Summary
Recent work in a variety of animal models, including mice, zebrafish, and macaques, as well as in humans, has led to a reassessment of several tenets of mycobacterial infection. In this review, we highlight new findings about the composition and dynamics of the tuberculous granuloma, the central host structure in mycobacterial infection, as well as inflammatory mediators that drive a successful anti-microbial response on one hand and pathological inflammation on the other. We highlight granuloma heterogeneity that emerges in the context of infection, the functional consequences of angiogenesis in tuberculous granulomas, and data that balanced inflammation in humans, with a central role for tumor necrosis factor, appears to play a key role in optimal defense against mycobacterial infection. These findings have suggested new and specific host-directed therapies that await further clinical exploration.
Keywords: bacterial disease, inflammation, in vivo imaging, monocytes/macrophages, tuberculosis
Introduction
The tuberculous granuloma, a central structure in mycobacterial infection, is the hallmark structure of tuberculosis (TB) (1). At its most elemental, the granuloma consists of tightly interdigitated macrophages that transform phenotypically and take on an epithelioid appearance (2). As the tuberculous granuloma progresses, other immune cell types join the nucleating granuloma in a coordinated response, but this complex structure often fails to eliminate infection. Historically, the granuloma was thought to be an exclusively host-protective response, and granuloma formation was posited to culminate in an impermeable ring around bacterial invaders. In this review, we highlight new findings about the nature of the tuberculous granuloma, both in its early stages, and as it recruits functional blood vessels that impact oxygen availability, pathogenesis and dissemination. Finally, we highlight findings about eicosanoids and inflammation. These lipid mediators, induced during infection with pathogenic mycobacteria, play an important role in controlling inflammatory balance. Genetic variation in eicosanoid pathways is associated with different host responses and outcomes. Understanding how mycobacteria manipulate the inflammatory balance and differences in human disease progression may provide new opportunities for host-directed therapeutic approaches.
Optical access to the granuloma
The ability to perform in vivo, real time imaging of granuloma dynamics and the adoption of new techniques and animal models have expanded traditional views of mycobacterial granulomas. To directly observe granuloma formation in vivo, we use optically transparent zebrafish larvae, which form early granulomas within days after infection with Mycobacterium marinum (3, 4).
Mycobacterium marinum is the closest genetic relative of the M. tuberculosis complex (5). M. marinum infections in humans cause a granulomatous infection called fish tank or aquarium tank granuloma, characterized by skin and soft tissue granulomas that can have strikingly similar pathology to those of tuberculosis, with necrotic areas and vascularization (6). In zebrafish, M. marinum infection is similarly characterized by the organized multicentric epithelioid granulomas characteristic of human tuberculosis (7, 8). The zebrafish larva is optically transparent and allows real-time visualization of granuloma formation using fluorescently-labelled bacteria and immune cells. This approach has yielded substantial insights about mycobacterial granuloma formation; within days of infection a given mycobacterium-infected macrophage recruits additional macrophages to form tightly organized aggregates (2, 3). These aggregates represent early granulomas; the participating macrophages undergo the hallmark epithelioid transformation characteristic of mature granulomas and activate mycobacterial gene expression programs that are selectively induced in the mature granulomas of adult animals but not in isolated macrophages (2, 3).
Initially, these granulomas are dynamic, with egress of infected macrophages and both apoptotic and necrotic cell death within (3, 4). Superinfecting strains of mycobacteria traffic into established granulomas, both in adult zebrafish and mice, consistent with a dynamic and relatively accessible structure during active disease (9, 10). Multiphoton intravital microscopy of mouse liver granulomas, both with the Mycobacterium bovis vaccine strain BCG and later with M. tuberculosis, has revealed dynamics of both macrophages and T cells within mouse mycobacterial granulomas (11, 12). Notably, an unusually small number of mycobacteria-specific T cells displaying any antigen-induced migration arrest, suggesting that properties of the mycobacterial granuloma limit effector T-cell function (12).
Granulomas and their progression are not uniform. Analysis of zebrafish granulomas suggests that even in the presence of antibiotics and overall reduction in bacterial burden, some granulomas can expand (13, 14). Many of the findings made using Mycobacterium marinum in its natural hosts have been recapitulated in mouse and non-human primate models infected with Mycobacterium tuberculosis, notably the heterogeneity of individual granulomas and the dynamics of granulomas, which can either expand or regress throughout the infection (15). In non-human primates, in addition to different granuloma types, microenvironments appear to exist within the granuloma itself and different macrophage subsets appear to populate individual granulomas; iNOS-positive macrophages tend to dominate near the necrotic core, while so-called alternatively activated macrophages or Type II macrophages are more common near the outer cuff (16).
Although epithielioid macrophages may define the nascent granuloma, many cell types participate and may serve important functions within. Dendritic cells and lymphocytes play key roles (17), and neutrophils also have been proposed to play important yet sometimes contradictory roles in a number of granuloma-associated processes including generation of reactive oxygen species (ROS), efferocytosis and immunopathology (18–20). There is a need to expand our understanding of infection and granuloma complexity still further; in the mouse model there is sequential transit of infected cell types over the course of infection, with significant bacterial residence in dendritic cells, recruited interstial macrophages and neutrophils (21) (22, 23). As adaptive immunity is induced, there are similarly important roles for CD4 T cells, which play key roles both in protection and immunopathology (17). Beyond this complex immune cell milieu in granulomas, other non-immune cell types play additional supporting roles. Fibroblasts are important in the deposition of collagen in fibrotic granulomas, one of multiple heterogeneous granuloma types that are observed (1)
Hypoxia
Within the heterogeneous granulomas that form, pathologists have long suggested that necrotic areas within some human granulomas may become hypoxic (24). Anecdotal observations about growth in areas of the lung with higher versus lower oxygen tension (reactivation most often occurs in the more oxygenated upper lobes of the human lung) as well as dramatic experimental effects on cell growth and metabolism of mycobacteria under low-oxygen conditions have spawned robust investigations of the effect of oxygen availability on mycobacterial growth and behavior (25, 26).
Hypoxia has long been posited to play an important role in mycobacterial pathogenesis, as a component of the granuloma and one that induces metabolic adaptations in bacteria (26–28). Adaptation to hypoxic and nutrient limiting conditions, as well as a functional host immune system, are major challenges for mycobacterial survival, and the induction of the DosR regulon appears to regulate a number of these responses, including exposure to reactive nitrogen species or carbon monoxide (29–31) (32).
One of the challenges in understanding the interactions of hypoxic conditions within the granuloma is that the granulomas of many standard and well-characterized animal models lack hypoxia. For example, the most commonly used C57Bl/6 mouse model appears to lack extensive hypoxia within the granulomas (33). In contrast, guinea pigs, rabbits, and macaques develop granulomas with hypoxic areas, as determined by pimonidazole staining. Notably, the drug metronidazole, which targets anaerobic bacteria (34), shows substantial efficacy in nonhuman primate models of TB (35), suggesting that in some hypoxic models, mycobacteria may be susceptible to metronidazole. In humans, trials of metronidazole in cases of multi-drug resistant tuberculosis were marginally effective, but halted because of excessive peripheral neuropathies, but this class of drugs may function in principle, and newer nitroimidazoles are being assessed (36).
Using the zebrafish model, we found both strong evidence of hypoxic granulomas in the adult, as assessed by pimonidazole staining and activation of hypoxia markers such as phd3 (37). Strikingly, in zebrafish, metronidazole was effective therapeutically when granulomas were largely hypoxic but not in non-hypoxic granulomas that form at different sites in the animals.
In cancer, there is both abundant literature and clinically approved therapy based on an intimate relationship between tumor hypoxia and angiogenesis (38, 39). Might there be analogous relationships and/or consequences in the case of the mycobacterial granuloma? A number of groups have noted vascularization of the tuberculous granuloma in animal models and in humans (40–43).
Granuloma-induced angiogenesis
Using the optical transparency of the zebrafish, we dissected the kinetics and dynamics of angiogenesis upon infection with Mycobacterium marinum (37). We found that angiogenesis coincided tightly with the formation of an early granuloma, at around four days post-infection (Fig. 1). Delay of granuloma induction using bacterial mutants with granuloma defects limited the appearance of angiogenesis, suggesting that these processes are tightly linked.
