Abstract
Background
Vibrio parahaemolyticus (V. parahaemolyticus) is a Gram-negative, halophilic bacterium recognized as one of the most important foodborne pathogen. When ingested, V. parahaemolyticus causes a self-limiting illness (Vibriosis), characterized mainly by watery diarrhoea. Treatment is usually oral rehydration and/or antibiotics in complicated cases. Since 1996, the pathogenic and pandemic V. parahaemolyticus O3:K6 serotype has spread worldwide, increasing the reported number of vibriosis cases. Thus, the design of new strategies for pathogen control and illness prevention is necessary. Lactobacillus sp. grouped Gram positive innocuous bacteria, part of normal intestinal microbiota and usually used as oral vaccines for several diarrheic diseases. Recombinants strains of Lactobacillus (RL) expressing pathogen antigens can be used as part of an anti-adhesion strategy where RL block the pathogen union sites in host cells. Thus, we aimed to express MAM-7 V. parahaemolyticus adhesion protein in Lactobacillus sp. to generate an RL that prevents pathogen colonization.
Results
We cloned the MAM-7 gene from V. parahaemolyticus RIMD 2210633 in Lactobacillus expression vectors. Recombinant strains (Lactobacillus rhamnosus pSEC-MAM7 and L. rhamnosus pCWA-MAM7) adhered to CaCo-2 cells and competed with the pathogen. However, the L. rhamnosus wild type strain showed the best capacity to inhibit pathogen colonization in vitro. In addition, LDH-assay showed that recombinant strains were cytotoxic compared with the wild type isogenic strain.
Conclusions
MAM-7 expression in lactobacilli reduces the intrinsic inhibitory capacity of L. rhamnosus against V. parahaemolyticus.
Keywords: Lactobacillus, V. parahaemolyticus, Adhesion, Recombinants probiotics
Background
Vibrio parahaemolyticus (V. parahaemolyticus) is a Gram-negative halophilic bacterium; one of the most important foodborne pathogens worldwide. Autochthonous to estuarine, marine and coastal environments, V. parahaemolyticus causes acute gastroenteritis by consumption of raw, undercooked and/or mishandled seafood [1]. The illness vibriosis is mainly characterized by diarrhoea. It is self-limiting and usually treated by oral rehydration. In some cases antibiotics are necessary [1]. Recently, the CDC reported an increment of 75 % in vibriosis cases in the US during 2013, compared with the period 2006–2008. During 2010–2013, the increment observed was 32 %. Considering that the CDC estimates that for every V. parahaemolyticus case reported, there are 142 cases not diagnosed, official data are clearly underestimated [http://www.cdc.gov/foodnet/data/trends/trends-2013-progress.html]. In Chile, according the Epidemiology Department of the Chilean Ministry of Health, during the summer of 2015, there was an increase of nearly 56 % of the number of cases in comparison with 2014 (Departamento de Epidemiología, Ministerio de Salud de Chile, 2015. http://epi.minsal.cl/). Thus, it is imperative to design new strategies for the control of this pathogen.
Pandemic V. parahaemolyticus O3:K6 strain was first detected in Osaka (Japan) in 1950, and since 1996 this serotype has been spread throughout India, Europe, Africa, North, Central and South America [1]. In addition to classical V. parahaemolyticus TDH and TRH virulence factors, O3:K6 exhibited specific genetic markers as toxRS/New and orf8 [2, 3]. Additionally, V. parahaemolyticus O3:K6 strain RIMD 2210633, was detected for the first time in Japan in 1996, encoded for Type 3 secretion system (T3SS) and a pathogenicity island (VPaI-7) [4].
In addition, an outer membrane protein, multivalent adhesion molecule (MAM) which includes MCE (from Mammalian cell entry domains) was recently described in V. parahaemolyticus [5, 6]. The protein, named MAM-7, can mediate pathogen attachment to mammalian cells even in the absence of other adhesion proteins [5, 6].
Because adhesion of V. parahaemolyticus to host cellular membranes is a crucial step for the delivery of pathogen toxins, MAM-7 could be considered as a virulence factor [7, 8]. Thus, interference with MAM-7-mediated adhesion can prevent or treat vibriosis.
Interference with the adhesion of the pathogen is known as anti-adhesion therapy (AAT). AAT includes disruption of host pathogen receptor biogenesis; analogues to compete with these receptors or antibodies block surface epitopes required for pathogen binding [9]. The analogues of adhesion can be used purified [10] or expressed (secreted or anchored in the outer membrane) in non-pathogenic bacteria. Non-pathogenic bacteria used for this purpose are those that form part of normal microbiota (as non-pathogenic Escherichia coli) or beneficial bacteria (probiotics) as members of acid lactic bacteria (i.e. Lactobacillus sp.).
Lactobacillus sp. are generally recognized as safe (GRAS), and can colonize human intestinal mucosa. In addition, Lactobacillus strains can produce inhibitory compounds such as organic acids, hydrogen peroxide and bacteriocins that have antimicrobial properties. For example, L. plantarum AS1 show antibacterial activity against several enteropathogens and inhibit V. parahaemolyticus adhesion to the human intestinal cell line HT-29 [11]. Additionally, mice treated with L. rhamnosus and L. brevis showed a significant reduction in intestinal fluid accumulation and villi damage when animals were challenged with the pathogen [12].
Given these characteristics, Lactobacillus strains are good candidates for mucosal vaccines and/or therapeutic delivery vehicles. In fact, recombinant lactobacilli expressing pathogen antigens can elicit mixed Th1/Th2 immune responses; induce specific antibodies, dendritic cell maturation and production of pro-inflammatory cytokines [13]. The most common strategy to generate recombinant lactobacilli is the cloning of a determined antigen-encoded gene in expression vectors [12]. One example is the use of expression vectors pSEC and pCWA to target heterologous proteins to different secreted or cell wall destinations in Lactococcus and Lactobacillus [14–16]. pSEC harbours a transcriptional fusion between the ribosome-binding site (RBSUsp45) and the signal peptide sequence (SPUsp45) of the usp45 gene, allowing antigen release into the medium. In addition, pCWA encoded for Streptococcus pyogenes M6 protein cell wall-anchor region CWAM6 [14–18].
