Abstract
Background
Patients with pulmonary metastases from colorectal cancer can benefit from surgical removal. However, the biological determinants of postsurgical outcome are not completely elucidated. We evaluated the role of host systemic inflammation status in this setting.
Methods
The modified Glasgow prognostic score (based on serum C-reactive protein and albumin levels) and the neutrophil-to-lymphocyte (NTL) ratio were obtained from 44 patients who received curative-intent metastasectomy, and were used as indicators of systemic inflammation status. We tested the impact of both of these parameters on overall survival (OS) and progression-free survival (PFS), as well as their correlation with other well-known prognosticators.
Results
Five-year PFS and OS rates were 18% and 49%, respectively. At univariate analysis, multiple metastases, disease-free interval <36 months, and a Glasgow score of 2 (P = 0.031) were significantly associated to a worse PFS rate. A NTL ratio >3 predicted disease progression in the short-term (P = 0.036), but the effect on late events was weaker (P = 0.079). Factors associated with worse OS were multiple metastasis (P = 0.002), elevated carcinoembryonic antigen (P = 0.009), a Glasgow score of 2 (P = 0.029), and a faster metastasis growth (P = 0.008). At Cox regression analysis, neither a Glasgow score of 2, nor elevated NTL ratio showed an independent effect on survival rates.
Conclusions
Systemic inflammation scores did not perform well as independent survival prognosticators in patients undergoing curative-intent pulmonary metastasectomy. Further investigation is warranted to evaluate whether these measurements could still be useful when restricting the analysis to specific patient subcategories or to diverse postoperative phases.
Keywords: Albumin, colorectal cancer, C-reactive protein, neutrophil-to-lymphocyte ratio, pulmonary metastasectomy
Introduction
Colorectal cancer is one of the most frequent malignant diseases, responsible for more than 100 000 annual deaths in European countries. It is estimated that about 5% of patients with resectable colorectal cancer will develop pulmonary metastases at some point in their clinical history.1 Despite the lack of level-A recommendations coming from randomized controlled trials, surgical metastasectomy has gained acceptance as a reliable treatment option in this setting, as it is expected to offer a long term control of the disease in up to 50% of patients.2 In the 1990s, an International Registry of Pulmonary Metastasis study showed that patients with single lesions and those with delayed onset of metastases have the best chance to benefit from surgery.3 Since then, many other studies have been addressing the question as to what factors might be associated with a better postoperative outcome in this setting.4–12 These studies focused mostly on clinical features of the metastatic disease itself, whereas the effect of host-related factors have been less systematically investigated.
In recent years, an increasing interest is being devoted to the prognostic role of systemic inflammation scores in surgical oncology. In particular, an increased C-reactive protein level and a deranged leukocyte count have been shown to be associated with a poor prognosis in patients with colorectal cancer at diverse stages of disease. In this study, we sought to evaluate whether these kinds of clinical parameters might help predict surgical outcomes in the specific subgroup of patients with metastases to the lung, who are offered curative-intent surgery.
Materials and methods
Study design
This study is a retrospective analysis of a prospective database of pulmonary metastasectomy, compiled at our institution since September 2003. Only patients who had undergone curative-intent metastasectomy were included in the study. Major exclusion criteria were the presence of acute conditions remarkably affecting the systemic inflammation status, and a follow-up shorter than 12 months. The decision to perform surgical metastasectomy was made in the context of a multidisciplinary meeting, and all patients were required to sign an informed consent form after a thorough discussion of the expected benefits and limitations of the planned treatment. The Institutional Review Board of the Policlinico Tor Vergata University approved the study, and waived patient consent for the use of anonymous data for research purposes.
Lab measurements
The degree of systemic inflammation status was expressed by means of the neutrophil-to-lymphocyte (NTL) ratio and the modified Glasgow prognostic score, which is based on preoperative serum levels of C-reactive protein and albumin. Patients with increased C-reactive protein (>10 mg/L) and low albumin status (<35 g/L) were given a score of 2. Patients with increased C-reactive protein and normal albumin status were given a score of 1. Patients with a normal C-reactive protein level were scored as 0, regardless of albumin status. For all patients, venous blood samples (5–10 mL) were taken at 7:00 am, one to five days prior to surgery, in the context of a routine preoperative workup. Full blood count was assessed by means of a fluorocytometric method (workstation: XE 2100, Sysmex, Japan), whereas serum C-reactive protein and albumin levels were obtained by colorimetric method (workstation: Vista Dimension 1500, Siemens Healthcare, Germany) after centrifugation at 3500–5000 rounds per minute. All of the lab procedures, devices, and staff members fulfilled the requirements for the ISO 2001 quality certification.
