Abstract
The aim of this laboratory method is to describe two approaches for the investigation of bone responses to mechanical loading in mice in vivo. The first is running exercise, because it is easily translatable clinically, and the second is axial compression of the tibia, because it is precisely controllable. The effects of running exercise, and in general physical activity, on bone tissue have been shown to be both direct through mechanical loading (ground impact and muscle tension) and indirect through metabolic changes. Therefore, running exercise has been considered the most convenient preclinical model for demonstrating the general idea that exercise is good for bone health, either early in age for increasing peak bone mass or later in age by slowing down bone loss. However, numerous combinations of protocols have been reported, which makes it difficult to formulate a simple take-home message. This laboratory method also provides a detailed description of in vivo direct mechanical axial compression of the mouse tibia. The effects of mechanical loading depend on the force (strain), frequency, waveform and duration of application, and they range from bone anabolism with low bone remodeling, inducing lamellar bone accumulation, to bone catabolism with high bone remodeling, leading to microdamage, woven bone formation and bone loss. Direct in vivo loading models are extensively used to study mechanotransduction pathways, and contribute by this way to the development of new bone anabolism treatments. Although it is particularly difficult to assemble an internationally adopted protocol description, which would give reproducible bone responses, here we have attempted to provide a comprehensive guide for best practice in performing running exercise and direct in vivo mechanical loading in the laboratory.
Introduction
Skeletal adaptations to mechanical demands maintain and/or optimize bone shapes and structures for locomotive functions.1 This principle, known as the ‘mechanostat theory', involves both bone modeling and remodeling.2,3 These two cellular processes are altered in osteoporosis, which has been defined as a failure of bone adaptation to loading.4 Physical activity has been the most encouraged lifestyle means to improve skeletal health through decreases bone remodeling with an uncoupling effect; decreasing bone resorption and increasing bone formation. However, despite the common idea that mechanical stimuli are good for bone, a literature overview rapidly reveals discordant results. Indeed, the use of different animal strains, ages and genders in the different research groups makes for poor reproducibility of the procedures.5 There is a clear lack of international recommendations and standardization of laboratory practice in this field.
In this report, we focus on the two most popular loading models in mice: treadmill running exercise and in vivo axial compression of the tibia. Note that all the authors' personal observations have been made in experiments with C57BL6. Of course, all animal procedures that may be based on this laboratory method must be reviewed and conducted under licenses approved by the local animal ethics committee.
Treadmill running exercise
In this section, we shortly describe the materials for treadmill running experiments, expose some aspects of animal compliance and describe typical protocols.
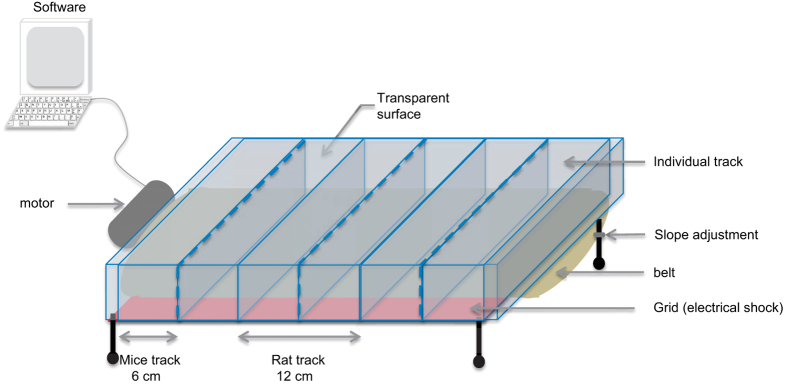
Treadmill machines consist of a belt rotation driven by a motor and controlled by software. Companies such as Columbus Instruments or TSE systems propose different types of treadmills (websites below1). All treadmills propose the same options, such as exercise duration, speed and acceleration (or deceleration). On some devices, the exercise intensity can be modulated by varying the inclination slope. The belt surface must have an optimal grip to prevent the animal from slipping, but without generating irritation by abrasion. Only one animal can be put per track, but treadmills can have several tracks depending on the size of the animal (up to 5 tracks for rats and obese mice, and 10 tracks for normal mice). A minimum of 6 cm for mice and 12 cm for rats is usually recommended by the treadmill manufacturers, footnote 1. The surface in front of the animals must be transparent and clean; otherwise, they seem to mistake it for a wall and do not run or run only intermittently. Aversive signals such as electrical shocks or compressed air are sometimes needed to force some animals to run. Most commercially available treadmills have a grid localized at the back of the tracks that can deliver electrical shocks of constant voltage (current from 0 to 2 mA, severity of the shock is proportional to current). In our typical protocol, shocks are administered when an animal remains steady at the back of the treadmill more than 10 s or when it returns to the back five times consecutively. A treadmill is described in Figure 1.
Figure 1.
Schematic representation of a homemade treadmill made in collaboration with Ficap. Treadmill consists of a belt, some individual track, a grid for electrical shock and software to control the speed and time of running. Dotted lines represent removable separator to adjust the width of the track depending on the animal's size.
Animals' compliance to treadmill running is crucial, as a minimum of 3–5 weeks of exercising is necessary to induce a bone mass/structure response.6,7,8 Rodents are more active in the dark, and good control of the light/dark cycle is therefore important in order to enable the rodent to run during their active cycle. In addition, one must ensure that baseline locomotor performances are equal before segregation of control and exercise groups. This procedure can be done in an open-field locomotor chamber or by using a simple exercise wheel installed into the cage to evaluate spontaneous locomotor activities. This evaluation is classically performed for 60 min/day for 5 consecutive days. Voluntary exercise has been also used to investigate the effect of exercise on bone metabolism. We do not discuss it here, because it usually requires a higher number of mice because of the heterogeneity of exercise practice in terms of distance, speed and daily running.9
Here, we present the minimum pertinent steps and considerations, which in our opinion are necessary to achieve maximum compliance. One can refer to Arnorld et al.10 for more details. Once baseline locomotor performances have been verified, a 1-week acclimation period is introduced to gradually familiarize the animals with the required exercise regimen. In the first acclimation phase, place each animal on individual tracks of the stationary treadmill for 5–10 min, and then immediately return the rodents to their home cages for another 5–10 min; repeat the procedure three times, for 2 consecutive days. This step familiarizes the animals with the treadmill apparatus. For the second acclimation phase, place the animals at the front of the track. Turn the treadmill onto a slow walking speed (6 m/min). Assist them as necessary by orienting or gently prodding them until they walk in the right direction. During this period, train them to walk on the treadmill without assistance. Perform five sessions of 5–10 min walk, with 5–10 min rest between two sessions. Typically, at the end of this phase, all C57BL6 mice are able to walk continuously at a speed of 6 m min−1 without assistance (footnote: the protocol may need some adaptations for other strains). For the last phase, perform 2 days of five walk/rest sessions/day, and increase the speeds by 1 m min−1 for each session so that at the end the animals run at a speed of 10 m min−1 for 10–15 min.