We evaluated the role of recombinant Lactobacillus strains containing pSEC-MAM7 and pCWA-MAM7 in the adhesion to human epithelial cell lines and their capacity to inhibit V. parahaemolyticus colonization in vitro.
Our results showed that L. rhamnosus wild type can inhibit and compete in a significantly more efficient manner than both the recombinant strains pSEC-MAM7 and pCWA-MAM7. In addition, these recombinant strains showed a significantly higher grade of cytotoxicity than the L. rhamnosus wild type. Thus, MAM-7 expression in lactobacilli does not improve the intrinsic inhibitory capacity of L. rhamnosus against V. parahaemolyticus.
Results
Recombinant L. rhamnosus strains
Primers were design from V. parahaemolyticus reported sequence RIMD 2210633 (GenBank: NC_004603). The PCR product of approx 2600 pb were cloned in pSEC and pCWA and used to transform L. rhamnosus. PCR was used to check the presence of MAM-7 gene in recombinant L. rhamnosus strains. To check if MAM-7 gene was transcribed, we performed an RT-PCR (Fig. 1). Figure 1 shows the amplicon of MAM-7 gene (Fig. 1a) and amplification of MAM-7 transcript (Fig. 1b) in both L. rhamnosus pSEC-MAM7 and L. rhamnosus pCWA-MAM7 strains.
Fig. 1.
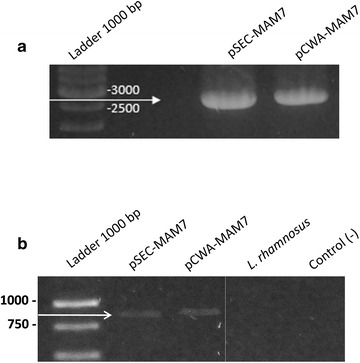
PCR and RT-PCR of MAM-7 from recombinants L. rhamnosus strains. a PCR of MAM-7 gene from L. rhamnosus pSEC-MAM-7 (pSEC-MAM7) and L. rhamnosus pCWA-MAM-7 (pCWA-MAM7) strains. b RT-PCR of MAM-7 transcript from L. rhamnosus pSEC-MAM-7 (pSEC-MAM7), L. rhamnosus pCWA-MAM-7 (pCWA-MAM7) and L. rhamnosus wild type (L. rhamnosus) strains
To assess if MAM-7 protein was expressed in both L. rhamnosus pSEC-MAM7 and L. rhamnosus pCWA-MAM7 strains, we performed an SDS-PAGE (Fig. 2) where it was expected a MAM-7 protein size of about 97 kDa. Figure 2 shows a weak band that corresponds to a protein of about 100 kDa (black arrows) suggesting the presence of MAM-7 in L. rhamnosus pSEC-MAM7 and L. rhamnosus pCWA-MAM7 strains. Thus, to check the presence of MAM-7 protein in recombinants strains, an immunodetection assay using anti-Vibrio (KPL) and anti-rabbit fluorescent (Molecular Probes, Thermo Fisher Scientific) antibodies was performed (Fig. 3). Figure 3 shows that while in L. rhamnosus wild type did not show fluorescence, both L. rhamnosus pSEC-MAM7 and L. rhamnosus pCWA-MAM7 strains showed fluorescent bacteria. The previous results strongly suggest that recombinant bacterial strains were expressing V. parahaemolyticus MAM-7 protein.
Fig. 2.
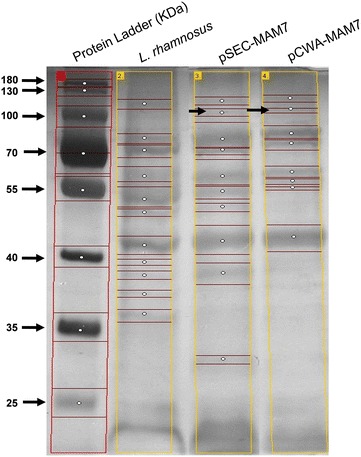
SDS-PAGE electrophoresis from recombinants L. rhamnsus strains. The figure shows the protein profile from L. rhamnosus pSEC-MAM-7 (pSEC-MAM7), L. rhamnosus pCWA-MAM-7 (pCWA-MAM7) and L. rhamnosus wild type (L. rhamnosus). Black arrows show a weakly band of approximately 100 kDa in recombinants strains. Bands of each lane were detected using GelAnalyzer and each white circle indicates a band
Fig. 3.
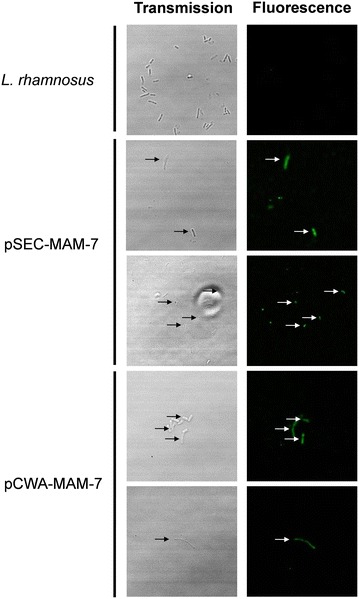
Expression of MAM-7 protein in L. rhamnosus. MAM-7 protein was detected using anti-Vibrio antibody and revealed with a secondary antibody alexa fluor® conjugated. The figures shows green fluorescence of alexa fluor® only in recombinant L. rhamnosus strains (L. rhamnosus pSEC-MAM-7 and L. rhamnosus pCWA-MAM-7). As a control, L. rhamnosus wild type was used
To assess if the expression of MAM-7 alters (or not) both L. rhamnosus pSEC-MAM7 and L. rhamnosus pCWA-MAM7 growth, a growth curve at 37 ℃ was performed (Fig. 4a). As shown in Fig. 4a, growth of recombinant strains was not negatively altered with the expression of MAM-7 compared with the wild type.
Fig. 4.