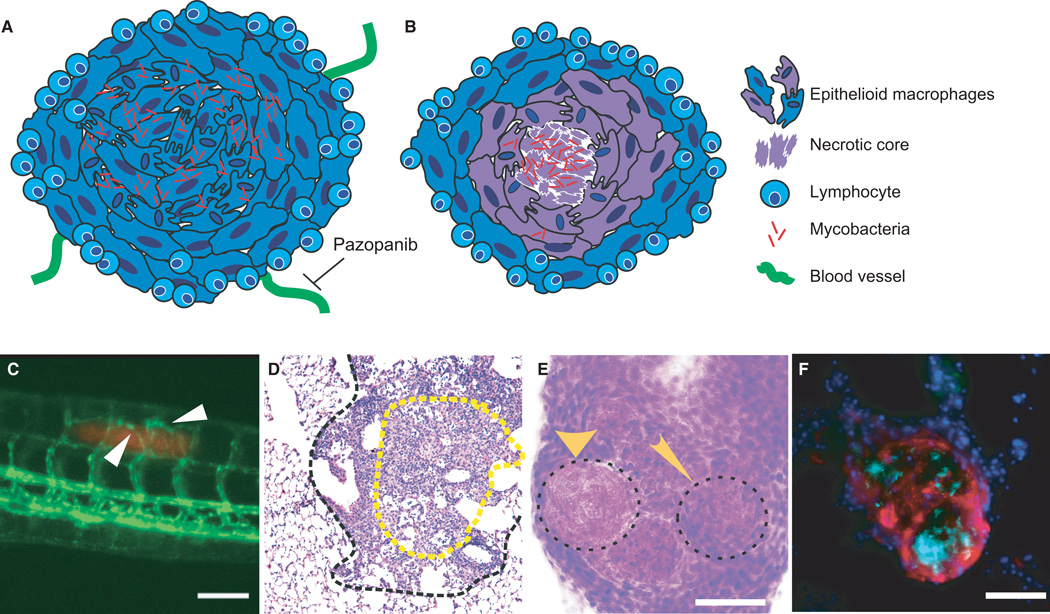
Fig. 1. Heterogeneous granuloma progression leads to morphologically and phenotypically distinct granuloma subtypes.
(A) Schematic of a normoxic, non-necrotic granuloma. A non-necrotic granuloma is comprised of a core of infected and uninfected epithelioid macrophages, with a cuff of lymphocytes. Vasculature extends toward the granuloma, and can be inhibited by the VEGFR inhibitor pazopanib. (B) Schematic of a necrotic, hypoxic granuloma. A mycobacteria-containing central core of necrotic cell debris is surrounded by layers of epithelioid macrophages circumscribed by a lymphocytic cuff. This granuloma is characterized by hypoxia (represented in purple) within the core. (C) Vascularization of early mycobacterial granulomas. Angiogenesis induced by granuloma formation in a 6-day post infection zebrafish larva infected with fluorescent M. marinum (red). Vasculature (flk1:gfp transgenic line) in green. White arrows point to blood vessels sprouting toward the site of infection. (D) H&E stain of a typical mouse granuloma. Outlined in a black dashed line is a granuloma from a C57Bl/6 mouse 25 weeks post-infection with Mycobacterium tuberculosis. The central region of epithelioid macrophages (eosin-rich region in yellow dotted line) is surrounded by a lymphocyte-rich cuff, with little evidence of a necrotic core or hypoxia. (E) H&E staining of adult zebrafish granuloma shows distinct necrotic and non-necrotic granulomas. A cryosectioned adult zebrafish infected with M. marinum for 2 weeks. The wide arrow marks a necrotic granuloma and the narrow arrow shows a non-necrotic granuloma, indicated by absence and presence of hematoxylin staining within the central core. (F) Necrotic granulomas are hypoxic in adult zebrafish. Section of an adult zebrafish infected with M. marinum 2 weeks post-infection, stained for pimonidazole (red), DAPI (blue), and M.marinum (cyan). Hypoxia, indicated by pimonidazole staining, is seen in and around a central mycobacteria-containing necrotic core. Scale bars in all panels are 100 µm.
What are the consequences to angiogenesis on infection? Theoretically, this host response could serve to aid bacterial replication by providing oxygen and nutrients or, alternatively, might be an exclusively host-beneficial response providing better access of immune cells to a center of infection.
In assessing the functional consequences of mycobacterial-induced angiogenesis, we initially focused on the role of vascularization in hypoxia, due to the evidence of hypoxic conditions within the granuloma, both in pimonidazole staining of animal models and in the efficacy of metronidazole, and the critical role that hypoxia is thought to play in the biology of mycobacteria. While vascularization is known to be a crucial mediator of tumor hypoxia, the functional role of mycobacterially induced angiogenesis on infection, and its possible relationship to oxygenation of the tuberculous granuloma has remained unclear. In investigating this infection-associated vascularization event, we sought to assess whether the vasculature that arises is a bystander effect driven by the extensive inflammation accompanying granuloma formation or is a central feature of mycobacterial pathogenesis.
The growth factor VEGF, a primary mediator of host vascularization, has been found to be induced in human tuberculosis patients (43, 44). We found that vascularization of zebrafish granulomas was accompanied by macrophage expression of VEGFA. To assess whether this VEGF induction was crucial to granuloma vascularization and bacterial growth, we took administered the FDA-approved VEGFR antagonist pazopanib (45, 46). In adult animals treated with pazopanib, both incipient and established infections could be treated therapeutically, with dramatic reductions in bacterial burdens (37). Second, we found that limiting granuloma vascularization diminished bacterial dissemination from established infections. Additionally, these treatments increased the proportion of hypoxic and sterilizing granulomas in adult zebrafish infections. Extension of these findings to other pre-clinical models, particularly those with hypoxic granulomas, will be exciting, but there are at least two additional important insights here into fundamental biology.
We conclude that a VEGF-mediated, granuloma-induced angiogenic program is ultimately beneficial to mycobacteria. Just as bacterial induction of granulomas may provide a new niche for cell-to-cell spread (described below), so does the induction of angiogenesis favor bacterial replication by providing an additional oxygen source.
By examining adult tissue sections of granulomas using fluorescently-labelled bacteria and assessing pimonidazole staining (Fig. 1), we could examine the relationship between angiogenesis, necrosis, and hypoxia. Notably, we found that even necrotic granulomas, typically associated with worse disease outcome, had decreased burden in animals treated with anti-angiogenic agents, with increased numbers of low-burden or sterilized granulomas and increased numbers of hypoxic granulomas (37). These low-burden granulomas have been observed and associated with sterilization in both non-human primate models and human lesions (15, 47).
While the clinical utility of these findings will depend on price decreases in anti-angiogenic agents and further exploration in pre-clinical models, they have, at the very least, provided insight into an additional aspect of granuloma biology and suggested a potential host-directed therapy that could be used adjunctively with existing antibiotics.
Bacterial manipulation of granulomas
A theme in these and other studies is that induction of granulomas and fundamental properties of the granuloma can be exploited by pathogenic mycobacteria for their own benefit. The mycobacterial cell wall component trehalose dimycolate plays multiple roles in pathogenesis, including promoting granuloma induction, inhibition of phagolysosome fusion, and induction of matrix metalloproteases (48, 49). Notably TDM has also been shown to act to induce angiogenesis in a rat corneal model (50), suggesting that it may also help to mediate the pro-bacterial angiogenic response described above.
The classic mycobacterial virulence locus RD1/ESX-1 plays a number of roles, including mediating access to the cytoplasm and induction of type I interferons (51–55). In the zebrafish larval model, the secreted ESAT-6 protein plays an important role in the induction of host MMP9 from epithelium, which helps to orchestrate granuloma formation. MMP9-deficient zebrafish have limited granuloma formation and are more resistant to infection, again suggesting a role for the early granuloma in promoting bacterial replication and spread (56). Similarly, MMP9 knockout mice show reduced granuloma formation and reduced dissemination (57). Thus, induction of a host pathway via a bacterial virulence determinant mediates both granuloma formation and promotes bacterial growth.
Cell wall lipids, which sit at the interface of bacteria and host, have recently been shown to play an important role in determining the outcome of infection by determining the repertoire of specific macrophage subsets that are recruited to initial infection site. Cambier et al. (58) have shown that cell surface associated phthiocerol dimycoceroserate (PDIM) masks activation of canonical TLR activation pathways that would normally recruit iNOS-expressing, microbicidal macrophages. In contrast, the expression of the phenolic glycolipid PGL is instrumental in recruitment of a macrophage subset through a CCR2 recruitment pathway (58). These findings in both zebrafish and mice suggest that pathogenic mycobacteria have evolved to manipulate the host immune response for their own survival and transmission.
