Abstract
The testicular nuclear receptor 4 (TR4) is a member of the nuclear receptor superfamily that mediates various biological functions with key impacts on metabolic disorders and tumor progression. Here we demonstrate that TR4 may play a positive role in prostate cancer (PCa) CD133+ stem/progenitor (S/P) cell invasion. Targeting TR4 with lentiviral silencing RNA significantly suppressed PCa CD133+ S/P cell invasion both in vitro and in vivo. Mechanism dissection found that TR4 transcriptionally regulates the oncogene EZH2 via binding to its 5′ promoter region. The consequences of targeting TR4 to suppress EZH2 expression may then suppress the expression of its downstream key metastasis-related genes including NOTCH1, TGFβ1, SLUG and MMP9. Rescue approaches via adding the EZH2 reversed the TR4-mediated PCa S/P cell invasion. Together, these results suggest that the TR4→EZH2 signaling may play a critical role in the PCa S/P cell invasion and may allow us to develop a better therapy to battle the PCa metastasis.
Keywords: TR4, EZH2, prostate cancer, Stem/Progenitor cells, metastasis
Introduction
Prostate cancer (PCa) continues to be the most common malignant tumor in men in the United States. Androgen/androgen receptor (AR) signals play important roles in PCa progression, and androgen deprivation therapy (ADT) is the standard therapy for the advanced PCa. However, most ADT eventually fails and PCa progresses into castration resistant PCa (CRPC) that is often accompanied with metastasis. The detailed mechanisms, however, remain unclear.
One key factor that may contribute to the metastasis development, despite the ongoing ADT treatment, is the increasing population of PCa stem/progenitor (S/P) cells, and targeting cancer S/P cells has been suggested as a potential novel therapy to suppress the PCa metastasis (1).
Testicular nuclear receptor 4 (TR4) may modulate many signals by interacting with other nuclear receptors including AR and estrogen receptor. Results from TR4 knockout (TR4KO) mice studies have shown that TR4 may play key roles to influence embryonic development, stem cell pluripotency and progression of several diseases including metabolic disorders and various tumors (2–6).
Enhancer of zeste homolog 2 (EZH2) is a member of the polycomb repressive complex 2 (PRC2), which includes suppressor of zeste 12 (SUZ12) and embryonic ectoderm development (EED). EZH2 may serve as a histone methyltransferase to methylate the histone H3 lysine 27 (H3K27) at target gene promoters that lead to epigenetic silencing. EZH2 interacts with SNAI1 and suppress E-cadherin to promote epithelial–mesenchymal transition (EMT) to influence the cancer metastasis. EZH2 is essential for embryonic development and stem cell pluripotency. Increased expression of EZH2 was found in a variety of human cancers including PCa (7) and its expression is positively linked with the progression and metastasis of PCa (8).
Here we demonstrate that targeting TR4 could suppress PCa CD133+ S/P cell invasion via inhibition of EZH2 expression and its downstream metastasis-related genes including NOTCH1, SLUG, TGFβ1 and MMP9.
Materials and Methods
Cell Culture
C4-2 cells were a gift from Dr. Jer-Tsong Hsieh of University of Texas Southwestern Medical Center in 2009 and were not authenticated by us. The CWR22Rv1 cell line was purchased from and authenticated by the American Type Culture Collection (ATCC, Manassas, VA) in May, 2010. PCa stem cells (PCSCs) were purchased from Celprogen (San Pedro, CA) in 2011 and were not authenticated by us. C4-2 and CWR22Rv1 cell lines were maintained in RPMI 1640 media containing penicillin (25 units/ml), streptomycin (25 g/ml), 1% L-glutamate, and 10% fetal bovine serum (FBS). Isolated CD133+ S/P cells and PCSC cells were incubated in DMEM/F12 media with 0.6% glucose, 10 mg/ml putrescine, 50 mg/ml insulin, 100 mg/ml apo-transferin, 0.03 mM sodium selenite, 2 mM progesterone, 5 mM HEPES, 0.1% sodium bicarbonate, 10 ng/ml bFGF and 20 ng/ml EGF.
Magnetic beads isolation of CD133+ cells
Isolation of CD133+ PCa cells was performed as previously described (9, 10). Briefly, magnetic beads (Invitrogen, Grand Island, NY) were conjugated with biotinylated CD133 antibody (Miltenyi Biotec, Cambridge, MA). Cells (2 × 107) were detached with 5 mM EDTA and incubated with beads, and separated by placing tubes in a magnetic field. The S/P markers of the sorted cells were confirmed by qPCR and immunofluorescence. Flow cytometric analysis indicated that >95% of the sorted cells are CD133+ cells (data not shown).
Quantitative real-time PCR
Total RNA was isolated by Trizol reagent (Invitrogen, Grand Island, NY), and 1 μg of total RNA was then subjected to reverse transcription using Superscript III transcriptase (Invitrogen, Grand Island, NY). Quantitative real-time PCR (qRT-PCR) was conducted using a Bio-Rad CFX96 system with SYBR green to determine the mRNA expression of specific genes. Expression levels were normalized to the expression of GAPDH and all reactions were run at least in triplicate.
Western Blot Analysis
Cells were washed with PBS and lysed in RIPA buffer. Proteins (30 μg) were separated on 10% or 15% SDS-PAGE gel and transferred to PVDF membranes (Millipore, Billerica, MA). Membranes were blocked in 5% non-fat milk in PBST for 1 hour at room temperature, and then incubated with diluted primary antibodies against GAPDH (Santa Cruz, #sc-166574), TR4 (Perseus Proteomics, #PP-H0107B-00), EZH2 (Cell Signaling, #5246P), NOTCH1 (Cell Signaling, #3439), TGFβ1 (Santa Cruz, #sc-146), SLUG (Cell Signaling, #9585), or MMP9 (Santa Cruz, #sc-10737) overnight at 4°C. Blots were incubated with HRP conjugated secondary antibody for 1 hour at room temperature, washed, and developed in the ECL system (Bio-Rad, Hercules, CA, USA). Quantitations of blots are shown in Fig. S1.