During the acclimation phases, score the animals' performance after each session, on the basis of, for example, the assistance needed, or constancy of running. For the assistance needed, assign a score of 4 when they do not require any assistance at all, a score of 3 when they require little assistance (for example, less than 25% of the time), 2 for moderate assistance (50%) and 1 for substantial assistance (75% and more). A simple criterion to evaluate constancy is the number of times that the animals touch the back grid. Upon completion of the acclimation phases, evaluate average performance scores and exclude noncompliant animals. For example, in our protocols, we exclude those with an assistance score below or equal to 1 or those that touched the back grid more than 10 times in a session.11,12 A summary of the treadmill acclimation is illustrated in a movie (Supplementary Movie 1).
Exercise running begins in the second week, once animals are familiarized with the setup and noncompliant animals have been excluded. Start with 3–5 min on a stationary treadmill, followed by warm-up: 5 min at a speed of 6–8 m min−1 and then another 5 min at a speed of 8–10 m min−1, before the running exercise itself. The inclination of the track, duration and speed are adjusted depending on the research question; for example, 14–16 m min−1 for 30 min with an inclination of 8° is a moderate exercise for adult C57BL6 mice.12 For resistance exercises, a 5-min cool down for 8–10 m min−1 is added at the end of the session to limit lactate accumulation.13
The exercise intensity is determined through repeated measurements of VO2max. VO2max is measured under a closed chamber or estimated from the maximum aerobic speed (Figure 2). The maximum aerobic speed test consists in increasing the speed at regular intervals of time until exhaustion. The test starts at 8 m min−1, and the speed is increased by 2 m min−1 every 2 min. Animals are considered exhausted when they touch the back of the treadmill five times consecutively. The last speed at which they ran is considered their maximum aerobic speed. Maximum aerobic speed increases with training; therefore, the test should be performed every month to adjust the speed and inclination of the regimen session in function of the actual maximum aerobic speed of the animal.
Figure 2.
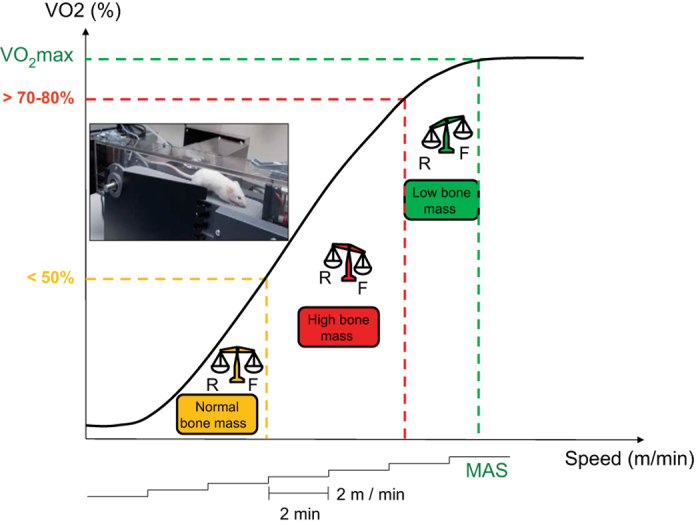
Effects of various exercise intensities on bone mass. To obtain maximum aerobic speed (MAS), running speed is increased by 2 m/min every 2 min. Below 50% VO2max, exercises have no significant effect on bone mass; from 50 to 80% VO2max: exercises increase bone mass; beyond 80% VO2max, exercises intensity have deleterious effect on bone mass. F, Formation; R, resorption (adapted from http://jcet.eu).
Physiological effects of running exercise can be measured through body weight loss between pre-exercise and post-exercise. It has been estimated that exercise in mice induced a mean weight loss of 3–6% compared with their pre-exercise body weight, whereas the controls group had a mean loss of 0–1%.14,15
Finally, there are a few secondary aspects of study designs that can still improve experimental outcomes. At each session, animals should be randomly assigned to running tracks to avoid potential track bias during exercise training. The treadmill belt should be cleaned with 50% ethanol after each individual session to erase all traces of olfactory stress, urine and feces that may distract the animals from running. Treadmill noise may also induce some stress; hence, sedentary (control) and exercise groups should stay in the same room during the running session. Similarly, control animals should be handled twice daily and placed on a stationary treadmill to mimic the stress induced by handling before and after running, and to the changes of the environments.
In vivo axial compression
In contrast to treadmill running, animals are anesthetized during in vivo loading; compliance is therefore not a concern. In addition, while in treadmill running forces are generated by the animals' muscles, in external loading models forces are generated by a mechanical apparatus. The forces are applied directly to a limb through contact points, inducing changes in endosteal, periosteal and trabecular bone, but not under physiological conditions. Various loading systems have been described previously: for example, four-point loading of the rat and later mouse tibia,16,17 bending of the rat tibia,18 axial loading of the mouse and rat ulna19,20 and axial loading of the mouse tibia.21 Although well controlled in terms of mechanical strains, four-point bending and cantilever-like bending rely upon direct pressure to the diaphyseal periosteum, which produces periostal woven bone under high loads. These models are therefore not recommended when assessing mechanically induced modeling on the periosteal surface.19
In contrast, axial loading models do not affect the periostum by direct contact pressure. The contact points are located at the joints, making these models the closest to natural locomotion in terms of strain distributions. The drawback of axial loading models, however, is that strains are distributed unevenly as a result of the bones' complex geometries.