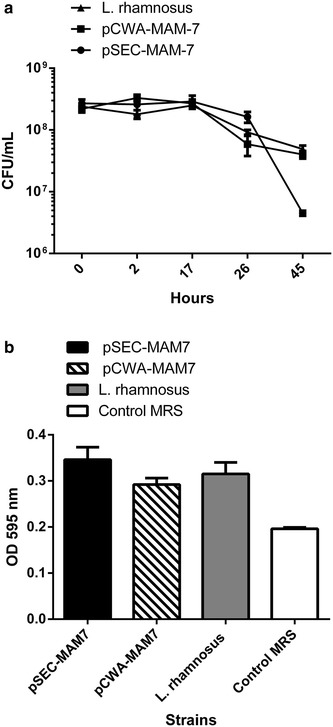
Effect of MAM-7 expression in L. rhamnosus in vitro growth. a Growth curve of L. rhamnosus pCWA-MAM-7 (black squares), L. rhamnosus pSEC-MAM-7 (black circles) recombinants strains and L. rhamnosus wild type strain (black triangle). b Biofilm formation of L. rhamnosus pSEC-MAM-7 (black column), L. rhamnosus pCWA-MAM-7 (striped column) recombinants strains and L. rhamnosus wild type strain (grey column). White column shows control with sterile MRS broth. The figure shows values expressed as the mean ± standard deviation of three full biological replicates, each time in technical triplicate
Adherent bacteria, such as L. rhamnosus, can colonize different environments as biofilms, where a self-produced extracellular matrix protects bacteria against environmental conditions [19]. Biofilm formation can be altered by the expression of the MAM-7 protein in L. rhamnosus recombinants strains. Therefore, we evaluated the biofilm formation capacity of L. rhamnosus recombinant and wild type strains using crystal violet staining protocol (Fig. 4b). Figure 4b not shows significant differences between recombinant and wild type strains. Hence, MAM-7 expression did not alter the intrinsic capacity of L. rhamnosus to form biofilm.
Adhesion of recombinants L. rhamnosus strains to epithelial cell lines
MAM-7 is an outer membrane protein involved in host cell early adhesion in Gram negative pathogens including V. parahaemolyticus [6]. Thus, our objective was to determine if the expression of MAM-7 from RIMD in L. rhamnosus promotes bacterial adhesion to both human epithelial cell lines CaCo-2 and HEp-2 (Fig. 5a). As shown in Fig. 5a, MAM-7 did not promote higher adhesion in recombinant L. rhamnosus strains than the L. rhamnosus wild type. Furthermore, L. rhamnosus pSEC-MAM7 (pSEC-MAM7) showed significantly lesser adhesion than L. rhamnosus pCWA-MAM7 (pCWA-MAM7) and the L. rhamnosus wild type. In addition, all L. rhamnosus strains (recombinants and wild type strains) were significantly more adhesive than RIMD.
Fig. 5.
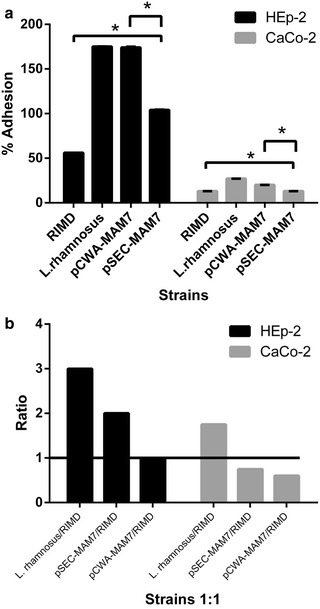
Adhesion and competition assays of V. parahaemolyticus and L. rhamnosus strains in epithelial cell lines. a Adhesion assay. The figure shows the adhesion percentage of L. rhamnosus strains and V. parahaemolyticus in epithelial cell line (HEp-2, black bars) and colon carcinoma cell line (CaCo-2, gray bars) monolayers. The strains used were V. parahaemolyticus RIMD 2210633 (RIMD), L. rhamnosus wild type (L. rhamnosus), L. rhamnosus/pCWA-MAM-7 (pCWA-MAM7) and L. rhamnosus/pSEC-MAM-7 (pSEC-MAM7). The results were expressed such as the percentage of adhesion at 3 h compared with initial inoculums of each strain at time zero. The figure shows values expressed as the mean ± standard deviation of three full biological replicates, each time in technical triplicate. *p < 0.001 (Student’s-Test). b Competition assay. The figure shows the results about the role of L. rhamnosus strains in the inhibition of V. parahaemolyticus from colonizing epithelial cell line (HEp-2, black bars) and colon carcinoma cell line (CaCo-2, gray bars) monolayers. The strains used were V. parahaemolyticus RIMD 2210633 (RIMD), L. rhamnosus wild type (L. rhamnosus), L. rhamnosus/pCWA-MAM-7 (pCWA-MAM7) and L. rhamnosus/pSEC-MAM-7 (pSEC-MAM7). Values expressed as a ration between the number of Lactobacillus strains UFC versus V. parahaemolyticus UFC after 3 h of monolayers 1:1 co-infection. The figure represents the result of three full biological replicates, each time in technical triplicate. The figure shows values expressed as the mean ± standard deviation of three full biological replicates, each time in technical triplicate. *p < 0.001 (Student’s-Test)
Inhibition of V. parahaemolyticus from colonizing HEp-2 and CaCo-2 cell lines
Competition assay: Krachler et al. demonstrated that E. coli BL21 expressing MAM-7 inhibits V. parahaemolyticus attachment in HeLa cells [7, 9]. Thus, we expected that recombinant L. rhamnosus strains inhibit V. parahaemolyticus attachment in HEp-2 and/or CaCo-2 cell lines. Figure 5b shows that all L. rhamnosus strains efficiently competed with RIMD in HEp-2 cell line but L. rhamnosus wild type inhibition was higher than the recombinant strains. In CaCo-2, only L. rhamnosus wild type inhibited RIMD adhesion. Hence, L. rhamnosus wild type inhibited the adherence of V. parahaemolyticus to HEp-2 and Caco-2 cell lines more efficiently than recombinants strains.
