Abstract
Background
In the era of intensity-modulated radiotherapy (IMRT), the role of neoadjuvant chemotherapy (NAC) for locoregionally advanced nasopharyngeal carcinoma (NPC) is under-evaluated. The aim of this study was to compare the efficacy of NAC plus IMRT and concurrent chemoradiotherapy (CCRT) plus adjuvant chemotherapy (AC) on locoregionally advanced NPC.
Methods
Between January 2004 and December 2008, 240 cases of locoregionally advanced NPC confirmed by pathologic assessment in Sun Yat-sen University Cancer Center were reviewed. Of the 240 patients, 117 received NAC followed by IMRT, and 123 were treated with CCRT plus AC. The NAC + IMRT group received a regimen that included cisplatin and 5-fluorouracil (5-FU). The CCRT + AC group received cisplatin concurrently with radiotherapy, and subsequently received adjuvant cisplatin and 5-FU. The survival rates were assessed by Kaplan–Meier analysis, and the survival curves were compared using a log-rank test. Multivariate analysis was conducted using the Cox proportional hazard regression model.
Results
The 5-year overall survival (OS), locoregional relapse-free survival (LRRFS), distant metastasis-free survival (DMFS), and disease-free survival (DFS) were 78.0, 87.9, 79.0, and 69.8%, respectively, for the NAC + IMRT group and 78.7, 84.8, 76.2, and 65.6%, respectively, for the CCRT + AC group. There were no significant differences in survival between the two groups. In multivariate analysis, age (<50 years vs. ≥50 years) and overall stage (III vs. IV) were found to be independent predictors for OS and DFS; furthermore, the overall stage was a significant prognostic factor for DMFS. Compared with the CCRT + AC protocol, the NAC + IMRT protocol significantly reduced the occurrence rates of grade 3–4 nausea–vomiting (6.5 vs. 1.5%, P = 0.023) and leukopenia (9.7 vs. 0.8%, P = 0.006).
Conclusions
The treatment outcomes of the NAC + IMRT and CCRT + AC groups were similar. Distant metastasis remained the predominant mode of treatment failure.
Keywords: Nasopharyngeal carcinoma, Intensity-modulated radiotherapy, Neoadjuvant chemotherapy, Concurrent chemoradiotherapy, Adjuvant chemotherapy
Background
Nasopharyngeal carcinoma (NPC) is endemic in South China and Southeast Asia, with an annual incidence of 15–50 cases per 100,000 [1]. NPC mortality in China, especially in South China, was also at high levels [2]. Radiotherapy is the primary treatment modality for NPC, and the outcome for patients with early-stage disease is usually favorable; however, the response of locoregionally advanced NPC to radiotherapy is unsatisfactory [3].
Combining chemotherapy and radiotherapy is a reasonable strategy for improving the long-term outcome of locoregionally advanced NPC. The Intergroup 0099 Study (IGS) was the first randomized, controlled trial to achieve a significant improvement in the 3-year overall survival (OS) for stages III-IVb NPC patients by adding concurrent and adjuvant chemotherapy to conventional radiotherapy [4]. Since then, this regimen has been deemed the standard of care for advanced NPC. However, there have been serious concerns regarding the applicability of the IGS results, and the outcomes of several randomized, controlled trials attempting to verify the efficacy of concurrent chemoradiotherapy (CCRT) plus adjuvant chemotherapy (AC) were conflicting [5–9]. With the advent of intensity-modulated radiotherapy (IMRT), local control has been substantially improved, and distant metastasis is now the main cause of treatment failure [10]. Further improvements in systemic control by the use of concurrent chemotherapy is unlikely because of drug-related toxic effects [7]; data from several published studies [11, 12] show that AC does not translate to significant improvements in prolonging OS and reducing distant metastatic rate (DMR). One strategy for treatment improvement is to change the sequence from concurrent-adjuvant to addition of neoadjuvant chemotherapy (NAC) prior to radiotherapy. It is important to address the efficacy of NAC because meta-analyses showed that NAC could significantly reduce both locoregional and distant failures [13, 14]. A pooled meta-analysis of two trials by Chua et al. [13] noted that modest improvements in relapse-free survival and disease-specific survival could be achieved by NAC. Another meta-analysis by Ouyang et al. [14] revealed significant treatment efficacy in terms of OS and DMR for NAC.
Because most of these studies were based on the non-IMRT technique, the role of NAC plus IMRT for locoregionally advanced NPC in the era of IMRT is unknown. The aim of this study was to compare the efficacy of NAC plus IMRT with CCRT plus AC on locoregionally advanced NPC.
Methods
Patients
Inclusion criteria were as follows: pathologically diagnosed non-keratinizing or undifferentiated carcinoma of the nasopharynx (World Health Organization [WHO] type II or III); age of 18–65 years; stages III-IVb disease according to the 2002 Union for International Cancer Control (UICC) Staging System; no evidence of distant metastasis; Karnofsky performance score ≥70; normal hematologic function [white blood cell (WBC) count ≥4.0 × 109/L, platelet (PLT) count ≥100 × 109/L]; normal hepatic function [total bilirubin (TBIL) and alanine aminotransferase (ALT) <2 times the normal values]; normal renal function [creatinine (Cr) <1.5 times the normal value]; receiving radical IMRT at initial diagnosis; and receiving NAC with cisplatin and 5-fluorouracil (5-FU) for two cycles or receiving concurrent chemotherapy with cisplatin for two cycles plus AC with cisplatin and 5-FU for two cycles.