Imaging
Imaging workup included multidetector computed tomography (CT) scans (collimation: 2.5 mm), and whole-body positron-emission tomography when appropriate. As per protocol, a second-look CT scan was planned whenever the last imaging assessment had been obtained more than 30 days before hospital admission. When available, the Specific Growth Ratio (SGR) for the metastatic lesions was calculated according to the following formula:
where V2 and V1 represent the approximate spheroid tumor volume of the most rapidly growing lesion on two interval CT scans. SGR is a reverse log function of the Tumor Doubling Time, but it has been shown to be more reliable than the latter for analytic purposes.13
Follow-up
Clinical examination was performed at 15 days and at three months after discharge. Subsequently, patients were seen at six-monthly intervals in a multidisciplinary fashion. The surveillance plan included serial blood checks, imaging assessment, and colonoscopy as appropriate.
Outcome measures and statistical methods
As a first step, we focused on eventual correlations between systemic inflammation scores and other known prognosticators of lung metastasectomy for colorectal cancer. These included age, gender, features of the metastatic lesions (i.e. number, central vs. peripheral location, size and unilateral vs. bilateral distribution), disease-free interval from primary tumor resection, features of the primary tumor (i.e. T-status and nodal involvement), active cigarette smoke exposure, and carcinoembryonic antigen (CEA) level. For analytic purposes, continuous variables were categorized according to usual reference thresholds, or taking the median as the cut-off (Table 1). A two-tailed Spearman test was used for the analysis.
Table 1.
Cut-off and criteria for variable categorization
| Variable | Cut-off or criterion |
|---|---|
| Age | >65 years |
| Number of metastases | Single versus multiple (>1) |
| Elevated CEA level | >5 ng/dL |
| Specific Growth Rate | >0.0054/day* |
| Elevated C-reactive protein level | >10 mg/L |
| Low albumin level | <35 g/L |
| Active cigarette smoke exposure | Active smoking until six months before metastatic onset |
| Centrally located metastases | Proximity to a segmental or lobar bronchus/vessel |
| Large size | ≥3 cm (longer axis) |
| Disease-free interval from primary tumor resection | <36 months |
| Nodal involvement of primary tumor | N1–3 (vs. N0) |
This value corresponds to a tumor doubling time of 128 days, according to the formula: tumor doubling time = ln(2)/specific growth rate. CEA, carcino-embryonic antigen.
We then evaluated the univariate effect of diverse factors on both overall survival (OS) and progression-free survival (PFS) rates, by means of a comparative analysis of Kaplan-Meier estimates. To this purpose, we used both the Breslow and Cox-Mantel tests, to highlight differences in early and late events, respectively. Factors showing a difference at P < 0.05 with either test, or at P < 0.1 with both of the tests were then included in a backward stepwise Cox-regression analysis in order to check for any independent effect on the outcome measures.
SGR measurement was not available for all patients, as a repeat CT scan protocol was adopted mainly in patients with an uncertain diagnosis or treatment plan. In order to explore the possible association between this measurement and the other tested variables, we generated five virtual datasets by means of a multiple-imputation method. Throughout the text, both the results obtained from the original dataset with missing values, and those obtained by the virtual datasets, are reported whenever SGR is taken in the analysis. All of the statistics were compiled using the SPSS package, version 20.0 (IBM, Chicago, Illinois, USA). A P-value of less than 0.05 was set as the significance threshold. All values are reported as median plus interquartile range (IQR), unless indicated otherwise.