Plasmids and lentivirus
TR4 shRNA sequence was cloned into pLKO.1 puro plasmid. To overexpress EZH2, EZH2 cDNA was cloned into PWPI vector. Lentivirus packing and production was the same as previously described (11). Human EZH2 full-length promoter (−2007~+313), along with truncated promoter (−1083~+313, −458~+313, −187~+313,) were amplified and cloned into pGL3-basic vector, respectively.
Invasion Assay
Growth factor reduced Matrigel (BD, #356234) was diluted with coating buffer (0.01M Tris, 0.7% NaCl, pH 8.0) at the radio of 1:15, coated onto the 8 μm transwell plates (Corning, #3422), and incubated at 37°C for 2 hours. Cells (105) were re-suspended with serum-free media and seeded in the upper chamber of transwells. Media with 10% FBS was put in the lower chamber. After 24 hours incubation, cells invaded to the lower part of the membrane were harvested, fixed with methanol, and stained with 0.1% Toluidine Blue. Invaded cells were counted under microscope. Triplicate experiments were performed.
3-D invasion assay
The 3-D invasion assay was as previously described (12). Briefly, 1×104 cells were suspended in 200 μl media containing 1% Matrigel (Trivigen, #3500-096-K) and then plated into the previously prepared collagen/Matrigel mixture coated plates. Media will be replenished every 3 days for 2 wks and then spheres and protrusions will be recorded under microscope.
ChIP Assay
ChIP experiments were performed following the Cold Spring Harbor ChIP protocol (13) with minor modifications. Cultured C4-2 PCa cells (2 × 107) were harvested. The cells were treated with 1% formaldehyde at room temperature for 10 min to cross-link DNA. After sonication (10 × 10 sec, 10% amplitude on a Branson Sonifier 450), cross-linked chromatin was precleared with protein A/G-agarose beads and then immunoprecipitated using anti-TR4-specific antibody or IgG overnight at 4°C. Supernatants from the no-antibody-added samples were used to measure total input chromatin. The chromatins were then incubated overnight at 65°C to reverse cross-links. DNA was then treated with RNase A, purified using gel extraction columns (OMEGA) and resuspended in 50 μL TE buffer. Then TR4 occupancy on chromatin was assessed by PCR with locus-specific primers.
Luciferase reporter gene assays
C4-2 cells were seeded in 24-well plates and were transfected with PGL3 reporter, pCMX/pCMX-TR4, and 1 ng pRL-TK using Lipofectamine 2000 (Invitrogen). Cell lysates were prepared with Passive Lysis Buffer (Promega, Madison, WI) 24 hrs after transfection, and luciferase activity was measured using the Dual Luciferase Reporter Assay (Promega, Madison, WI). pRL-TK served as internal control. The assays were run in triplicate.
Orthotopic-injected mice model
Male 6- to 8-week-old nude mice were purchased from NCI (Bethesda, MD, USA). Mice were divided into 2 groups include the control group and the shRNA group. CW22Rv1 S/P cells (2 × 105) with shTR4 or Scramble (scr) control mixed with Matrigel (BD) were orthotopically injected into both anterior prostates. IVIS system was used to monitor the tumor size and metastasis 4 weeks after injection. Mice were then sacrificed; tumor tissue samples were fixed, processed into paraffin tissue sections and used for immunohistochemistry.
Immunohistochemistry
Tumor tissues were fixed in 4% neutral buffered para-formaldehyde and embedded in paraffin. The primary antibodies of the TR4 (Perseus Proteomics, #PP-H0107B-00, 1:50), EZH2 (Cell Signaling, #5246P, 1:100) were used for staining. The primary antibody was recognized by the biotinylated secondary antibody (Vector, Burlingame, CA), and visualized by VECTASTAIN ABC peroxidase system and peroxidase substrate DAB kit (Vector). The positive staining signals were semiquantitated by ImageJ software.
Statistical analysis
Values were expressed as mean ± S.D. The Student’s t and ANOVA tests were used to calculate P values. P values were two-sided, and considered statistically significant when <0.05.
Results
Higher expression of TR4 in PCa CD133+ S/P cells
Recent studies found that higher S/P cell population in tumors may play important roles in tumor initiation, invasion and recurrence, and targeting S/P cells may represent another powerful therapy to suppress PCa (14). Importantly, recent studies also suggested that CRPC might function through increased S/P cell population to promote the CRPC metastasis (15), and TR4 has also been reported to play important roles in PCa progression (16). We were therefore interested in examining the potential linkage of TR4 in PCa S/P cells-enhanced metastasis.
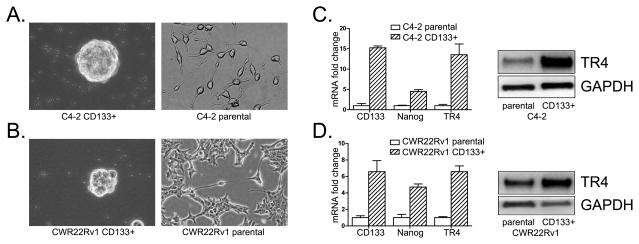
We used the CD133+ as the marker of PCa S/P cells since increasing evidence indicated that PCa S/P cells are characterized with high expression of CD133 (10, 17, 18), and CD133+ cancer stem cells are responsible for cellular migration and metastasis in cancer (19, 20), including PCa (21, 22). We first sorted out PCa S/P cells from PCa C4-2 and CWR22Rv1 cells via CD133+ antibody (9, 10), and results revealed CD133+ sorted S/P cells have distinct morphologies (Fig. 1A, B) with higher expression of the S/P markers of CD133 and Nanog (Fig. 1C, D, left panels) as compared to parental cells. Importantly, we also found higher expression of TR4 in CD133+ S/P cells at protein and mRNA level (Fig. 1C, D), and knocked down TR4 in CD133+ S/P cells led to morphology change to more differentiated PCa cells (Fig. S2).
Fig. 1. Higher expression of TR4 in PCa stem/progenitor cells.
