Commentary on: Worthley DL et al.1 `Gremlin 1 identifies a skeletal stem cell with bone, cartilage, and reticular stromal potentia' and Chan CKF et al.2 `Identification and Specification of the Mouse Skeletal Stem Cell'.
In no small part due to the popularity of so-called ‘mesenchymal stem cells' or MSCs as a potential therapeutic for a panoply of diseases (313 open clinical trials worldwide, clinicaltrials.gov, accessed on October 2015), it is commonly accepted that skeletal tissues (fat, cartilage and bone) arise from a common progenitor or stem cell. This single-origin stem cell concept is primarily derived from the paradigm of the organization of the hematopoietic system, where one cell can generate the entirety of the blood/immune cell systems through branching linear pathways. Although the hematopoietic system is often held as a model for stem cell hierarchy, a number of functional differences exist between hematopoiesis and skeletal differentiation that might suggest an alternate stem cell model where discrete individualized skeletal progenitor cells yield endochondral bone, intramembranous bone, cartilage and fat, as well as being critical contributors to specialized niche sites for hematopoietic stem cells and various hematopoietic progenitor populations. However, despite a number of proposed stem cell identities and models, elucidation of differentiation control mechanisms and the identity of skeletal stem cell populations in the bone has remained challenging.1,2,3,4,5,6,7 Two papers by Worthley et al.1 and Chan et al.2 in a recent issue of Cell help to advance the skeletal field by providing novel candidates for the skeletal stem cell in vivo and insight into the structure and control of skeletal stem cell hierarchy.
A multipotent bone marrow progenitor cell population was suggested by Freidenstein's work8 and later clearly defined to have self-renewal by Sacchetti et al. in the CD146-expressing human cell population.9 In the mouse, efforts by Méndez-Ferrer et al.4 with Nestin-expressing cells and Sean Morrison's group with Leptin receptor-expressing cells (LepR+)3,10 identified markers that fulfilled the definition of an in vivo skeletal stem cell (SSC) and played a critical role in hematopoietic stem cell maintenance and health. However, Nestin-expressing cells have not been proven to yield single-cell multipotency or self-renewal in postnatal mice.4 In young adult mice, robust leptin receptor expression was limited to perivascular (sinusoidal and arterial) stromal cells and not to more differentiated mesenchymally derived cells – including Col2.3-green fluorescent protein osteoblastic cells, Aggrecan+ cartilage cells or Perilipin+ bone marrow fat cells.3 Although fate mapping cells derived from these LepR+ cells using Lepr-cre reporter mice showed very limited numbers of osteoblasts/osteocytes that originated from them at 2 months, the numbers markedly increased with age, especially in trabecular bone. Likewise they saw LepR+ cells contributing to the bone marrow adipocyte population, also increasing in percentage with age. However, they saw little contribution of LepR+ cells to normal cartilage development even with aging.3,10,11 Additional candidate markers such as Mx1-Cre,6 Osx-Cre11 or Prx1-Cre/CXCL1212 have been promising as enriched clonal populations or hematopoiesis maintaining sites but do not fulfill the in vivo requirements of skeletal progenitor cells.
To overcome these challenges, the recent pair of papers in Cell approached the identification and characterization of SSCs from disparate but complimentary angles. Worthley et al.1 used the secreted bone morphogenetic protein (BMP) family antagonist, and vascular endothelial growth factor receptor-2 (VEGFR2) agonist, Gremlin-1 (Grem1), to label their putative skeletal progenitor cells. Gremlin-1 is important in bone formation, and as an antagonist for the cell proliferation and differentiation regulating BMPs it is a uniquely functional choice for a skeletal progenitor cell gene. In contrast, Chan et al.2 used a morphological approach to identify long bone sites of clonal generation where skeletal progenitor cell activity could be inferred. They identified SSCs in these locations based on cell surface marker expression and microarray analysis of osteoblastic Alpha V integrin-expressing stem cells and identified eight distinct subpopulations of SSC with different developmental fates. They also evaluated secreted factors for the directed differentiation and reprogramming of SSCs and committed osteo-progenitors.