Radiotherapy
All patients were immobilized in the supine position with a head, neck, and shoulder thermoplastic mask. Two sets of images, with and without contrast, were obtained from the computed tomography (CT) simulator for treatment planning. All patients were scanned with serial 3-mm slices from the vertex through the clavicles. Inverse IMRT planning was performed using the Corvus system, version 3.0 (Peacock, Nomos, Deer Park, IL, USA), and a dynamic, multileaf, intensity-modulating collimator (MIMiC; Nomos Corp., Sewickly, PA, USA) was used for planning and treatment. The gross tumor volumes of the nasopharynx (GTVnx) and positive neck lymph nodes (GTVnd) were delineated according to our previously described institutional treatment protocol [15], which is in agreement with the International Commission on Radiation Units and Measurements Reports 50 [16] and Reports 62 [17]. The first clinical tumor volume (CTV1) was defined as the GTVnx plus a margin of 5–10 mm for potential microscopic spread, including the entire nasopharyngeal mucosa plus a 5-mm submucosal volume. The second CTV (CTV2) was defined by adding a margin of 5–10 mm to CTV1 and included the following regions, which needed prophylactic irradiation: retropharyngeal lymph node regions, the clivus, skull base, pterygoid fossae, parapharyngeal space, inferior sphenoid sinus, posterior edge of the nasal cavity, maxillary sinuses, and the lymphatic drainage area. The planning target volume (PTV) for GTVs and CTVs were generated automatically by adding a 5-mm margin after delineation of tumor targets according to the immobilization and localization uncertainties. The prescribed dose was 68–70 Gy to the PTV of the GTVnx (PTVnx), 64–68 Gy to the PTV of the GTVnd (PTVnd), 60 Gy to the PTV of the CTV1 (PTV1), and 54 Gy to the PTV of the CTV2 (PTV2) in 30 fractions. All patients were treated with one fraction daily over 5 days per week.
Chemotherapy
For the NAC + IMRT group, two NAC cycles, consisting of cisplatin (80 mg/m2 intravenous infusion on day 1) and 5-FU (4 g/m2 daily as a 120-h intravenous infusion on days 1–5), were administered at an interval of 3 weeks before radiotherapy.
For the CCRT + AC group, two concurrent chemotherapy cycles, consisting of cisplatin (80 mg/m2 intravenous infusion on days 1 and 22), were administered at an interval of 3 weeks during radiotherapy; 2 subsequent AC cycles, consisting of cisplatin (80 mg/m2 intravenous infusion on day 1) and 5-FU (4 g/m2 daily as a 120-h intravenous infusion on days 1–5), were administered at an interval of 3 weeks after the completion of radiotherapy.
Patient assessment and follow-up
Clinical evaluation of the efficacy of therapy was based on the Response Evaluation Criteria in Solid Tumors (RECIST) v1.1 [18]. Acute and late adverse events were assessed according to the Common Terminology Criteria for Adverse Events (CTCAE) v3.0 [19]. Patients were evaluated weekly during radiotherapy and every 3 months for the first 3 years, every 6 months for the fourth and fifth years, and every year thereafter. Follow-up evaluation included physical examination of the head and neck, nasopharyngeal endoscopy, chest radiography, abdominal ultrasound, Epstein–Barr virus (EBV) serological testing, and magnetic resonance imaging (MRI) or CT of the head and neck region. CT scans of the abdominopelvic cavity or chest, bone scans, and positron emission tomography scans were conducted when clinically indicated.
Statistical analysis
The SPSS 16.0 statistical software (SPSS Inc., Chicago, IL, USA) was used. The Chi square analysis was used to compare occurrence rates of adverse events and categorical variables. The means of continuous variables were compared using the Student’s t test. The study endpoints of OS, LRRFS, DMFS, and DFS were determined by patient death, relapse of a local or nodal tumor, occurrence of distant metastasis, and occurrence of relapse or distant metastasis respectively. The time-to-event for each endpoint was calculated from the date of completion of treatment to the occurrence date of the event using the Kaplan–Meier method. Statistical differences in endpoints were estimated using the log-rank test. The multivariate analysis was conducted by the Cox proportional hazard regression model. A two-tailed P value of less than 0.05 was considered significant.
Results
Patient characteristics
Between January 2004 and December 2008, clinical data of 240 NPC patients treated in the Sun Yat-sen University Cancer Center who met all of the criteria were retrospectively analyzed. Of these patients, 175 were males and 65 were females. The median age was 44 years. Of the 240 patients, 117 received NAC followed by IMRT, and 123 were treated with CCRT plus AC. No significant differences were found between the two groups in baseline characteristics (Table 1).
Table 1.