Exclusion assay: To asses if V. parahaemolyticus could adhere to CaCo-2 already colonized with L. rhamnosus wild type or recombinant strains, we performed an exclusion assay in this cell line (Fig. 6). All L. rhamnosus strains significantly excluded RIMD from the CaCo-2 cell line. However, the L. rhamnosus wild type was significantly better than recombinant strains in excluding the pathogen. In fact, when RIMD was incubated with CaCo-2, a 16 % of adhesion was obtained. However, when we pre-colonized CaCo-2 with L. rhamnosus wild type, only 1 % of the adhered pathogen was recovered.
Fig. 6.

Competitive exclusion of L. rhamnosus to protect CaCo-2 cell line from V. parahaemolyticus colonization. The figure shows the results about the role of L. rhamnosus strains in the inhibition of V. parahaemolyticus from colonizing colon carcinoma cell line (CaCo-2) monolayers. The strains used were V. parahaemolyticus RIMD 2210633 (RIMD), L. rhamnosus wild type (L. rhamnosus), L. rhamnosus pCWA-MAM-7 (pCWA-MAM7) and L. rhamnosus pSEC-MAM-7 (pSEC-MAM7). Values are expressed as the adhesion percentage of V. parahaemolyticus after 3 h of L. rhamnosus wild type (L. rhamnosus– RIMD), pCWA-MAM-7 (pCWA-MAM7– RIMD) or pSEC-MAM-7 (pSEC-MAM7– RIMD) strains monolayer colonization. V. parahaemolyticus adhesion, without previous Lactobacillus treatment, is expressed as “Adhesion” bar. The figure shows values expressed as the mean ± standard deviation of three full biological replicates, each time in technical triplicate. *p < 0.001 (Student’s-Test)
Cytotoxic effect of L. rhamnosus expressing MAM-7
It has been reported that MAM-7 mediates V. parahaemolyticus TDH- independent cytotoxicity [6]. Thus, we wanted to know if Lactobacillus recombinant strains could generate cytotoxicity in CaCo-2 cell lines. Both pSEC-MAM7 and pCWA-MAM7 were significantly more cytotoxic than L. rhamnosus wild type (Fig. 7). However, cytotoxicity levels were significantly lower than the positive control. Our results suggest that the presence of MAM-7 in L. rhamnosus can induce cytotoxicity in the CaCo-2 cell line.
Fig. 7.
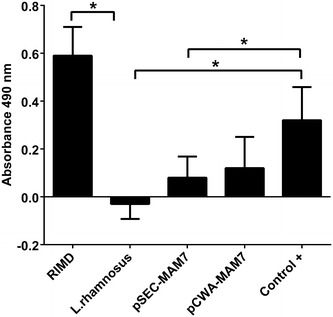
Cytotoxicity of V. parahaemolyticus and recombinants L. rhamnosus strains in CaCo-2 cell line. The figure shows LDH level release as absorbance at 490 nm of V. parahaemolyticus and Lactobacillus strains in colon carcinoma cell line (CaCo-2) monolayers. The strains used were V. parahaemolyticus RIMD 2210633 (RIMD), L. rhamnosus wild type (L. rhamnosus), L. rhamnosus pCWA-MAM-7 (pCWA-MAM7) and L. rhamnosus pSEC-MAM-7 (pSEC-MAM7). As a positive control (Control+), CaCo-2 were treated with Tritón ×100. The figure represents the result of three full biological replicates, each time in technical triplicate. *p < 0.001 (Student’s-Test)
Discussion
We evaluated the anti-adhesion capacity of L. rhamnosus recombinant strains against V. parahaemolyticus compared to the wild type, in HEp-2 and CaCo-2 epithelial cell lines.
Adhesion of recombinant L. rhamnosus strains was not better than that of L. rhamnosus wild type. In fact, in CaCo-2, an epithelial cell line that mimics epithelial cells of human colon, recombinant strains adhesion levels were significantly lower. L. rhamnosus are some of the most adhesive Lactobacillus species in CaCo-2 and HEp-2 cell lines [20, 21] and their adhesive capacity is mediated by numerous and varied surface proteins (such as S-layers proteins) and other structural molecules that form part of their surface architecture [22–24]. However, in Gram-negative bacteria, MAM-7 mce domains mediate attachment to host tissues by interaction with host fibronectin and membrane lipid phosphatidic acid (PA) [6]. Thus, bacteria that have the ability to bind to host fibronectin can be inhibited by the presence of MAM-7. Such is the case of the Gram-positive pathogen Staphylococcus aureus, which employs adhesins to attach and invade host cells through the interaction with fibronectin and can be inhibited by MAM-7 derived peptides [9, 25]. Recently, it was found that L. rhamnosus possesses a mucus-binding factor (MBF) that binds to mucin, laminin, collagen and fibronectin [26, 27]. Thus, the presence of attached or secreted MAM-7 in cell cultures could inhibited recombinant L. rhamnosus adhesion to epithelial cell lines and/or negatively interfere with intrinsic adhesion mechanisms of L. rhamnosus.
In our study, the presence of MAM-7 in L. rhamnosus induced cytotoxicity in CaCo-2 cell lines, suggesting that V. parahaemolyticus MAM-7 may trigger death in this adenocarcinoma cell line. In addition, Lim et al. demonstrated that V. parahaemolyticus MAM-7 is sufficient to disrupt the epithelial barrier and promote cell lysis. In HeLa as well as in the CaCo-2 cell line, MAM-7 triggered disruption of the epithelial barrier and a marked decreased in transepithelial electrical resistance (TER) [28].
V. parahaemolyticus MAM-7 recognizes host membrane PA lipids and can cluster on cell surface triggering the activation of the small GTPase RhoA [28]. In the same way, activation of Rho-associated serine/threonine kinase (ROCK) was observed downstream RhoA [28]. ROCK protein signalling can acts both a pro- and anti-apoptotic fashion depends of cell type, cell context and microenvironment [29]. ROCK family contains two members: ROCK1 and ROCK2 [30]. ROCK1 is related with cellular blebbing and blebbing is a conserved event of the apoptotic execution phase [29, 31]. Given that release of lactate dehydrogenase is related with either apoptosis or necrosis events, the level of LDH activity as a result of MAM-7 expression in L. rhamnosus could be related with apoptotic processes related with ROCK activation. Although there are a number of studies of pro-survival role of ROCK, ROCK proteins are essential for multiple aspects of both the intrinsic and extrinsic apoptotic processes [29]. The molecular mechanisms that modulate these pleiotropic roles are largely unknown. Thus, Lactobacillus expression of MAM-7 could have a role inducing apoptosis in CaCo-2 carcinoma cell line.