The stem cell population Worthley et al. have identified does not itself fulfill the requirement to be the earliest multipotent mesenchymal stem cell, as it does not normally differentiate into an adipogenic lineage. However, it is a SSC that is in line with an earlier concept from Gerard Karsenty's group of an osteochondroprogenitor cell.13 As Grem1 cells also differentiate into stromal reticular cells and self-renew, they named them osteochondroreticular (OCR) cells. Worthley et al. present convincing evidence that their Grem1-derived OCR cell population is not only expressed developmentally in endochondral cartilage and bone over time but also has contributions to bone healing and the ability to self renew after serial transplantation. They further proposed that Grem1-derived cells might contribute to local MSC-like stem cells in peripheral tissues and tested this idea by evaluating their role in intestinal crypts where a sheath of MSC-like cells is located. Worthley et al. frame their search for the SSC in the context of a gene of known importance in cellular differentiation and are able to trace the in vivo differentiation of the cells through the long bones. However, despite exhibiting single-cell multi-lineage potential and much higher clonogenicity than nestin-expressing cells, this approach to identifying a SSC remains limited, as it only positively labels a small subset of the total cells that differentiate into bone and contribute to healing.
Morphological approach by Chan et al. first found the long bone stem cell niche using rainbow clone generation. Noting that the bulk of clonal formation occurs in the growth plate, the authors isolate cells from this location and use a series of surface receptor expression markers and functional outcomes to define distinct populations of cells. However, they were unable to identify the in vivo location of their surface receptor-defined SSC populations versus the more committed progenitors. In contrast, Worthley et al could clearly locate Grem1 OCRs in a region that was distinct from previously reported perisinusoidal SSCs.1,2,3,4,10,12 They then used gene expression microarrays to define secreted factor receptors that would be differential for these populations and could have a role in regulating the SSC behavior. By taking a population-based approach, they identified BMP-2 as a secreted factor that could expand the SSC population and VEGF as a factor whose inhibition pushed committed bone-directed progenitor cells to a cartilage-forming fate in vivo. They were able to achieve cartilage formation ectopically by coadministration of BMP-2 and a VEGF-inhibitor, which is relevant given Grem1's antagonism of BMP-2 and agonist action on VEGFR2 driving osteogenesis.1,2 Interestingly, the Thy+ progenitor (Thy) and B-cell lymphocyte stromal progenitor (BLSP) SSC populations described by Chan et al. express noggin and Thy expresses Gremlin 2. Both molecules are BMP family antagonists, whereas Gremlin 2, like Grem1, is a VEGFR agonist. This potentially links them to Grem1 OCR cells of Worthley et al. (Figure 1a). In addition, the Thy SSC population has striking similarities to the LepR+ and CXCL12-abundant reticular (CAR) SSC populations and may share identity or represent separate SSC populations that overlap with each other (Figure 1b). For example, Thy, LepR+ and CAR SSCs are involved in hematopoietic stem cell (HSC) niche site maintenance and express high levels of CXCL12, which is required for BMP-2 receptor recognition of its ligand BMP-2 and activation, and also express the leptin receptor.3,6,12,14 The perisinusoidal CAR cells are critical to osteoprogenitor generation, which is in line with the emerging osteogenic roles for the LepR+ and Thy populations.3,6,12,14 In contrast, the Grem1 SSCs appear to be distinct from LepR+ or CAR SSCs in part based on their non-sinusoidal niche location in the bone marrow (Figure 1b). In addition, it is not clear whether Grem1 SSCs are involved in the HSC niche or express significant levels of CXCL12, which has been proposed to be associated with early mesenchymal progenitors.3,6,12 As such further study is needed to clarify the relationship of these proposed SSCs to each other during development and growth and aging. Although these autocrine and paracrine factors have been extensively researched for their cell differentiation roles, Chan et al.'s morphological and microarray-based approach provides methodological rigor and initial steps to viewing SSC differentiation as a systems biology problem with multiple interacting components. Overall, their approach of seeking functional and surface receptor-based identification of an adult stem cell population and identification of multiple discrete SSC progenitors derived from it with distinct niches and functions that were supported by subsequent investigation into signaling pathways involved with stem cell niche maintenance and differentiation, points the field to a more holistic view of the study of the bone.
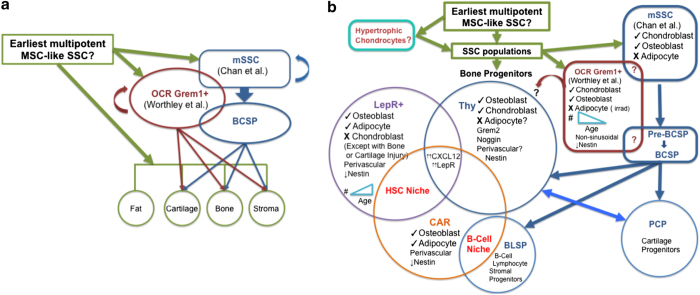
Figure 1.