Eicosanoid mediators in mycobacterial infections
Over the last decade, a number of lipid mediators of inflammation including the eicosanoid family have been implicated in mycobacterial infection. The eicosanoids are a broad group of signaling lipids derived from arachidonic acid that include the leukotrienes, lipoxins, thromboxanes and prostaglandins. The initial observation of the role of eicosanoids in mycobacterial infection came from mice deficient in the enzyme Alox5, an enzyme crucial both for leukotriene production from arachidonic acid and for production of anti-inflammatory lipoxins, which exhibited altered control of mycobacterial infection (59), with more resistance to high-dose infections. Since then, a network of eicosanoid mediators has emerged that play important roles in infection outcome, in both animal and cell culture models. The lipoxins are anti-inflammatory eicosanoids derived from arachidonic acid, through either 12- or 15-lipoxygenases (60), and which are induced during mycobacterial infection in both mice (with M. tuberculosis) and zebrafish (with M. marinum) (59, 61–63). Lipoxins appear to be largely host-detrimental in mycobacterial infection, and promote necrotic cell death, which favors unrestrained bacterial growth (61–64). In cell culture models and in mice, the interplay between the lipoxin LXA4 and the prostaglandin PGE2 determines mode of cell death, with necrotic cell death favoring robust bacterial growth (61, 62, 65). In zebrafish, we identified a mutation in the lta4h gene, which sits at the crossroads between the pro-inflammatory leukotriene LTB4 and the anti-inflammatory lipoxins (66). The lta4h deficiency shunted an LTA4 intermediate into an excess lipoxin state (63, 64). Hence, the observed hypersusceptibility to infection resulted from excess production of the anti-inflammatory lipoxins, which translated into limitation of TNF induction in vivo. TNF, discussed below, is absolutely critical in host defense, and so the limitation of this pro-inflammatory response resulted in necrotic cell death and extracellular bacterial growth. Genetic variants in these pathways appear to have important effects on human mycobacterial infections (63, 64) and measured differences in eicosanoid levels correlate with disease presentation (67). What has become clear in all these studies are that complex networks of eicosanoids feed back on one another. In a mouse model with IL-1 deficiency or with excess type I interferon production, inhibition of Alox5 via a chemical inhibitor, zileuton, resulted in increased survival and decreased burden. Mechanistically, inhibition of this relatively upstream enzyme appears to shunt eicosanoid production toward PGE2, thus establishing a favorable eicosanoid balance (67).
TNF’s two sides
In all animal models studied, the proinflammatory cytokine TNF is a key determinant of susceptibility to mycobacterial infection. However, attempts to characterize TNF as either wholly host-protective or pro-bacterial have failed. Instead, as we outline in the following section the emerging experimental picture has indicated that there exists an optimal level of TNF that results in a balanced inflammatory state within the host. Through a diverse range of mechanisms, deviations of TNF levels from this optimal state result in either increased bacterial proliferation from loss of mycobacterial restriction or deleterious inflammation and extensive damage of host tissues.
As a well characterized mediator of inflammatory signaling, TNF has long been suspected to play a role in mycobacterial infection. Early studies found that TNF release followed Mtb infection and that TNF drove many host processes thought to be crucial for mycobacterial control (68–70). However, the observation that enshrined TNF as one of the most clinically relevant host restriction genes was the finding that TNF blocking agents used in the treatment of unrelated inflammatory diseases exacerbated active TB and drove reactivation of latent TB within treated patients (71, 72). Supporting this crucial role of TNF in tuberculosis patients, later studies found that reactivation of TB is directly related to the amount of free TNF available, a topic (reviewed in 24, 73).
While the data from human patient populations clearly indicates that TNF levels are clinically relevant in restriction of Mtb, animal studies have enabled researchers to identify the diverse mechanisms by which TNF controls mycobacterial infection. While the first studies of the role of TNF in mouse Mtb infection models preceded findings in human patient populations, these findings mirrored those in humans: mice treated with a TNF antibody or genetically deficient for the TNF receptor (TNFR1−/−) were both found to be hypersusceptible to TB infection with markedly increased bacterial numbers in lungs and other organs. However, while there was no change in granuloma numbers in TNF-deficient animals, the loss of TNF resulted in extensive tissue damage and necrosis within both granulomas and surrounding tissue that likely contributed to the early death of TNFR-deficient animals (74). In agreement with loss of TNF leading to granuloma necrosis, TNFR knockout mice infected with M. avium exhibited extensive necrosis within the granuloma despite similar bacterial numbers between TNFR-deficient and wildtype animals (75). These findings in the mouse model are also observed in the zebrafish model. Zebrafish depleted for TNFR1 form granulomas even more quickly than wildtype fish, yet fail to restrict mycobacterial growth (76). The optical clarity of zebrafish enabled the direct observation of the earliest steps of macrophage recruitment and bacterial uptake, demonstrating that macrophage recruitment was not inhibited by lack of TNFR. Instead, the lack of mycobacterial restriction leads to excessively high bacterial burden within individual macrophages, resulting in macrophage necrosis, granuloma disintegration and extracellular growth of infecting mycobacteria as was observed in mouse models. TNF is required to reduce bacterial burden through a number of proposed mechanisms involving macrophage activation, often synergistically with Interferon-gamma activation (77); downstream of TNFR1 activation is transcriptional activation of a number of key defense genes that act cell-autonomously including antimicrobial peptides, autophagy genes, IRGM proteins, and GBPs (77). However, the lack of an adaptive immune system in these zebrafish larvae suggests that TNF is required in the context of innate immunity alone.
While the previous findings indicated that TNF is needed to restrict bacterial growth and prevent granuloma breakdown, data also indicated that excessive TNF levels were deleterious. In support of TNF mediating bacterial restriction, it was found that TNF deficiency in low dose wildtype BCG-infected animals resulted in large, disorganized granulomas that could be rescued by expressing TNF within the infecting BCG. However, hinting at the pleiotropic effects of TNF, infecting wildtype or TNF-deficient animals with a high dose of TNF expressing BCG lead to early death of infected animals despite the effective restriction of mycobacteria within these animals (78). More recently, the deleterious effects of high TNF levels has been investigated in the zebrafish model as discussed below.
The link between TNF neutralization and tuberculosis has been further investigated by studies performed in a non-human primate model of TB (79). The macaque model recapitulates many characteristics of the medical definition of human latent TB infections and can remain latently infected for years (80). Monkeys with latent infections treated with TNF neutralizing agents were more likely to develop more granulomas, exhibit weight loss, and test positive for actively growing bacteria (79). During acute active infection, TNF ablation was found to cause many more granulomatous lesions compared to acute infection in wildtype animals. These data not only validate the clinical observation that human patients taking TNF-neutralizing drugs are at a higher risk to reactivate TB, but also show that TNF inhibition also exacerbates active infection. These studies in the mouse and macaque inextricably link TNF with all stages of mycobacterial disease.
Until recently, the mouse model has been limited by its non-necrotizing granulomas, making the mouse model of TB rather unlike human infections. With the C3HeB/FeJ mice, this model of infection recapitulates human disease with necrotic centers, hypoxic tissue, and smaller bacterial loads (81). With this mouse model, the sterilizing activities of LTBI treatments were investigated, as well as the progression from latent to active TB. In this model, TNF neutralization led to increased bacterial burden, disorganized granulomas, and increased mortality (82). These new models, along with the in vivo zebrafish:Mm model and the non-human primates, will continue to further our understanding of the role that this pleiotropic cytokine, TNF, plays in the dynamic tuberculous granuloma.
TNF and cell death modalities
In addition to the stimulation of cell-autonomous immunity, TNF has been long-known for its ability to trigger caspase activation and apoptosis of the target cell, a process which has clear impact on mycobacterial pathogenesis (83). TNF binds two receptors, TNFR1 and TNFR2: TNFR1 is characterized for the presence of death domains in its intracellular portion while TNFR2 lacks these domains. Nevertheless, both TNFRs can recruit death domain-containing proteins (e.g. TRADD, FADD, and caspase 8) after binding TNF. Such recruitment ends with caspase 8 autocatalysis and conversion into the caspase 8 active form. Activated caspase 8 is an initiator caspase that cleaves different cellular components to amplify the apoptotic signal, particularly through activation of the effector caspase-3 to induce cell death (84). Extrinsic apoptosis depends on ligand-receptor interaction and is conserved among model organisms, with homologs for FasL, apo2L and TRAIL (85) and caspase 8 (86). Specifically, Fas and TNFR stimulation of zebrafish caspase 8-expressing cells induces caspase 8 activation and apoptosis (86).
