Abstract
The mechanism(s) by which certain small peptides and peptide mimics carry large cargoes across membranes through exclusively non-covalent interactions has been difficult to resolve. Here, we use the droplet-interface bilayer as a platform to characterize distinct mechanistic differences between two such carriers: Pep-1 and a guanidinium-rich peptide mimic we call D9. While both Pep-1 and D9 can carry an enzyme, horseradish peroxidase (HRP) across a lipid bilayer, we found that they do so by different mechanisms. Specifically, Pep-1 requires voltage or membrane asymmetry while D9 does not. In addition, D9 can facilitate HRP transport without pre-forming a complex with HRP. By contrast, complex formation is required by Pep-1. Both carriers are capable of forming pores in membranes but our data hints that these pores are not responsible for cargo transport. Overall, D9 appears to be a more potent and versatile transporter when compared to Pep-1 because D9 does not require an applied voltage or other forces to drive transport. Thus, D9 might be used to deliver cargo across membranes under conditions where Pep-1 would be ineffective.
Keywords: Droplet-interface bilayer (DIB), Pep-1, arginine-rich peptide, guanidine, membrane translocation, protein transduction domain mimic (PTDM)
Graphical abstract
INTRODUCTION
Cell-penetrating peptides (CPPs) and protein transduction domains (PTDs) facilitate the translocation of large molecules into cells[1]. Most are covalently linked to their cargo and enter cells through endocytosis[2–3]. However, a small subset possesses two remarkable traits. Specifically, peptides such as Pep-1[4–6] and polyarginine (polyR) [7–12] do not need to be covalently tethered to cargo. In addition, these carriers appear to deliver their cargo directly to the cytoplasm, bypassing endocytosis. The mechanism(s) by which non-covalent, nonendocytotic carriers deliver cargo has proven difficult to resolve and remains an active area of research.
As an example, consider the differences observed in the translocation action of Pep-1 and guanidinium-rich domains such as polyarginine. Pep-1 is believed to work by first forming a complex with its cargo through electrostatic and/or hydrophobic interactions[5, 13]. A ratio of 20:1 Pep-1:cargo is typical of a translocation scheme. Studies at 4°C, where energy-dependent endocytosis is inactivated, show that Pep-1 can carry cargo into the cytoplasm[14]. However, at 37°C endocytosis is the predominant transport mode[14]. Experiments using artificial membranes (vesicles) at room temperature showed that Pep-1-mediated protein transport across lipid bilayers required negatively charged lipids and a transmembrane voltage gradient[6]. Some have suggested that Pep-1 forms pores in membranes that facilitate the trafficking of molecular cargo[15–18]. This is supported by the fact that Pep-1 induces pore-like defects in bilayers that are clearly visible in ionic current measurements[18]. Models of Pep-1’s mechanism, based on pore formation, have been proposed but others have countered this idea suggesting that Pep-1 simply disintegrates the membrane[19–20].
Pep-1 consists of three domains: a tryptophan rich hydrophobic region, a flexible linker and a lysine-rich domain. In addition, the ends of the peptide are capped by acetyl and cysteamide groups, meaning that in neutral buffer Pep-1 exists as a disulfide-linked dimer[5]. This structure contrasts sharply with guanidinium rich carriers such as polyRs, where the structure is uniform from end to end. As with Pep-1, polyR cargo is transported directly to the cytoplasm in some cases and there is also some evidence that shows endocytosis is dominant unless translocation is performed at low temperature[14]. Others argue that molecular transport is dependent on both energy and the presence of certain lipid domains in the membrane[21]. The notion of pore formation induced by guanidinium-rich species is also contentious. PolyR and synthetic mimics do induce membrane curvature in model bilayers[22]. However, fluorescent dye molecules outside cells do not enter the cytoplasm when the cell is exposed to arginine-rich TAT peptides, something that would be expected if TAT formed pores[23]. By contrast, experiments showed that low molecular weight (3kDa) dextran molecules within giant unilamellar vesicles (GUVs) were released when the membrane was exposed to TAT[24]. Larger molecular weights remained trapped, leading to the expectation that pores were between 1.3 and 2 nm in diameter. Molecular simulations also support the idea that guanidinium-rich carriers form pores in lipid bilayers[12, 25].
It is important to note that guanidinium-rich carriers have been studied in two distinct modes. In the first, carriers and cargo are mixed to induce cargo delivery across a membrane[9, 21, 26– 27]. In a recent example, a protein transduction domain mimic (PTDM) in the form of a guanidinium functionalized polymer was used to deliver siRNA into T cells[26]. In the second, carrier and cargo are on opposite sides of the bilayer, such as carrier-mediated release of contents from vesicles[9, 24]. One intriguing idea that has emerged from this mode is the importance of anions in transport. Previous reports show that hydrophobic anions (termed activators) facilitate the movement of polyarginine across lipid bilayers[27]. Importantly, the guanidinium groups have high anion affinity (contrast to the low affinity of lysine residues in Pep-1) and thus may permeate the membrane upon charge neutralization. Here, pore formation is not a prerequisite for translocation, nor is there any need to preform a complex since cargo and carrier are on opposite sides of the membrane. From this, a very interesting scenario emerges. Guanidiniumrich carriers enter the membrane bound to one ion and upon reaching the other side, exchange anionic cargo. The new complex crosses back to extract the cargo. The idea that guanidiniumrich species might “reach across” membranes to withdraw anions (termed interface-directed translocation) has been demonstrated with small cargoes[9].