The apparatus for axial loading of a mouse tibia is usually fully or largely custom-made, as, to the authors' knowledge, there are no commercially available systems. Here we review the various material aspects of these devices and provide some details about the systems that we have previously used with satisfaction.
The choice of an actuator is important, as it influences the type of loading (waveform) and the type of feedback that can be expected. The three main actuator categories are rotary motors, hydraulic/electromagnetic testing machines and moving coil actuators. In addition to the actuator, it is important to have load and displacement sensors in order to have real-time control of the experiment and record loading histories to possibly exclude outliers.
Rotary motors are cheap and simple to use, but they require dedicated mechanical systems to convert rotation into axial movements.22 The resulting displacement or force waveforms are usually pseudo-sinusoidal, and one has to tweak the mechanical design to change them, which can be cumbersome. On the other end of the price scale, hydraulic and electromagnetic testing machines (such as Instron, MTS, Bose, Zwick and so on) are also used for in vivo loading.23,24 With testing machines, any waveform can be programmed. Displacement feedback is already integrated, but an additional load sensor (usually a strain gauge) is necessary to record forces. These machines are very expensive and not found everywhere.
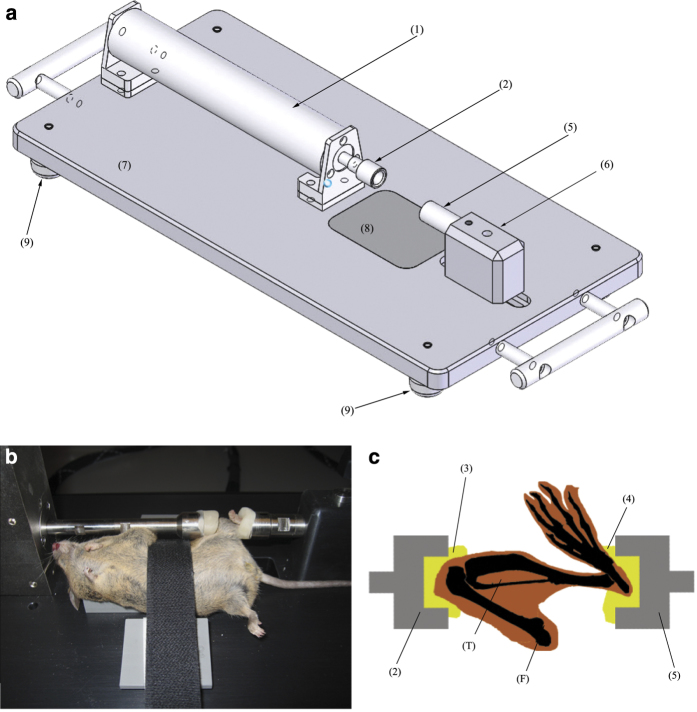
Because they are compact, versatile and have a lower price tag, our favorite actuators are moving coil actuators.11,25,26 These actuators have a piston riding on a linear bearing carriage, inside a magnet assembly. A coil and an optical encoder are mounted on the piston. When current flows in the coil, a reaction force is produced, which causes the piston to slide along the guide. As the piston moves, a detector reads the distance traveled from the optical encoder.2 These systems allow for very simple and compact experimental designs (Figure 3), because they include natively both force measurement (through current read) and displacement sensor (through the optical encoder). For example, the CAL36 (SMAC-MCA, Carlsbad, CA, USA) can develop forces up to 40 N, with a range of motion of 15 mm at a resolution of 1 to 5 μm. An external controller drives the linear coil actuator. The program code, or script, is uploaded from a personal computer onto the controller in the form of a text file. Scripts are written in a dedicated machine language, which requires experience in programming and basic ballistic knowledge, but it grants a very precise control of the actuator's load and movement (position, speed and acceleration are hard-coded).3 Once the script is saved in the controller, the system is independent from the computer. This is advantageous, because a system crash or freeze of the computer will not affect the actuator actions. The script can be updated easily between loading sessions, which means that the same actuator can be used for various applications.
Figure 3.
Experimental setup for in vivo axial loading. (a) a compact design based on a cylindrical linear coil actuator; (b) a C57BL6 cadaver position in the machine for sake of illustration; and (c) the anatomical positioning of the tibia during loading. The linear coil actuator (1) applies load through the knee cup (2), which is filled with modeling clay (3) shaped to receive the flexed knee. The load is transmitted through the tibia (T) to the ankle hold in place by the ankle cup (5), filled with modeling clay (4). The ankle cup is mounted to an adjustable slider (6) to adjust the length of the system to each animal. All the parts are mounted on an aluminum baseplate (7). The baseplate is anodized, and thus it resists better to cleaning agents. The baseplate has a radiolucent window (8) made of composite where the animal lies, to allow radiographic imaging during loading. We advise mounting the machine on dampening feet (9) to reduce vibrations.
Linear coil actuators are designed to work around the center of their stroke, where friction is minimal and encoder precision is maximal; therefore, we suggest that the ankle support be free to be adjusted over a range of a few centimeters, and the knee cup be attached to the actuator. This helps setting up the animal in place comfortably (for the operator) and adjusting the machine dimensions to the animal size, while leaving the actuator's working position unchanged.
During in vivo axial loading, forces are applied to the flexed knee and ankle joint placed in concave cups. As both these joints are very flexible, and the skin covering them moves almost freely, both joints must be well secured in the cups to avoid slip-offs. The joints need to be locked during loading to avoid movements of bone relative to each other, which would damage the ligaments and cartilage. The knee should be flexed as much as possible and the ankle joint in maximal dorsi-flexion (Figures 3b–c). The surfaces of contact should be hard enough to transmit loads without damping but without high-pressure points that may injure soft tissues. A solution is to use custom-molded pads that are made of oven-hardening modeling clay (for example, FIMO professional 8004, STAEDTLER Mars GmbH, Nuernberg, Germany). This material comes in multiple colors, which can be practical when multiple users are using the same loading system. To prepare the contact pads, the knee and ankle cups are filled with clay. An anesthetized animal is placed in the loading system, and a moderate force is applied onto the ankle cup until the joint pads are shaped to the flexed knee and ankle. The cups are placed for 30 min in an oven preheated at 110 °C (230 °F) for hardening and then cooled down. An axial compression device for the mouse tibia is described in Figure 3.