The role of apoptosis in mycobacterial infection in vivo is multifaceted (87). In zebrafish larvae infected with virulent mycobacteria, apoptosis plays an important role in spread of bacteria within the granuloma (4). Similarly, in mice, strains with increased apoptosis correlated with increased bacterial growth (88). In cell culture models of mycobacterial disease, which may miss out on the full complexity of granuloma formation in vivo, virulent strains of Mtb tend to induce necrosis while attenuated strains are associated with apoptosis (89–91). Infection with attenuated strains of Mtb exhibit increased expression of TNFR1, increased apoptotic cells, and decreased bacterial replication (92). Interestingly, virulent as well as attenuated strains of Mtb can induce similar levels of TNF (93). Virulent mycobacteria themselves express anti-apoptotic gene products, and apoptosis may be neither entirely host-driven nor host-beneficial (91, 94). Apoptosis also is posited to be important in packaging infecting mycobacteria for efferocytosis as well as in the development of T-cell responses (18, 20, 65, 95),
There is almost universal agreement, however, that macrophage necrosis is detrimental to the host. In mycobacterial infection, insufficient TNF induction can lead to inadequate control of bacterial replication and macrophage necrosis, liberating the infecting bacteria to grow extracellularly, unencumbered by intracellular defenses. Recent findings have suggested that high levels of TNF can also result in host cell necrotic death, via a programmed necrosis pathway. Thus, TNF can serve as a double-edged sword, and either too little or too much can promote mycobacterial pathogenesis and/or immunopathology.
Genetic control of inflammatory balance
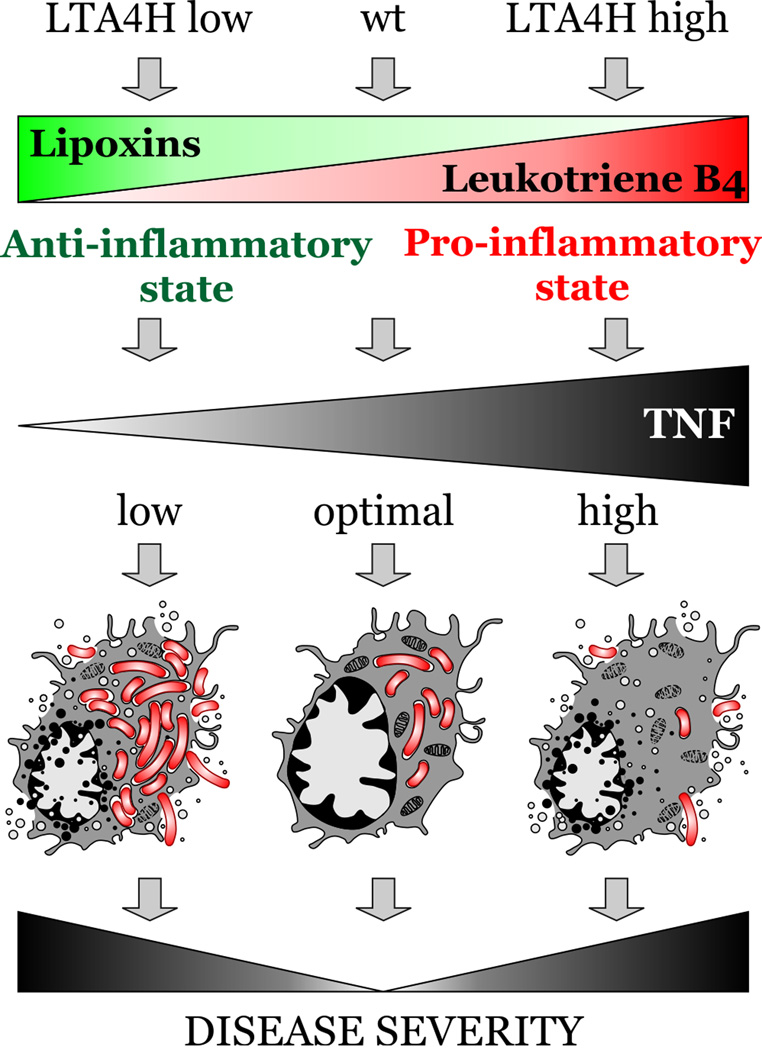
The availability of TNF during mycobacterial infection has been associated with the outcome of both active and inactive disease (24, 73). In the zebrafish model, low TNF and high TNF can both lead to increased infection burden (63, 64, 96, 97). Might findings from animal models and clinical observations be relevant to human genetic variants that impinge on TNF? We identified a common promoter polymorphism at the human LTA4H locus, the orthologue of which we had identified in the zebrafish forward genetic screen as a key regulator of TNF levels. This variant was associated with increased expression of LTA4H transcript. We then interrogated three human cohorts (two tuberculosis cohorts in Vietnam and a leprosy cohort in Nepal) and found an association of the heterozygous genotype (one low-activity allele, and one high-activity allele) with protection from more severe disease. Low-activity homozygotes, predicted to produce more lipoxin and less TNF, developed more severe disease than the balanced heterozygotes. Similarly, high-activity homozygotes, predicted to produce more pro-inflammatory leukotrienes, also developed more severe disease than their heterozygous counterparts (Fig. 2).
Fig. 2. LTA4H genotype associates with disease severity in humans and zebrafish.
A polymorphism in LTA4H is associated with increased severity of TB in humans. In zebrafish, both high- and low-activity LTA4H genotypes result in more severe disease, but for distinct reasons, with high-burden necrosis in the low inflammatory state and induction of necroptosis in the high inflammatory condition.
If this hypothesis is correct, then the two classes of homozygotes had fundamentally different inflammatory states. By examining a TB meningitis cohort that had participated in a previous clinical trial of the broadly acting anti-inflammatory agent dexamethasone as adjunctive therapy (98), we could examine whether there were differences in treatment responsiveness based on this single nucleotide promoter variant. Overall, patients with TB meningitis receiving dexamethasone showed improved survival over patients receiving placebos, and this trial established adjunctive dexamethasone therapy as the standard of care for TB meningitis (98). Remarkably, when these patients were stratified by host genotype, the entirety of the dexamethasone effect derived from the single class of high-activity homozygotes (63). Replication of these findings in new, larger cohorts is underway (G. Thwaites, personal communication). The association of tuberculosis disease severity with the LTA4H promoter variant in Vietnam also appears to hold true for populations in Nepal, where there is an association with more severe forms of leprosy (64), and China, where there is an association with TB meningitis (99), also with a heterozygous advantage.
High TNF state: mechanisms of necroptosis
High inflammatory states associated with a high activity LTA4H allele in humans, rather than controlling intracellular mycobacterial growth better, trigger cellular pathways that culminate with the death of mycobacterium-infected macrophages and increased disease severity. In a zebrafish model of high LTA4H activity, we demonstrated more severe disease in the high inflammatory state, and the increased bacterial burden was mediated through excess TNF. Partial ablation of TNF could rescue LTA4H high susceptibility. Reciprocally, injection of soluble recombinant TNF rescues susceptibility in LTA4H low animals (63). We decided to exploit the zebrafish model for TB in order to dissect the molecular pathways by which excess TNF promotes susceptibility to TB.
In humans, both LTA4H low- and high-activity genotypes share worse outcomes in patients with TB meningitis than those patients with balanced inflammation (63). In the zebrafish, the same states are similarly susceptible, presenting the same phenotype: infected macrophage lysis and exuberant extracellular bacterial growth (63, 100). However, while macrophages from LTA4H/TNF low larvae are unable to control intracellular mycobacteria, those from LTA4H/TNF high presented increased bactericidal capacity and fewer intracellular mycobacteria (63). Although there was increased bacterial control initially, excess TNF induced mitochondrial reactive oxygen species (ROS) production in infected macrophages. These ROS kill both intracellular bacteria and the infected macrophage (100) (Fig. 3). Both excess TNF and mycobacterial infection are required to trigger mitochondrial ROS production.