Here, we conducted a series of translocation studies using either a lysine-rich carrier (Pep-1) or a guanidinium-rich carrier (D9) and compared the results. We found that, unlike Pep-1, guanidinium-based carriers trafficked molecular cargo without the need for an applied voltage or asymmetric membrane. Moreover, the D9 carrier was able to “extract” protein cargo from the opposite side of a membrane, thereby highlighting a unique capability of the carrier.
METHODS AND MATERIALS
Materials
Pep-1 (Ac-KETWWETWWWEWSQPKKKRKV-Cya) was obtained from Active Motif (Carlsbad, CA). Horseradish peroxidase (HRP, peroxidase type XII from horseradish) and hexadecane were obtained from Sigma-Aldrich. Maleic anhydride, furan, 4-dimethylaminopyridine (DMAP), 1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC), 1,3-di-boc-2-(2-hydroxyethyl)guanidine, N-hydroxysuccinimide, norbornene acid, 4-chloro-7-nitrobenzofurazan, N-boc-ethylenediamine, N,Ndiisopropylethylamine (DIPEA), ethyl vinyl ether, trifluoroacetic acid (TFA), methanol (MeOH), ethyl acetate (EtOAc), pentane, N,N-dimethylformamide (DMF), hexanes and tetrahydrofuran (THF) were obtained as reagent grade from Aldrich, Fisher Scientific, Fluka or Acros and used as received. 3rd generation Grubbs catalyst (Dichloro-di(3-bromopyridino)-N,N’-Dimesitylenoimidazolino-Ru=CHPh; G3) was synthesized as described previously by Grubbs and coworkers.1 Dichloromethane (CH2Cl2) (HPLC grade, Fisher Scientific) was distilled from CaH2 under nitrogen. Spectra/Por® Biotech Cellulose Ester (CE) dialysis membranes with a molecular weight cutoff (MWCO) of 100–500 g/mol were purchased from Spectrum Medical Industries. The Amplex Red enzymatic substrate for HRP was obtained from Invitrogen. Low melting point agarose, hydrogen peroxide and HEPES buffer were obtained from Fisher Scientific. 1,2-diphytanoyl-sn-glycero-3-phosphocholine (DPhPC), 1,2-diphytanoyl-sn-glycero-3-phospho-(1'-rac-glycerol) (sodium salt) (DPhPG), and egg yolk phosphatidylcholine (EYPC) lipids were purchased from Avanti Polar Lipids (Alabaster, AL). and 5(6)-carboxyfluorescein (CF) was purchased from Fluka.
Preparation of DPhPC and DPhPC/DPhPG Vesicles. All vesicle solutions were prepared in 10mM HEPES and 150mM NaCl buffered to pH 7.4 with sodium hydroxide. 2 mM DPhPC vesicle solution was prepared by air-drying an aliquot of DPhPC in pentane until the solvent was removed, followed by further drying in a vacuum desiccator for 30 minutes. Lipids were resuspended in buffer and extruded 21 times through a polycarbonate filter with 100 nm track-etched pores (Millipore) using a mini-extruder (Avanti). For negatively charged vesicles, a 2 mM solution was prepared with a 9:1 molar ratio of DPhPC to DPhPG.
Preparation of Pep-1 complexes and D9-NBD/HRP mixtures. A 500 µM stock solution of Pep-1 was prepared by dissolution in 18 MΩ water and was then stored as 5 µL aliquots in lobind eppendorf tubes at −20°C. A 50 µM stock solution of HRP was prepared by dissolution in 18 MΩ water and was then stored as aliquots at −20°C. 50 mM Amplex Red was prepared by dissolution in DMSO and stored at −4°C. Pep-1/HRP complexes were prepared fresh from the stock solutions prior to each experiment. First, Pep-1 and HRP were diluted in water to 40 µM and 2 µM, respectively. Second, equal volumes of these dilutions were mixed and incubated at room temperature for 30 minutes. Finally, a 2 µL aliquot of complex was mixed with 18 µL of vesicle solution to create the solution used as the source droplet. The final concentrations of Pep-1 and HRP in the complex containing droplet were 2 µM and 100 nM, respectively
Preparation of D9-NBD/HRP complexes. A 2 mM stock solution of D9-NBD was stored in DMSO as 50 µL aliquots. A 50 µM stock solution of HRP was prepared by dissolution in 18 MΩ water and stored in aliquots at −20 °C. D9-NBD/HRP complexes were prepared fresh from the stock solutions prior to each experiment. First, D9-NBD and HRP were diluted in water. Second, equal volumes of these dilutions were mixed and incubated at room temperature for 30 minutes. In experiments where the D9-NBD and HRP were present in opposing droplets, the D9-NBD and HRP were incubated separately with a solution of only water replacing the other compound. Finally, a 2 µL aliquot of complex was mixed with 18 µL of vesicle solution to create the solution used as the source droplet. The final concentrations of D9-NBD and HRP in the source droplet were in a 20:1 ratio in all trials.
Electrophysiology. The electrodes were connected to a patch-clamp amplifier (Axopatch 200B; Axon Instruments). The currents were filtered with a low-pass Bessel filter (80 dB/decade) with a corner frequency of 2 kHz and then digitized with a DigiData 1400 series A/D converter (Axon Instruments) at a sampling frequency of 5 kHz. The oil reservoir and amplifying headstage were enclosed in a metal box, which served as a Faraday cage.