The loading waveform and schedule are crucial, as they determine the type of biological response of the bone tissue. Six main parameters should be considered when defining a loading waveform (and reported when publishing data): waveform shape, peak strain, strain rate, number of cycles, frequency and pauses/time-offs. These parameters were well reviewed by Meakin et al.27 In brief, experiments have shown clear correlations between peak strain and new bone formation and between strain rate and new bone formation rate. Simply put, higher strains and higher strain rates seem (within a reasonable range) to induce higher osteogenic responses.23,28 However, there still lacks a consensus on loading waveforms and schedule between research groups, and therefore it is not straightforward to decide on a loading protocol from the available publications. To help the reader, we have compiled waveforms, loading parameters, schedules and their corresponding bone responses from selected reference publications in Table 1.
Table 1. Collection of reference publications using in vivo axial loading, with details of loading waveforms, loads, strains and observed effects on bone.
| Species | Strain | Gender (M/F) | Site | Age (weeks) | Waveform | Peak load (N) | Peak strain (μɛ) | Strain rate (μɛ s−1) | Pulse period (s) | Rest period (s) | Frequency (Hz) | Nb cycles/session | Schedule | Observations (Only WT/non-treated/controls) | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rats | Spargue Dawley | F | Ulna | 10±2a | Sine | 15 and 20 | 3125 | NA | NA | NA | 10, 20 | 1200, 12 000 | Day 5, 6, 8, 11, 12, 13, 14 and 15 | First introduction of in vivo axial loading. Osteogenic response with 20N: decelerated modeling drift, increased cross-sectional area, increase in periostal formation. New bone formation proportional to surface strains. Small or no repsonse with 15N. | Torrance et al.19 |
| Rats | Fisher | F | Ulna | 32 | Haversine | 13.3 | 2400 | NA | NA | NA | 2 | ∼8000 | Day 1 | Periostal bone formation at 6, 12 and 18 days. Stiffness and toughness loss of 50% and 70%, respectively at day 0, equivalent to controls at day 12, increased +20% at day 18. | Hsieh and Silva,40 |
| Mice | C57Bl/J6 | F | Tibia | 8, 12 or 20 | NA | 2 to 13 | NA | 0.002 | NA | 10 | 2 | 40 | 3 Days per week for 2 weeks | Between 8 and 12 N, significant increased periosteal inter-label; load-related increase in cortical new bone formation. Response to 13N equivalent to 12 N. No effect below 8 N. | De Souza et al.21 |
| Mice | C57BL/6J | M | Tibia | 14±1 | Trapeze | 12 | 1500 | NA | 0.1 | 10 | 0.1 | 40 | 3 Days per week for 2 weeks sacrificed 3 days later | Increased bone mineral density and biomechanical properties of long bones; increased bone formation activity, particularly at the periosteum. Enhanced trabecular and cortical microarchitecture. | Bonnet et al.11 |
| Mice | C57BL6 | M | Tibia | 17±1 | Square | 8 | 1900 | 80 000 | 0.25 | 0.25 | 2 | 120 | Day 1, 3, 5, 8 and 102 × per day | Localized increase of cortical thickness and bone area; Stiffness of tibia increased by 16% compared with control, but no effect on ultimate force and postyield energy to failure. | Stadelmann et al.25 |
| Mice | C57BL/6 C3H/HeJ and DBA/2J | F | Ulna Tibia | 16 | Haversine | Strain adjusted | 2000 | NA | NA | NA | 2 | 99 | 3 Days per week for 3 weeks | Tibiaes showed greater MS, MAR and BFR than ulna at equivalent strains. No significant breed-related differences found in response to loading. | Kuruvilla et al.45 |
| Mice | C57BL/6 | F | Tibia | 17 | Triangle | 2 to 14 | 700 to 5000 | up to ∼ 75 000 | 0.05 | 10 | 0.1 | 40 | Day 5, 7, 9, 11, 13, 15, 17, and 19 | Woven bone formation at the proximal and midshaft on lateral and posterior surfaces tibia with 5000 μɛ loading. Strength adaptation of tibia to peak dynamic loads proporational to peak load. | Sugiyama et al.23 |
| Mice | C57BL/6 | F | Tibia | 19 | Trapeze | 13.5 | 1400 | ∼50 000b | 0.05 | 10 | 0.1 | 40 | 3 Days per week for 2 weeks | Increased proximal trabecular bone fraction, proximal cortical volume and midshaft cortical volume in tibia. Increased midshaft cortical bone volume in fibula. Woven bone formation. No systemic effects. | Sugiyama et al.43 |
| Mice | C57BL/6 | F | Tibia | 19 | Trapeze | 13.5 | 1800 | ∼50 000c | 0.05 | 10 | 0.1 | 40 | 3 Days per week for 2 weeks | In cortical bone, decreased osteocyte sclerostin, increased bone formation and bone volume proximal but not distal. In trabecular bone, decreased osteocyte sclerostin and increased bone volume in secondary spongiosa. | Moustafa et al.46 |
| Mice | C57BL/6 | F | Tibia | 16 | Haversine | 5, 7 and 9 | 1833 | NA | NA | NA | 2 | 360 | 3 Days per week for 4 weeks | In cortical bone, load-related response, up to 42% increase in midshaft pMOI with 9N. In trabecular bone, no response with 5 and 7N. With 9N, proximal BV/TV increased by 31%. | Weatherholt et al.47 |
| Mice | C57Bl/6 | F | Tibia | 26 | NA | 6 and 11.5 | 1200 and 2100 | NA | NA | 0.1 | 4 | 1200 | 5 Days per week for 2 weeks (Days 1–5, 8–12)d | With 11.5N, trabecular mass increased by 54% through trabecular thickening, cortical area increased by 41% through medullary contraction and periosteal expansion. No effect with 6N. | Lynch et al.48 |
| Mice | C57Bl/6 | F | Tibia | 8 | Haversine bouts (4 cycles followed by 3s pause) | 8.8, 10.6, and 12.4 | 1700, 2050 and 2400 | NA | NA | 3 | 2 | 220 | 14 day period with a day of rest after every third day of loading, resulting in 9 loading days | Load-related improved bone architecture. No effect with 8.8 N. With 10.6N improved cortical and trabecular bone, improved mechanical properties. With 12.4 N half of animals show woven bone response. | Berma et al.49 |
| Mice | BALB/c | M | Tibia | 28 and 88 | Triangle | 8, 10, and 12 | 3100 | 48N s−1 | 0 | 10 | ∼0.1 | ∼12 000 | 5 Days per week for 1 weeks (Mon-Fr)e | Strong anabolic response on midshaft endocortex and periosteum for both age groups. Aged mice show greater endocortical response than young adult. | Brodt et al.50 |
| Mice | BALB/c | F | Tibia | 8, 16, 28 and 48 | Triangle | 7.5, 10, 8.5 and 11 | 1300 | 48N s−1 | 0 | 10 | ∼ 0.1 | 60 | 3 Days per week for 1 or 6 weeks | After 1 week, no effect in 8-w-old mice but increased osteoblast/matrix genes in older mice. After 6 weeks, increased cortical bone volume at all ages. | Silva et al.51 |
| Mice | C57BL/6 and BALB/c | F | Tibia | 16 | Triangle | 10 | 2800 in BL6 2350 in BALBc | NA | NA | 100.1 | 26.7 | 60 (WashU) 1200 (Cornell) | 3 Days per week for 6 weeks | Compared WashU and Cornell/HSS protocols. Anabolic cortical bone response with both, but Cornell/HSS more rapid response (+ 13%). Mouse strain had no influence. | Holguin et al.52 |
Abbreviations: BV/TV, bone volume/total volume; F, female; M, male; NA, not applicable.