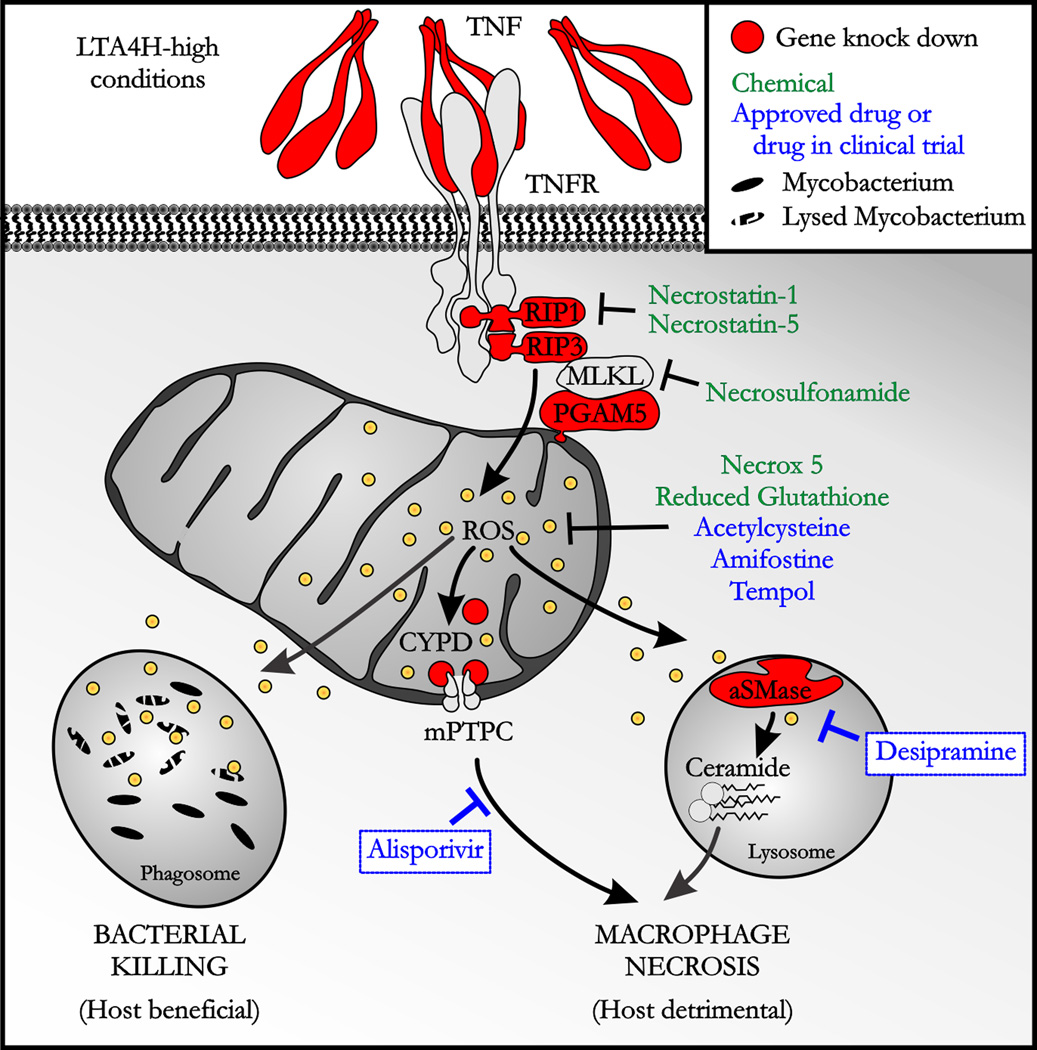
Fig. 3. TNF excess triggers both host-beneficial and bacteria-beneficial molecular pathways.
A schematic of chemical and genetic manipulations of TNF-induced pathways that mediate bacterial killing and macrophage necrosis. Abbreviations: TNFR, TNF receptor; RIP, receptor-interacting protein; MLKL, mixed lineage kinase-like; ROS, reactive oxygen species, CYPD, cyclophilin D; mPTPC, mitochondrial permeability transition pore complex; aSMase, acid sphingomyelinase.
In studying the different pathways that TNF triggers to induce cell death, we discarded apoptosis (63) and autophagy (F.J.R. and L. Ramakrishnan, unpublished data) and showed that excess TNF was inducing RIP1-RIP3-dependent programmed necrosis (also called necroptosis). The RIP1-RIP3 pair has been shown to promote TNF-induced necrosis in human, mouse and zebrafish cell lines (101–103). RIP1-RIP3 form part of the called necroptosome complex, a protein complex that might target the mitochondrion by associating with a mitochondrial serine/threonine-protein phosphatase (PGAM5), a protein associated with the outer mitochondrial membrane. Interestingly, ablation of RIP1 or RIP3 and use of ROS scavengers in wildtype animals did not modify infection outcome in the zebrafish, suggesting that the pathway by which TNF triggers macrophage necrosis operates only in conditions of TNF excess (63).
We next interrogated the pathway for the elements promoting necrosis downstream of mitochondrial ROS production. A common step during mitochondrial-dependent cell death is mitochondrial ROS production and mitochondrial permeability transition pore complex (mPTPC) formation culminating with mitochondrial disruption. Some studies in the literature pointed to the redox and calcium sensor cyclophilin D (CYPD) located in the mitochondrial matrix as an attractive candidate as a modulator of the mPTPC (104–106). We demonstrated by genetic knockdown that CYPD was required for the necrosis of infected macrophages in the high TNF state (Fig. 3). Moreover, we showed that CYPD acts downstream of mitochondrial ROS production in the pathways as CYPD-deficient animals in a LTA4H high background showed decreased susceptibility yet retained mitochondrial ROS production and increased bactericidal capacity (63). We might expect CYPD deficient/LTA4H high animals to be resistant to Mm infection. However, they were as susceptible as wildtype fish and they still presented infected macrophage lysis while being better at killing intracellular bacteria. In asking if a partial CYPD ablation was the cause of macrophage lysis, we found that mitochondrial ROS production activates the lysosomal acid sphingomyelinase (aSMase) leading to ceramide production (Fig. 3). aSMase has been implicated in cell death and specifically in programmed necrosis downstream of RIP1 (105).
Genetic or chemical inhibition of elements upstream of mitochondrial ROS production in the pathway or the use of ROS scavenger agents completely abrogates the necrotic pathway and abrogates increased bactericidal capacity, macrophage lysis, and overall hypersusceptibility (63). However, downregulation of CYPD and aSMase, both elements located downstream of mitochondrial ROS production, restores macrophage survival while keeping increased bactericidal capacity thus converting hypersusceptibility in resistance in the LTA4H high state.
The relevance of detailing this necrotic pathway is the possibility of targeting new elements with specific drugs with a narrower spectrum of action than glucocorticoids such as dexamethasone used in cases of TB meningitis. There are some available and approved ROS scavenger drugs such as acetylcysteine, amifostine and tempol that would restore wildtype conditions in the zebrafish and might be used in humans (Fig. 3). However, the major finding of this study is the possibility of using even more specific drugs downstream of mitochondrial ROS production what might allow the infected macrophages to live longer with while retaining the ability to kill mycobacteria better. We used desipramine, an FDA-approved drug that promotes proteolytic degradation of aSMase (107), and alisporivir, an inhibitor of CYPD, in clinical trials to treat hepatitis C (108). We showed synergy in decreasing susceptibility in LTA4H high zebrafish, pointing to these two molecules as a potential adjunctive treatment for TB patients with the LTA4H/TNF high genotype.
Conclusions: prospects for host-directed therapies
Mycobacterial genes co-evolved with their hosts to exploit host immune processes that have traditionally been viewed as part of a productive response to infection. Processes such as granuloma formation and granuloma-induced angiogenesis provide niches and/or conditions in which mycobacteria can replicate and disseminate, eventually leading to successful host-to-host transmission.
Virulent mycobacteria have been remarkably recalcitrant to elimination using traditional antibiotics. Identifying the host processes that mycobacteria have become dependent on enables the rational development of host directed therapies for tuberculosis. We have shown how this approach has identified potential therapeutic compounds including FDA-approved compounds that target angiogenesis, host eicosanoids, and pathways downstream of hyperinflammatory states.
Acknowledgements
We thank Stefan Oehlers, Sunhee Lee, Kristen Smith, Dana Sisk, and Joseph Saelens (Duke University) for the images in Fig, 1. We are grateful for funding from a National Science Foundation Graduate Research Fellowship (M.A.M.), a postdoctoral fellowship from the educational ministry of Spain (F.J.R), a postdoctoral fellowship (PF-13-223-01-MPC) from the American Cancer Society (M.R.C.), and a Mallinckrodt Scholar Award, a Searle Scholar Award, a Vallee Foundation Young Investigator Award, an NIH Director’s New Innovator Award 1DP2-OD008614, and the Duke University Center for AIDS Research (D.M.T.).
Footnotes
The authors declare no conflict of interest.