Conclusion
L. rhamnosus can inhibit adhesion of V. parahaemolyticus in human epithelial cell lines. Expression of V. parahaemolyticus MAM-7 adhesin in L. rhamnosus decreases the intrinsic capacities of L. rhamnosus to inhibit V. parahaemolyticus in vitro.
Methods
Bacterial strains and culture conditions
V. parahaemolyticus (RIMD 2210633) serotype O3:K6 was obtained from Instituto de Salud Pública, (ISP, Chile). The strain was routinely grown in Luria–Bertani broth supplemented with 1,5 % NaCl (LBS) (Merck) and cultured with agitation at 37 ℃ for 12–24 h. L. rhamnosus LL1 was previously isolated from fermented dairy food, and was grown in de Man Rogosa and Sharpe broth (MRS, Merck) at 5–10 % CO2 (capnophilically) and at 37 ℃ without agitation for 48 h.
L. rhamnosus recombinants strains
The MAM-7 gene was amplified from genomic DNA from V. parahaemolyticus using the primers MAM7-F (CCGATATCTCCGTATGTGCCTGATGTTAAGAGGA) and MAM7-R (CCAATGCATCAAGGGCTTAGGAATTGGCGTT) designed from V. parahaemolyticus reported sequence RIMD 2210633 (GenBank: NC_004603.1). PCR amplification was performed using 1.25 U of Dream Taq® (Fermentas), 40 ng of genomic DNA from RIMD, and 65 ℃ of annealing temperature (30 cycles). The amplification product was cloned in pSEC and pCWA plasmids at EcoRV and NsiI restriction sites to yield the plasmids pSEC-MAM7 and pCWA-MAM7. Resulting recombinant vectors were used to transform L. rhamnosus according to Serror et al. [32]. Presence of MAM-7 gene in L. rhamnosus strains was confirmed by PCR amplification and restriction endonuclease analyses. Expression of MAM-7 from each plasmid was assessed by RT-PCR using primers MAMRT-F (GCTCTACCTGACGACAACAAAGT) and MAMRT-R (TTCCTATCGGTGCGGTCTAC) (Fig. 1) and Western blot (Data not shown).
Protein extraction
40 mL of MRS cultures of each recombinant L. rhamnosus and wild type strains was centrifuged at top speed for 2 min and washed once with PBS 1X (Gibco). Bacterial pellets were resuspended in digestion buffer (50 mM Tris–HCL pH 8,0; 5 mM MgCl2; 50 mM EDTA; 10 mg/mL lysozyme) and incubated by 3 h at 37 ℃. Glass pearls were then added and lysates were vortexed for 120 s. Then, the lysates were centrifuged at 7000xg for 15 min. Supernatants were recovered and sonicated in ice three times for 10 s each. Proteins were precipitated with 10 % trichloroacetic acid (TCA, Merck) for 12 h and then centrifuged at top speed at 4 ℃ for 20 min. Supernatants were discarded, and pellets were washed once with cold acetone and centrifuged at top speed at 4 ℃ for 5 min. Obtained pellets were resuspended with buffer (100 mM Tris–HCl pH 8,0) and stored at −20 ℃ until their use.
Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE)
To evaluate the presence of MAM-7 protein, a SDS-PAGE was performed. A polyacrylamide gel (with 10 % acrylamide in separating gel) was prepared according to standard protocol. 50 μg of each protein sample was loaded and gel was run in a Mini-PROTEAN chamber at 100 V for 2 h (BioRad) in pH 8,3 Tris-glicine-SDS buffer. The gel was stained with silver using the Pierce Silver Stain Kit (Thermo Fisher Scientific) according the instruction of manufacturer. Gel electrophoresis bands were analyzed using freeware 1D gel electrophoresis image analysis software GelAnalyzer (http://www.gelanalyzer.com/index.html).
Fluorescent immunodetection assay
To asses if recombinant L. rhamnosus strains expressed MAM-7, bacterial samples were placed onto coverslips previously treated with poly-l-lysine (Sigma) for 20 min at room temperature. Each coverslip with the sample was dried at 37 ℃ and fixed with 3 % paraformaldehyde (PFA, Merck) for 15 min at room temperature. Then, coverslips were put on 24-well cell culture plate (TrueLine) and washed three times with PBS 1 × (Gibco). Samples were then blocked using 1 % albumin bovine serum (BSA, Calbiochem®) for 1 h at room temperature in humidity chamber. Each sample was incubated with 50 μL of 1:50 anti-Vibrio primary antibody (BacTrace anti-vibrio species antibody, KPL) for 12 h at 4 ℃. Once time had passed, each sample was washed with PBS 1 × (Gibco) and then, incubated with 50 μL of 1:500 goat anti-rabbit secondary antibody alexa fluor® 488 conjugate (molecular probes) in darkness and humidity chamber for 2 h. After that, samples were washed three times with PBS (Gibco) and coversilps were mounted over microscope slides with Dako fluorescent mounting medium (Fluoromont-g Emsdiasum) for 1 h in darkness. Image adquisition was performed in Leica TCS SP8, DMI6000 CS (Leica Microsystems). Adquisition was performed with argon laser, HC PL APO CS2 63x/1.40 oil objective, and optic slices were set at 0.895 μm.
Growth curves
To assess if MAM-7 expression alters the growth of L. rhamnosus recombinants strains, Lactobacillus strains were cultivated in MRS broth (wild type), or MRS supplemented with chloramphenicol (recombinants strains) at 5–10 % CO2 at 37 ℃. Then, an aliquot of each culture was aseptically obtained at 0, 2, 17, 26 and 45 h and serially diluted in MRS. The bacterial concentration was determined according the number of CFU/mL of MRS. The assay was performed in triplicate.