aEstimated from Charles River growth chart based on reported weight of 200–220 g.
bEstimated from reported data.
cSame experiment as Sugiyama et al. -> We assume same strain.
dAlso known as Cornell/HSS protocol.
eAlso known as WashU protocol.
In the in vivo loading context, the peak strain magnitude is determined by the maximum force and by mechanical properties of the bone, whereas the strain rate is determined by the loading waveform and dampening of the system. Higher forces will induce higher responses, but one has to be careful not to exceed the yield point, the limit above which permanent damage occurs. Note that the yield point is not easy to determine noninvasively; therefore, an educated guess has to be made based on a representative sample of cadaveric bones. If large variations in shape are present in the animal sample, it may be necessary to adapt the maximum force for each specimen to generate equivalent strains. These custom forces are proportional to the tibia stiffness, which can be determined by micro-computed tomography-based finite element modeling. These finite element models are validated with experimental data from strain gages placed directly onto loaded bones.26,29
The number of cycles also affects bone response: a small number (less than 100) is enough to activate osteogenic responses,30 and a high number (thousands) is necessary to induce fatigue microdamage.31 Note that loading duration is related to the number of cycles x frequency; therefore, at equivalent frequency, higher numbers will require longer anesthesia that may have adverse side effects (weight loss and others) when repeated regularly. In addition, as mechanosensitivity decreases with loading duration, resting times between cycles and time-offs between loading sessions can be included for optimal osteogenic effects.32,33
Finally, the optimal loading frequency of bone is still debated: low magnitude/high frequency or high magnitude/low frequency are both claimed to induce osteogenic responses, with lamellar or woven bone formation and varying indexes of bone formation.34,35,36
The interactions between all of these parameters, together with pharmacological agents and/or mice strains, are difficult to predict, and pilot studies should be run to evaluate the best loading protocols beforehand in special situations. Moreover, depending on the actuator's control loop feedback (proportional-integral-derivative) and on the dampening characteristics of bone and soft tissues, the real waveform may vary slightly from the one programmed, particularly when higher strain rates are programmed (Figure 4). It is therefore a good idea to measure the actual strain experienced by the tibia in cadaveric bones with microstrain gages, similar to what was described earlier.26 The strain gage signal can then be used to fine-tune the actuator's PID or the waveform script.
Figure 4.
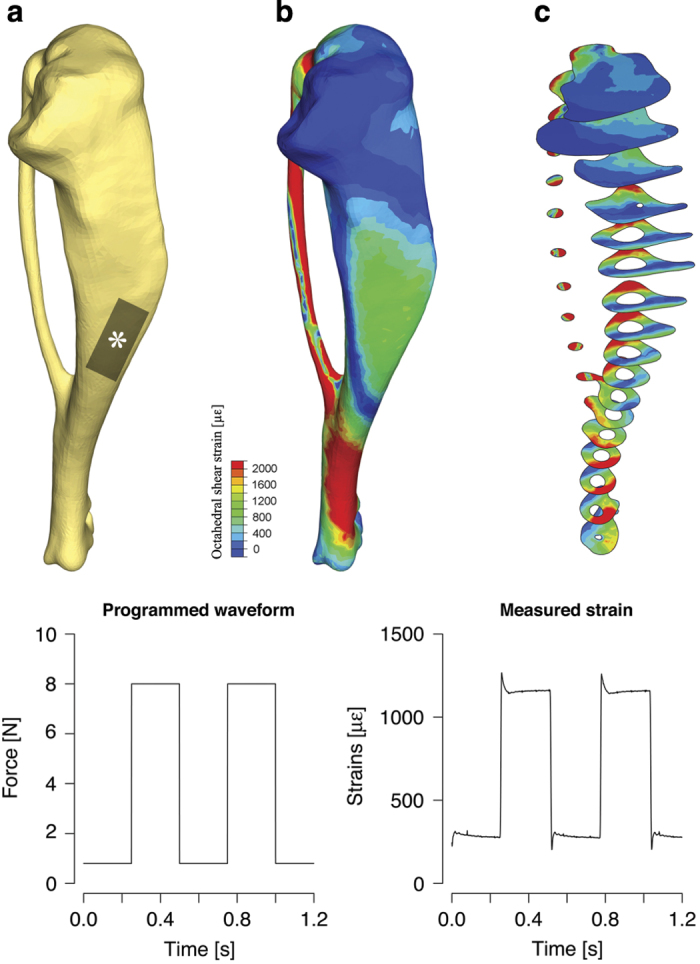
In homogeneities of spatial and temporal strains, distribution must be considered when designing an experiment using in vivo axial loading. (a) a typical shape of the mouse tibia (3D rendering of micro-computed tomography scan). The various bends of the tibia deflect forces under axial loading, resulting in an inhomogeneous spatial distribution of strains, as shown with finite-element models: Strains are distributed inhomogeneously over the tibia surface (b), but also within a single cross-section (c). (Bottom) Comparison between programmed waveform and the actual strains waveform measured locally (position of strain gage shown with *, as described in Akhter et al.17
Another useful script to implement is the soft landing. This routine moves the actuator's axis until contact with the animal's knee slowly with very limited force and then applies a defined preload. Precise force and speed control eliminate any damage to the knee.4 We typically use 10% of the maximum load for preload, but it is a rule of thumb.