Results
Baseline findings and surgical results
Between September 2003 and January 2011, pulmonary resections for metastatic colorectal cancer were performed in 47 patients. Two patients were ineligible for the study because of immunosuppresive therapy for renal transplantation and non-radical surgery, respectively. Another patient was started on imatinib soon after multiple videothoracoscopic metastasectomy because of chronic myelocytic leukemia, therefore, he was excluded from the study because of the possible anti-metastatic effect of this drug. Baseline characteristics of the 44 patients included in the study are displayed in Table 2. The surgeries performed included 25 videothoracoscopic and 19 open resections. The decision on a surgical approach was basically made on the basis of anatomic features of the metastasis, therefore, a muscle-sparing thoracotomy was preferred in case of larger and more deeply located lesions. Overall, there were 37 non-anatomical resections, five lobectomies, and two anatomical segmentectomies. Among patients with bilateral lesions (n = 12), eight were treated in the same surgical session by means of a hand-assisted, transxiphoid videothoracoscopic approach,14 while the remaining patients received a staged operation with a median interval of 1.5 (IQR: 1–2) months. Lobar specific mediastinal lymph-node dissection was performed in cases of centrally located lesions, multiple metastases, and/or whenever a nodal enlargement was found intraoperatively. In total, this was performed in 12 patients, with a node-positive rate of 25% (n = 3). All operations were performed by, or under the supervision of the same surgeon. The thirty-day mortality and morbidity rates were 0% and 20.4% (9 patients, 2 of whom experienced more than an event), respectively. Perioperative complications included hemothorax requiring surgical revision (n = 1), acute lung injury/acute respiratory distress syndrome (n = 2), acute kidney injury (n = 3), atrial fibrillation (n = 2), and prolonged air-leak (>5 days, n = 4). Nine patients received iterative resections for local recurrences, with a median interval from first metastasectomy of nine (IQR: 7–11) months. Liver metastases occurred at any point in the clinical history in 17 patients (38.6%). These were treated by radiofrequency ablation (n = 5), surgical resection (n = 7), or a combination of these (n = 4). Median follow-up was 36 (IQR: 18–62) months. Recurrence patterns are detailed in Table 3. During follow up, no patient developed second primary cancers, and none died from non-cancer related causes.
Table 2.
Baseline patient features
| Factor | Result (median or rate) |
|---|---|
| Age (years) | 64 [IQR: 58–72] |
| Age >65 years | 28/45 (61%) |
| Gender (M/F) | 30/14 |
| C-reactive protein (mg/dL) | 5 [IQR: 2–8] |
| Albumin (g/dL) | 3.5 [IQR: 3.1–3.9] |
| CEA level (ng/dL) | 4 [2–6] |
| Neutrophil-to-lymphocyte ratio | 2.2 [IQR: 1.4-3-1] |
| Neutrophil-to-lymphocyte ratio (>3) | 9/44 (20.4%) |
| Modified Glasgow Prognostic Score (0–1-2) | 32/7/5 |
| Primary tumor T status (1–2 vs. 3–4) | 8/36 |
| Primary tumor N status (0 vs. 1–2) | 16/28 |
| Active smoking habit | 14/44 (31%) |
| Adjuvant chemotherapy* | 40/44 (90%) |
| Central vs. peripheral metastasis | 14/30 |
| Metastasis size >3 cm | 12/44 (27%) |
| Single versus multiple metastasis | 22/22 |
| Number of metastasis | 1.5 [IQR: 1–2] |
| Disease-free interval (months) | 26 [IQR: 14–38] |
CEA, carcino-embryonic antigen; IQR, interquartile range.
Table 3.
Progression patterns in the study group
| Progression pattern (34 patients; 37 events) | Number (Rate) |
|---|---|
| Multisite pulmonary dissemination with “snow-storm” appearance | 8 (21%) |
| Bulky mediastinal masses | 5 (13.5%) |
| Brain metastases | 3 (8.1%) |
| Colorectal recurrence | 5 (13.5%) |
| Malignant pleural effusion | 4 (10.8%) |
| Multiorgan metastatization | 12 (32.4%) |
Limited pulmonary relapses amenable for redo metastasectomy are not included. Some patients presented more than one feature.
Correlations
There was no association between the categorized NTL ratio and the modified Glasgow prognostic score (R: 0.136, P = 0.37). NTL ratio (R: 0.345, P = 0.022), not the modified Glasgow prognostic score, was positively associated with categorized CEA level. A borderline, positive correlation was found between active cigarette smoke exposure and elevated C-reactive protein level alone (R: 0.229, P = 0.051). No association was found between systemic inflammation scores and categorized SGR, nor with any other usual prognosticator included in the analysis. Interesting correlations, not linked to the main objective of the study, were found between nodal involvement of the primary tumor and metastatization to liver (R: 0.353, P = 0.014), as well as between active cigarette smoke and centrally-located metastases (R: 0.365, P = 0.016). Male gender had a shorter disease-free interval from primary tumor (R: 0.460, P = 0.002). Patients aged >65 years were more likely to have single lesions (R: −388, P = 0.008), but this finding could mirror a selection bias because of more restrictive indication criteria to metastasectomy in older subjects.