References
- 1.Russell DG. Who puts the tubercle in tuberculosis? Nature reviews Microbiology. 2007;5:39–47. doi: 10.1038/nrmicro1538. [DOI] [PubMed] [Google Scholar]
- 2.Ramakrishnan L. Revisiting the role of the granuloma in tuberculosis. Nat Rev Immunol. 2012;12:352–366. doi: 10.1038/nri3211. [DOI] [PubMed] [Google Scholar]
- 3.Davis JM, Clay H, Lewis JL, Ghori N, Herbomel P, Ramakrishnan L. Real-time visualization of mycobacterium-macrophage interactions leading to initiation of granuloma formation in zebrafish embryos. Immunity. 2002;17:693–702. doi: 10.1016/s1074-7613(02)00475-2. [DOI] [PubMed] [Google Scholar]
- 4.Davis JM, Ramakrishnan L. The role of the granuloma in expansion and dissemination of early tuberculous infection. Cell. 2009;136:37–49. doi: 10.1016/j.cell.2008.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stinear TP, et al. Insights from the complete genome sequence of Mycobacterium marinum on the evolution of Mycobacterium tuberculosis. Genome Res. 2008;18:729–741. doi: 10.1101/gr.075069.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Min KW, Ko JY, Park CK. Histopathological spectrum of cutaneous tuberculosis and non-tuberculous mycobacterial infections. J Cutan Pathol. 2012;39:582–595. doi: 10.1111/j.1600-0560.2012.01903.x. [DOI] [PubMed] [Google Scholar]
- 7.Cosma CL, Swaim LE, Volkman H, Ramakrishnan L, Davis JM. Zebrafish and frog models of Mycobacterium marinum infection. Current protocols in microbiology. 2006;Chapter 10(Unit 10B):12. doi: 10.1002/0471729256.mc10b02s3. [DOI] [PubMed] [Google Scholar]
- 8.Swaim LE, Connolly LE, Volkman HE, Humbert O, Born DE, Ramakrishnan L. Mycobacterium marinum infection of adult zebrafish causes caseating granulomatous tuberculosis and is moderated by adaptive immunity. Infection and immunity. 2006;74:6108–6117. doi: 10.1128/IAI.00887-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cosma CL, Humbert O, Ramakrishnan L. Superinfecting mycobacteria home to established tuberculous granulomas. Nature immunology. 2004;5:828–835. doi: 10.1038/ni1091. [DOI] [PubMed] [Google Scholar]
- 10.Cosma CL, Humbert O, Sherman DR, Ramakrishnan L. Trafficking of superinfecting Mycobacterium organisms into established granulomas occurs in mammals and is independent of the Erp and ESX-1 mycobacterial virulence loci. The Journal of infectious diseases. 2008;198:1851–1855. doi: 10.1086/593175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Egen JG, Rothfuchs AG, Feng CG, Winter N, Sher A, Germain RN. Macrophage and T cell dynamics during the development and disintegration of mycobacterial granulomas. Immunity. 2008;28:271–284. doi: 10.1016/j.immuni.2007.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Egen JG, Rothfuchs AG, Feng CG, Horwitz MA, Sher A, Germain RN. Intravital imaging reveals limited antigen presentation and T cell effector function in mycobacterial granulomas. Immunity. 2011;34:807–819. doi: 10.1016/j.immuni.2011.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adams KN, et al. Drug tolerance in replicating mycobacteria mediated by a macrophage-induced efflux mechanism. Cell. 2011;145:39–53. doi: 10.1016/j.cell.2011.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takaki K, Cosma CL, Troll MA, Ramakrishnan L. An in vivo platform for rapid high-throughput antitubercular drug discovery. Cell reports. 2012;2:175–184. doi: 10.1016/j.celrep.2012.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin PL, et al. Sterilization of granulomas is common in active and latent tuberculosis despite within-host variability in bacterial killing. Nature medicine. 2014;20:75–79. doi: 10.1038/nm.3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mattila JT, et al. Microenvironments in tuberculous granulomas are delineated by distinct populations of macrophage subsets and expression of nitric oxide synthase and arginase isoforms. Journal of immunology. 2013;191:773–784. doi: 10.4049/jimmunol.1300113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mayer-Barber K, Barber DL. Innate and Adaptive Cellular Immune Responses to Mycobacterium tuberculosis Infection. Cold Spring Harbor perspectives in medicine. 2015 doi: 10.1101/cshperspect.a018424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang CT, Cambier CJ, Davis JM, Hall CJ, Crosier PS, Ramakrishnan L. Neutrophils exert protection in the early tuberculous granuloma by oxidative killing of mycobacteria phagocytosed from infected macrophages. Cell host & microbe. 2012;12:301–312. doi: 10.1016/j.chom.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Desvignes L, Ernst JD. Interferon-gamma-responsive nonhematopoietic cells regulate the immune response to Mycobacterium tuberculosis. Immunity. 2009;31:974–985. doi: 10.1016/j.immuni.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martin CJ, et al. Efferocytosis is an innate antibacterial mechanism. Cell host & microbe. 2012;12:289–300. doi: 10.1016/j.chom.2012.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Repasy T, et al. Intracellular bacillary burden reflects a burst size for Mycobacterium tuberculosis in vivo. PLoS pathogens. 2013;9:e1003190. doi: 10.1371/journal.ppat.1003190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Srivastava S, Ernst JD, Desvignes L. Beyond macrophages: the diversity of mononuclear cells in tuberculosis. Immunol Rev. 2014;262:179–192. doi: 10.1111/imr.12217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wolf AJ, et al. Mycobacterium tuberculosis infects dendritic cells with high frequency and impairs their function in vivo. Journal of immunology. 2007;179:2509–2519. doi: 10.4049/jimmunol.179.4.2509. [DOI] [PubMed] [Google Scholar]
- 24.Barry CE, 3rd, et al. The spectrum of latent tuberculosis: rethinking the biology and intervention strategies. Nature reviews Microbiology. 2009;7:845–855. doi: 10.1038/nrmicro2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rustad TR, Sherrid AM, Minch KJ, Sherman DR. Hypoxia: a window into Mycobacterium tuberculosis latency. Cellular microbiology. 2009;11:1151–1159. doi: 10.1111/j.1462-5822.2009.01325.x. [DOI] [PubMed] [Google Scholar]
- 26.Rittershaus ES, Baek SH, Sassetti CM. The normalcy of dormancy: common themes in microbial quiescence. Cell host & microbe. 2013;13:643–651. doi: 10.1016/j.chom.2013.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wayne LG, Hayes LG. An in vitro model for sequential study of shiftdown of Mycobacterium tuberculosis through two stages of nonreplicating persistence. Infection and immunity. 1996;64:2062–2069. doi: 10.1128/iai.64.6.2062-2069.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gomez JE, McKinney JD. M. tuberculosis persistence, latency, and drug tolerance. Tuberculosis. 2004;84:29–44. doi: 10.1016/j.tube.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 29.Kumar A, et al. Heme oxygenase-1-derived carbon monoxide induces the Mycobacterium tuberculosis dormancy regulon. J Biol Chem. 2008;283:18032–18039. doi: 10.1074/jbc.M802274200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shiloh MU, Manzanillo P, Cox JS. Mycobacterium tuberculosis senses host-derived carbon monoxide during macrophage infection. Cell host & microbe. 2008;3:323–330. doi: 10.1016/j.chom.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ohno H, et al. The effects of reactive nitrogen intermediates on gene expression in Mycobacterium tuberculosis. Cellular microbiology. 2003;5:637–648. doi: 10.1046/j.1462-5822.2003.00307.x. [DOI] [PubMed] [Google Scholar]
- 32.Galagan JE, et al. The Mycobacterium tuberculosis regulatory network and hypoxia. Nature. 2013;499:178–183. doi: 10.1038/nature12337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Via LE, et al. Tuberculous granulomas are hypoxic in guinea pigs, rabbits, and nonhuman primates. Infection and immunity. 2008;76:2333–2340. doi: 10.1128/IAI.01515-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wayne LG, Sramek HA. Metronidazole is bactericidal to dormant cells of Mycobacterium tuberculosis. Antimicrobial agents and chemotherapy. 1994;38:2054–2058. doi: 10.1128/aac.38.9.2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lin PL, et al. Metronidazole prevents reactivation of latent Mycobacterium tuberculosis infection in macaques. Proceedings of the National Academy of Sciences of the United States of America. 2012 doi: 10.1073/pnas.1121497109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carroll MW, et al. Efficacy and safety of metronidazole for pulmonary multidrug-resistant tuberculosis. Antimicrobial agents and chemotherapy. 2013;57:3903–3909. doi: 10.1128/AAC.00753-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oehlers SH, et al. Interception of host angiogenic signalling limits mycobacterial growth. Nature. 2014 doi: 10.1038/nature13967. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Folkman J. Role of angiogenesis in tumor growth and metastasis. Seminars in oncology. 2002;29:15–18. doi: 10.1053/sonc.2002.37263. [DOI] [PubMed] [Google Scholar]