Practical protocol for in vivo axial loading
Axial loading of the mouse tibia is applied through the knee and ankle joint of an anesthetized animal. For small loads (below 8 N for a C57BL6 mouse for example), simple isoflurane anesthesia is adequate, but for higher loads it is advised to add analgesia. For intense load regimes or fatigue loading (both induce pain), we recommend the following protocol: Induction in a box with 5% isoflurane, analgesia with Methadone (Methadon Streuli injectable solution 10 mg ml−1, Streuli Pharma AG, Switzerland) and Carprofen (Rimadyl ad us. vet., injectable solution, Zoetis Schweiz GmbH, Zürich, Switzerland), respectively, 5 mg per kg body weight (BW) intraperitoneally and 5 mg per kg BW subcutaneously 30 min before loading, and maintenance at 1.5–2% isoflurane via mask during loading. Other anesthesia options were well described by Sophocleous and Idris.37 The mouse is placed on a heat pad during the experiment to prevent heat loss. One loading cycle is performed to verify the stability of the tibia and the pain level of the animal. Anesthesia level and/or analgesia are adapted until the animal does not react to loading anymore. Only then, the full procedure starts.
Fatigue loading experiments require specific attention. In general, the goal of in vivo fatigue loading is to generate fatigue microdamage to the tibia without full fracture. However, accumulation of microdamage leads inevitably to failure after some time, and the specific moment or cycle number when it happens is unpredictable. For example, we used six female adult C57BL6 loaded at 1.5–15 N, 1.5 Hz, triangle waveform, and the fractures happened between 300 cycles for the shortest and 15 000 cycles for the longest (unpublished data). Luckily, the occurrence of fracture is generally preceded by a rapid loss of stiffness. Therefore, to prevent fracture occurrence, the program can be set to constantly monitor the tibial deformation and to stop when it reaches a preset limit. These limits are defined empirically: for example, 30%38,39 to 60% of the deformation.40 We use a limit at +40% of tibia deformation at 400 cycles, and never experienced full fractures in vivo so far.
Finally, for postprocessing of the bones (micro-computed tomography, histology), it is important to remember that strain distributions in the tibia are inhomogeneous (Figure 4) and that they vary between animals in function of their specific sizes, shapes and mechanical properties. Intense periosteal bone formation following mechanical loading occurs primarily in regions where strains are high, and lower levels of bone formation (or even resorption) occur where strains are low. Therefore, we strongly suggest scanning the tibias with micro-computed tomography and to refer to finite element simulations of whole tibia under loading to define regions of interest before postprocessing.
Advantages and pitfalls
Exercise running
Running exercise on treadmill integrates all the physiological effects of exercise observed in humans:1 direct impact on bone, through mechanical stimulus translated into biological signals by mechanoreceptors;2 indirect impact on bone through improvement of muscle mass and strength; and/or by inducing changes in hormonal levels (calciotropic hormone) and energetic metabolism (adipokine, myokine and so on).
However, treadmill running is not an ideal model to investigate the direct bone signaling pathways following mechanical loading, as it integrates several confounding factors coming from fat, muscle, liver metabolism and so on. To our best knowledge, nobody was so far able to discriminate the effect of loading from the metabolic component during physical activity. However, several studies have already demonstrated a direct correlation between strain gradients (measured with strain gages in situ) and levels of bone formation (measured with calcein labeling) in adult roosters after high-speed running,41,42 which supports the existence of a direct effect of loading during running. Such direct strain measurements may, however, not yet be feasible in mice, as currently available strain gages and electronics are so cumbersome that they would perturb the animal.20,21,23 Once miniaturized strain gage systems will be available, such a direct link may be experimentally observed in mice too.
The fundamental disadvantage of treadmill running is that bone strains are not controlled; hence, comparisons between experiments are difficult even if the same protocol of exercise is applied. Treadmill running has practical constraints. Larger numbers of animals are necessary to compensate for the excluded noncompliant animals. These exclusions are, in a sense, a selection bias because only the compliant subpopulation is studied. Finally, the acclimation period and the running sessions are very time-consuming, as supervision is required.
Axial compression
In vivo axial loading is a good model to investigate direct signaling pathways of bone mechanotransduction, as strains can be perfectly controlled, and even mapped spatially. The various parameters of loading can even be varied independently. It has been shown that bone response to in vivo loading is purely local;43 therefore, the contralateral bone can be used as internal control, which improves statistics without the need for larger groups.
However, in vivo axial loading is also subject to confounding factors—for example, metabolic effects of repeated anesthesia and analgesia, or knee osteoarthritis following overload.44 In addition, the concepts are not transposable to the rat tibia, as the loads that are necessary to generate osteogenic strains in rat tibia would cause soft tissue injuries at the contact points. Finally, the clinical relevance of in vivo axial loading is quite limited, as it does not encompass twisting, muscle function or fat metabolism known to actively regulate bone metabolism particularly in response to exercise.
Conclusion
Treadmill running and in vivo axial loading are two powerful techniques to induce mechanical stimuli in murine bones. Both methods have their advantages and limitations, and this paper should help the reader choose a model and design a study. We tried our best to give the reader the necessary information (animal handling, material sources, literature references) to get started and to set up similar experiments in a laboratory. Such a publication, with more practical details than a research manuscript, is a step toward standardization of procedures and reporting. It should hopefully increase reproducibility between research groups.
Supplementary Material
Acknowledgments
We thank Dr Dieudonne for his assistance in realizing the video and M Colas Renald in video editing (http://www.renaldcolas.fr/,e-mail: colas.renald@gmail.com).
Footnotes
The authors declare no conflict of interest.