We examined the membrane activity of D9-NBD both in the presence and absence of HRP (Figure S7). Regardless of the polarity of the applied potential or the presence of HRP, transient pores formed in the DIB membrane. Typically, more “\pore forming activity is observed in the first half of the trace when compared to the second half. By 10 minutes, the conductance of the membrane approaches zero pA.
RESULTS AND DISCUSSION
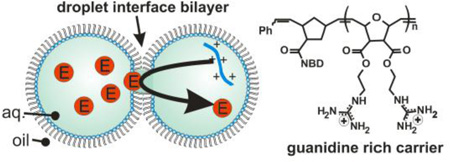
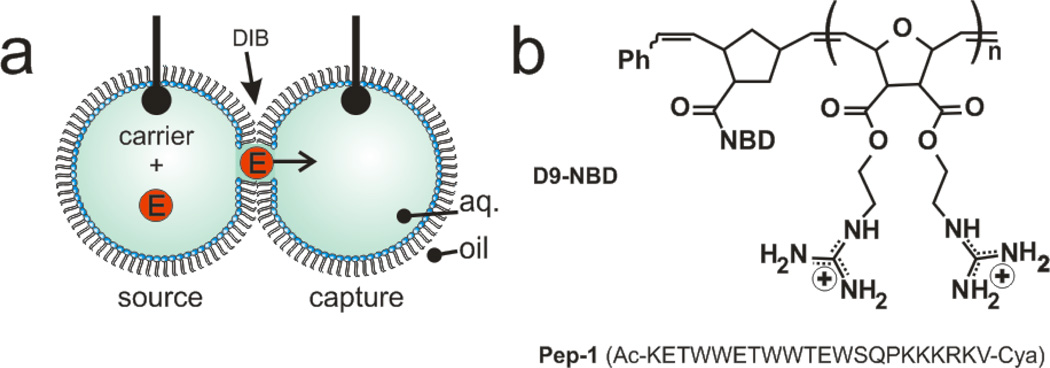
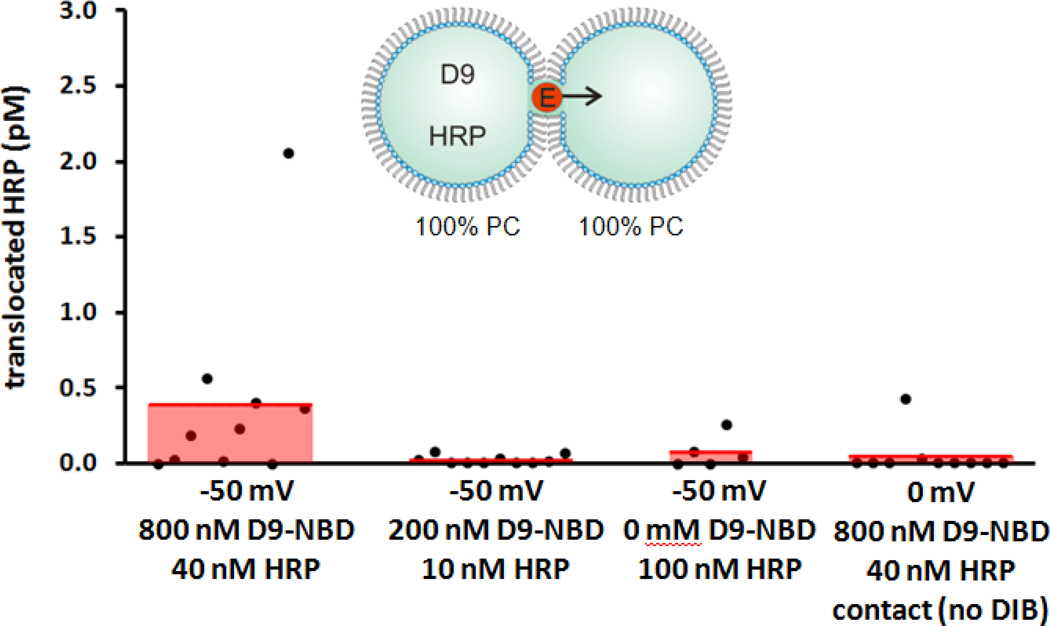
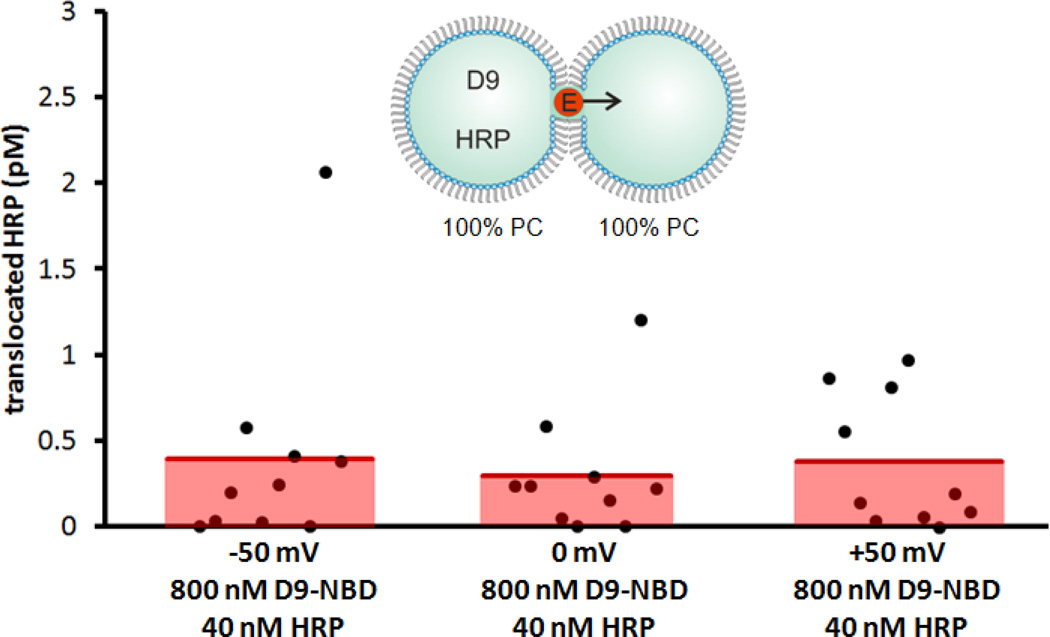
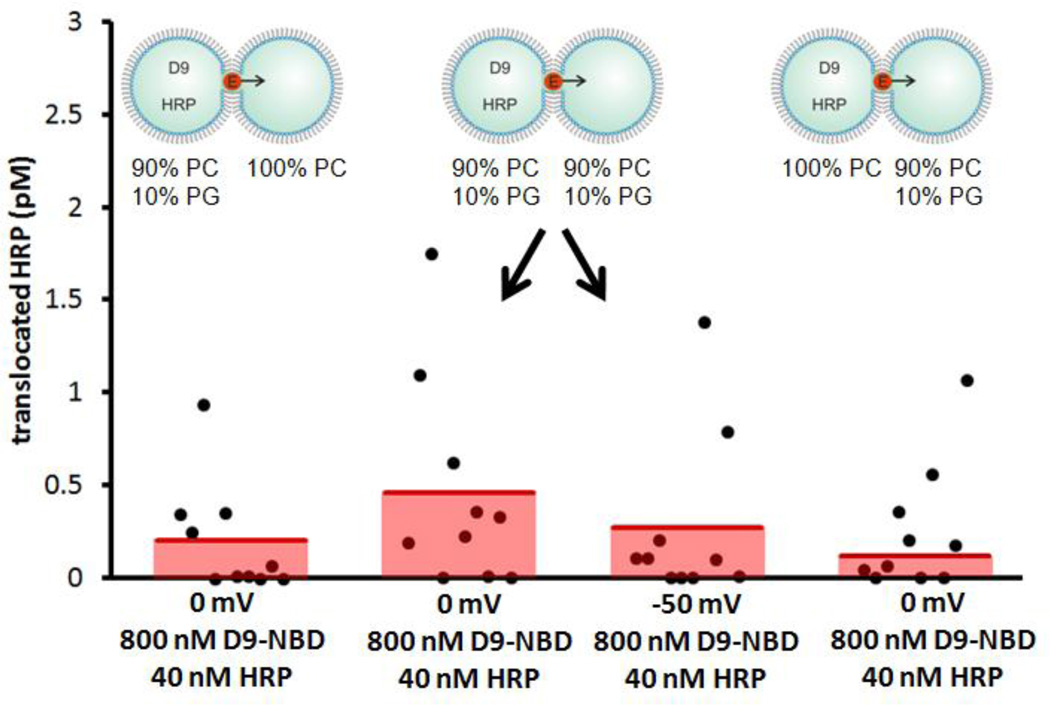
Here, we employ droplet-interface bilayers (DIBs) as model membranes to investigate the nuances of carrier-mediated translocation. Briefly, monolayer-encased aqueous droplets are contacted under oil to form a lipid bilayer[28–29]. Electrodes embedded within the droplets permit monitoring of the bilayer by ionic current recording during translocation. In these experiments, the enzyme horseradish peroxidase (HRP) is used as the cargo. HRP (44 kDa) translocation from the source droplet to the capture droplet is facilitated both by carrier molecules and experimental conditions such as lipid composition, symmetry or applied voltage (Fig. 1a). In all DIB experiments, membranes were composed of DPhPC with or without a mole fraction of DPhPG lipids. By manipulating the PC:PG headgroup ratio of each leaflet, asymmetric membranes were created. Diphytanoyl lipid tails were used in these experiments since they do not undergo phase transitions near the temperatures used in our experiments[30]. Captured enzymes were subsequently analyzed by a fluorogenic assay. We have previously used this approach to examine HRP transport mediated by the cell-penetrating peptide Pep-1[31]. Now we turn our attention to a guanidinium rich, poly-arginine inspired polymer as a model carrier in an effort to understand the relationship between carrier chemistry and translocation mechanism. D9-NBD is a monodisperse, guanidinium-rich 9-mer synthesized by ring-opening metathesis polymerization[26, 32–34]. This method is ideal because it is fast, efficient (high monomer conversion), and yields polymers with well-controlled molecular weights and dispersities. The detailed synthetic procedures are provided in the supporting information. At one end, a nitrobenzoxadiazole (NBD) fluorophore