Progression-free survival
The five-year PFS rate was 18% (mean survival: 36.4 months; 95% confidence interval [CI]: 26.8–45.1). At univariate analysis, multiple metastasis (>1), disease-free interval <36 months, and a modified Glasgow prognostic score of 2 were significantly associated with a worse outcome in both the Breslow and Cox-Mantel tests (P = 0.011/P = 0.008; P = 0.021/P = 0.037; and P = 0.029/P = 0.031, respectively). A NTL ratio >3 was associated with disease progression in the short-term (Breslow test: P = 0.036, Fig. 1), but the effect on late events was weaker (Cox-Mantel test: P = 0.079). Nodal involvement of the primary tumor appeared to be weakly associated with worse outcomes, regardless of the timing (P = 0.058 at both tests). The effect of CEA level on both short and long-term progression events was also of borderline significance (P = 0.067 and P = 0.097, respectively). Surprisingly, patients with centrally located metastases displayed a better long-term outcome (P = 0.013). At Cox-regression analysis (Table 4), disease-free interval <36 months, multiple metastasis, and centrally-located metastases, but not the Glasgow score nor the categorized NTL ratio displayed an independent prognostic effect on PFS.
Figure 1.
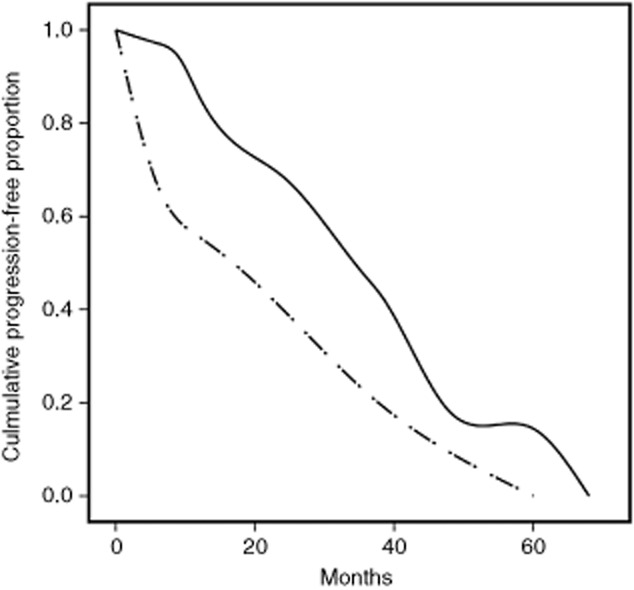
Kaplan-Meier plots of progression-free survival according to categorized neutrophil-to-lymphocyte ratio (NTL). Life-tables from year one to five are as follows: NTL = < 3 (continuous line): 94%, 85%, 68%, 43%, 18%; number at risk: 35; 32.5, 19, 12, 4; censored: 2, 5, 1, 0, 0. NTL > 3 (dashed line): 67%, 44%, 44%, 22%, 22%; number at risk: 9, 6, 4, 2, 1; censored: 0, 0, 2, 0, 0.
Table 4.
Progression-free survival: results of multivariate analysis
| Factor | P-value | Hazard ratio | 95% CI |
|---|---|---|---|
| DFI <36 months | 0.001 | 5.4 | 1.9–14.8 |
| Multiple metastasis | 0.001 | 4.5 | 1.8–10.9 |
| Centrally-located metastasis | 0.012 | 0.2* | 0.08–0.74 |
CI, confidence interval; DFI, disease-free interval.
Protective effect.
Overall survival
The five-year OS rate was 49% (mean: 62 months; standard error of the mean: 8.7). Factors associated with worse outcome in the Breslow and Cox-Mantel tests were multiple metastasis (P = 0.006/P = 0.002), elevated CEA level (P = 0.018/P = 0.009), and a modified Glasgow prognostic score >0 (P = 0.031/P = 0.029, Fig. 2). The NTL ratio was weakly associated with early (P = 0.055), but not with late (P = 0.12) deaths. Cox regression analysis showed an independent negative prognostic effect for multiple metastasis (P = 0.008, hazard ratio [HR]: 4.9, 95% CI: 1.5-16.0), whereas the modified Glasgow prognostic score was excluded from the model at the second step.
Figure 2.