Characteristics of the 240 patients with locoregionally advanced nasopharyngeal carcinoma (NPC)
| Characteristic | NAC + IMRT [cases (%)] | CCRT + AC [cases (%)] | χ2 | P |
|---|---|---|---|---|
| Total | 117 | 123 | ||
| Age (years) | 0.199 | 0.656 | ||
| <50 | 83 (70.9) | 84 (68.3) | ||
| ≥50 | 34 (29.1) | 39 (31.7) | ||
| Gender | 1.148 | 0.284 | ||
| Male | 89 (76.1) | 86 (69.9) | ||
| Female | 28 (23.9) | 37 (30.1) | ||
| T stage | 1.029 | 0.794 | ||
| T1 | 4 (3.4) | 4 (3.3) | ||
| T2 | 11 (9.4) | 14 (11.4) | ||
| T3 | 57 (48.7) | 65 (52.8) | ||
| T4 | 45 (38.5) | 40 (32.5) | ||
| N stage | 3.578 | 0.311 | ||
| N0 | 22 (18.8) | 27 (22.0) | ||
| N1 | 40 (34.2) | 50 (40.6) | ||
| N2 | 46 (39.3) | 42 (34.1) | ||
| N3 | 9 (7.7) | 4 (3.3) | ||
| Clinical stage | 2.256 | 0.133 | ||
| III | 65 (55.6) | 80 (65.0) | ||
| IV | 52 (44.4) | 43 (35.0) | ||
| WHO histology | 0.313 | 0.576 | ||
| II | 13 (11.1) | 11 (8.9) | ||
| III | 104 (88.9) | 112 (91.1) |
NAC neoadjuvant chemotherapy, IMRT intensity-modulated radiotherapy, CCRT concurrent chemoradiotherapy, AC adjuvant chemotherapy, WHO World Health Organization
Treatment and compliance
All patients completed the full course of radiotherapy. In the NAC + IMRT group, all patients completed two cycles of NAC. In the CCRT + AC group, all patients completed two cycles of concurrent chemotherapy and two cycles of AC.
Clinical response
After two cycles of NAC with cisplatin plus 5-FU, five patients (4.3%) had a complete response (CR), and 93 (79.5%) had a partial response (PR) in the NAC + IMRT group. Three months after radiotherapy, the effective rates (CR plus PR) were 98.3% (115/117) in the NAC + IMRT group and 98.4% (121/123) in the CCRT + AC group (P = 1.000).
Patterns of treatment failure
The failure patterns, locoregional or distant, in the NAC + IMRT and CCRT + AC groups were similar (Table 2).
Table 2.
Patterns of disease failure in patients treated with NAC plus IMRT vs. CCRT plus AC
| Failure pattern | NAC + IMRT [cases (%)] | CCRT + AC [cases (%)] | P |
|---|---|---|---|
| Locoregional relapse only | 11 (9.4) | 12 (9.8) | 0.926 |
| Distant metastases only | 22 (18.8) | 26 (21.1) | 0.651 |
| Both locoregional relapse and distant metastases | 2 (1.7) | 6 (4.9) | 0.314 |
| Death | 28 (23.9) | 37 (30.1) | 0.284 |
Abbreviations as in Table 1
Acute and late toxicities
Acute toxicities during radiotherapy were well tolerated by the entire group. Compared with the CCRT + AC group, the NAC + IMRT group had significantly reduced occurrence rates of grades 3–4 nausea–vomiting (6.5 vs. 1.5%, P = 0.023) and leukopenia (9.7 vs. 0.8%, P = 0.006). No significant differences in anemia, thrombocytopenia, liver dysfunction, and mucositis were found between the two groups (Table 3). The most common late toxicities were xerostomia, hear loss, skin dystrophy, subcutaneous fibrosis, and temporal lobe injury (TLI). Ten patients (4.2%) developed TLI. Among them, 4 had radiation injuries in unilateral temporal lobes, and 6 in bilateral temporal lobes. No significant differences in late toxicities were found between the two groups. However, in the CCRT + AC group, 4 patients (3.3%) had grades 3–4 hear loss, and 2 (1.6%) had grades 3–4 TLI.
Table 3.
Treatment-related toxicities in patients with locoregionally advanced NPC treated with NAC plus IMRT vs. CCRT plus AC
| Toxicity | NAC + IMRT [cases (%)] |
CCRT + AC [cases (%)] |
χ 2 | P |
|---|---|---|---|---|
| Grade 3/4 acute toxicities | ||||
| Leukopenia | 1 (0.9) | 12 (9.8) | 7.618 | 0.006 |
| Anemia | 0 | 3 (2.4) | 1.252 | 0.263 |
| Thrombocytopenia | 1 (0.9) | 4 (3.3) | 0.719 | 0.397 |
| Hepatotoxicity | 0 | 1 (0.8) | 0.000 | 1.000 |
| Nausea–vomiting | 2 (1.7) | 10 (8.1) | 5.204 | 0.023 |
| Mucositis | 5 (4.3) | 8 (6.5) | 0.582 | 0.445 |
| Late toxicities | ||||
| Skin dystrophy | 37 (31.6) | 51 (41.5) | 2.500 | 0.114 |
| Subcutaneous fibrosis | 26 (22.2) | 29 (23.6) | 0.062 | 0.803 |
| Xerostomia | 68 (58.1) | 85 (69.1) | 3.132 | 0.077 |
| Hear loss | 54 (46.2) | 64 (52.0) | 0.829 | 0.363 |
| Temporal lobe injury | 4 (3.4) | 6 (4.9) | 0.059 | 0.809 |
Abbreviations as in Table 1
Survival analysis
The 5-year OS, LRRFS, DMFS, and DFS rates were 78.0, 87.9, 79.0, and 69.8%, respectively, for the NAC + IMRT group, and 78.7, 84.8, 76.2, and 65.6%, respectively, for the CCRT + AC group. There were no significant differences in survival between the two groups (Fig. 1). Subset analyses revealed that the differences in OS, LRRFS, DMFS, and DFS were significant in all subsets (Table 4).