C4-2 (A) and CWR22Rv1 (B) PCa S/P cells were sorted by MACS with CD133 antibody. Sorted stem cells were examined by qPCR with CD133 and Nanog stem markers and TR4 primers. Parental cells were used as control. Triplicate experiments were performed, and mean values ± S.D. (error bars) are presented. Result shows the sorted CD133+ S/P cells had significantly higher stem cell markers and TR4 level (C, D, left panels). Western blot data show that PCa CD133+ S/P cells had higher TR4 level (C, D, right panels) compared to parental cells.
Together, results from Fig. 1A–D demonstrate the higher expression of TR4 in PCa S/P cells.
TR4 promotes PCa CD133+ S/P cells metastasis
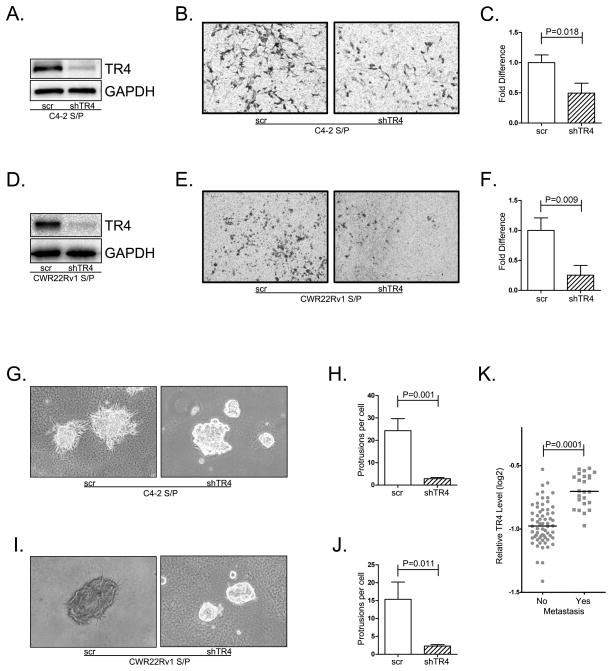
To examine whether increased TR4 in CD133+ S/P cells may impact their influence on PCa metastasis, we knocked-down TR4 and assayed the influence on cell invasion using chamber transwell invasion assays. The results revealed that knocked-down TR4 (Fig. 2A) led to suppressing the C4-2 CD133+ S/P cell invasion (Fig. 2B, C). Similar results were also obtained when we replaced CD133+ S/P cells derived from C4-2 cells with those from CWR22Rv1 cells (Fig. 2D–F).
Fig. 2. TR4 role in mediating PCa metastasis.
TR4 was successfully knocked down in both C4-2 and CWR22Rv1 CD133+ S/P cells (A, D). Then both TR4 knocked-down (shTR4) and control S/P cells (scr) were used for invasion assays. Triplicate experiments were performed, and mean values ± S.D. (error bars) are presented. The shTR4 groups had less invasion ability than control groups (B, E). C–F shows the quantitative data. Then the invasion ability change was measured by the 3D invasion assay that shows the similar results (G–J). K. gene array data of 88 PCa patients from the Oncomine database shows that the patients with metastatic PCa have a significantly higher TR4 level than the localized PCa.
We then used another 3D invasion assay to further confirm the previous results, and results again revealed that knocking-down TR4 with TR4-shRNA suppressed PCa C4-2 or CWR22Rv1 CD133+ S/P cell invasion (Fig. 2 G–J).
Using NCBI GEO databases(GEO dataset accession GSE6919) (23) to analyze the human PCa sample array with TR4 expression also indirectly suggested that PCa tissues with metastasis have a higher TR4 expression than those localized PCa tissues (Fig. 2K).
Together, results from in vitro cell lines studies (Fig. 2A–J) and human PCa sample survey (Fig. 2K) all suggest that higher TR4 expression in S/P cells may link to the S/P cell ability to metastasize and targeting TR4 leads to suppressed PCa CD133+ S/P cell invasion.
TR4 regulates EZH2 and its metastasis-related genes in PCa S/P cells
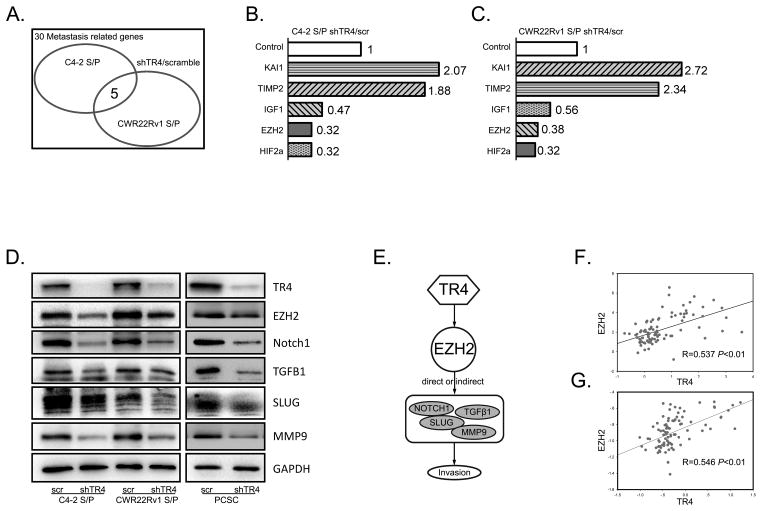
To dissect the mechanism by which TR4 alters the S/P cells-mediated PCa cell invasion in the C4-2 and CWR22Rv1 CD133+ S/P cells, we screened those genes that were reported to link to PCa metastasis (Fig. 3A, supplementary table 1). The results revealed 5 overlapping potential key genes with KAI1 and TIMP2 increased and IGF, HIF2α, and EZH2 decreased upon knocking down of TR4 (Fig. 3B, C). We decided to focus on EZH2 as it plays important roles in cancer S/P cells and its expression was significantly decreased in both C4-2 and CWR22Rv1 CD133+ S/P cells.
Fig. 3. TR4 regulates EZH2 and its downstream genes.