is attached for visualization. D9-NBD was originally designed to mimic the prototypical CPP, TAT. This PTDM, with two guanidines per repeat unit, was shown to be more effective at cellular internalization than its corresponding one-guanidine counterpart[33]. To test D9-NBD’s translocation ability, 8 µM D9-NBD and 400 nM HRP were incubated together for 30 minutes and then diluted 10-fold with a solution containing 2 mM lipid vesicles. Next, 200 nL of this mixture was suspended from the electrically grounded electrode to form the source droplet. A droplet of vesicles was suspended from the working electrode to create the capture droplet and the two were contacted to form a DIB. In this trial, the DIB was composed of pure phosphatidylcholine (PC) lipids and the final concentrations in the source droplet were 800 nM D9-NBD and 40 nM HRP. Following a 10 minute translocation period at an applied potential of −50 mV, an average of 0.4 pM of HRP was detected in the capture droplet by fluorogenic assay (Fig. 2)[31]. This amount is similar to our previous studies, where Pep-1 (2 µM) carried HRP (100 nM) across the DIB during a 45 minute interval to yield an average of 1.1 pM translocated enzyme[31]. Note that the D9-NBD trials occurred for a shorter interval and lower concentration of carrier and cargo, thus we surmise that D9-NBD is a more potent carrier than Pep-1. We used 800 nM D9-NBD for these experiments because higher concentrations easily ruptured the DIB membrane. We also examined the concentration dependence of D9-NBD HRP transport. A four-fold dilution of the D9-NBD HRP mixture or absence of carrier greatly reduced translocation. As a control, the 800 nM D9-NBD 40 nM HRP trial was repeated but with one important change. The droplets were contacted only briefly and then separated (no DIB formed) and the source droplet analyzed for enzyme activity. In 10 trials, no enzyme activity was detected, showing that translocation occurs only across the DIB membrane and not by other means. Pep-1mediated transport is facilitated by the application of a transmembrane potential or lipid charge asymmetry[31]. We were curious to see if D9-NBD translocation also required similar conditions. In contrast to Pep-1, we found that approximately the same amount of HRP is carried across the DIB by D9-NBD regardless of the applied potential polarity (Fig. 3). In addition, while