References
- Lee TC, Taylor D. Bone remodelling: should we cry Wolff? Ir J Med Sci 1999; 168: 102–105. [DOI] [PubMed] [Google Scholar]
- Robling AG, Burr DB, Turner CH. Skeletal loading in animals. J Musculoskelet Neuronal Interact 2001; 1: 249–262. [PubMed] [Google Scholar]
- Frost HM. Bone's mechanostat: A 2003 update. Anat Rec 2003; 275A: 1081–1101. [DOI] [PubMed] [Google Scholar]
- Rubin J, Rubin C, Jacobs CR. Molecular pathways mediating mechanical signaling in bone. Genes Dev 2006; 367: 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnet N, Ferrari SL. Exercise and the skeleton: how it works and what it really does. IBMS BoneKEy 2010; 7: 235–248. [Google Scholar]
- Bourrin S, Ghaemmaghami F, Vico L, Chappard D, Gharib C, Alexandre C. Effect of a five-week swimming program on rat bone: a histomorphometric study. Calcif Tissue Int 1992; 51: 137–142. [DOI] [PubMed] [Google Scholar]
- Notomi T, Okazaki Y, Okimoto N, Saitoh S, Nakamura T, Suzuki M. A comparison of resistance and aerobic training for mass, strength and turnover of bone in growing rats. Eur J Appl Physiol 2000; 83: 469–474. [DOI] [PubMed] [Google Scholar]
- Mori T, Okimoto N, Sakai A, Okazaki Y, Nakura N, Notomi T et al. Climbing exercise increases bone mass and trabecular bone turnover through transient regulation of marrow osteogenic and osteoclastogenic potentials in mice. J Bone Miner Res 2003; 18: 2002–2009. [DOI] [PubMed] [Google Scholar]
- Styner M, Thompson WR, Galior K, Uzer G, Wu X, Kadari S et al. Bone marrow fat accumulation accelerated by high fat diet is suppressed by exercise. Bone 2014; 64: 39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold JC, Salvatore MF. Getting to compliance in forced exercise in rodents: a critical standard to evaluate exercise impact in aging-related disorders and disease. J Vis Exp 2014; 90: 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnet N, Standley KN, Bianchi EN, Stadelmann V, Foti M, Conway SJ et al. The matricellular protein Periostin is required for Sclerostin inhibition and the anabolic response to mechanical loading and physical activity. J Biol Chem 2009; 284: 35939–35950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu H, Desvergne B, Ferrari S, Bonnet N. Impaired musculoskeletal response to age and exercise in PPARβ-/- diabetic mice. Endocrinology 2014; 155: 4686–4696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menzies P, Menzies C, McIntyre L, Paterson P, Wilson J, Kemi OJ. Blood lactate clearance during active recovery after an intense running bout depends on the intensity of the active recovery. J Sports Sci 2010; 28: 975–982. [DOI] [PubMed] [Google Scholar]
- Kelly SA, Nehrenberg DL, Hua K, Garland Jr T, Pomp D. Exercise, weight loss, and changes in body composition in mice: phenotypic relationships and genetic architecture. Physiol Genomics 2011; 43: 199–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenmoehl J, Albrecht E, Komolka K, Schering L, Langhammer M, Hoeflich A et al. Irisin is elevated in skeletal muscle and serum of mice immediately after acute exercise. Int J Biol Sci 2014; 10: 338–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner CH, Akhter MP, Raab DM, Kimmel DB, Recker RR. A noninvasive, in vivo model for studying strain adaptive bone modeling. Bone 1991; 12: 73–79. [DOI] [PubMed] [Google Scholar]
- Akhter M, Cullen D, Pedersen E, Kimmel D, Recker R. Bone response to in vivo mechanical loading in two breeds of mice. Calcif Tissue Int 1998; 63: 442–449. [DOI] [PubMed] [Google Scholar]
- Gross TS, Srinivasan S, Liu CC, Clemens TL, Bain SD. Noninvasive loading of the murine tibia: an in vivo model for the study of mechanotransduction. J Bone Miner Res 2002; 17: 493–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrance AG, Mosley JR, Suswillo RF, Lanyon LE. Noninvasive loading of the rat ulna in vivo induces a strain-related modeling response uncomplicated by trauma or periostal pressure. Calcif Tissue Int 1994; 54: 241–247. [DOI] [PubMed] [Google Scholar]
- Lee K, Maxwell A, Lanyon L. Validation of a technique for studying functional adaptation of the mouse ulna in response to mechanical loading. Bone 2002; 31: 407–412. [DOI] [PubMed] [Google Scholar]
- De Souza R, Matsuura M, Eckstein F, Rawlinson S, Lanyon L, Pitsillides A. Non-invasive axial loading of mouse tibiae increases cortical bone formation and modifies trabecular organization: a new model to study cortical and cancellous compartments in a single loaded element. Bone 2005; 37: 810–818. [DOI] [PubMed] [Google Scholar]
- Zacchetti G, Wiskott A, Cugnoni J, Botsis J, Ammann P. External mechanical microstimuli modulate the osseointegration of titanium implants in rat tibiae. Biomed Res Int 2013; 2013: 234093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama T, Meakin LB, Browne WJ, Galea GL, Price JS, Lanyon LE. Bones' adaptive response to mechanical loading is essentially linear between the low strains associated with disuse and the high strains associated with the lamellar/woven bone transition. J Bone Miner Res 2012; 27: 1784–1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama T, Saxon LK, Zaman G, Moustafa A, Sunters A, Price JS et al. Mechanical loading enhances the anabolic effects of intermittent parathyroid hormone (1-34) on trabecular and cortical bone in mice. Bone 2008; 43: 238–248. [DOI] [PubMed] [Google Scholar]
- Stadelmann V, Bonnet N, Pioletti DP. Combined effects of zoledronate and mechanical stimulation on bone adaptation in an axially loaded mouse tibia. Clin Biomech 2011; 26: 101–105. [DOI] [PubMed] [Google Scholar]
- Stadelmann VA, Hocke J, Verhelle J, Forster V, Merlini F, Terrier A et al. 3D strain map of axially loaded mouse tibia: a numerical analysis validated by experimental measurements. Comput Methods Biomech Biomed Engin 2008; 12: 95–100. [DOI] [PubMed] [Google Scholar]