Fig. 1.
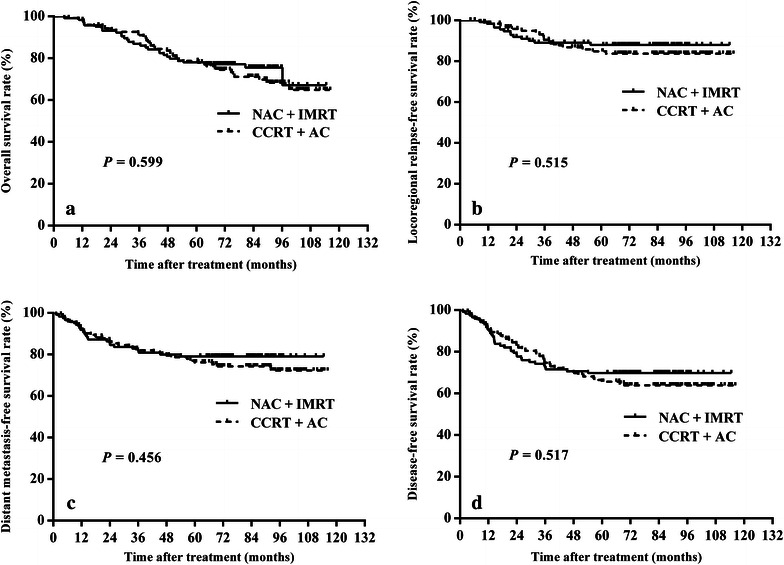
Kaplan–Meier estimates of the survival of patients with locoregionally advanced nasopharyngeal carcinoma (NPC) treated by neoadjuvant chemotherapy (NAC) plus intensity-modulated radiotherapy (IMRT) vs. concurrent chemoradiotherapy (CCRT) plus adjuvant chemotherapy (AC). a overall survival; b locoregional relapse-free survival; c distant metastasis-free survival; d disease-free survival. There were no significant differences in survival between the two groups
Table 4.
Subset analyses on tumor control in patients with locoregionally advanced NPC treated by NAC plus IMRT vs. CCRT plus AC
| Stage | OS | LRRFS | DMFS | DFS | ||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | |
| Overall | 1.14 (0.70–1.87) | 0.599 | 1.27 (0.62–2.59) | 0.515 | 1.22 (0.72–2.08) | 0.456 | 1.16 (0.74–1.81) | 0.517 |
| III | 1.52 (0.68–3.39) | 0.308 | 2.23 (0.79–6.24) | 0.119 | 1.62 (0.69–3.80) | 0.259 | 1.62 (0.83–3.16) | 0.155 |
| IV | 1.04 (0.55–1.96) | 0.912 | 0.65 (0.21–1.98) | 0.440 | 1.11 (0.55–2.21) | 0.777 | 0.92 (0.50–1.70) | 0.789 |
| T1–2 | 0.70 (0.14–3.48) | 0.663 | 50.52 (0–6.22 × 108) | 0.386 | 0.48 (0.11–2.17) | 0.332 | 0.65 (0.16–2.60) | 0.537 |
| T3–4 | 1.23 (0.73–2.07) | 0.430 | 1.25 (0.61–2.57) | 0.548 | 1.41 (0.80–2.49) | 0.237 | 1.27 (0.79–2.02) | 0.325 |
| N0–1 | 1.17 (0.60–2.28) | 0.638 | 1.38 (0.55–3.47) | 0.487 | 1.12 (0.54–2.32) | 0.766 | 1.08 (0.60–1.94) | 0.797 |
| N2–3 | 1.11 (0.52–2.37) | 0.783 | 1.01 (0.31–3.30) | 0.990 | 1.42 (0.66–3.07) | 0.370 | 1.27 (0.64–2.52) | 0.487 |
OS overall survival, LRRFS locoregional relapse-free survival, DMFS distant metastasis-free survival, DFS disease-free survival, HR hazard ratio, CI confidence interval. Other abbreviations as in Table 1
Prognostic factors
The various potential prognostic factors for predicting LRRFS, DMFS, DFS, and OS rates, including gender, age, WHO histology, T stage, N stage, overall stage, and treatment, were evaluated in univariate and multivariate analysis. In univariate analysis, age and overall stage were significant prognostic factors for OS, DMFS, and DFS (Table 5). In multivariate analysis, age and overall stage were found to be the independent predictors for OS and DFS, and overall stage was a significant prognostic factor for DMFS (Table 6). No significant prognostic factors were found for LRRFS in either univariate or multivariate analysis.
Table 5.