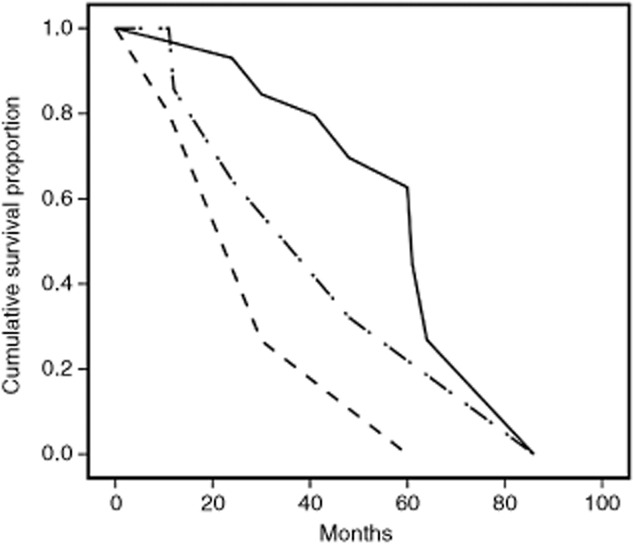
Kaplan-Meier analysis of overall survival according to modified Glasgow prognostic score m(GPS). Life-tables from year one to five are as follows: mGPS = 0 (continuous line): 97%, 97%, 85%, 80%, 69%; number at risk: 32, 28, 24, 18, 14; censored: 0, 6, 2, 3, 4. mGPS = 1 (dashed line): 100%, 83%, 63%, 63%, 31%; number at risk: 7, 6, 4, 2.5, 2; censored: 0, 2, 0, 1, 0. mGPS = 2 (dash-dot line): 84%, 80%, 27%, 27%, 0%; number at risk: 5, 3.5, 3, 1, 1; censored: 0, 1, 0, 0, 0.
Effect of specific growth rate
In the original dataset with missing values, the univariate impact of categorized SGR on OS was significant at P = 0.008 (median survival: 86 and 60 months in low and high-rate groups, respectively). At multivariate analysis, this variable showed a borderline independent effect (P = 0.057, HR: 4.9, CI: 0.94-25.5, correction method: pairwise deletion). A similar result was reproduced in only one out of the five virtual datasets (best prediction model: P = 0.047, HR: 2.7, CI 1.1–7.5).
Discussion
In recent years, surgical resection has gained acceptance as a treatment option in selected patients with colorectal cancer metastatic to the lung. Nonetheless, there is no robust evidence to support this practice,15 and it is still unclear whether the reported benefits of metastasectomy should be truly attributable to a treatment effect or to a less aggressive disease behavior in the subgroup of surgical candidates. However, given that metastases, by definition, represent a systemic disease, the basic idea is that surgical extirpation might serve as an adjuvant means to reduce the tumor burden. Host-related factors, including circulating cytokines, would then tip the balance in favor of tumor dormancy or progression, by interacting with both the immune-surveillance system and cancer growth mechanisms.16 A thorough understanding of these factors and their inclusion in clinical studies would be, therefore, of paramount importance in order to optimize indications and postoperative patient management in this setting.
In this regard, we aimed to evaluate whether a deranged systemic inflammation status could be associated with a more aggressive evolution of metastatic disease after complete resection, thereby working as an outcome predictor. To this purpose, we focused on two inexpensive and commonly used parameters, namely the NTL ratio and the modified Glasgow prognostic score. Our results showed that, despite the fact that these parameters are associated with a favorable long-term outcome at univariate analysis, they did not perform well as independent prognosticators when included in a multivariate model.
The efficacy of C-reactive protein based scores as independent survival predictors in colorectal cancer and other malignant and non-malignant diseases has been widely reported in previous studies.17–28 The largest and most relevant study in this field is that of Proctor et al.18 They first reported the prognostic value of the modified Glasgow prognostic score on more than 27 000 patients with diverse primary cancers at a P-level <0.001. Thereafter, other groups applied the same parameter to specific types of cancers and diverse clinical scenarios. In particular, the C-reactive protein and the Glasgow prognostic score were shown to have prognostic value in metastatic colorectal cancer patients who were managed either surgically28 or within a multimodality strategy.20,21,23,27 However, in patients with a limited extent of disease, these parameters may be somewhat less reliable. In this regard, Sugimoto et al. found that the prognostic value of a modified Glasgow prognostic score in patients with resectable colorectal cancer is stage specific and substantially invalidated by the presence of nodal involvement.26 Fujii et al. have recently shown that, although the C-reactive protein level was associated with tumor depth in patients with resectable colorectal cancer, it did not have any impact on disease-free survival.22 Similar considerations apply as far as the neutrophil-to-lymphocyte ratio is concerned. Indeed, although numerous studies have focused on the efficacy of this clinical measure in predicting the outcome of colorectal cancer patients in general,29–33 some discordant findings do exist. In particular, Jankova et al.34 failed to find an independent association between an elevated NTL ratio and cancer-related survival rates. These results suggest that, although systemic inflammation parameters remain important prognosticators in cancer patients, their relevance could be somewhat limited when applied to more restricted subgroups, especially those with good performance status and limited tumor burden.34
The analysis of a prospectively collected database gave us the opportunity to explore some hypotheses other than the main objective of the study. We found a relationship between active cigarette smoking and the likelihood of having centrally-located metastases. Although the association between cigarette smoking and pulmonary metastatization is a known topic of surgical oncology, we are not aware whether such a finding has been previously reported. A possible explanation could be that, in active smokers, persistent central airway inflammation could create a favorable microenvironment to metastatic seeding. If confirmed by further studies, this finding could suggest the need for a tailored postsurgical follow-up protocol for these patients.