- 39.Naumov GN, et al. A model of human tumor dormancy: an angiogenic switch from the nonangiogenic phenotype. Journal of the National Cancer Institute. 2006;98:316–325. doi: 10.1093/jnci/djj068. [DOI] [PubMed] [Google Scholar]
- 40.Tsai MC, et al. Characterization of the tuberculous granuloma in murine and human lungs: cellular composition and relative tissue oxygen tension. Cellular microbiology. 2006;8:218–232. doi: 10.1111/j.1462-5822.2005.00612.x. [DOI] [PubMed] [Google Scholar]
- 41.Aly S, Laskay T, Mages J, Malzan A, Lang R, Ehlers S. Interferon-gamma-dependent mechanisms of mycobacteria-induced pulmonary immunopathology: the role of angiostasis and CXCR3-targeted chemokines for granuloma necrosis. J Pathol. 2007;212:295–305. doi: 10.1002/path.2185. [DOI] [PubMed] [Google Scholar]
- 42.Ulrichs T, et al. Differential organization of the local immune response in patients with active cavitary tuberculosis or with nonprogressive tuberculoma. The Journal of infectious diseases. 2005;192:89–97. doi: 10.1086/430621. [DOI] [PubMed] [Google Scholar]
- 43.Matsuyama W, et al. Increased serum level of vascular endothelial growth factor in pulmonary tuberculosis. Am J Respir Crit Care Med. 2000;162:1120–1122. doi: 10.1164/ajrccm.162.3.9911010. [DOI] [PubMed] [Google Scholar]
- 44.Matsuyama W, et al. Expression of vascular endothelial growth factor in tuberculous meningitis. J Neurol Sci. 2001;186:75–79. doi: 10.1016/s0022-510x(01)00515-9. [DOI] [PubMed] [Google Scholar]
- 45.Podar K, et al. The small-molecule VEGF receptor inhibitor pazopanib (GW786034B) targets both tumor and endothelial cells in multiple myeloma. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:19478–19483. doi: 10.1073/pnas.0609329103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fong TA, et al. SU5416 is a potent and selective inhibitor of the vascular endothelial growth factor receptor (Flk-1/KDR) that inhibits tyrosine kinase catalysis, tumor vascularization, and growth of multiple tumor types. Cancer Res. 1999;59:99–106. [PubMed] [Google Scholar]
- 47.Opie EL, Aronson JD. Tubercle bacilli in latent tuberculosis lesions and in lung tissue without tuberculosis lesions. Arch Pathol Lab Med. 1927;4:1–21. [Google Scholar]
- 48.Welsh KJ, Hunter RL, Actor JK. Trehalose 6,6'-dimycolate--a coat to regulate tuberculosis immunopathogenesis. Tuberculosis. 2013;93(Suppl):S3–S9. doi: 10.1016/S1472-9792(13)70003-9. [DOI] [PubMed] [Google Scholar]
- 49.Sakamoto K, et al. Mycobacterial trehalose dimycolate reprograms macrophage global gene expression and activates matrix metalloproteinases. Infection and immunity. 2013;81:764–776. doi: 10.1128/IAI.00906-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Saita N, Fujiwara N, Yano I, Soejima K, Kobayashi K. Trehalose 6,6'-dimycolate (cord factor) of Mycobacterium tuberculosis induces corneal angiogenesis in rats. Infection and immunity. 2000;68:5991–5997. doi: 10.1128/iai.68.10.5991-5997.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stamm LM, et al. Mycobacterium marinum escapes from phagosomes and is propelled by actin-based motility. The Journal of experimental medicine. 2003;198:1361–1368. doi: 10.1084/jem.20031072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hsu T, et al. The primary mechanism of attenuation of bacillus Calmette-Guerin is a loss of secreted lytic function required for invasion of lung interstitial tissue. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:12420–12425. doi: 10.1073/pnas.1635213100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gao LY, Guo S, McLaughlin B, Morisaki H, Engel JN, Brown EJ. A mycobacterial virulence gene cluster extending RD1 is required for cytolysis, bacterial spreading and ESAT-6 secretion. Mol Microbiol. 2004;53:1677–1693. doi: 10.1111/j.1365-2958.2004.04261.x. [DOI] [PubMed] [Google Scholar]
- 54.Stanley SA, Johndrow JE, Manzanillo P, Cox JS. The Type I IFN response to infection with Mycobacterium tuberculosis requires ESX-1-mediated secretion and contributes to pathogenesis. Journal of immunology. 2007;178:3143–3152. doi: 10.4049/jimmunol.178.5.3143. [DOI] [PubMed] [Google Scholar]
- 55.Manzanillo PS, Shiloh MU, Portnoy DA, Cox JS. Mycobacterium tuberculosis activates the DNA-dependent cytosolic surveillance pathway within macrophages. Cell host & microbe. 2012;11:469–480. doi: 10.1016/j.chom.2012.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Volkman HE, Pozos TC, Zheng J, Davis JM, Rawls JF, Ramakrishnan L. Tuberculous granuloma induction via interaction of a bacterial secreted protein with host epithelium. Science. 2010;327:466–469. doi: 10.1126/science.1179663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Taylor JL, et al. Role for matrix metalloproteinase 9 in granuloma formation during pulmonary Mycobacterium tuberculosis infection. Infection and immunity. 2006;74:6135–6144. doi: 10.1128/IAI.02048-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cambier CJ, et al. Mycobacteria manipulate macrophage recruitment through coordinated use of membrane lipids. Nature. 2014;505:218–222. doi: 10.1038/nature12799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bafica A, et al. Host control of Mycobacterium tuberculosis is regulated by 5-lipoxygenase-dependent lipoxin production. The Journal of clinical investigation. 2005;115:1601–1606. doi: 10.1172/JCI23949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Serhan C, Ward P, Gilroy D. Fundamentals of Inflammation. Cambridge University Press; 2010. [Google Scholar]
- 61.Chen M, et al. Lipid mediators in innate immunity against tuberculosis: opposing roles of PGE2 and LXA4 in the induction of macrophage death. The Journal of experimental medicine. 2008;205:2791–2801. doi: 10.1084/jem.20080767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Divangahi M, et al. Mycobacterium tuberculosis evades macrophage defenses by inhibiting plasma membrane repair. Nature immunology. 2009 doi: 10.1038/ni.1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tobin DM, et al. Host genotype-specific therapies can optimize the inflammatory response to mycobacterial infections. Cell. 2012;148:434–446. doi: 10.1016/j.cell.2011.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tobin DM, et al. The lta4h locus modulates susceptibility to mycobacterial infection in zebrafish and humans. Cell. 2010;140:717–730. doi: 10.1016/j.cell.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Divangahi M, Desjardins D, Nunes-Alves C, Remold HG, Behar SM. Eicosanoid pathways regulate adaptive immunity to Mycobacterium tuberculosis. Nature immunology. 2010;11:751–758. doi: 10.1038/ni.1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Haeggstrom JZ. Leukotriene A4 hydrolase/aminopeptidase, the gatekeeper of chemotactic leukotriene B4 biosynthesis. J Biol Chem. 2004;279:50639–50642. doi: 10.1074/jbc.R400027200. [DOI] [PubMed] [Google Scholar]
- 67.Mayer-Barber KD, et al. Host-directed therapy of tuberculosis based on interleukin-1 and type I interferon crosstalk. Nature. 2014;511:99–103. doi: 10.1038/nature13489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rook GA, Taverne J, Leveton C, Steele J. The role of gamma-interferon, vitamin D3 metabolites and tumour necrosis factor in the pathogenesis of tuberculosis. Immunology. 1987;62:229–234. [PMC free article] [PubMed] [Google Scholar]
- 69.Silva CL, Faccioli LH, Rocha GM. The role of cachectin/TNF in the pathogenesis of tuberculosis. Brazilian journal of medical and biological research = Revista brasileira de pesquisas medicas e biologicas / Sociedade Brasileira de Biofisica [et al] 1988;21:489–492. [PubMed] [Google Scholar]
- 70.Hoffman M, Weinberg JB. Tumor necrosis factor-alpha induces increased hydrogen peroxide production and Fc receptor expression, but not increased Ia antigen expression by peritoneal macrophages. Journal of leukocyte biology. 1987;42:704–707. doi: 10.1002/jlb.42.6.704. [DOI] [PubMed] [Google Scholar]
- 71.Gardam MA, et al. Anti-tumour necrosis factor agents and tuberculosis risk: mechanisms of action and clinical management. The Lancet Infectious diseases. 2003;3:148–155. doi: 10.1016/s1473-3099(03)00545-0. [DOI] [PubMed] [Google Scholar]
- 72.Gomez-Reino JJ, Carmona L, Valverde VR, Mola EM, Montero MD, Group B. Treatment of rheumatoid arthritis with tumor necrosis factor inhibitors may predispose to significant increase in tuberculosis risk: a multicenter active-surveillance report. Arthritis and rheumatism. 2003;48:2122–2127. doi: 10.1002/art.11137. [DOI] [PubMed] [Google Scholar]