Pep-1 could not transport HRP across a pure PC DIB at 0 mV applied potential, D9-NBD spontaneously carried HRP across a neutrally charged membrane with no voltage (Fig. 3). Since membrane charge asymmetry can drive Pep-1 mediated translocation in the absence of voltage[31], we also examined the effect of lipid asymmetry on D9-NBD mediated HRP transport. Translocation experiments were performed using DIBs that contained 10 mol% of negatively charged phosphatidylglycerol (PG) and 90% PC lipids in one or both leaflets. Unlike Pep-1, D9-NBD based translocation of HRP showed no significant dependence on charge distribution in the DIBs (Fig. 4). The addition of a voltage bias did not increase translocation across an asymmetric DIB (Fig. 4). The lack of dependence of D9-NBD facilitated HRP transport on either voltage or membrane asymmetry suggests that D9 and perhaps other guanidinium-rich carriers may function by a different mechanism than Pep-1.
Figure 1.
Carrier-mediated transport across a droplet-interface bilayer (DIB). (a) Carriers transport an enzyme (HRP) from the source to the capture droplet during a fixed interval. Electrodes within each droplet are used to record the ionic current and membrane capacitance. Following translocation the capture droplet is analyzed for enzyme activity via a fluorescence assay. (b) An NBD-labeled diguanidine polymer (9-mer) or Pep-1 was used to facilitate transport.
Figure 2.
Concentration dependence of D9-NBD on HRP translocation across PC DIBs. The left data set (i) demonstrates D9-NBD’s activity as a protein carrier. Controls show reduced transport at (ii) lower concentration (iii) absence of carrier. Finally, droplets were contacted, then detached before DIB formation (iv). This demonstrated that merely contacting droplets does not transfer HRP. The third data set from the left was previously published30 and is included here for comparison.
Figure 3.
D9-NBD facilitated transport as a function of voltage. Regardless of the presence or polarity of applied potential, little variation in the quantity of translocated HRP was observed.
Figure 4.
D9-NBD facilitated transport as a function of membrane charge symmetry. 10 mol% of a PG lipid was added to either or both sides of DIBs to create negatively charged leaflet; the remaining lipids were neutral PC. Similar amounts of HRP were transported regardless of charge asymmetry.