Univariate analysis of prognostic factors for patients with locoregionally advanced NPC
| Variate | 5-year survival rate (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| OS | P | LRRFS | P | DMFS | P | DFS | P | |
| Gender | 0.173 | 0.587 | 0.077 | 0.224 | ||||
| Male | 75.8 | 87.1 | 75.3 | 65.9 | ||||
| Female | 88.0 | 83.3 | 85.8 | 74.0 | ||||
| Age (years) | 0.002 | 0.489 | 0.025 | 0.007 | ||||
| <50 | 82.8 | 87.0 | 81.5 | 73.1 | ||||
| ≥50 | 68.6 | 84.6 | 68.4 | 55.6 | ||||
| WHO histology | 0.362 | 0.564 | 0.526 | 0.549 | ||||
| Type II | 87.1 | 90.3 | 83.3 | 74.5 | ||||
| Type III | 77.5 | 85.8 | 76.9 | 66.9 | ||||
| T stage | 0.213 | 0.070 | 0.773 | 0.299 | ||||
| T1–2 | 84.4 | 96.4 | 81.3 | 78.1 | ||||
| T3–4 | 77.4 | 84.6 | 76.9 | 66.0 | ||||
| N stage | 0.934 | 0.475 | 0.414 | 0.994 | ||||
| N0–1 | 80.8 | 85.2 | 79.4 | 67.2 | ||||
| N2–3 | 75.1 | 87.8 | 74.9 | 68.2 | ||||
| Overall stage | <0.001 | 0.518 | 0.001 | 0.004 | ||||
| III | 85.6 | 87.7 | 84.9 | 74.7 | ||||
| IV | 66.5 | 83.5 | 65.3 | 56.2 | ||||
| Treatment | 0.599 | 0.515 | 0.456 | 0.517 | ||||
| NAC + IMRT | 78.0 | 87.9 | 79.0 | 69.8 | ||||
| CCRT + AC | 78.7 | 84.8 | 76.2 | 65.6 | ||||
Table 6.
Multivariate analysis of prognostic factors for patients with locoregionally advanced NPC
| Variable | OS | LRRFS | DMFS | DFS | ||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | |
| Gender (males vs. females) | 0.63 (0.32–1.26) | 0.191 | 1.25 (0.55–2.85) | 0.588 | 0.50 (0.22–1.11) | 0.087 | 0.71 (0.39–1.30) | 0.265 |
| Age (<50 vs. ≥50 years) | 1.92 (1.17–3.15) | 0.010 | 1.31 (0.62–2.77) | 0.486 | 1.59 (0.93–2.72) | 0.092 | 1.70 (1.08–2.67) | 0.022 |
| WHO histology (Type II vs. Type III) | 1.87 (0.67–5.21) | 0.229 | 1.54 (0.36–6.50) | 0.561 | 1.59 (0.57–4.44) | 0.379 | 1.22 (0.77–1.92) | 0.433 |
| T stage (T1–2 vs. T3–4) | 1.39 (0.52–3.67) | 0.512 | 5.69 (071–45.81) | 0.102 | 1.00 (0.39–2.59) | 0.999 | 1.36 (0.58–3.19) | 0.475 |
| N stage (N0–1 vs. N2–3) | 1.36 (0.79–2.37) | 0.270 | 1.13 (0.51–2.47) | 0.768 | 1.59 (0.88–2.87) | 0.126 | 1.29 (0.78–2.13) | 0.322 |
| Overall stage (III vs. IV) | 2.50 (1.47–4.26) | 0.001 | 1.05 (0.51–2.17) | 0.897 | 2.54 (1.41–4.58) | 0.002 | 1.80 (1.12–2.89) | 0.016 |
| Treatment (NAC + IMRT vs. CCRT + AC) | 1.23 (0.74–2.03) | 0.426 | 1.34 (0.65–2.77) | 0.436 | 1.35 (0.78–2.32) | 0.281 | 1.40 (0.60–3.25) | 0.397 |
Discussion
For locoregionally advanced NPC, the present study demonstrated that NAC plus IMRT and CCRT plus AC resulted in similar outcomes in terms of 5-year OS, LRRFS, DMFS, and DFS rates. A subgroup analysis indicated that the effect of concurrent and adjuvant chemotherapy on OS, LRRFS, DMFS, and DFS was insignificant in all subsets.
CCRT has been considered the standard of care for locoregionally advanced NPC [20]. Although the efficacy of chemotherapy delivered concurrently with conventional radiotherapy has been repeatedly proven [4, 6, 21], the most effective combination of chemotherapy and IMRT has not been well established.
Three retrospective studies have evaluated the contribution of chemotherapy for patients with NPC treated with IMRT [22–24]. Lin et al. [22] reported that IMRT following NAC for locoregionally advanced NPC provided a favorable outcome in terms of 3-year local/regional control, metastasis-free survival (MFS), DFS, and OS; furthermore, their results suggested that concurrent chemotherapy offered no significant value for further improvement of local and regional control to IMRT following NAC. Su et al. [23] demonstrated that patients with locoregionally advanced NPC had similar OS, MFS, and DFS when treated with IMRT-based modalities, including IMRT alone, NAC plus IMRT, IMRT plus AC, CCRT alone, NAC plus CCRT, and CCRT plus AC. Another retrospective study assigned 276 patients with locoregionally advanced NPC to compare IMRT alone with NAC plus IMRT, CCRT alone, and NAC plus CCRT [24]. The results revealed that the addition of concurrent or neoadjuvant-concurrent chemotherapy to IMRT prolonged relapse-free survival (RFS) or DFS for patients with locoregionally advanced NPC, whereas NAC provided no significant benefit for OS, MFS, RFS, and DFS.
In the current retrospective cohort study, NAC plus IMRT produced a superb outcome similar to CCRT plus AC in terms of locoregional control in patients with locoregionally advanced NPC (87.9 vs. 84.8%, P = 0.515). We hypothesized that the application of IMRT would improve the local control rate, which may “counteract” the effect of CCRT on improving the local control rate and survival rate. In addition, NAC could reduce hypoxia in the primary site and metastatic lymph nodes by shrinking the tumor, which could increase radio-sensitivity and increase locoregional control [25]. After 2 cycles of NAC with cisplatin plus 5-FU, 5 patients (4.3%) had CR, and 93 (79.5%) had PR in the NAC + IMRT group.