(a) Shows the possible overlap of OCR Grem1 and BCSP Progenitor populations. Cells derived from Gremlin-1-expressing osteochondroreticular (OCR Grem1+) cells give rise to a subset of chondrogenic and osteogenic cells early in development but appear to decline with age. Mouse skeletal stem cells (mSSC) also give rise to a subset of chondrogenic and osteogenic cells. They appear to give rise to distinct populations of SSCs that may occupy different bone marrow (BM) niches first differentiating into intermediate potential progenitor pools, the pre-bone, cartilage and stromal progenitors (pre-BCSPs), then the Bone, Cartilage and Stromal Progenitors (BCSPs). In turn, the BCSPs are proposed to give rise to two stromal cell progenitor populations and other SSC progenitors that give rise to bone, cartilage and fat cells. On the basis of the cell fate profiles and molecular expression, there may be an overlap between some of these newly identified progenitor subsets. (b) Shows the potential relationships between different Skeletal Stem Cell (SSC) populations and their cell fates. The BCSP progenitor population gives rise to a pro-chondrogenic progenitor (PCP), a B-cell lymphocyte stromal progenitor (BLSP), which appears to be important for the B-cell niche, and an osteogenic Thy+ progenitor (Thy) that can be pushed to shift to a PCP phenotype and generate chondrogenic cells. The CXCL12-abundant reticular (CAR) SSC progenitors are known to be osteogenic and adipogenic and are involved in two distinct stem/progenitor cell niches the hematopoietic stem cell (HSC) niche and the B-cell progenitor niche. The osteogenic and adipogenic progenitor populations derived from leptin receptor-expressing cells (LepR+) are also seen in the HSC niche and may overlap with the CAR progenitor, and even the Thy progenitor, populations, and, in contrast to the OCR Grem1+-derived cells, their numbers increase with age. It is unclear whether cells derived from hypertrophic chondrocytes contribute to any of the other progenitor populations.
Importantly, although the SSC populations identified by different groups seem to be limited in potency under normal development, and homeostasis, in pathogenic situations such as fracture repair, aging and other injuries, these potency limitations may not be inflexible. For example, following irradiation Grem1+-derived cells that normally are not fated to become adipocytes can do so. However, there is an open question about why the distinct SSC progenitor subsets that Chan et al. identified, like the Grem1-derived cells identified by Worthley et al., do not seem to be able to normally form adipocytes or myocytes. This is curious given that the Thy SSC line appears to be quite similar to the LepR+ and CAR cells, which can form adipocytes (Figure 1b). Therefore, it would be of interest to see whether following irradiation, or with age, adipocytes could be derived from the Thy SSCs or the other related SSC subsets. In an earlier report using Lepr-cre Col2.3 dual-labeled mice, Zhou et al.3 showed that LepR+-derived osteoblasts are rare in fetal bone but postnatally increased progressively with age, going from 3–10% at 2 months to up to and 81% at 14 months. The same type of age-associated increase in adipocytes was also seen. In contrast, although LepR+ cells did not give rise to chondrocytes developmentally, they did so after mechanical injury to articular cartilage and following tibial fracture in 2-month-old mice. LepR+-derived chondrocytes accounted for 46% of the soft callous chondrocytes 2 weeks out and 45% of osteoblasts (and 85% at 8 weeks) suggesting that they were critical in ‘adult' endochondral and intermembranous bone formation with injury.2 Therefore, this switch to a cartilage fate is not recapitulating the LepR+ development program with injury/repair but is resetting the fate of these cells to include a new fate, just as the Grem1 SSCs could become adipogenic following irradiation injury. This is in line with the observation of Chan et al. that SSC subsets with distinct skeletal fates, like PCP and Thy, could be shifted to a different fate based on the changes in niche ligand levels and SSC receptor function (Figure 1b). The early mouse SSC (mSSC) population identified by Chan et al. rapidly, but only transiently, increases by about 10-fold 1-week post fracture, presumably generating a number of different SSC progenitors with distinct fate potentials. However, what specific contributions the different mSSC-derived SSC lineages made to the repair process was not described. Though on the basis of the ability of the different SSC populations to form bone and cartilage in explant surgeries, the assumption is that they would also do so as part of the normal fracture repair process. It would be interesting to determine whether, in addition to the normally non-chondrogenic LepR+ SSCs, the CAR-like Thy+ SSCs, or other specific SSC lineages, give rise to callous chondrocytes and osteoblasts. Fracture of adult Grem1+ reporter mice 1 week after tamoxifen induction showed that the Grem1+ OCR cells contributed 28% of callous osteoblasts and 14% of chondrocytes. In line with the idea that committed SSCs can alter their ‘normal' fate, Chan et al. demonstrated that manipulation of the BMP-2 and VEGF signaling pathways could push chondrogenic fated SSCs to an osteogenic path and bone committed SSCs to form cartilage. This suggests that, although there may be multiple distinct SSC lineages to meet numerous stem and progenitor cell niche maintenance needs together with the needs to provide committed skeletal progenitors to give rise to bone, cartilage and adipose cells, the SSCs may actually be dynamic and plastic in response to changes in their niche environments. Therefore, the boundaries between different SSCs, and their potencies, may be alterable as part of normal skeletal homeostasis and following aging, injury or disease.