A. 30 PCa metastasis-related genes were screened by qPCR assay. B–C. Five out of the 30 metastasis related genes changed dramatically in both the C4-2 and CWR22Rv1 CD133+ S/P cells with EZH2 expression decreased most significantly. D. Western blots were performed to check EZH2 and it downstream genes change after knocking-down TR4. EZH2, NOTCH1, SLUG, TGFβ1 and MMP9 were significantly decreased in the three different cell lines include C4-2 and CWR22Rv1 CD133+ S/P cells and PCa stem cell line PCSC. Triplicate experiments were performed. E. Shows the integrated signaling pathways. F–G. Show the positive correlation between TR4 and EZH2 in clinical human PCa samples.
Early studies suggested that EZH2 might function through modulation of several key metastasis-associated genes including NOTCH1, SLUG, TGFβ1 and MMP9 to promote cancer cell invasion (24–26). We first demonstrated that knocking-down TR4 suppressed the expression of both EZH2 and these metastasis-related genes in C4-2 CD133+ S/P cells (Fig. 3D). Similar results were also obtained when we replaced C4-2 CD133+ S/P cells with other two S/P cell lines (CWR22Rv1 CD133+ S/P and PCSC) (Fig. 3D). PCSC is a PCa stem cell line originally obtained from a PCa patient and immortalized by Celprogen (San Pedro, CA).
Together, results from Fig. 3A–D suggest that TR4 may function through modulation of EZH2-related signals (see cartoon, Fig. 3E) to influence the PCa S/P cell invasion.
Using NCBI GEO databases to analyze the human PCa sample array, we also found the positive correlation of TR4 and EZH2 in 94 PCa samples (GEO dataset accession GSE35988) (27) (Fig. 3F). Similarly, analysis of another dataset (GEO dataset accession GSE6919) revealed the positive correlation of TR4 and EZH2 in 89 human PCa samples (Fig. 3G).
TR4 regulates EZH2 expression at the transcriptional level
TR4 is a transcription factor and can modulate its downstream target genes via binding to the TR4 response element (TR4RE) of its downstream genes (28). To further dissect the molecular mechanism by which TR4 affects EZH2 expression, we first searched for potential TR4REs (Fig. 4A) and found 3 putative TR4REs within the EZH2 promoter region (−1698~−1683; −1055~−1041; +149~+163) using JASPAR database (Fig. 4B). Since it is commonly accepted that only binding sites in the conserved region are functional, we checked the sequence and found all three sites are in the evolutionary conserved region. We then applied the ChIP assay and found that TR4 only bound to the first 2 putative TR4REs (Fig. 4C). We then linked the EZH2 promoter (−2007~+313) into pGL-3 basic luciferase reporter and examined TR4 influence on its activity. As shown in Fig. 4D, TR4 enhanced EZH2 promoter activity in a dose-dependent manner.
Fig. 4. TR4 transcriptionally regulates EZH2.
A. TR4RE motif sequences. B. Three putative TR4REs predicted by JASPAR from EZH2 promoter. C. ChiP assay showed TR4 protein binding to the chromatin at the first and second putative TR4RE in EZH2 promoter. D. Promoter luciferase reporter assay showed that TR4 could promote EZH2 promoter activity in a dose dependent manner. E. Luciferase reporter assay of series truncation of EZH2 promoter. At the loss of the first TR4RE, TR4 could no longer induce EZH2 promoter activity. F. ChIP assay of the first TR4RE of EZH2 promoter were performed in TR4 knocked-down cells. Results showed that the signal was significantly decreased. Luc assays were run in triplicate.
Since TR4RE1 and 2 are only about 600 nucleotides apart, the limitation of ChIP technology makes it very difficult to determine whether TR4 is recruited to both sites or only one of these two sites. We further confirmed above results using a series of truncations of the EZH2 promoter reporter and results showed that truncation without the first TR4RE failed to induce EZH2 promoter activity (Fig. 4E), suggesting this TR4RE may play essential roles to mediate the TR4 modulation of EZH2 expression. This conclusion is further confirmed via ChIP assay showing knocking-down TR4 with TR4-shRNA significantly suppressed the binding to the first TR4RE (Fig. 4F)
Taken together, results from both ChIP and luciferase reporter assays all conclude that TR4 may induce EZH2 expression by binding directly to the EZH2 promoter region.
TR4 promotes PCa S/P cell invasion via inducing EZH2
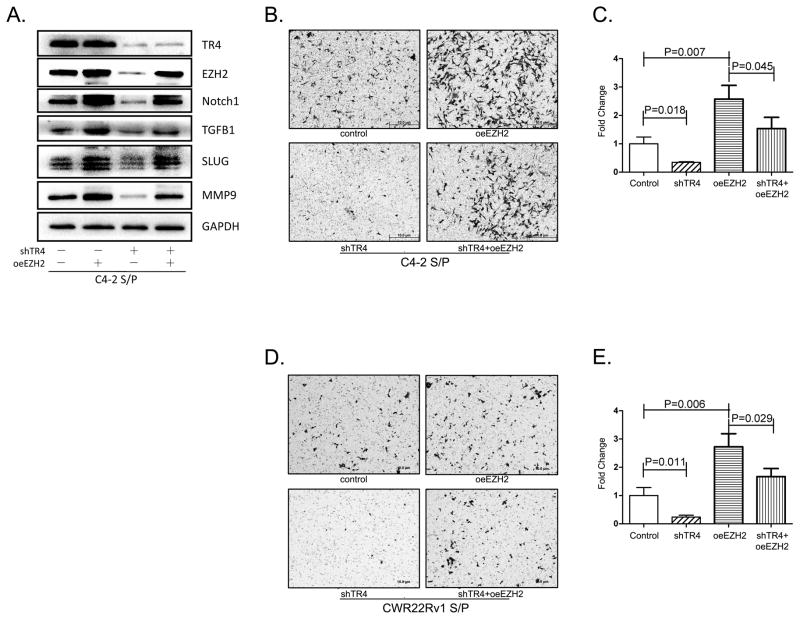
To link TR4 induced EZH2 expression to TR4 capacity to promote PCa S/P cell invasion, we applied the interruption approach via overexpression of EZH2 using lentivirus system to see if this could reverse the TR4-shRNA suppression effect. The Western blot results revealed that adding EZH2 could reverse (rescue) the suppression effect of TR4-shRNA on EZH2 and its downstream metastasis-related genes (Fig. 5A). Importantly, adding EZH2 also partially reversed the TR4 ability to influence the CD133+ S/P cell invasion both in C4-2 (Fig. 5B, C) and CWR22Rv1 cells (Fig. 5D, E).