Biofilm formation
To assess if MAM-7 expression alters intrinsic properties of Lactobacillus as biofilm formation, wild type and recombinant Lactobacillus strains were cultivated in MRS broth (wild type), or MRS supplemented with chloramphenicol (recombinants strains) at 5–10 % CO2 at 37 ℃ for 24 h. After the time, optical density at 600 nm (OD600nm) was measured. A volume of 100 μL of bacterial cultures adjusted at OD600nm 0, 2 were used to inoculate sterile 96-well plates (TrueLine) (108 cells/well) and grown at 5–10 % CO2 at 37 ℃ for 5 days. Sterile MRS broth was used as a control.
Crystal violet staining
To assess biofilm formation, crystal violet staining protocol was used. Cells suspensions were removed from test wells before washing three times with PBS. 100 μL of methanol (Winkler) was added to each test well and was incubated for 15 min at room temperature. After this treatment, methanol was discarded before drying. 100 μL of 2 % (w/v) crystal violet solution was added and wells were further incubated at room temperature for 5 min. After this incubation, the non-bound dye was removed and wells were washed with H2O. Bound crystal violet was dissolved using 33 % (v/v) of glacial acetic acid (Winkler). The resulting dissolved dye was measured at a wavelength of 590 nm using NanoQuant Infinite M200 PRO (TECAN).
Adhesion assay
To assess bacterial adhesion to epithelial cells, both HEp-2 and CaCo-2 cell lines were grown in 96-well plates (TrueLine) (105 cells/well) at 37 ℃ with 5 % CO2/95 % air atmosphere in DMEM High Glucose (HyClone). V. parahaemolyticus RIMD were grown overnight in LBS, while L. rhamnosus wild type and recombinant strains were grown overnight in a capnophilic environment in MRS broth (wild type), or MRS supplemented with chloramphenicol (recombinants strains) at 37 ℃. Bacterial cultures were harvested by centrifugation prior to colonisation of the cellular monolayers (approx. 108 UFC/mL of each strain). After 3 h of co-incubation at 37 ℃ in 5 % CO2 to allow bacteria to adhere to eukaryotic cells, monolayers were washed twice with phosphate-buffered saline (PBS, Gibco). Cells were then lysed with Tritón X-100 (1 %, in PBS), and the quantity of bacteria was determined. Quantitative adhesion assay values were calculated as follows:
% Adhesion = 100 × [(UFC mL−1 at 3 h post-colonization)/(UFC mL−1 added)]. The output is the result of three independent assays and each experiment was made in triplicate.
Competition assay
In this assay, L. rhamnosus strains (108 CFU/mL) and V. parahaemolyticus (108 CFU/mL) were added simultaneously to CaCo-2 and HEp-2 cell lines. The strains were then co-incubated for 3 h at 37 ℃ with 5 % CO2/95 % air atmosphere. After the incubation period, non-adhered bacteria were removed by washing with PBS, and adhered bacterial cells were recovered by lysis of eukaryotic cells with Tritón X-100 (1 %, in PBS). Bacterial count was expressed as: Ratio = [(UCF mL−1L. rhamnosus strain)/(UFC mL−1V. parahaemolyticus)] at 3 h. The output is the result of three independent assays and each experiment was made in triplicate.
Exclusion assay
For exclusion assays, approximately 108 CFU/mL of Lactobacillus strains (wild type or recombinant strains) were added to confluent CaCo-2 monolayers in 96-well plates, in presence of 5 % CO2/95 % air atmosphere at 37 ℃. After 3 h of co-incubation to allow bacteria adhere to eukaryotic cells, monolayers were washed twice with PBS (Gibco) to remove non-adhered bacteria. Then, 108 CFU/mL of V. parahaemolyticus was added to CaCo-2 already colonized by L. rhamnosus strains, which allowed further incubation of 3 h in presence of 5 % CO2/95 % air atmosphere at 37 ℃. After the co-incubation period, non-adhered bacteria were removed by washing with PBS, and bacteria were recovered by lysis of eukaryotic cells with Tritón X-100 (1 %, in PBS). Bacterial counts were expressed as percentage of adhered V. parahaemolyticus, as mentioned earlier. The output is the result of three independent assays and each experiment was made in triplicate.
LDH cytotoxicity assay
To assess if the expression of MAM-7 in L. rhamnosus can induce cytotoxicity in CaCo-2 cells, an LDH-Cytotxicity assay was performed. V. parahaemolyticus RIMD were grown overnight in LBS, while L. rhamnosus wild type and recombinant strains were grown overnight in a capnophilic environment in MRS broth (wild type), or MRS supplemented with chloramphenicol (recombinants strains) at 37 ℃. Bacterial cultures were harvested by centrifugation prior to colonisation of the cellular monolayers (approx. 108 UFC/mL of each strain). After 4 h supernatants from CaCo-2 cells colonized by V. parahaemolyticus or L. rhamnosus strains was assayed using the Cyto Tox® NonRadiactive cytotoxicity assay (Promega), which measures the extracellular release of LDH into media by dead cells, according manufacturer’s instructions. As a positive control, CaCo-2 were treated with Tritón X-100 for 45 min.
The absorbance values of treated cells were expressed at 490 nm after correcting for background from media without cells. The output is the result of three independent assays, and each experiment was carried out in triplicate.
Statistics
Results are expressed as average ± SD of an individual experiment performed in triplicate. P values were calculated according to Student’s t test, and values p < 0.05 or p < 0.01 were considered statistically significant.
Authors’ contributions
SB and CMB designed studies and performed the cloning of the MAM-7 gene in L. rhamnosus strains. CS analyzed the recombinants strains. AE and JQ performed SDS-PAGE and immunofluorescent assay and ANT performed adhesion, competition and exclusion assays; as well as cellular culture maintenance. ANT also performed cytotoxicity assays, wrote the paper and created the figures. All authors read and approved the final manuscript.
Acknowledgements
We thank Luis G Bermúdez-Humarán PhD for the donation of pSEC and pCWA plasmids, Reinaldo Figueroa for their technical support in the use of microscope and imaging and Patricio Manque PhD lab for the donation of secondary antibody and solutions for immunofluorescence assay.
