Fang J, Gan DK, Zheng SH, et al. A case-control study of the risk factors for lung cancer among Chinese women who have never smoked. J Hygiene Res. 2006;35:464–467. (In Chinese.) [PubMed] [Google Scholar]
- Hill SE, Blakely T, Kawachi I, Woodward A. Mortality among lifelong nonsmokers exposed to secondhand smoke at home: cohort data and sensitivity analyses. Am J Epidemiol. 2006;165:530–540. doi: 10.1093/aje/kwk043. [DOI] [PubMed] [Google Scholar]
- Tse LA, Yu ITS, Au JSK, et al. Environmental tobacco smoke and lung cancer among Chinese nonsmoking males: might adenocarcinoma be the culprit? Am J Epidemiol. 2009;169:533–541. doi: 10.1093/aje/kwn385. [DOI] [PubMed] [Google Scholar]
- Sun TD, Li L, Shi NF, Zhang X, Zhong HX, Zen YQE. A 40-year cohort study on cancer mortality among female workers with manual spinning of chrysotile asbestos. J Hygiene Res. 2003;32:511–513. (In Chinese.) [PubMed] [Google Scholar]
- Xiang YB, Gao YT. Polychotomous logistic regression analysis for lung cancer among nonsmoking women in Shanghai, P. R. China. Chin J Health Stat. 2005;22:66–70. [Google Scholar]
- Yu IT, Chiu YL, Au JS, Wong TW, Tang JL. Dose-response relationship between cooking fumes exposures and lung cancer among Chinese nonsmoking women. Cancer Res. 2006;66:4961–4967. doi: 10.1158/0008-5472.CAN-05-2932. [DOI] [PubMed] [Google Scholar]
- Li J, Zhang Y, Li Y, et al. Descriptive study on the epidemiology of lung cancer in coal-producing area in eastern Yunnan, China. Chin J Lung Cancer. 2011;14:107–119. doi: 10.3779/j.issn.1009-3419.2011.02.02. (In Chinese.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis WD, Brambilla E, Müller-Hermelink HK, Harris CC, editors. WHO Classification of Tumours. Pathology and Genetics: Tumours of the Lung, Pleura, Thymus and Heart. Lyon, France: IARC Press; 2004. p. 10. , eds. [Google Scholar]
- Zou XN, Lin DM, Wan X, et al. Histological subtypes of lung cancer in Chinese males from 2000 to 2012. Biomed Environ Sci. 2014;27:3–9. doi: 10.3967/bes2014.010. [DOI] [PubMed] [Google Scholar]
- US Department of Health and Human Services. 2001. Risks Associated with Smoking Cigarettes with Low Machine-measured Yields of Tar and Nicotine. Smoking and Tobacco Control. US Department of Health and Human Services, Public Health Service, National Institutes of Health, National Cancer Institute, Bethesda (MD) Monograph No. 13. [DOI] [PubMed]
- Harris JE, Thun MJ, Mondul AM, Calle EE. Cigarette tar yields in relation to mortality from lung cancer in the cancer prevention study II cohort, 1982–8. BMJ. 2004;328:72–79. doi: 10.1136/bmj.37936.585382.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bialous SA, Yach D. Whose standard is it, anyway? How the tobacco industry determines the international organization for standardization (ISO) standards for tobacco and tobacco products. Tob Control. 2001;10:96–104. doi: 10.1136/tc.10.2.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King BA, Dube SR, Homa DM. Smoking-free rules and secondhand smoke exposure in homes and vehicles among US adults, 2009–2010. Prev Chronic Dis. 2013;10:E79. doi: 10.5888/pcd10.120218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei ZQ, Yang J, Chu GH, Hu QY. Review, current situation and prospect of the course of reducing tar in domestic cigarettes. Tob Sci Technol Inspect Stand. 2003;5:29–31. [Google Scholar]
- Zi Y. Trend of Low-tar Cigarette Development in Market. [Cited 4 Jan 2014.] Available from URL: http://www.tobaccochina.com/management/market/stratagem/20113/20113158398_453357.shtml.
- World Health Organization. 2008. WHO Report on the Global Tobacco Epidemic, 2008: the MPOWER package. Geneva,
- Yang GH, Hu AG. Tobacco Control and the Future in China – Evaluative Report from the Domestic and International Experts on the Tobacco Use and Tobacco Control in China. Beijing: Economic Daily Press; 2011. . (In Chinese.) [Google Scholar]
- Yang GH. Monitoring epidemic of tobacco use, promote tobacco control. Biomed Environ Sci. 2010;23:420–421. doi: 10.1016/S0895-3988(11)60001-9. [DOI] [PubMed] [Google Scholar]
- Xiao L, Yang Y, Li Q, Wang CX, Yang GH. Population-based survey of secondhand smoke exposure in China. Biomed Environ Sci. 2010;23:430–436. doi: 10.1016/S0895-3988(11)60003-2. [DOI] [PubMed] [Google Scholar]
- Wang CP, Ma SJ, Xu XF, Wang JF, Mei CZ, Yang GH. The prevalence of household second-hand smoke exposure and its correlated factors in six counties of China. Tob Control. 2012;18:121–126. doi: 10.1136/tc.2008.024836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinese Center for Disease Control and Prevention. Report on Chronic Disease Risk Factor Surveillance in China 2010. Beijing: Military Medical Science Press; 2009. pp. 34–40. . (In Chinese.) [Google Scholar]
- Yang G. Marketing “less harmful, low-tar” cigarettes is a key strategy of the industry to counter tobacco control in China. Tob Control. 2014;23:167–172. doi: 10.1136/tobaccocontrol-2012-050691. [DOI] [PMC free article] [PubMed] [Google Scholar]