- Meakin LB, Price JS, Lanyon LE. The contribution of experimental in vivo models to understanding the mechanisms of adaptation to mechanical loading in bone. Front Endocrinol 2014; 5: 154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor JA, Lanyon LE, MacFie H. The influence of strain rate on adaptive bone remodelling. J Biomech 1982; 15: 767–781. [DOI] [PubMed] [Google Scholar]
- Prasad J, Wiater BP, Nork SE, Bain SD, Gross TS. Characterizing gait induced normal strains in a murine tibia cortical bone defect model. J Biomech 2010; 43: 2765–2770. [DOI] [PubMed] [Google Scholar]
- Rubin CT, Lanyon LE. Regulation of bone formation by applied dynamic loads. J Bone Joint Surg Am 1984; 66: 397–402. [PubMed] [Google Scholar]
- Kennedy OD, Herman BC, Laudier DM, Majeska RJ, Sun HB, Schaffler MB. Activation of resorption in fatigue-loaded bone involves both apoptosis and active pro-osteoclastogenic signaling by distinct osteocyte populations. Bone 2012; 50: 1115–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robling AG, Burr DB, Turner CH. Partitioning a daily mechanical stimulus into discrete loading bouts improves the osteogenic response to loading. J Bone Miner Res 2000; 15: 1596–1602. [DOI] [PubMed] [Google Scholar]
- Saxon LK, Robling AG, Alam I, Turner CH. Mechanosensitivity of the rat skeleton decreases after a long period of loading, but is improved with time off. Bone 2005; 36: 454–464. [DOI] [PubMed] [Google Scholar]
- Shi H-F, Cheung W-H, Qin L, Leung AH-C, Leung K-S. Low-magnitude high-frequency vibration treatment augments fracture healing in ovariectomy-induced osteoporotic bone. Bone 2010; 46: 1299–1305. [DOI] [PubMed] [Google Scholar]
- Pounder NM, Harrison AJ. Low intensity pulsed ultrasound for fracture healing: a review of the clinical evidence and the associated biological mechanism of action. Ultrasonics 2008; 48: 330–338. [DOI] [PubMed] [Google Scholar]
- Manske SL, Good CA, Zernicke RF, Boyd SK, Garatachea N. High-frequency, low-magnitude vibration does not prevent bone loss resulting from muscle disuse in mice following botulinum toxin injection. PLoS ONE 2012; 7: e36486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sophocleous A, Idris AI. Rodent models of osteoporosis. BoneKEy Rep 2014; 3: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnet N, Gineyts E, Ammann P, Conway SJ, Garnero P, Ferrari S. Periostin deficiency increases bone damage and impairs injury response to fatigue loading in adult mice. PLoS ONE 2013; 8: e78347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danova NA, Colopy SA, Radtke CL, Kalscheur VL, Markel MD, Vanderby R et al. Degradation of bone structural properties by accumulation and coalescence of microcracks. Bone 2003; 33: 197–205. [DOI] [PubMed] [Google Scholar]
- Hsieh Y-F, Silva MJ. In vivo fatigue loading of the rat ulna induces both bone formation and resorption and leads to time-related changes in bone mechanical properties and density. J Orthop Res 2002; 20: 764–771. [DOI] [PubMed] [Google Scholar]
- Judex S, Gross TS, Zernicke RF. Strain gradients correlate with sites of exercise-induced bone-forming surfaces in the adult skeleton. J Bone Miner Res 1997; 12: 1737–1745. [DOI] [PubMed] [Google Scholar]
- Gross TS, Edwards JL, McLeod KJ, Rubin CT. Strain gradients correlate with sites of periosteal bone formation. J Bone Miner Res 1997; 12: 982–988. [DOI] [PubMed] [Google Scholar]
- Sugiyama T, Price JS, Lanyon LE. Functional adaptation to mechanical loading in both cortical and cancellous bone is controlled locally and is confined to the loaded bones. Bone 2010; 46: 314–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christiansen BA, Anderson MJ, Lee CA, Williams JC, Yik JH, Haudenschild DR. Musculoskeletal changes following non-invasive knee injury using a novel mouse model of post-traumatic osteoarthritis. Osteoarthritis Cartilage 2012; 20: 773–782. [DOI] [PubMed] [Google Scholar]
- Kuruvilla S, Fox S, Cullen D, Akhter M. Site specific bone adaptation response to mechanical loading. Journal Musculoskelet Neuronal Interact 2008; 8: 71–78. [PubMed] [Google Scholar]
- Moustafa A, Sugiyama T, Prasad J, Zaman G, Gross TS, Lanyon LE et al. Mechanical loading-related changes in osteocyte sclerostin expression in mice are more closely associated with the subsequent osteogenic response than the peak strains engendered. Osteoporos Int 2012; 23: 1225–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weatherholt AM, Fuchs RK, Warden SJ. Cortical and trabecular bone adaptation to incremental load magnitudes using the mouse tibial axial compression loading model. Bone 52: 372–3792013; . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch ME, Main RP, Xu Q, Schmicker TL, Schaffler MB, Wright TM et al. Tibial compression is anabolic in the adult mouse skeleton despite reduced responsiveness with aging. Bone 2011; 49: 439–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman AG, Clauser CA, Wunderlin C, Hammond MA, Wallace JM. Structural and mechanical improvements to bone are strain dependent with axial compression of the tibia in female C57BL/6 mice. PLoS ONE 2015; 10: e0130504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodt MD, Silva MJ. Aged mice have enhanced endocortical response and normal periosteal response compared with young-adult mice following 1 week of axial tibial compression. J Bone Miner Res 2010; 25: 2006–2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva MJ, Brodt MD, Lynch MA, Stephens AL, Wood DJ, Civitelli R et al. Tibial loading increases osteogenic gene expression and cortical bone volume in mature and middle-aged mice. PLoS ONE 2012; 7: e34980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holguin N, Brodt MD, Sanchez ME, Kotiya AA, Silva MJ. Adaptation of Tibial Structure and Strength to Axial Compression Depends on Loading History in Both C57BL/6 and BALB/c Mice. Calcif Tissue Int 2013; 93: 211–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.