In our study, patients who received anatomical lung resection showed a better PFS. This finding could reflect a bias. Indeed, it is reasonable that a decision for more aggressive surgery, as dictated by anatomical reasons, was made only for patients falling into an ideal low-recurrence risk subgroup. In this regard, there is no evidence to support the routine employ of anatomical resections in pulmonary metastasectomy, therefore, we continue to consider parenchyma-sparing procedures to be the standard of treatment whenever they are technically feasible.35 Measurements of tumor growth have often been indicated as one of the most important tools in clinical studies on colorectal cancer metastasis. Recently, Poullis et al.36 have presented an elegant algorithm by which the evolution of metastatic disease and the chance of definitive cure could be estimated by taking into the account the tumor doubling time and disease-free interval. Such a model could be employed as a useful tool in assessing and understanding the benefits of pulmonary metastases in both retrospective and prospective studies. Therefore, we have been prompted to include the SGR in our study. The SGR had an independent effect on OS rate that was not found on PFS. It was also interesting that the SGR was not significantly associated with any of the usual predictors of lung metastasectomy, nor with any systemic inflammation parameter.
Limitations of the study have been partly discussed. They include the relatively small sample size, which did not allow stratification of the results for diverse risk factors, and the non-systematic nature of imaging-based tumor growth measurement. Furthermore, the assumption of proportional HR was not tested, thus possibly resulting in a somewhat misleading data interpretation. In particular, this was the case for NTL ratio, which displayed a much stronger association with survival rates when restricting the analysis to early events. A larger and stratified analysis is, therefore, welcome to confirm or reinforce our results, taking into consideration different patients' subcategories, as well as different postoperative time intervals.
Conclusion
In conclusion, we have reported that simple and inexpensive indicators of systemic inflammation are associated with worse survival rates in the setting of pulmonary metastasectomy for colorectal cancer. Although our data were insufficient in locating an independent effect, we think that these factors should be included in future studies. In particular, the interactions between systemic inflammation, anticancer immunosurveillance, and tumor dormancy/progression cycles should be thoroughly investigated, under the light of concurrent advances in multimodality treatment protocols and strategies.
Disclosure
No authors report any conflict of interest.
References
- Watanabe K, Saito N, Sugito IM, Ito M, Kobahyashi A, Nishizawa Y. Incidence and predictive factors of pulmonary metastases after curative resection for colorectal cancer. Ann Surg Oncol. 2013;20:1374–1380. doi: 10.1245/s10434-012-2747-y. [DOI] [PubMed] [Google Scholar]
- Zisis C, Tsakiridis K, Kougioumtzi I, et al. The management of advanced colorectal cancer: management of the pulmonary metastases. J Thorac Dis. 2013;5(Suppl. 4):S383–S388. doi: 10.3978/j.issn.2072-1439.2013.06.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedel G, Pastorino U, Buyse M, et al. Resection of lung metastases: long-term results and prognostic analysis based on 5206 cases – the International Registry of Lung Metastases. Zentralbl Chir. 1999;124:96–103. (In German.) [PubMed] [Google Scholar]
- Iida T, Nomori H, Shiba M, et al. Prognostic factors after pulmonary metastasectomy for colorectal cancer and rationale for determining surgical indications: a retrospective analysis. Ann Surg. 2013;257:1059–1064. doi: 10.1097/SLA.0b013e31826eda3b. [DOI] [PubMed] [Google Scholar]