Worthley et al. propose that separate SSC progenitors develop and maintain skeletal tissues in a temporal-spatial and lineage-specific manner. This could include the Grem1+ OCR stem cells as one of the critical cells during development, postnatally and into adulthood providing homeostatic regulation. However, one or more SSCs, such as the LepR+, or CAR cells, take on an increasing larger role with age. mSSC-derived SSC subpopulations of Chan et al. may also fit with this concept. This harkens back to the Frenette group's idea of distinct waves of SSCs at different stages of bone development.11 Chan et al. also propose that changes in the SSC niche microenvironment can regulate skeletal progenitor fates by influencing lineage commitment into bone or cartilage, including the differentiation of one SSC population into another. As such inappropriate ossification, or chondrogenesis, or blockade of their appropriate induction could occur. This emerging paradigm of multiple parallel, and linked, SSCs populations with distinct temporal-spatial patterns and function has important therapeutic implications. For example, if more clonogenic and robust chondrogenic and osteogenic Grem1+-derived SSC populations decrease with age, whereas more adipogenic and less clonogenic LepR+ (or CAR or Thy+ SSC) populations increase, then overall bone and cartilage homeostasis may be disrupted. In bone repair following a fracture multiple SSC progenitor populations may normally be enlisted (for example, Grem1, mSSC-derived SSCs like Thy, and PCP, LepR+ or CAR), each having overlapping but different cell fate and functionality. This could change with age and as a consequence such a shift in the SSC population profiles might explain the impairment in fracture healing seen with age. This could be a critical factor in osteoporosis, osteoarthritis and other age-associated skeletal pathologies.
To finally clarify, if there is a postnatal SSC that can give rise to all of the SSC progenitors, or if the different progenitors can switch between types with aging, pathologies or changing niche environments, and to get a better comparison to determine the relationships between the different identified SSCs will require a better understanding of in vitro and in vivo conditions and parameters used by investigators. For instance, what are the consequences of different SSC isolation methods (for example, BM flush, explant or tissue digestion, classic adherent plating with negative and positive selection, or direct surface marker targeting using FACS or antibody-based pull down),15 culture conditions including oxygen tension (low niche O2 levels vs room O2 level), extracellular matrix coatings that control integrin and other receptor-mediated signaling/gene expression, and fetal bovine serum (FBS) percentage or defined media in distinguishing putative SSCs? Worthley et al. point out that they used standard adherent culture conditions (MEMα, 10% FBS and room O2 levels), whereas others do not, and that this might explain discrepancies between labs and the differences in the SSC populations and their fates. Indeed Chan et al. used media similar to Worthley's for their different SSCs but cultured them at the 2% MSC niche O2 level, which is known to alter gene expression and function with significant impacts on proliferation and differentiation on MSC-like cells.16 Further, in a fascinating twist the source of the SSC progenitor populations may now also include CXCL12-expressing hypertrophic chondrocytes derived from the epiphyseal growth plate.17,18 These cells instead of dying may dedifferentiate into SSCs, which then appear to redifferentiate into osteoblasts that can contribute to a significant portion of trabecular and cortical bone, not only throughout development but also in adult bone formation, including fracture repair. This is particularly of interest given the identification by Chan et al. of the growth plate as being a major source of the clonal populations that give rise to bone, cartilage and stromal tissue.7
These complimentary studies define novel functional markers for skeletal progenitor cell populations and provide initial steps to understand the cell populations involved in adult bone homeostasis and fracture repair. Interestingly, they both find SSC populations that do not efficiently generate adipose tissue (Figure 1a). Their findings open a line of questioning about the possibility of a non-traditional hierarchy for bone generation, with multiple tissue-specific SSCs in adult bone. In addition, the intersection of their findings with BMP and VEGF signaling serves to highlight the importance of these pathways as central to stem cell proliferation and differentiation. As the understanding of SSC in vivo location, proliferation and differentiation into bone and cartilage continues to improve, there is greater potential to understand diseases of the skeleton and to design enhanced therapies for bone and cartilage defects.