Fig. 5. TR4 promotes invasion in PCa CD133+ S/P cells via inducing EZH2.
A. Western blot shows EZH2 and its downstream genes decreased with knocking-down TR4, which can be partially reversed by overexpression of EZH2. B. C4-2 CD133+ S/P cells invasion assay showed shTR4 decreased invasion ability. Effect of decreased invasion ability via knocking down of TR4 was partially reversed by overexpressing EZH2. C. The quantitative data. D–E. the similar results in CWR22Rv1 CD133+ S/P cells. Triplicate experiments were performed.
Together, results from Fig. 5A–E suggest that targeting TR4 with TR4-shRNA may suppress PCa CD133+ S/P cell invasion via modulation of EZH2 signaling.
Targeting TR4 suppresses PCa S/P tumor metastasis in in vivo mouse model
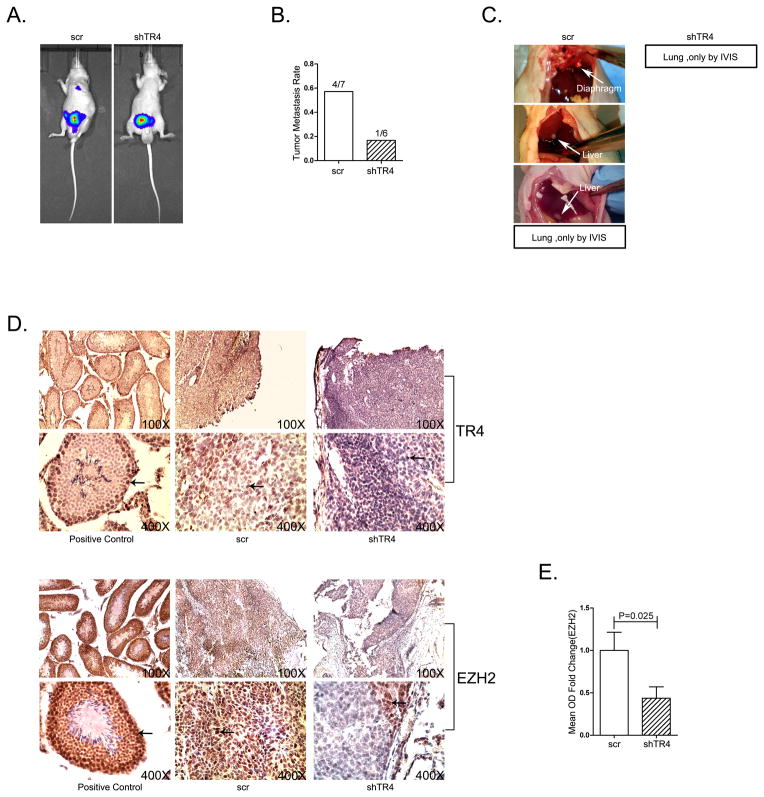
All the above in vitro data concluded that targeting TR4 could suppress PCa CD133+ S/P cell invasion via alteration of EZH2. To further confirm this in vitro conclusion in vivo, we applied an orthotopically xenografted PCa S/P cells mouse model. CWR22Rv1 cells stably transfected with firefly luciferase reporter gene were sorted by MACS with CD133 antibody and infected with either TR4-shRNA or scramble RNA (scr). 2×105 sorted CD133+ S/P cells were orthotopically xenografted into anterior prostates of nude mice, with 12 mice each group. There were seven mice in the scr group and six in the shTR4 group who developed primary tumor. In the scr group, four out of seven mice developed metastatic sites include liver, diaphragm, and lung. In the shTR4 group, only one mouse developed lung metastasis. Both of the lung metastasis in the two groups can only be seen under IVIS scan. The results revealed that mice with knocked-down TR4 in S/P cells had much fewer metastatic tumors detected (Fig. 6A, B, C), suggesting TR4 indeed is the key player to promote PCa S/P cell invasion.
Fig 6. Targeting TR4 suppresses PCa S/P tumor metastasis in vivo.
A. Luc-CWR22Rv1 CD133+ cells with shTR4 or control were implanted into nude mice with 12 mice in each group. The tumor growth and metastasis were detected by IVIS system. B. Quantitative data of tumor metastases detected by IVIS system and autopsy. In the scr group, 4/7 mice developed metastatic sites include liver, diaphragm, and lung. In the shTR4 group, only one mouse out of six developed lung metastasis. C. Gross appearance of metastases in control group, white arrows point out metastasis. Both of the lung metastasis in the two groups can only be seen under IVIS scan. D. Tumor samples were collected for IHC staining with E. quantitative data.
We then analyzed the expression of TR4 and EZH2 using IHC staining in the in vivo mouse model and found that EZH2 expression is much lower in the knocked-down TR4 tumors as compared to vehicle control (Fig. 6D, E).
Together, results from Fig. 6A–E in vivo mouse model studies are in agreement with the above in vitro cell lines studies and demonstrated that targeting TR4 could suppress PCa S/P cell invasion via regulation of EZH2 signals.
Discussion
Accumulating evidence show that CD133 is an important stem/progenitor marker in PCa (10, 17, 29). Vander et al. (18) reported that clonogenic ability of sorted CD133+ CWR22Rv1 cells is 2.4 times greater than the parental cells and average colony size was two times larger by 10 day. More importantly, the increased CD133+ cell population led to metastasis and poor prognosis in PCa patients (21, 22). Here we further proved that TR4 could alter the PCa CD133+ S/P cell invasion via modulating the EZH2-related metastasis gene expression.