- Gonzales M, Poncet A, Combescure C, Robert J, Ris HB, Gervaz P. Risk factors for survival after lung metastasectomy in colorectal cancer patients: a systematic review and meta-analysis. Ann Surg Oncol. 2013;20:572–579. doi: 10.1245/s10434-012-2726-3. [DOI] [PubMed] [Google Scholar]
- Surrouille I, Mordant P, Maggiori L, et al. Long-term survival after hepatic and pulmonary resection of colorectal cancer metastases. J Surg Oncol. 2013;108:220–224. doi: 10.1002/jso.23385. [DOI] [PubMed] [Google Scholar]
- Jarabo JR, Fernández E, Calatayud J, et al. More than one pulmonary resections or combined lung-liver resection in 79 patients with metastatic colorectal carcinoma. J Surg Oncol. 2011;104:781–786. doi: 10.1002/jso.22007. [DOI] [PubMed] [Google Scholar]
- Blackmon SH, Stephens EH, Correa AM, et al. Predictors of recurrent pulmonary metastases and survival after pulmonary metastasectomy for colorectal cancer. Ann Thorac Surg. 2012;94:1802–1809. doi: 10.1016/j.athoracsur.2012.07.014. [DOI] [PubMed] [Google Scholar]
- Watanabe K, Nagai K, Kobayashi A, Sugito M, Saito N. Factors influencing survival after complete resection of pulmonary metastases from colorectal cancer. Br J Surg. 2009;96:1058–1065. doi: 10.1002/bjs.6682. [DOI] [PubMed] [Google Scholar]
- Younes RN, Abrao F, Gross J. Pulmonary metastasectomy for colorectal cancer: long term survival and prognostic factors. Int J Surg. 2013;11:244–248. doi: 10.1016/j.ijsu.2013.01.003. [DOI] [PubMed] [Google Scholar]
- Iisaza T, Suzuki M, Yoshida S, et al. Prediction of prognosis and surgical indications for pulmonary metastasectomy from colorectal cancer. Ann Thorac Surg. 2006;82:254–260. doi: 10.1016/j.athoracsur.2006.02.027. [DOI] [PubMed] [Google Scholar]
- Inoue M, Ohta M, Iuchi K, et al. Benefits of surgery for patients with pulmonary metastases for colorectal carcinoma. Ann Thorac Surg. 2004;78:238–244. doi: 10.1016/j.athoracsur.2004.02.017. [DOI] [PubMed] [Google Scholar]
- Mehara E, Forssell-Aronsson E, Ahlman H, Bernhardt P. Specific growth rate versus doubling time for quantitative characterization of tumor growth rate. Cancer Res. 2007;67:3970–3975. doi: 10.1158/0008-5472.CAN-06-3822. [DOI] [PubMed] [Google Scholar]
- Mineo TC, Ambrogi V, Paci M, Iavicoli N, Pompeo E, Nofroni I. Transxiphoid bilateral palpation in video-assisted thoracoscopic lung metastasectomy. Arch Surg. 2001;136:783–788. doi: 10.1001/archsurg.136.7.783. [DOI] [PubMed] [Google Scholar]
- Treasure T, Fallowfield L, Lees B, Farewell V. Pulmonary metastasectomy in colorectal cancer: the PulMiCC trial. Thorax. 2012;67:185–187. doi: 10.1136/thoraxjnl-2011-200015. [DOI] [PubMed] [Google Scholar]
- Giancotti FG. Mechanisms governing metastatic dormancy and reactivation. Cell. 2013;155:750–764. doi: 10.1016/j.cell.2013.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizuka M, Kita J, Shimoda M, et al. Systemic inflammation response predicts postoperative outcome in patients with liver metastases from colorectal cancer. J Surg Oncol. 2009;100:38-42. doi: 10.1002/jso.21294. [DOI] [PubMed] [Google Scholar]
- Proctor MJ, Morrison DS, Tawlar D, et al. An inflammation-based prognostic score (mGPS) predicts cancer survival independent of tumour site: a Glasgow Inflammation Outcome Study. Br J Cancer. 2011;15:726–734. doi: 10.1038/sj.bjc.6606087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin M, Huang J, Zhu J, Shen H. Elevated pre-treatment levels of high sensitivity C-reactive protein as a potential prognosticator in patients with colorectal cancer. Exp Ther Med. 2013;6:1369–1374. doi: 10.3892/etm.2013.1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuchi M, Kuwabara K, Tsuji Y, et al. C-reactive protein is a negative independent factor in patients with stage IV colorectal cancer undergoing oxaliplatin-based chemotherapy. Anticancer Res. 2013;33:5051–5055. [PubMed] [Google Scholar]
- Ikeguchi M, Shimoda R, Yamamoto M, Maeta Y, Ashida K, Saito H. Scoring of prognostic parameters in patients with unresectable advanced or recurrent colorectal cancer undergoing chemotherapy. Yonago Acta Med. 2013;56:69–72. [PMC free article] [PubMed] [Google Scholar]