Also, we thank Escuela de Tecnología Médica, Facultad de Medicina, Universidad Mayor, by the primary antibody anti-Vibrio for immunofluorescence assay.This work was supported by a FIDUM 100500 grant (ANT) from the Universidad Mayor (Chile).
Competing interests
The authors declare that they have no competing interests.
Abbreviations
- RL
recombinant Lactobacillus strains
- AAT
anti-adhesion therapy
- GRAS
generally recognized as safe
- V. parahaemolyticus
Vibrio parahaemolyticus
- L. plantarum
Lactobacillus plantarum
- L. brevis
Lactobacillus brevis
- L. rhamnosus
Lactobacillus rhamnosus
- RIMD
V. parahaemolyticus RIMD 2210633 serotype O3:K6
- pSEC-MAM7
pSEC plasmid containing V. parahaemolyticus MAM-7 gene
- pCWA-MAM7
pCWA plasmid containing V. parahaemolyticus MAM-7 gene
Footnotes
Sebastian Beltran and Cristian A. Munoz-Bergmann contributed equally to this work
Contributor Information
Sebastian Beltran, Email: sebastian.beltran.v@gmail.com.
Cristian A. Munoz-Bergmann, Email: cbergmannmunoz@gmail.com
Ana Elola-Lopez, Email: elola.ana@gmail.com.
Javiera Quintana, Email: javieranqr@gmail.com.
Cristopher Segovia, Email: cristopher.segovia@gmail.com.
Annette N. Trombert, Email: annette.trombert@umayor.cl
References
- 1.Velazquez-Roman J, León-Sicairos N, de Jesus Hernández-Díaz L, Canizalez-Roman A. Pandemic Vibrio parahaemolyticus O3:K6 on the American continent. Frontiers in cellular and infection. Microbiology. 2014;3:110–124. doi: 10.3389/fcimb.2013.00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Whitaker WB, Parent MA, Boyd A, Richards GP, Boyd EF. The Vibrio parahaemolyticus ToxRS regulator is required for stress tolerance and colonization in a novel orogastric streptomycin-induced adult murine model. Infect Immun. 2012;80(5):1834–1845. doi: 10.1128/IAI.06284-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nasu H, Iida T, Sugahara T, Yamaichi Y, Park KS, Yokoyama K, Makino K, Shinagawa H, Honda T. A filamentous phage associated with recent pandemic Vibrio parahaemolyticus O3:K6 strains. J Clin Microbiol. 2000;38(6):2156–2161. doi: 10.1128/jcm.38.6.2156-2161.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ceccarelli D, Hasan NA, Huq A, Colwell RR. Distribution and dynamics of epidemic and pandemic Vibrio parahaemolyticus virulence factors. Front Cell Infect Microbiol. 2013;3:97–106. doi: 10.3389/fcimb.2013.00097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krachler AM, Ham H, Orth K. Outer membrane adhesion factor multivalent adhesion molecule 7 initiates host cell binding during infection by gram-negative pathogens. Proc Natl Acad Sci USA. 2011;108(28):11614–11619. doi: 10.1073/pnas.1102360108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krachler AM, Orth K. Functional characterization of the interaction between bacterial adhesin multivalent adhesion molecule 7 (MAM7) protein and its host cell ligands. J Biol Chem. 2011;286(45):38939–38947. doi: 10.1074/jbc.M111.291377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krachler AM, Ham H, Orth K. Turnabout is fair play: use of the bacterial Multivalent Adhesion Molecule 7 as an antimicrobial agent. Virulence. 2012;3(1):68–71. doi: 10.4161/viru.3.1.18172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elola-Lopez A, Esquivel MJ, Muñoz-Bergmann C, Beltrán S, Osorio CG, Trombert AN. PCR restriction fragment lenght polymorphism analyses of V. parahaemolyticus MAM-7 virulence gene in clinical and environmental strains. Electron J Biol. 2015;11(3):119–125. [Google Scholar]
- 9.Krachler AM, Orth K. Targeting the bacteria-host interface: strategies in anti-adhesion therapy. Virulence. 2013;4(4):284–294. doi: 10.4161/viru.24606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Falcioni T, Papa S, Campana R, Mannello F, Casaroli A, Burattini S, Baffone W. Flow cytometric evaluation of Vibrio parahaemolyticus adhesion inhibition to human epithelial cells. Cytometry B Clin Cytom. 2005;66(1):25–35. doi: 10.1002/cyto.b.20056. [DOI] [PubMed] [Google Scholar]
- 11.Satish Kumar R, Kanmani P, Yuvaraj N, Paari KA, Pattukumar V, Arul V. Lactobacillus plantarum AS1 binds to cultured human intestinal cell line HT-29 and inhibits cell attachment by enterovirulent bacterium Vibrio parahaemolyticus. Lett Appl Microbiol. 2011;53(4):481–487. doi: 10.1111/j.1472-765X.2011.03136.x. [DOI] [PubMed] [Google Scholar]
- 12.Yang ZQ, Jin CJ, Gao L, Fang WM, Gu RX, Qian JY, Jiao XA. Alleviating effects of Lactobacillus strains on pathogenic Vibrio parahaemolyticus-induced intestinal fluid accumulation in the mouse model. FEMS Microbiol Lett. 2013;339(1):30–38. doi: 10.1111/1574-6968.12050. [DOI] [PubMed] [Google Scholar]
- 13.Trombert A. Recombinant lactic acid bacteria as delivery vectors of heterologous antigens: the future of vaccination? Beneficial Microbes. 2015;6(3):1–12. doi: 10.3920/BM2014.0068. [DOI] [PubMed] [Google Scholar]