Nuclear receptors have been reported to act as regulators of cancer S/P cell metabolism (30). Genome-wide gene expression data suggest that TR4 is expressed at a higher level in S/P cells (30), which is consistent with our data showing that PCa CD133+ S/P cells have higher TR4 expression, and that may be important in mediating PCa biological progression. Yang et al. also found TR4 was highly expressed in PCSC cells, the established PCa stem cell line from Celprogen, as compared to C4-2 CD133-cells (11). Up-regulation of EZH2 has been reported to increase the population of cancer S/P cells in other tumors (31–35), while targeting EZH2 using antagonist or silencing RNA decreased the self-renewal ability and tumorigenesis (36, 37). These findings also fit well with our findings here showing that TR4 may function through modulation of EZH2 signaling to mediate PCa S/P cell invasion.
Recent studies indicated that EMT might play key roles in the cancer stem cells (CSCs) function. Both EMT and CSCs play critical roles in tumor metastasis and they have biological similarities (38–40). These EMT-phenotype cells share molecular characteristics with CSCs (41). On the other hand, signaling pathways involved in the regulation of stem cell function also can trigger EMT programs (42), and EMT inducers can also play key roles in the maintenance of CSCs (43). Ding et al. found that TR4 could promote PCa metastasis via the CCL2/CCR2 signaling, the key player of EMT (16). In this study we further found that TR4 could promote PCa CD133+ S/P cells invasion via regulation of EZH2, which is important in both CSCs and EMT. These results therefore suggest that TR4 may promote PCa metastasis through both EMT-dependent pathway and CD133+ S/P cells.
In this study we demonstrated that TR4 might promote PCa S/P cell invasion. This is in contrast to an early study showing TR4 might suppress PCa initiation (6), suggesting that TR4 might play dual roles with a suppressor role in PCa initiation and a promoter role in PCa S/P cells invasion. Mechanism dissection suggested that TR4 might inhibit PCa initiation via positively regulating DNA damage-repair related gene ATM, thus enhancing DNA repair to prevent tumorigenesis. In contrast, TR4 could also promote PCa S/P cell invasion via modulation of EZH2 and its downstream metastasis-related genes. Other nuclear receptors, including the AR, may also play dual yet opposite roles in tumor initiation vs. tumor metastasis in PCa (44). Furthermore, BRCA1 was reported as a tumor suppressor (45) in PCa initiation but confers radiation resistance to PCa (46).
Early studies indicated that TR4 might be regulated by certain molecules including metformin (47), thiazolidinedione-rosiglitazone, and polyunsaturated fatty acids (PUFAs) (28). Since metformin has been linked to suppress the PCa progression (48–50), it will be interesting to see if metformin can also suppress PCa S/P cell invasion via modulation of TR4 activity.
In summary, the studies presented herein demonstrate that TR4 might be able to function through modulation of EZH2 expression to alter its downstream signals to enhance the PCa S/P cell-mediated invasion. Targeting TR4 may becoming a new potential therapeutic approach to better suppress PCa S/P cell invasion.
Supplementary Material
Acknowledgments
This study was supported by NIH grants (CA122840 and CA156700) for C Chang, George Whipple Professorship Endowment for C Chang, the Taiwan Department of Health Clinical Trial for C Chang, Research Center of Excellence (DOH99-TD-B-111-004 to China Medical University, Taichung, Taiwan) for C Chang, NSFC (Grant No. 81472776) for D Yang.
Abbreviations
- PCa
prostate cancer
- ADT
androgen deprivation therapy
- S/P cell
stem/progenitor cell
- CRPC
castration resistant prostate cancer
- TR4RE
TR4 response element
Footnotes
Conflict of interest
The authors declare no competing interests.
References
- 1.Lang SH, Frame FM, Collins AT. Prostate cancer stem cells. The Journal of pathology. 2009;217:299–306. doi: 10.1002/path.2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shyr C-R, Kang H-Y, Tsai M-Y, Liu N-C, Ku P-Y, Huang K-E, et al. Roles of testicular orphan nuclear receptors 2 and 4 in early embryonic development and embryonic stem cells. Endocrinology. 2009;150:2454–62. doi: 10.1210/en.2008-1165. [DOI] [PubMed] [Google Scholar]
- 3.Collins LL, Lee Y-F, Ting H-J, Lin W-J, Liu N-C, Meshul CK, et al. The roles of testicular nuclear receptor 4 (TR4) in male fertility-priapism and sexual behavior defects in TR4 knockout mice. Reprod Biol Endocrinol. 2011;9:138. doi: 10.1186/1477-7827-9-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lin S-J, Ho H-C, Lee Y-F, Liu N-C, Liu S, Li G, et al. Reduced osteoblast activity in the mice lacking TR4 nuclear receptor leads to osteoporosis. Reprod Biol Endocrinol. 2012;10:43. doi: 10.1186/1477-7827-10-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee Y-F, Liu S, Liu N-C, Wang R-S, Chen L-M, Lin W-J, et al. Premature aging with impaired oxidative stress defense in mice lacking TR4. American Journal of Physiology-Endocrinology and Metabolism. 2011;301:E91–E8. doi: 10.1152/ajpendo.00701.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin S-J, Lee SO, Lee Y-F, Miyamoto H, Yang D-R, Li G, et al. TR4 nuclear receptor functions as a tumor suppressor for prostate tumorigenesis via modulation of DNA damage/repair system. Carcinogenesis. 2014;35:1399–406. doi: 10.1093/carcin/bgu052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simon JA, Lange CA. Roles of the EZH2 histone methyltransferase in cancer epigenetics. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis. 2008;647:21–9. doi: 10.1016/j.mrfmmm.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 8.Varambally S, Dhanasekaran SM, Zhou M, Barrette TR, Kumar-Sinha C, Sanda MG, et al. The polycomb group protein EZH2 is involved in progression of prostate cancer. Nature. 2002;419:624–9. doi: 10.1038/nature01075. [DOI] [PubMed] [Google Scholar]