Although the use of IMRT has produced significant improvements in LRRFS, effective treatment of distant metastases remains an important problem to be solved. In the present study, 23 patients experienced a local or regional relapse, and 48 developed a distant metastasis. Distant metastasis remained the predominant mode of treatment failure, which was consistent with the results of other reports [26, 27]. Our results demonstrated that NAC plus IMRT achieved similar DMFS compared with CCRT plus AC (79.0 vs. 76.2%, P = 0.456). The main goal of NAC is to eradicate distant micrometastases [28]. Given the high distant failure rate associated with NPC, it is logical to expect a decline in distant failure with the use of NAC. A recent meta-analysis that emphasized the use of NAC revealed that NAC could effectively enhance OS and reduce DMR [14]. However, both the drug dose and course necessary to eradicate all distant micrometastases are still unknown. As a result, we recommend further investigation on the optimal regimen of NAC for locoregionally advanced NPC.
A multivariable analysis for prognostic factors was also performed in the present study. OS and DFS were both independently affected by age and overall stage, and DMFS was only affected by overall stage. It is well known that overall stage is undoubtedly the most important prognostic factor for predicting the survival of NPC patients [29, 30].
Despite of the proven efficacy of chemotherapy delivered concurrently with conventional radiation, the combined treatment strategy comes with substantial adverse effects. In the pivotal INT-0099 trial, the proportions of patients who could complete the scheduled concurrent and adjuvant chemotherapy were only 63 and 55%, respectively, due to excess toxicity [4]. Similar results on treatment-related toxicities have been documented in retrospective and randomized studies [6, 21, 31].
In the present study, the group of patients who received CCRT plus AC had significantly more severe leukopenia and nausea–vomiting than patients who received NAC plus IMRT. In the CCRT + AC group, most of the grades 3–4 nausea–vomiting occurred during the CCRT phase. Compared with the CCRT + AC group, the NAC + IMRT group had less gastrointestinal and hematologic toxicities. Studies by Lin et al. [22] and Sun et al. [32] also demonstrated that the total occurrence rates of grade 3 or 4 acute toxicities in patients receiving concurrent chemotherapy was higher than those who received IMRT alone. No significant differences in late toxicities were found between the two groups. In our study, in the NAC + IMRT group, skin dystrophy, subcutaneous fibrosis, xerostomia, and TLI were mild (grades 1–2); however, in the CCRT + AC group, 4 patients (3.3%) had grades 3–4 hear loss, and 2 (1.6%) had grades 3–4 TLI. One possible explanation for milder toxicities in the NAC + IMRT group may be that NAC was likely to reduce primary tumor volume for patients with intracranial invasion, and re-planning of the delineation for tumor volume after NAC could better protect critical normal tissue and reduce IMRT-associated adverse events [33].
These results demonstrated that a regimen of CCRT plus AC that significantly increases the probability and severity of treatment-related adverse events might not be essential for the treatment of NPC if NAC and IMRT are used instead. We hypothesize that omission of concurrent-adjuvant chemotherapy may be possible, and effective disease control in the primary area and neck lymph nodes can be achieved with improvement in radiation technology and use of sequential chemotherapy. Further randomized clinical trials are necessary for the establishment of the most effective combination of chemotherapy and IMRT to improve the prognosis of patients with locoregionally advanced NPC.
Conclusions
The treatment outcomes of NAC plus IMRT and CCRT plus AC for locoregionally advanced NPC were similar. Distant metastasis remained the predominant mode of treatment failure. The most effective combination of chemotherapy and IMRT needs to be established through further randomized clinical trials.
Authors’ contributions
WQ participated in the design of the study, performed the statistical analysis, and drafted the manuscript. PH participated in the design of the study and performed the statistical analysis. JS participated in the acquisition of data. HX participated in the acquisition of data. CZ participated in the design of the study and performed the statistical analysis. KC conceived of the study, and participated in its design and coordination, and helped to draft the manuscript. All authors read and approved the final manuscript.
Acknowledgements
This work was supported by grants from Sun Yat-sen University Clinical Research 5010 Program (No. 2015010), the Fundamental Research Funds for the Central Universities (No. 15ykpy36), Clinical Research of Special Funds of Wu Jieping Medical Foundation (no. 320.6750.14270).
Competing interests
The authors declare that they have no competing interests.
Footnotes
Wen-Ze Qiu and Pei-Yu Huang contributed equally to this article
Contributor Information
Wen-Ze Qiu, Email: qiuwenze2007@163.com.
Pei-Yu Huang, Email: huangpy@sysucc.org.cn.
Jun-Li Shi, Email: 245155346@qq.com.
Hai-Qun Xia, Email: xiahq@sysucc.org.cn.
Chong Zhao, Email: zhaochong@sysucc.org.cn.
Ka-Jia Cao, Email: caokajia@163.com.