- Fujii T, Yajima R, Tabe Y, et al. Elevated C-reactive protein is associated with the tumor depth of invasion but not with disease recurrence in stage II and III colorectal cancer. Hepatogastroenterology. 2013;60:1343–1347. doi: 10.5754/hge11214. [DOI] [PubMed] [Google Scholar]
- Dréanic J, Maillet M, Dhooge M, et al. Prognostic value of the Glasgow Prognostic Score in metastatic colorectal cancer in the era of anti-EGFR therapies. Med Oncol. 2013;30:656. doi: 10.1007/s12032-013-0656-y. [DOI] [PubMed] [Google Scholar]
- Toiyama Y, Fujikawa H, Koike Y, et al. Evaluation of preoperative C-reactive protein aids in predicting poor survival in patients with curative colorectal cancer with poor lymph node assessment. Oncol Lett. 2013;5:1881–1888. doi: 10.3892/ol.2013.1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasaku C, Shimada M, Kurita N, et al. Impact of C-reactive protein on prognosis of patients with colorectal carcinoma. Hepatogastroenterology. 2013;60:507–511. doi: 10.5754/hge11425. [DOI] [PubMed] [Google Scholar]
- Sugimoto K, Komiyama H, Kojima Y, Goto M, Tomiki Y, Sakamoto K. Glasgow prognostic score as prognostic factor in patients undergoing curative surgery for colorectal cancer. Dig Surg. 2012;29:503–509. doi: 10.1159/000346002. [DOI] [PubMed] [Google Scholar]
- Inoue Y, Iwata T, Okugawa Y, et al. Prognostic significance of a systemic inflammatory response in patients undergoing multimodality therapy for advanced colorectal cancer. Oncology. 2013;84:100–107. doi: 10.1159/000343822. [DOI] [PubMed] [Google Scholar]
- Ishizuka N, Nagata H, Takagi K, Iwasaki Y, Kubota K. Inflammation-based prognostic system predicts survival after surgery for stage IV colorectal cancer. Am J Surg. 2013;205:22–28. doi: 10.1016/j.amjsurg.2012.04.012. [DOI] [PubMed] [Google Scholar]
- Li MX, Liu XM, Zhang JF, et al. Prognostic role of neutrophil-to-lymphocyte ratio in colorectal cancer: a systematic review and meta-analysis. Int J Cancer. 2014;134:2403–2413. doi: 10.1002/ijc.28536. [DOI] [PubMed] [Google Scholar]
- Shibutani M, Maeda K, Nagahara H, et al. A high preoperative neutrophil-to-lymphocyte ratio is associated with poor survival in patients with colorectal cancer. Anticancer Res. 2013;33:3291–3294. [PubMed] [Google Scholar]
- He W, Yin C, Guo G, et al. Initial neutrophil to lymphocyte ratio is superior to platelet lymphocyte ratio as an adverse prognostic and predictive factor in metastatic colorectal cancer. Med Oncol. 2013;30:439. doi: 10.1007/s12032-012-0439-x. [DOI] [PubMed] [Google Scholar]
- Chiang SF, Hung HY, Tang R, et al. Can neutrophil-to-lymphocyte ratio predict the survival of colorectal cancer patients who have received curative surgery electively? Int J Colorectal Dis. 2012;27:1347–1357. doi: 10.1007/s00384-012-1459-x. [DOI] [PubMed] [Google Scholar]
- Malietzis G, Giacometti M, Askari A, et al. A preoperative neutrophil to lymphocyte ratio of 3 predicts disease-free survival after curative elective colorectal cancer surgery. Ann Surg. 2013 doi: 10.1097/SLA.0000000000000216. . doi: 10.1097/SLA.0000000000000216. [DOI] [PubMed] [Google Scholar]
- Jankova L, Dent OF, Chan C, Chapuis P, Clarke SJ. Preoperative neutrophil/lymphocyte ratio predicts overall survival but does not predict recurrence or cancer-specific survival after curative resection of node-positive colorectal cancer. BMC Cancer. 2013;13:442. doi: 10.1186/1471-2407-13-442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mineo TC, Ambrogi V, Tonini G, Nofroni I. Pulmonary metastasectomy: might the type of resection affect survival? J Surg Oncol. 2001;76:47–52. doi: 10.1002/1096-9098(200101)76:1<47::aid-jso1008>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Poullis M, Littler J, Gosney J. Biology of colorectal pulmonary metastasis: implications for surgical resection. Interact Cardiovasc Thorac Surg. 2012;14:140–142. doi: 10.1093/icvts/ivr050. [DOI] [PMC free article] [PubMed] [Google Scholar]