- 9.Lee SO, Ma Z, Yeh C-R, Luo J, Lin T-H, Lai K-P, et al. New therapy targeting differential androgen receptor signaling in prostate cancer stem/progenitor vs. non-stem/progenitor cells. Journal of molecular cell biology. 2013;5:14–26. doi: 10.1093/jmcb/mjs042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miki J, Furusato B, Li H, Gu Y, Takahashi H, Egawa S, et al. Identification of putative stem cell markers, CD133 and CXCR4, in hTERT-immortalized primary nonmalignant and malignant tumor-derived human prostate epithelial cell lines and in prostate cancer specimens. Cancer research. 2007;67:3153–61. doi: 10.1158/0008-5472.CAN-06-4429. [DOI] [PubMed] [Google Scholar]
- 11.Yang D-R, Ding X-F, Luo J, Shan Y-X, Wang R, Lin S-J, et al. Increased chemosensitivity via targeting testicular nuclear receptor 4 (TR4)-Oct4-interleukin 1 receptor antagonist (IL1Ra) axis in prostate cancer CD133+ stem/progenitor cells to battle prostate cancer. Journal of Biological Chemistry. 2013;288:16476–83. doi: 10.1074/jbc.M112.448142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xiang B, Muthuswamy SK. Using Three-Dimensional Acinar Structures for Molecular and Cell Biological Assays. Methods in enzymology. 2006;406:692–701. doi: 10.1016/S0076-6879(06)06054-X. [DOI] [PubMed] [Google Scholar]
- 13.Carey MF, Peterson CL, Smale ST. Chromatin immunoprecipitation (ChIP) Cold Spring Harbor Protocols. 2009;2009 doi: 10.1101/pdb.prot5279. pdb. prot5279. [DOI] [PubMed] [Google Scholar]
- 14.Maitland NJ, Collins AT. Prostate cancer stem cells: a new target for therapy. Journal of Clinical Oncology. 2008;26:2862–70. doi: 10.1200/JCO.2007.15.1472. [DOI] [PubMed] [Google Scholar]
- 15.Luo J, Ok Lee S, Liang L, Huang CK, Li L, Wen S, et al. Infiltrating bone marrow mesenchymal stem cells increase prostate cancer stem cell population and metastatic ability via secreting cytokines to suppress androgen receptor signaling. Oncogene. 2014;33:2768–78. doi: 10.1038/onc.2013.233. [DOI] [PubMed] [Google Scholar]
- 16.Ding X, Yang D-R, Lee SO, Chen Y-L, Xia L-Q, Lin S-J, et al. TR4 nuclear receptor promotes prostate cancer metastasis via up-regulation of CCL2/CCR2 signaling. International Journal of Cancer. 2014 doi: 10.1002/ijc.29049. n/a-n/a. [DOI] [PubMed] [Google Scholar]
- 17.Collins AT, Berry PA, Hyde C, Stower MJ, Maitland NJ. Prospective identification of tumorigenic prostate cancer stem cells. Cancer research. 2005;65:10946–51. doi: 10.1158/0008-5472.CAN-05-2018. [DOI] [PubMed] [Google Scholar]
- 18.Vander Griend DJ, Karthaus WL, Dalrymple S, Meeker A, DeMarzo AM, Isaacs JT. The role of CD133 in normal human prostate stem cells and malignant cancer-initiating cells. Cancer research. 2008;68:9703–11. doi: 10.1158/0008-5472.CAN-08-3084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reggiani Bonetti L, Migaldi M, Boninsegna A, Fanali C, Farina M. Expression of CD133 Correlates with Tumor Stage, Lymph Node Metastasis and Recurrence in Oral Squamous Cell Carcinoma. J Cancer Sci Ther. 2014;6:094–8. [Google Scholar]
- 20.Hermann PC, Huber SL, Herrler T, Aicher A, Ellwart JW, Guba M, et al. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell stem cell. 2007;1:313–23. doi: 10.1016/j.stem.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 21.Mehra N, Penning M, Maas J, Beerepoot LV, van Daal N, van Gils CH, et al. Progenitor marker CD133 mRNA is elevated in peripheral blood of cancer patients with bone metastases. Clinical Cancer Research. 2006;12:4859–66. doi: 10.1158/1078-0432.CCR-06-0422. [DOI] [PubMed] [Google Scholar]
- 22.Eaton CL, Colombel M, van der Pluijm G, Cecchini M, Wetterwald A, Lippitt J, et al. Evaluation of the frequency of putative prostate cancer stem cells in primary and metastatic prostate cancer. The Prostate. 2010;70:875–82. doi: 10.1002/pros.21121. [DOI] [PubMed] [Google Scholar]
- 23.Yu YP, Landsittel D, Jing L, Nelson J, Ren B, Liu L, et al. Gene expression alterations in prostate cancer predicting tumor aggression and preceding development of malignancy. Journal of Clinical Oncology. 2004;22:2790–9. doi: 10.1200/JCO.2004.05.158. [DOI] [PubMed] [Google Scholar]
- 24.Moore HM. The Role of EZH2 in Breast Cancer Progression and Metastasis. 2013. [Google Scholar]
- 25.Rao Z-Y, Cai M-Y, Yang G-F, He L-R, Mai S-J, Hua W-F, et al. EZH2 supports ovarian carcinoma cell invasion and/or metastasis via regulation of TGF-β1 and is a predictor of outcome in ovarian carcinoma patients. Carcinogenesis. 2010;31:1576–83. doi: 10.1093/carcin/bgq150. [DOI] [PubMed] [Google Scholar]
- 26.Shin YJ, Kim J-H. The role of EZH2 in the regulation of the activity of matrix metalloproteinases in prostate cancer cells. PloS one. 2012;7:e30393. doi: 10.1371/journal.pone.0030393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grasso CS, Wu Y-M, Robinson DR, Cao X, Dhanasekaran SM, Khan AP, et al. The mutational landscape of lethal castration-resistant prostate cancer. Nature. 2012;487:239–43. doi: 10.1038/nature11125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xie S, Lee Y-F, Kim E, Chen L-M, Ni J, Fang L-Y, et al. TR4 nuclear receptor functions as a fatty acid sensor to modulate CD36 expression and foam cell formation. Proceedings of the National Academy of Sciences. 2009;106:13353–8. doi: 10.1073/pnas.0905724106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Trerotola M, Rathore S, Goel HL, Li J, Alberti S, Piantelli M, et al. CD133, Trop-2 and alpha 2 beta 1 integrin surface receptors as markers of putative human prostate cancer stem cells. American journal of translational research. 2010;2:135–44. [PMC free article] [PubMed] [Google Scholar]