References
- 1.Wee JT, Ha TC, Loong SL, Qian CN. Is nasopharyngeal cancer really a “Cantonese cancer”? Chin J Cancer. 2010;29(5):517–526. doi: 10.5732/cjc.009.10329. [DOI] [PubMed] [Google Scholar]
- 2.Wei KR, Zheng RS, Zhang SW, Liang ZH, Ou ZX, Chen WQ. Nasopharyngeal carcinoma incidence and mortality in China in 2010. Chin J Cancer. 2014;33(8):381–387. doi: 10.5732/cjc.014.10086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ma J, Mai HQ, Hong MH, Cui NJ, Lu TX, Lu LX, et al. Is the 1997 AJCC staging system for nasopharyngeal carcinoma prognostically useful for Chinese patient populations? Int J Radiat Oncol Biol Phys. 2001;50(5):1181–1189. doi: 10.1016/S0360-3016(01)01537-1. [DOI] [PubMed] [Google Scholar]
- 4.Al-Sarraf M, LeBlanc M, Giri PG, Fu KK, Cooper J, Vuong T, et al. Chemoradiotherapy versus radiotherapy in patients with advanced nasopharyngeal cancer: phase III randomized Intergroup study 0099. J Clin Oncol. 1998;16(4):1310–1317. doi: 10.1200/JCO.1998.16.4.1310. [DOI] [PubMed] [Google Scholar]
- 5.Kwong DL, Sham JS, Au GK, Chua DT, Kwong PW, Cheng AC, et al. Concurrent and adjuvant chemotherapy for nasopharyngeal carcinoma: a factorial study. J Clin Oncol. 2004;22(13):2643–2653. doi: 10.1200/JCO.2004.05.173. [DOI] [PubMed] [Google Scholar]
- 6.Wee J, Tan EH, Tai BC, Wong HB, Leong SS, Tan T, et al. Randomized trial of radiotherapy versus concurrent chemoradiotherapy followed by adjuvant chemotherapy in patients with American Joint Committee on Cancer/International Union against cancer stage III and IV nasopharyngeal cancer of the endemic variety. J Clin Oncol. 2005;23(27):6730–6738. doi: 10.1200/JCO.2005.16.790. [DOI] [PubMed] [Google Scholar]
- 7.Lee AW, Tung SY, Chua DT, Ngan RK, Chappell R, Tung R, et al. Randomized trial of radiotherapy plus concurrent-adjuvant chemotherapy vs radiotherapy alone for regionally advanced nasopharyngeal carcinoma. J Natl Cancer Inst. 2010;102(15):1188–1198. doi: 10.1093/jnci/djq258. [DOI] [PubMed] [Google Scholar]
- 8.Lee AW, Tung SY, Chan AT, Chappell R, Fu YT, Lu TX, et al. A randomized trial on addition of concurrent-adjuvant chemotherapy and/or accelerated fractionation for locally-advanced nasopharyngeal carcinoma. Radiother Oncol. 2011;98(1):15–22. doi: 10.1016/j.radonc.2010.09.023. [DOI] [PubMed] [Google Scholar]
- 9.Chen Y, Sun Y, Liang SB, Zong JF, Li WF, Chen M, et al. Progress report of a randomized trial comparing long-term survival and late toxicity of concurrent chemoradiotherapy with adjuvant chemotherapy versus radiotherapy alone in patients with stage III to IV B nasopharyngeal carcinoma from endemic regions of China. Cancer. 2013;119(12):2230–2238. doi: 10.1002/cncr.28049. [DOI] [PubMed] [Google Scholar]
- 10.Lai SZ, Li WF, Chen L, Luo W, Chen YY, Liu LZ, et al. How does intensity-modulated radiotherapy versus conventional two-dimensional radiotherapy influence the treatment results in nasopharyngeal carcinoma patients? Int J Radiat Oncol Biol Phys. 2011;80(3):661–668. doi: 10.1016/j.ijrobp.2010.03.024. [DOI] [PubMed] [Google Scholar]
- 11.Peng G, Wang T, Yang KY, Zhang S, Zhang T, Li Q, et al. A prospective, randomized study comparing outcomes and toxicities of intensity-modulated radiotherapy vs. conventional two-dimensional radiotherapy for the treatment of nasopharyngeal carcinoma. Radiother Oncol. 2012;104(3):286–293. doi: 10.1016/j.radonc.2012.08.013. [DOI] [PubMed] [Google Scholar]
- 12.Liang Z, Zhu X, Li L, Qu S, Liang X, Liang Z, et al. Concurrent chemoradiotherapy followed by adjuvant chemotherapy compared with concurrent chemoradiotherapy alone for the treatment of locally advanced nasopharyngeal carcinoma: a retrospective controlled study. Curr Oncol. 2014;21(3):e408–e417. doi: 10.3747/co.21.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chua DT, Ma J, Sham JS, Mai HQ, Choy DT, Hong MH, et al. Long-term survival after cisplatin-based induction chemotherapy and radiotherapy for nasopharyngeal carcinoma: a pooled data analysis of two phase III trials. J Clin Oncol. 2005;23(6):1118–1124. doi: 10.1200/JCO.2005.12.081. [DOI] [PubMed] [Google Scholar]
- 14.Ouyang PY, Xie C, Mao YP, Zhang Y, Liang XX, Su Z, et al. Significant efficacies of neoadjuvant and adjuvant chemotherapy for nasopharyngeal carcinoma by meta-analysis of published literature-based randomized, controlled trials. Ann Oncol. 2013;24(8):2136–2146. doi: 10.1093/annonc/mdt146. [DOI] [PubMed] [Google Scholar]
- 15.Zhao C, Han F, Lu LX, Huang SM, Lin CG, Deng XW, et al. Intensity modulated radiotherapy for local-regional advanced nasopharyngeal carcinoma. Ai Zheng. 2004;23(11 Suppl):1532–1537. [PubMed] [Google Scholar]
- 16.ICRU report 50. Available at: http://www.icru.org/home/reports/prescribing-recording-and-reporting-photon-beam-therapy-report-50.