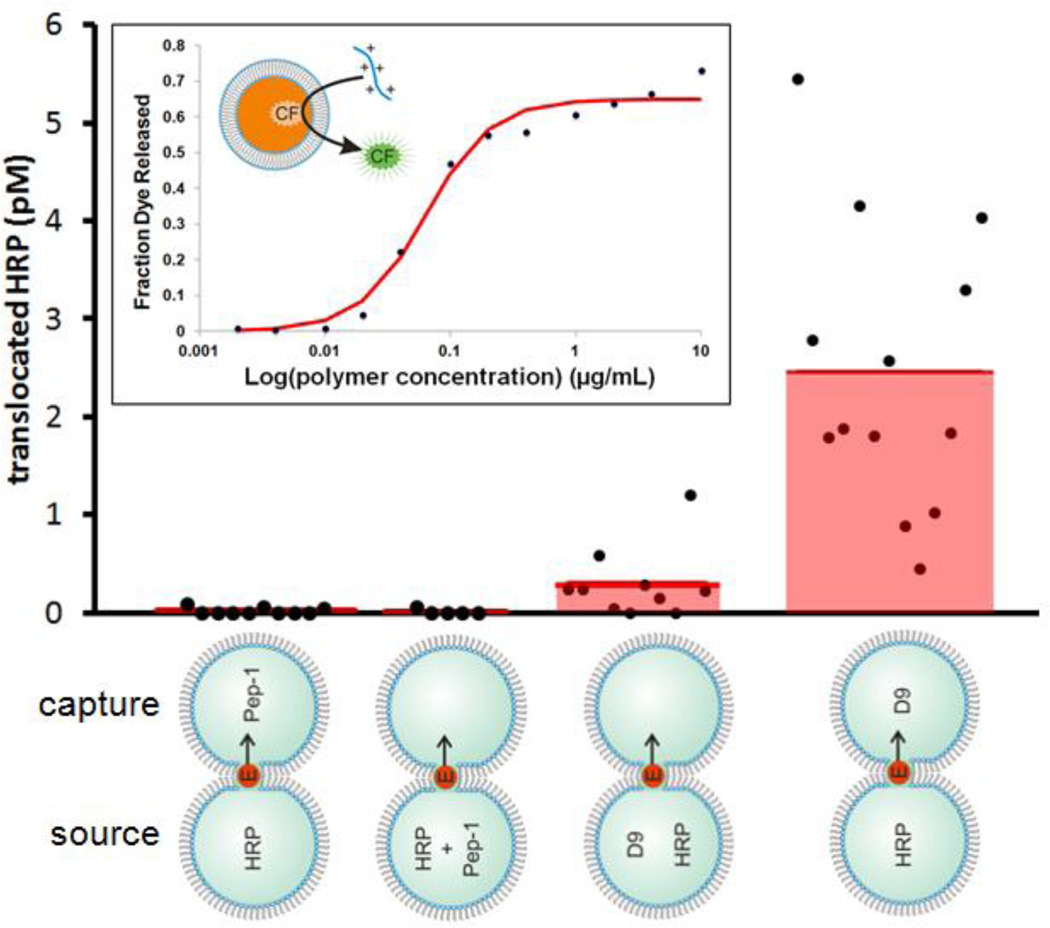
To explore these differences further, we examined the relationship between complex formation and carrier mediated transport using D9-NBD and Pep-1. In these experiments, the cargo and carrier were placed either in the same droplet or in opposite droplets. For Pep-1, HRP transport was not observed in either case. However, D9-NBD showed a remarkable increase in HRP transport when placed on the opposite side of the DIB from HRP (Fig. 5). Further, DLS measurements indicate that D9-NBD does not adsorb to HRP when they are incubated together (Table S2). Two important ideas emerge from these results. First, interactions between D9 and HRP, such as an incubation period, are not a prerequisite for translocation. This contrasts with Pep-1 where several literature reports emphasize the importance of both incubation and the peptide to cargo molar ratio. Second, transport is enhanced when the cargo and carrier are separated. Guanidinium-rich peptides have already been shown to “reach across” vesicle membranes to extract bulky anions[9]. D9-NBD is also capable of extracting bulky anions, in this case carboxyfluorescein, from vesicles in a concentration-dependent manner (Fig. 5, inset). Our data suggests that this idea might be extended to larger entities such as entire proteins. Following several of the DIB experiments presented here, we imaged the capture droplet by fluorescence microscopy to measure how much D9-NBD crossed over after translocation.
Figure 5.
Transport as a function of complex formation. Either Pep-1 or D9-NBD was used to transport HRP either from the same or the opposite side of the DIB. All trials were conducted at 0 mV. Remarkably, D9-NBD facilitates significantly more translocation when arranged opposite to the source droplet. No transport is observed with Pep-1. The second data set was published previously30 and the third data set is copied from Fig. 1 for comparison. Inset: 50 mM carboxyfluorescein was extracted from lipid vesicles as a function of D9-NBD concentration.
However, very little D9-NBD was detected in the capture droplets (except where D9-NBD and HRP were in separate droplets) (Fig. S8). This does not necessarily indicate that D9 cannot cross the DIB, but at least it does not rapidly equilibrate across the membrane. Models like carpet, toroidal pore and barrel stave are insufficient to explain the transport of molecular cargo observed in our experiments with Pep-1 and D9 as carriers[16, 35–36]. We note that both Pep- 1[31] and D9 form pores in DIB membranes in the absence and presence of cargo (Fig. S7). This suggests that the requirement for complex formation (as with Pep-1)[5] may not be related to the formation of pores in the membrane. If so, perhaps translocation does not take place by passing through carrier-lined pores but instead by some other means. For example, the hydrophobic regions of either D9 or Pep-1 could interact with the hydrophobic core of the membrane while the positive regions neutralize negatively-charged patches on the cargo. The lysine-rich Pep-1 might bind negative residues on cargo more weakly than guanidinium-rich D9, which might explain the need for voltage or lipid charge asymmetry when using Pep-1. So far, the DIB experiments have revealed that these two pore-forming carriers work very differently under well-defined conditions and that further investigation is needed to understand these differences.
Here, we have shown that the guanidinium-rich D9-NBD polymer can facilitate transport of a protein cargo across a lipid bilayer without the requirement of negative charge or voltage. In addition, its ability to transport this protein is enhanced when the carrier-cargo is not pre-mixed demonstrating that pre-formed complexes are not required for transport. The DIB system is ideally suited for probing the mechanistic details of various carriers and enables well-defined, independent variables to be studied in isolation which are more difficult to accomplish in many bulk studies. Given the complex landscape of CPP related transport, or internalization, we are motivated to continue studying these systems as broadly as possible. The growing use of CPPs and related molecules in molecular delivery applications highlights the need for more fundamental studies of the delivery mechanism. Learning how to develop more effective carriers is vital to researchers in many different fields.
Supplementary Material
Highlights.