- 30.Simandi Z, Cuaranta-Monroy I, Nagy L. Seminars in cell & developmental biology. Vol. 2013. Elsevier; 2013. Nuclear receptors as regulators of stem cell and cancer stem cell metabolism; pp. 716–23. [DOI] [PubMed] [Google Scholar]
- 31.Au SL, Wong CC, Lee JM, Fan DN, Tsang FH, Ng IO, et al. Enhancer of zeste homolog 2 epigenetically silences multiple tumor suppressor microRNAs to promote liver cancer metastasis. Hepatology. 2012;56:622–31. doi: 10.1002/hep.25679. [DOI] [PubMed] [Google Scholar]
- 32.Cai MY, Hou JH, Rao HL, Luo RZ, Li M, Pei XQ, et al. High expression of H3K27me3 in human hepatocellular carcinomas correlates closely with vascular invasion and predicts worse prognosis in patients. Mol Med. 2011;17:12–20. doi: 10.2119/molmed.2010.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mallen-St Clair J, Soydaner-Azeloglu R, Lee KE, Taylor L, Livanos A, Pylayeva-Gupta Y, et al. EZH2 couples pancreatic regeneration to neoplastic progression. Genes Dev. 2012;26:439–44. doi: 10.1101/gad.181800.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hu S, Yu L, Li Z, Shen Y, Wang J, Cai J, et al. Overexpression of EZH2 contributes to acquired cisplatin resistance in ovarian cancer cells in vitro and in vivo. Cancer Biol Ther. 2010;10:788–95. doi: 10.4161/cbt.10.8.12913. [DOI] [PubMed] [Google Scholar]
- 35.Fussbroich B, Wagener N, Macher-Goeppinger S, Benner A, Falth M, Sultmann H, et al. EZH2 depletion blocks the proliferation of colon cancer cells. PloS one. 2011;6:e21651. doi: 10.1371/journal.pone.0021651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chase A, Cross NC. Aberrations of EZH2 in cancer. Clinical Cancer Research. 2011;17:2613–8. doi: 10.1158/1078-0432.CCR-10-2156. [DOI] [PubMed] [Google Scholar]
- 37.Qi W, Chan H, Teng L, Li L, Chuai S, Zhang R, et al. Selective inhibition of Ezh2 by a small molecule inhibitor blocks tumor cells proliferation. Proceedings of the National Academy of Sciences. 2012;109:21360–5. doi: 10.1073/pnas.1210371110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hollier BG, Evans K, Mani SA. The epithelial-to-mesenchymal transition and cancer stem cells: a coalition against cancer therapies. Journal of mammary gland biology and neoplasia. 2009;14:29–43. doi: 10.1007/s10911-009-9110-3. [DOI] [PubMed] [Google Scholar]
- 39.Mani SA, Guo W, Liao M-J, Eaton EN, Ayyanan A, Zhou AY, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–15. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Santisteban M, Reiman JM, Asiedu MK, Behrens MD, Nassar A, Kalli KR, et al. Immune-induced epithelial to mesenchymal transition in vivo generates breast cancer stem cells. Cancer research. 2009;69:2887–95. doi: 10.1158/0008-5472.CAN-08-3343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kong D, Li Y, Wang Z, Sarkar FH. Cancer stem cells and epithelial-to-mesenchymal transition (EMT)-phenotypic cells: are they cousins or twins? Cancers. 2011;3:716–29. doi: 10.3390/cancers30100716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Polyak K, Weinberg RA. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nature Reviews Cancer. 2009;9:265–73. doi: 10.1038/nrc2620. [DOI] [PubMed] [Google Scholar]
- 43.Ouyang G, Wang Z, Fang X, Liu J, Yang CJ. Molecular signaling of the epithelial to mesenchymal transition in generating and maintaining cancer stem cells. Cellular and Molecular Life Sciences. 2010;67:2605–18. doi: 10.1007/s00018-010-0338-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Niu Y, Altuwaijri S, Lai K-P, Wu C-T, Ricke WA, Messing EM, et al. Androgen receptor is a tumor suppressor and proliferator in prostate cancer. Proceedings of the National Academy of Sciences. 2008;105:12182–7. doi: 10.1073/pnas.0804700105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fan S, Wang J-A, Yuan R, Ma YX, Meng Q, Erdos MR, et al. BRCA1 as a potential human prostate tumor suppressor: modulation of proliferation, damage responses and expression of cell regulatory proteins. Oncogene. 1998;16:3069–82. doi: 10.1038/sj.onc.1202116. [DOI] [PubMed] [Google Scholar]
- 46.Moskwa P, Buffa FM, Pan Y, Panchakshari R, Gottipati P, Muschel RJ, et al. miR-182-mediated downregulation of BRCA1 impacts DNA repair and sensitivity to PARP inhibitors. Molecular cell. 2011;41:210–20. doi: 10.1016/j.molcel.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim E, Liu N-C, Yu I-C, Lin H-Y, Lee Y-F, Sparks JD, et al. Metformin Inhibits Nuclear Receptor TR4–Mediated Hepatic Stearoyl-CoA Desaturase 1 Gene Expression With Altered Insulin Sensitivity. Diabetes. 2011;60:1493–503. doi: 10.2337/db10-0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sahra IB, Laurent K, Giuliano S, Larbret F, Ponzio G, Gounon P, et al. Targeting cancer cell metabolism: the combination of metformin and 2-deoxyglucose induces p53-dependent apoptosis in prostate cancer cells. Cancer research. 2010;70:2465–75. doi: 10.1158/0008-5472.CAN-09-2782. [DOI] [PubMed] [Google Scholar]
- 49.He X-X, Tu S, Lee M-H, Yeung S-C. Thiazolidinediones and metformin associated with improved survival of diabetic prostate cancer patients. Annals of Oncology. 2011;22:2640–5. doi: 10.1093/annonc/mdr020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ben Sahra I, Tanti JF, Bost F. The combination of metformin and 2-deoxyglucose inhibits autophagy and induces AMPK-dependent apoptosis in prostate cancer cells. Autophagy. 2010;6:670–1. doi: 10.4161/auto.6.5.12434. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.