- 73.Marino S, et al. Differences in reactivation of tuberculosis induced from anti-TNF treatments are based on bioavailability in granulomatous tissue. PLoS computational biology. 2007;3:1909–1924. doi: 10.1371/journal.pcbi.0030194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Flynn JL, et al. Tumor necrosis factor-alpha is required in the protective immune response against Mycobacterium tuberculosis in mice. Immunity. 1995;2:561–572. doi: 10.1016/1074-7613(95)90001-2. [DOI] [PubMed] [Google Scholar]
- 75.Ehlers S, Benini J, Kutsch S, Endres R, Rietschel ET, Pfeffer K. Fatal granuloma necrosis without exacerbated mycobacterial growth in tumor necrosis factor receptor p55 gene-deficient mice intravenously infected with Mycobacterium avium. Infection and immunity. 1999;67:3571–3579. doi: 10.1128/iai.67.7.3571-3579.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Clay H, Volkman HE, Ramakrishnan L. Tumor necrosis factor signaling mediates resistance to mycobacteria by inhibiting bacterial growth and macrophage death. Immunity. 2008;29:283–294. doi: 10.1016/j.immuni.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.MacMicking JD. Cell-Autonomous Effector Mechanisms against Mycobacterium tuberculosis. Cold Spring Harbor perspectives in medicine. 2014;4 doi: 10.1101/cshperspect.a018507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bekker LG, Moreira AL, Bergtold A, Freeman S, Ryffel B, Kaplan G. Immunopathologic effects of tumor necrosis factor alpha in murine mycobacterial infection are dose dependent. Infection and immunity. 2000;68:6954–6961. doi: 10.1128/iai.68.12.6954-6961.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lin PL, et al. Tumor necrosis factor neutralization results in disseminated disease in acute and latent Mycobacterium tuberculosis infection with normal granuloma structure in a cynomolgus macaque model. Arthritis and rheumatism. 2010;62:340–350. doi: 10.1002/art.27271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Capuano SV, 3rd, et al. Experimental Mycobacterium tuberculosis infection of cynomolgus macaques closely resembles the various manifestations of human M. tuberculosis infection. Infection and immunity. 2003;71:5831–5844. doi: 10.1128/IAI.71.10.5831-5844.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Driver ER, et al. Evaluation of a mouse model of necrotic granuloma formation using C3HeB/FeJ mice for testing of drugs against Mycobacterium tuberculosis. Antimicrobial agents and chemotherapy. 2012;56:3181–3195. doi: 10.1128/AAC.00217-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dutta NK, Illei PB, Jain SK, Karakousis PC. Characterization of a novel necrotic granuloma model of latent tuberculosis infection and reactivation in mice. The American journal of pathology. 2014;184:2045–2055. doi: 10.1016/j.ajpath.2014.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rath PC, Aggarwal BB. TNF-induced signaling in apoptosis. Journal of clinical immunology. 1999;19:350–364. doi: 10.1023/a:1020546615229. [DOI] [PubMed] [Google Scholar]
- 84.Wallach D, Kang TB, Kovalenko A. The extrinsic cell death pathway and the elan mortel. Cell death and differentiation. 2008;15:1533–1541. doi: 10.1038/cdd.2008.41. [DOI] [PubMed] [Google Scholar]
- 85.Eimon PM, et al. Delineation of the cell-extrinsic apoptosis pathway in the zebrafish. Cell death and differentiation. 2006;13:1619–1630. doi: 10.1038/sj.cdd.4402015. [DOI] [PubMed] [Google Scholar]
- 86.Sakata S, et al. Conserved function of caspase-8 in apoptosis during bony fish evolution. Gene. 2007;396:134–148. doi: 10.1016/j.gene.2007.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Srinivasan L, Ahlbrand S, Briken V. Interaction of Mycobacterium tuberculosis with host cell death pathways. Cold Spring Harbor perspectives in medicine. 2014;4 doi: 10.1101/cshperspect.a022459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Aguilo JI, et al. ESX-1-induced apoptosis is involved in cell-to-cell spread of Mycobacterium tuberculosis. Cellular microbiology. 2013;15:1994–2005. doi: 10.1111/cmi.12169. [DOI] [PubMed] [Google Scholar]
- 89.Chen M, Gan H, Remold HG. A mechanism of virulence: virulent Mycobacterium tuberculosis strain H37Rv, but not attenuated H37Ra, causes significant mitochondrial inner membrane disruption in macrophages leading to necrosis. Journal of immunology. 2006;176:3707–3716. doi: 10.4049/jimmunol.176.6.3707. [DOI] [PubMed] [Google Scholar]
- 90.Keane J, Remold HG, Kornfeld H. Virulent Mycobacterium tuberculosis strains evade apoptosis of infected alveolar macrophages. Journal of immunology. 2000;164:2016–2020. doi: 10.4049/jimmunol.164.4.2016. [DOI] [PubMed] [Google Scholar]
- 91.Velmurugan K, et al. Mycobacterium tuberculosis nuoG is a virulence gene that inhibits apoptosis of infected host cells. PLoS pathogens. 2007;3:e110. doi: 10.1371/journal.ppat.0030110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rodrigues MF, et al. Tumour necrosis factor receptors and apoptosis of alveolar macrophages during early infection with attenuated and virulent Mycobacterium bovis. Immunology. 2013;139:503–512. doi: 10.1111/imm.12097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Keane J, et al. Infection by Mycobacterium tuberculosis promotes human alveolar macrophage apoptosis. Infection and immunity. 1997;65:298–304. doi: 10.1128/iai.65.1.298-304.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hinchey J, et al. Enhanced priming of adaptive immunity by a proapoptotic mutant of Mycobacterium tuberculosis. The Journal of clinical investigation. 2007;117:2279–2288. doi: 10.1172/JCI31947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Blomgran R, Desvignes L, Briken V, Ernst JD. Mycobacterium tuberculosis inhibits neutrophil apoptosis, leading to delayed activation of naive CD4 T cells. Cell host & microbe. 2012;11:81–90. doi: 10.1016/j.chom.2011.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tobin DM, Ramakrishnan L. TB: the Yin and Yang of lipid mediators. Current opinion in pharmacology. 2013;13:641–645. doi: 10.1016/j.coph.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tobin DM, Roca FJ, Ray JP, Ko DC, Ramakrishnan L. An enzyme that inactivates the inflammatory mediator leukotriene b4 restricts mycobacterial infection. PloS one. 2013;8:e67828. doi: 10.1371/journal.pone.0067828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Thwaites GE, et al. Dexamethasone for the treatment of tuberculous meningitis in adolescents and adults. The New England journal of medicine. 2004;351:1741–1751. doi: 10.1056/NEJMoa040573. [DOI] [PubMed] [Google Scholar]
- 99.Yang J, Chen J, Yue J, Liu L, Han M, Wang H. Relationship between human LTA4H polymorphisms and extra-pulmonary tuberculosis in an ethnic Han Chinese population in Eastern China. Tuberculosis. 2014 doi: 10.1016/j.tube.2014.08.014. [DOI] [PubMed] [Google Scholar]
- 100.Roca FJ, Ramakrishnan L. TNF dually mediates resistance and susceptibility to mycobacteria via mitochondrial reactive oxygen species. Cell. 2013;153:521–534. doi: 10.1016/j.cell.2013.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cho YS, et al. Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell. 2009;137:1112–1123. doi: 10.1016/j.cell.2009.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.He S, et al. Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-alpha. Cell. 2009;137:1100–1111. doi: 10.1016/j.cell.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 103.Holler N, et al. Fas triggers an alternative, caspase-8-independent cell death pathway using the kinase RIP as effector molecule. Nature immunology. 2000;1:489–495. doi: 10.1038/82732. [DOI] [PubMed] [Google Scholar]
- 104.Vandenabeele P, Declercq W, Van Herreweghe F, Vanden Berghe T. The role of the kinases RIP1 and RIP3 in TNF-induced necrosis. Sci Signal. 2010;3:re4. doi: 10.1126/scisignal.3115re4. [DOI] [PubMed] [Google Scholar]
- 105.Vandenabeele P, Galluzzi L, Vanden Berghe T, Kroemer G. Molecular mechanisms of necroptosis: an ordered cellular explosion. Nat Rev Mol Cell Biol. 2010;11:700–714. doi: 10.1038/nrm2970. [DOI] [PubMed] [Google Scholar]
- 106.Baines CP, et al. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature. 2005;434:658–662. doi: 10.1038/nature03434. [DOI] [PubMed] [Google Scholar]
- 107.Elojeimy S, et al. New insights on the use of desipramine as an inhibitor for acid ceramidase. FEBS Lett. 2006;580:4751–4756. doi: 10.1016/j.febslet.2006.07.071. [DOI] [PubMed] [Google Scholar]
- 108.Quarato G, et al. The cyclophilin inhibitor alisporivir prevents hepatitis C virus-mediated mitochondrial dysfunction. Hepatology. 2012;55:1333–1343. doi: 10.1002/hep.25514. [DOI] [PubMed] [Google Scholar]