We compared transport of ability guanidinium-rich vs lysine-rich membrane carriers
Droplet-interface bilayer was used to quantitate protein transport across membrane
Guanidinium-rich carrier did not require force gradients to drive transport
Lysine-rich carrier required voltage or asymmetry to drive transport
Remarkably, guanidinium-rich carrier able to extract protein from across membrane
ACKNOWLEDGMENT
Support from the National Science Foundation under Grant No. (CAREER 1253565, M.A.H. and CHE-0910963, G.N.T.) and the National Institute of Health (National Re-search Service Award T32 GM08515) is gratefully acknowledged.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions
Lein, deRonde and Sgolastra performed the experiments and polymer synthesis. All authors contributed to the preparation of this manuscript.
REFERENCES
- 1.Langel U. Handbook of cell-penetrating peptides. 2nd ed. Boca Raton: CRC Press; 2007. [Google Scholar]
- 2.Kaplan IM, Wadia JS, Dowdy SF. Cationic TAT peptide transduction domain enters cells by macropinocytosis. J. Control. Release. 2005;102:247–253. doi: 10.1016/j.jconrel.2004.10.018. [DOI] [PubMed] [Google Scholar]
- 3.Palm-Apergi C, Lonn P, Dowdy SF. Do cell-penetrating peptides actually "penetrate" cellular membranes? Mol. Ther. 2012;20:695–697. doi: 10.1038/mt.2012.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morris MC, Depollier J, Mery J, Heitz F, Divita G. A peptide carrier for the delivery of biologically active proteins into mammalian cells. Nat. Biotechnol. 2001;19:1173–1176. doi: 10.1038/nbt1201-1173. [DOI] [PubMed] [Google Scholar]
- 5.Morris MC, Deshayes S, Heitz F, Divita G. Cell-penetrating peptides: from molecular mechanisms to therapeutics. Biol. Cell. 2008;100:201–217. doi: 10.1042/BC20070116. [DOI] [PubMed] [Google Scholar]
- 6.Henriques ST, Costa H, Castanho M. Translocation of beta-galactosidase mediated by the cell-penetrating peptide pep-1 into lipid vesicles and human HeLa cells is driven by membrane electrostatic potential. Biochemistry. 2005;44:10189–10198. doi: 10.1021/bi0502644. [DOI] [PubMed] [Google Scholar]
- 7.Sakai N, Matile S. Anion-mediated transfer of polyarginine across liquid and bilayer membranes. J. Am. Chem. Soc. 2003;125:14348–14356. doi: 10.1021/ja037601l. [DOI] [PubMed] [Google Scholar]
- 8.Fuchs SM, Raines RT. Pathway for polyarginine entry into mammalian cell. Biochemistry. 2004;43:2438–2444. doi: 10.1021/bi035933x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nishihara M, Perret F, Takeuchi T, Futaki S, Lazar AN, Coleman AW, Sakai N, Matile S. Arginine magic with new counterions up the sleeve. Org. Biomol. Chem. 2005;3:1659–1669. doi: 10.1039/b501472g. [DOI] [PubMed] [Google Scholar]
- 10.Sakai N, Takeuchi T, Futaki S, Matile S. Direct observation of anion-mediated translocation of fluorescent oligoarginine carriers into and across bulk liquid and anionic bilayer membranes. ChemBioChem. 2005;6:114–122. doi: 10.1002/cbic.200400256. [DOI] [PubMed] [Google Scholar]
- 11.Schmidt N, Mishra A, Lai GH, Wong GCL. Arginine-rich cell-penetrating peptides. FEBS Letters. 2010;584:1806–1813. doi: 10.1016/j.febslet.2009.11.046. [DOI] [PubMed] [Google Scholar]
- 12.Huang K, Garcia AE. Free Energy of Translocating an Arginine-Rich Cell-Penetrating Peptide across a Lipid Bilayer Suggests Pore Formation. Biophys. J. 2013;104:412–420. doi: 10.1016/j.bpj.2012.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deshayes S, Morris M, Heitz F, Divita G. Delivery of proteins and nucleic acids using a non-covalent peptide-based strategy. Adv. Drug. Dev. Rev. 2008;60:537–547. doi: 10.1016/j.addr.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 14.Loudet A, Han J, Barhoumi R, Pellois JP, Burghardt RC, Burgess K. Non-covalent delivery of proteins into mammalian cells. Org. Biomol. Chem. 2008;6:4516–4522. doi: 10.1039/b809006h. [DOI] [PubMed] [Google Scholar]
- 15.Deshayes S, Gerbal-Chaloin S, Morris MC, Aldrian-Herrada G, Charnet P, Divita G, Heitz F. On the mechanism of non-endosomial peptide-mediated cellular delivery of nucleic acids. BBA - Biomembranes. 2004;1667:141–147. doi: 10.1016/j.bbamem.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 16.Deshayes S, Heitz A, Morris MC, Charnet P, Divita G, Heitz F. Insight into the mechanism of internalization of the cell-penetrating carrier peptide Pep-1 through conformational analysis. Biochemistry. 2004;43:1449–1457. doi: 10.1021/bi035682s. [DOI] [PubMed] [Google Scholar]