Footnotes
The authors declare no conflict of interest.
References
- Worthley DL, Churchill M, Compton JT, Tailor Y, Rao M, Si Y et al. Gremlin 1 identifies a skeletal stem cell with bone, cartilage, and reticular stromal potential. Cell 2015; 160: 269–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan CKF, Seo EY, Chen JY, Lo D, McArdle A, Sinha R et al. Identification and Specification of the Mouse Skeletal Stem Cell. Cell 2015; 60: 285–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou BoO, Yue R, Murphy Malea M, Peyer JG, Morrison Sean J. Leptin-receptor-expressing mesenchymal stromal cells represent the main source of bone formed by adult bone marrow. Cell Stem Cell. 2014; 15: 154–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Méndez-Ferrer S, Michurina TV, Ferraro F, Mazloom AR, Macarthur BD, Lira SA et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature 2010; 466: 829–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinho S, Lacombe J, Hanoun M, Mizoguchi T, Bruns I, Kunisaki Y et al. PDGFRα and CD51 mark human nestin+ sphere-forming mesenchymal stem cells capable of hematopoietic progenitor cell expansion. J Exp Med 2013; 210: 1351–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutu DL, François M, Galipeau J. Inhibition of cellular senescence by developmentally regulated FGF receptors in mesenchymal stem cells. Blood 2011; 117: 6801–6812. [DOI] [PubMed] [Google Scholar]
- Park D, Spencer JA, Koh BI, Kobayashi T, Fujisaki J, Clemens TL et al. Endogenous bone marrow MSCs are dynamic, fate-restricted participants in bone maintenance and regeneration. Cell Stem Cell. 2012; 10: 259–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen M, Friedenstein AJ. Stromal stem cells: marrow-derived osteogenic precursors. Ciba Found Symp 1988; 136: 42–60. [DOI] [PubMed] [Google Scholar]
- Sacchetti B, Funari A, Michienzi S, Di Cesare S, Piersanti S, Saggio I et al. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell 2007; 131: 324–336. [DOI] [PubMed] [Google Scholar]
- Ding L, Morrison SJ. Haematopoietic stem cells and early lymphoid progenitors occupy distinct bone marrow niches. Nature 2013; 495: 231–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizoguchi T, Pinho S, Ahmed J, Kunisaki Y, Hanoun M, Mendelson A et al. Osterix marks distinct waves of primitive and definitive stromal progenitors during bone marrow development. Dev Cell 2014; 29: 340–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenbaum A, Hsu Y-MS, Day RB, Schuettpelz LG, Christopher MJ, Borgerding JN et al. CXCL12 in early mesenchymal progenitors is required for haematopoietic stem-cell maintenance. Nature 2013; 495: 227–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducy P, Zhang R, Geoffroy VR, Ridall AL, Karsenty Gr. Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation. Cell 1997; 89: 747–754. [DOI] [PubMed] [Google Scholar]
- Herberg S, Fulzele S, Yang N, Shi X, Hess M, Periyasamy-Thandavan S et al. Stromal cell-derived factor-1β potentiates bone morphogenetic protein-2-stimulated osteoinduction of genetically engineered bone marrow-derived mesenchymal stem cells in vitro. Tissue Eng Part A 2013; 19: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoshani O, Ravid O, Massalha H, Aharonov A, Ovadya Y, Pevsner-Fischer M et al. Cell isolation induces fate changes of bone marrow mesenchymal cells leading to loss or alternatively to acquisition of new differentiation potentials. Stem Cells 2014; 32: 2008–2020. [DOI] [PubMed] [Google Scholar]
- Pattappa G, Thorpe SD, Jegard NC, Heywood HK, de Bruijn JD, Lee DA. Continuous and uninterrupted oxygen tension influences the colony formation and oxidative metabolism of human mesenchymal stem cells. Tissue Eng Part C Methods 2013; 19: 68–79. [DOI] [PubMed] [Google Scholar]
- Zhou X, von der Mark K, Henry S, Norton W, Adams H, de Crombrugghe B. Chondrocytes transdifferentiate into osteoblasts in endochondral bone during development, postnatal growth and fracture healing in mice. PLoS Genet 2014; 10: e1004820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Tsang KY, Tang HC, Chan D, Cheah KSE. Hypertrophic chondrocytes can become osteoblasts and osteocytes in endochondral bone formation. Proc Natl Acad Sci USA 2014; 111: 12097–12102. [DOI] [PMC free article] [PubMed] [Google Scholar]