- 14.Bermúdez-Humarán LG, Langella P, Cortes-Perez NG, Gruss A, Tamez-Guerra RS, Oliveira SC, Saucedo-Cardenas O, Montes de Oca-Luna R, Le Loir Y. Intranasal immunization with recombinant Lactococcus lactis secreting murine interleukin-12 enhances antigen-specific Th1 cytokine production. Infect Immun. 2003;71(4):1887–1896. doi: 10.1128/IAI.71.4.1887-1896.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bermúdez-Humarán LG, Cortes-Perez NG, Le Loir Y, Alcocer-González JM, Tamez-Guerra RS, de Oca-Luna RM, Langella P. An inducible surface presentation system improves cellular immunity against human papillomavirus type 16 E7 antigen in mice after nasal administration with recombinant lactococci. J Med Microbiol. 2004;53(Pt 5):427–433. doi: 10.1099/jmm.0.05472-0. [DOI] [PubMed] [Google Scholar]
- 16.Cauchard S, Bermúdez-Humarán LG, Blugeon S, Laugier C, Langella P, Cauchard J. Mucosal co-immunization of mice with recombinant lactococci secreting VapA antigen and leptin elicits a protective immune response against Rhodococcus equi infection. Vaccine. 2011;30(1):95–102. doi: 10.1016/j.vaccine.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 17.Enouf V, Langella P, Commissaire J, Cohen J, Corthier G. Bovine rotavirus nonstructural protein 4 produced by Lactococcus lactis is antigenic and immunogenic. Appl Environ Microbiol. 2001;67(4):1423–1428. doi: 10.1128/AEM.67.4.1423-1428.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Langella P, Le Loir Y. Heterologous protein secretion in Lactococcus lactis: a novel antigen delivery system. Braz J Med Biol Res. 1999;32(2):191–198. doi: 10.1590/S0100-879X1999000200007. [DOI] [PubMed] [Google Scholar]
- 19.Lebeer S, Verhoeven TL, Perea Vélez M, Vanderleyden J, De Keersmaecker SC. Impact of environmental and genetic factors on biofilm formation by probiotic strain Lactobacillus rhamnosus GG. App Environ Microbiol. 2007;73(21):6768–6775. doi: 10.1128/AEM.01393-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tuomola EM, Salminen SJ. Adhesion of some probiotic and dairy Lactobacillus strains to Caco-2 cell cultures. Int J Food Microbiol. 1998;41(1):45–51. doi: 10.1016/S0168-1605(98)00033-6. [DOI] [PubMed] [Google Scholar]
- 21.Mack DR, Michail S, Wei S, McDougall L, Hollingsworth MA. Probiotics inhibit enteropathogenic E. coli adherence in vitro by inducing intestinal mucin gene expression. Am J Physiol. 1999;276(4 Pt 1):G941–G950. doi: 10.1152/ajpgi.1999.276.4.G941. [DOI] [PubMed] [Google Scholar]
- 22.Polak-Berecka M, Waśko A, Paduch R, Skrzypek T, Sroka-Bartnicka A. The effect of cell surface components on adhesion ability of Lactobacillus rhamnosus. Antonie Van Leeuwenhoek. 2014;106(4):751–762. doi: 10.1007/s10482-014-0245-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sengupta R, Altermann E, Anderson RC, McNabb WC, Moughan PJ, Roy NC. The role of cell surface architecture of lactobacilli in host-microbe interactions in the gastrointestinal tract. Mediators Inflamm. 2013;2013:237921. doi: 10.1155/2013/237921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang W, Wang H, Liu J, Zhao Y, Gao K, Zhang J. Adhesive ability means inhibition activities for Lactobacillus against pathogens and S-layer protein plays an important role in adhesion. Anaerobe. 2013;22:97–103. doi: 10.1016/j.anaerobe.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 25.Raja RH, Raucci G, Hook M. Peptide analogs to a fibronectin receptor inhibit attachment of Staphylococcus aureus to fibronectin-containing substrates. Infect Immun. 1990;58(8):2593–2598. doi: 10.1128/iai.58.8.2593-2598.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nishiyama K, Nakamata K, Ueno S, Terao A, Aryantini NP, Sujaya IN, Fukuda K, Urashima T, Yamamoto Y, Mukai T. Adhesion properties of Lactobacillus rhamnosus mucus-binding factor to mucin and extracellular matrix proteins. Biosci Biotechnol Biochem. 2015;79(2):271–279. doi: 10.1080/09168451.2014.972325. [DOI] [PubMed] [Google Scholar]
- 27.Shi T, Nishiyama K, Nakamata K, Aryantini NP, Mikumo D, Oda Y, Yamamoto Y, Mukai T, Sujaya IN, Urashima T, Fukuda K. Isolation of potential probiotic Lactobacillus rhamnosus strains from traditional fermented mare milk produced in Sumbawa Island of Indonesia. Biosci Biotechnol Biochem. 2012;76(10):1897–1903. doi: 10.1271/bbb.120385. [DOI] [PubMed] [Google Scholar]
- 28.Lim J, Stones DH, Hawley CA, Watson CA, Krachler AM. Multivalent adhesion molecule 7 clusters act as signaling platform for host cellular GTPase activation and facilitate epithelial barrier dysfunction. PLoS Pathogen. 2014;10(9):e1004421. doi: 10.1371/journal.ppat.1004421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Street C, Bryan B. Rho kinase proteins-pleiotropic modulators of cell survival and apoptosis. Anticancer Res. 2011;31(11):3645–3657. [PMC free article] [PubMed] [Google Scholar]
- 30.Shi J, Wei L. Rho kinase in the regulation of cell death and survival. Arch Immunol Ther Exp (Warsz) 2007;55(2):61–75. doi: 10.1007/s00005-007-0009-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sebbagh M, Renvoizé C, Hamelin J, Riché N, Bertoglio J, Bréard J. Caspase-3-mediated cleavage of ROCK I induces MLC phosphorylation and apoptotic membrane blebbing. Nat Cell Biol. 2001;3:346–352. doi: 10.1038/35070019. [DOI] [PubMed] [Google Scholar]
- 32.Serror P, Sasaki T, Ehrlich SD, Maguin E. Electrotransformation of Lactobacillus delbrueckii subsp. bulgaricus and L. delbrueckii subsp. lactis with various plasmids. Appl Environ Microbiol. 2002;68(1):46–52. doi: 10.1128/AEM.68.1.46-52.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]