- 17.Deshayes S, Morris MC, Divita G, Heitz F. Interactions of amphipathic CPPs with model membranes. BBA - Biomembranes. 2006;1758:328–335. doi: 10.1016/j.bbamem.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 18.Deshayes S, Plenat T, Charner P, Divita G, Molle G, Heitz F. Formation of transmembrane ionic channels of primary amphipathic cell-penetrating peptides. Consequences on the mechanism of cell penetration. BBA - Biomembranes. 2006;1758:1846–1851. doi: 10.1016/j.bbamem.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 19.Henriques ST, Quintas A, Bagatolli LA, Homble F, Castanho MA. Energy-independent translocation of cell-penetrating peptides occurs without formation of pores. A biophysical study with pep-1. Mol. Membr. Biol. 2007;24:282–293. doi: 10.1080/09687860601142936. [DOI] [PubMed] [Google Scholar]
- 20.Henriques ST, Castanho MA. Translocation or membrane disintegration? Implication of peptide-membrane interactions in pep-1 activity. J. Pept. Sci. 2008;14:482–487. doi: 10.1002/psc.1003. [DOI] [PubMed] [Google Scholar]
- 21.Xu Y, Liu BR, Lee HJ, Shannon KB, Winiarz JG, Wang TC, Chiang HJ, Huang YW. Nona-Arginine Facilitates Delivery of Quantum Dots into Cells viaMultiple Pathways. J. Biomed. Biotechnol. 2010 doi: 10.1155/2010/948543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schmidt NW, Lis M, Zhao K, Lai GH, Alexandrova AN, Tew GN, Wong GCL. Molecular Basis for Nanoscopic Membrane Curvature Generation from Quantum Mechanical Models and Synthetic Transporter Sequences. J. Am. Chem. Soc. 2012;134:19207–19216. doi: 10.1021/ja308459j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ter-Avetisyan G, Tuennemann G, Nowak D, Nitschke M, Herrmann A, Drab M, Cardoso MC. Cell Entry of Arginine-rich Peptides Is Independent of Endocytosis. J. Biol. Chem. 2009;284:3370–3378. doi: 10.1074/jbc.M805550200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ciobanasu C, Siebrasse JP, Kubitscheck U. Cell-Penetrating HIV1 TAT Peptides Can Generate Pores in Model Membranes. Biophys. J. 2010;99:153–162. doi: 10.1016/j.bpj.2010.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Herce HD, Garcia AE, Litt J, Kane RS, Martin P, Enrique N, Rebolledo A, Milesi V. Arginine-Rich Peptides Destabilize the Plasma Membrane, Consistent with a Pore Formation Translocation Mechanism of Cell-Penetrating Peptides. Biophys. J. 2009;97:1917–1925. doi: 10.1016/j.bpj.2009.05.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tezgel AO, Gonzalez-Perez G, Telfer JC, Osborne BA, Minter LM, Tew GN. Novel Protein Transduction Domain Mimics as Nonviral Delivery Vectors for siRNA Targeting NOTCH1 in Primary Human T cells. Molec. Ther. 2013;21:201–209. doi: 10.1038/mt.2012.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takeuchi T, Kosuge M, Tadokoro A, Sugiura Y, Nishi M, Kawata M, Sakai N, Matile S, Futaki S. Direct and rapid cytosolic delivery using cell-penetrating peptides mediated by pyrenebutyrate. ACS Chem. Biol. 2006;1:299–303. doi: 10.1021/cb600127m. [DOI] [PubMed] [Google Scholar]
- 28.Holden MA, Needham D, Bayley H. Functional bionetworks from nanoliter water droplets. J. Am. Chem. Soc. 2007;129:8650–8655. doi: 10.1021/ja072292a. [DOI] [PubMed] [Google Scholar]
- 29.Bayley H, Cronin B, Heron A, Holden MA, Hwang W, Syeda R, Thompson J, Wallace MI. Droplet interface bilayers. Mol. Biosystems. 2008;4:1191–1208. doi: 10.1039/b808893d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hung WC, Chen FY, Huang HW. Order-disorder transition in bilayers of diphytanoyl phosphatidylcholine. Biochim Biophys Acta. 2000;1467:198–206. doi: 10.1016/s0005-2736(00)00221-2. [DOI] [PubMed] [Google Scholar]
- 31.Huang J, Lein M, Gunderson C, Holden MA. Direct quantitation of peptide-mediated protein transport across a droplet-interface bilayer. J. Am. Chem. Soc. 2011;133:15818–15821. doi: 10.1021/ja2046342. [DOI] [PubMed] [Google Scholar]
- 32.Gabriel GJ, Madkour AE, Dabkowski JM, Nelson CF, Nusslein K, Tew GN. Synthetic Mimic of Antimicrobial Peptide with Nonmembrane-Disrupting Antibacterial Properties. Biomacromolecules. 2008;9:2980–2983. doi: 10.1021/bm800855t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tezgel AO, Telfer JC, Tew GN. De Novo Designed Protein Transduction Domain Mimics from Simple Synthetic Polymers. Biomacromolecules. 2011;12:3078–3083. doi: 10.1021/bm200694u. [DOI] [PubMed] [Google Scholar]
- 34.Som A, Reuter A, Tew GN. Protein Transduction Domain Mimics: The Role of Aromatic Functionality. Angew. Chem. Int. Ed. 2012;51:980–983. doi: 10.1002/anie.201104624. [DOI] [PubMed] [Google Scholar]
- 35.Bradshaw JP. Cationic antimicrobial peptides - Issues for potential clinical use. Biodrugs. 2003;17:233–240. doi: 10.2165/00063030-200317040-00002. [DOI] [PubMed] [Google Scholar]
- 36.Brogden KA. Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nat. Rev. Microbiol. 2005;3:238–250. doi: 10.1038/nrmicro1098. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.