- 17.ICRU report 62. Available at: http://www.icru.org/home/reports/prescribing-recording-and-reporting-photon-beam-therapy-report-62.
- 18.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45(2):228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 19.Common terminology criteria for adverse events v3.0. Available at: http://www.eortc.be/services/doc/ctc/ctcaev3.pdf.
- 20.Pfister DG, Ang KK, Brizel DM, Burtness BA, Busse PM, Caudell JJ, et al. Head and neck cancers, version 2.2013 featured updates to the NCCN guidelines. J Natl Compr Cancer Netw. 2013;11(8):917–923. doi: 10.6004/jnccn.2013.0113. [DOI] [PubMed] [Google Scholar]
- 21.Chan AT, Teo PM, Ngan RK, Leung TW, Lau WH, Zee B, et al. Concurrent chemotherapy–radiotherapy compared with radiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: progression-free survival analysis of a phase III randomized trial. J Clin Oncol. 2002;20(8):2038–2044. doi: 10.1200/JCO.2002.08.149. [DOI] [PubMed] [Google Scholar]
- 22.Lin S, Lu JJ, Han L, Chen Q, Pan J. Sequential chemotherapy and intensity-modulated radiation therapy in the management of locoregionally advanced nasopharyngeal carcinoma: experience of 370 consecutive cases. BMC Cancer. 2010;10:39. doi: 10.1186/1471-2407-10-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Su SF, Han F, Zhao C, Huang Y, Chen CY, Xiao WW, et al. Treatment outcomes for different subgroups of nasopharyngeal carcinoma patients treated with intensity-modulated radiation therapy. Chin J Cancer. 2011;30(8):565–573. doi: 10.5732/cjc.010.10547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ji X, Xie C, Hu D, Fan X, Zhou Y, Zheng Y. Survival benefit of adding chemotherapy to intensity modulated radiation in patients with locoregionally advanced nasopharyngeal carcinoma. PLoS One. 2013;8(2):e56208. doi: 10.1371/journal.pone.0056208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nordsmark M, Overgaard M, Overgaard J. Pretreatment oxygenation predicts radiation response in advanced squamous cell carcinoma of the head and neck. Radiother Oncol. 1996;41(1):31–39. doi: 10.1016/S0167-8140(96)91811-3. [DOI] [PubMed] [Google Scholar]
- 26.Wang R, Wu F, Lu H, Wei B, Feng G, Li G, et al. Definitive intensity-modulated radiation therapy for nasopharyngeal carcinoma: long-term outcome of a multicenter prospective study. J Cancer Res Clin Oncol. 2013;139(1):139–145. doi: 10.1007/s00432-012-1313-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu F, Wang R, Lu H, Wei B, Feng G, Li G, et al. Concurrent chemoradiotherapy in locoregionally advanced nasopharyngeal carcinoma: treatment outcomes of a prospective, multicentric clinical study. Radiother Oncol. 2014;112(1):106–111. doi: 10.1016/j.radonc.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 28.Ma BB, Chan AT. Recent perspectives in the role of chemotherapy in the management of advanced nasopharyngeal carcinoma. Cancer. 2005;103(1):22–31. doi: 10.1002/cncr.20768. [DOI] [PubMed] [Google Scholar]
- 29.Wei WI, Sham JS. Nasopharyngeal carcinoma. Lancet. 2005;365(9476):2041–2054. doi: 10.1016/S0140-6736(05)66698-6. [DOI] [PubMed] [Google Scholar]
- 30.Xu T, Zhu G, He X, Ying H, Hu C. A phase III randomized study comparing neoadjuvant chemotherapy with concurrent chemotherapy combined with radiotherapy for locoregionally advanced nasopharyngeal carcinoma: updated long-term survival outcomes. Oral Oncol. 2014;50(2):71–76. doi: 10.1016/j.oraloncology.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 31.Cheng SH, Jian JJ, Tsai SY, Yen KL, Chu NM, Chan KY, et al. Long-term survival of nasopharyngeal carcinoma following concomitant radiotherapy and chemotherapy. Int J Radiat Oncol Biol Phys. 2000;48(5):1323–1330. doi: 10.1016/S0360-3016(00)00779-3. [DOI] [PubMed] [Google Scholar]
- 32.Sun X, Su S, Chen C, Han F, Zhao C, Xiao W, et al. Long-term outcomes of intensity-modulated radiotherapy for 868 patients with nasopharyngeal carcinoma: an analysis of survival and treatment toxicities. Radiother Oncol. 2014;110(3):398–403. doi: 10.1016/j.radonc.2013.10.020. [DOI] [PubMed] [Google Scholar]
- 33.Yoshizaki T, Kondo S, Murono S, Endo K, Tsuji A, Nakanishi Y, et al. Progress and controversy for the role of chemotherapy in nasopharyngeal carcinoma. Jpn J Clin Oncol. 2015;45(3):244–247. doi: 10.1093/jjco/hyu212. [DOI] [PubMed] [Google Scholar]


