Abstract
Irisin was originally recognized as a hormone-like myokine secreted as a product of fibronectin type III domain containing 5 from skeletal muscle in response to exercise both in mice and humans. The first role attributed to Irisin was its ability to induce trans-differentiation of white adipose tissue into brown, but we recently demonstrated that Irisin also has a central role in the control of bone mass, even at lower concentration than required to induce the browning response. Considering how physical exercise is important for the development of an efficient load-bearing skeleton, we can now consider this myokine as one of the molecules responsible for the positive correlation between exercise and healthy bone, linking to the well-established relationship between muscle and bone. Recombinant Irisin (r-Irisin), administered at low dose in young mice, increases cortical bone mineral density and positively modifies bone geometry. Irisin exerts its effect prevalently on osteoblast lineage by enhancing differentiation and activity of bone-forming cells, through the increase in activating transcription factor 4 expression. Low-dose r-Irisin also increases osteopontin and decreases sclerostin synthesis but did not affect Uncoupling protein 1 expression in white adipose tissue, whose upregulation is known to cause browning of fat, when Irisin is administered at a higher dose. These findings offer an explanation to the positive outcome on the skeleton triggered by skeletal muscle during physical activity and prove that the bone tissue is more sensitive than the adipose tissue to the Irisin action.
Introduction
The benefits of exercise are widely recognized, so that physical activity is considered the best non-pharmacologic treatment for pathologies such as diabetes, cardiovascular disease, osteoporosis and obesity.1 Notably, it is extensively reported that physical exercise has beneficial effects on bone mineral density (BMD), in particular during childhood and adolescence.2 In adult, decrease in physical activity may lead to a progressive loss of bone mineral content, raising the incidence of osteoporotic fractures.3 Even worse, disuse and weightlessness can remarkably affect bone physiology: astronauts lose bone mass 10 times faster than women in early menopause;4 patients in vegetative state have high bone turnover and low BMD, which translates into a clinically relevant problem, as 20% of these patients develop spontaneous fractures.5
An existing intimate relationship between skeletal muscle and bone has been established. Thus, several studies indicated that higher muscle mass is closely related to increased BMD and reduced fracture risk in post-menopausal women. Conversely, age-related muscle loss may be the main factor causing age-associated bone loss.6 Furthermore, muscle and bone are simultaneously influenced by pathological states, such as glucocorticoid excess and vitamin D deficiency.7 Despite this close association between muscle and bone, the effect on the skeleton created by muscle contractions has been mainly explained as the ability of osteocytes, the bone antenna-cells, to perceive signals produced by mechanical stimulation and shear stress due to fluid flow.8
However, during the last decade, several lines of evidence have established that skeletal muscle is an endocrine organ producing and releasing myokines, which might be effectors of the positive outcome on the body triggered by physical activity. These myokines create a communication network in an autocrine/paracrine manner, not only within the muscle but also toward distant target tissues in an endocrine manner. For instance, it was recently found that contracting muscle cells secrete the metabolite β-aminoisobutyric acid, which mediates signaling processes in the white adipose tissue (WAT) and liver, inducing weight loss and increasing glucose tolerance in mice.9 Of note, the newly identified myokine Irisin, produced by skeletal muscle after physical exercise, has recently drawn the attention as candidate therapeutic target to treat metabolic diseases.10 Bostrom et al. observed that overexpression of PGC1α in mice muscle during exercise stimulated the production of the membrane protein fibronectin type III domain containing protein 5 (FNDC5), subsequently cleaved as Irisin and released to bloodstream.10 The authors showed that Irisin was able to induce the so-called ‘browning response'11,12,13,14 in WAT. During this phenomenon, a trans-differentiation program of white adipocytes15,16 or de novo beige/brite cell formation13,15,17,18 is induced by Irisin to shift from a WAT phenotype to a brown adipose tissue (BAT)-like phenotype, showing morphology, gene expression pattern and mitochondrial respiratory activity similar to those of classical BAT.10
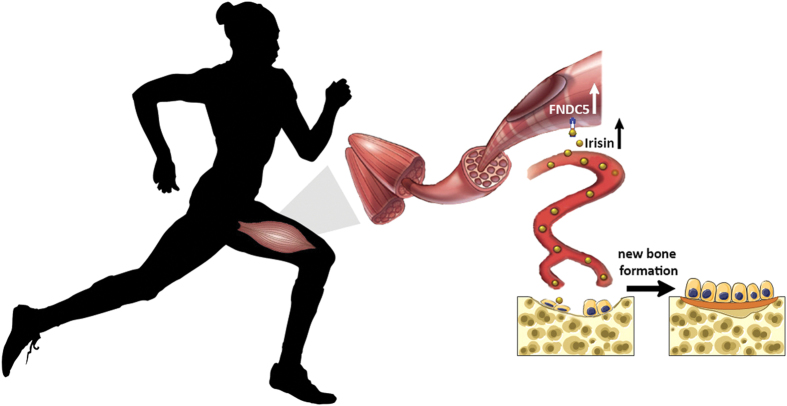
However, latest evidence has questioned the primary biological role of Irisin secretion by skeletal muscle. In fact, more recently, we demonstrated that Irisin also targets the bone tissue directly and, if administered at a lower dose than used to induce browning of white fat, it drives positive effects on cortical mineral density and improves bone strength and geometry in mice.19 This recent result highlighted a new biological significance of Irisin, which might be a molecular link responsible for the yet poorly characterized bone–muscle unit (Figure 1).
Figure 1.
The myokine Irisin, produced by skeletal muscle during physical activity, acts directly on osteoblasts by stimulating their differentiation and activity, thereby improving bone quality and strength.
Irisin, the messenger of healthy bone–muscle unit
The first evidence showing that the myokine Irisin was linked to bone and muscle connection arose from our first study in which we demonstrated that medium enriched of Irisin, synthesized by cultured skeletal muscle cells of exercised mice, was able to enhance the differentiation of bone marrow stromal cells into mature osteoblasts in vitro.20
More recently, the key evidence of a direct action of Irisin on bone has emerged by in vivo studies demonstrating that a low dose of recombinant Irisin (r-Irisin), which has no effect on fat, improves cortical bone mass and bone strength. We showed that tibiae of young male mice treated with r-Irisin exhibited a +7.15% increase in cortical BMD compared with those of mice injected with vehicle.19 Moreover, the administration of Irisin also changed cortical bone geometry, as observed by the increase in mineral apposition rate, bone formation rate, periosteal circumference and polar moment of inertia. The increase in BMD and the improvement of bone geometrical architecture are recognized parameters used to define high bone quality.21,22,23 Cross-sectional geometrical properties have been extensively used to measure long bone resistance to axial torsion. Recently, the polar moment of inertia was preferred rather than cross-sectional geometry because it better measures resistance of a long bone to torsion around a particular axis, taking into account the cross-sectional area but also the distribution of bone tissue around the neutral axis.24 Tibia of Irisin-treated mice showed a greater polar moment of Inertia (+19%) than control mice, indicating that Irisin treatment might maximize bone to become more structurally efficient for bending and torsion, in order to induce an optimal stress transfer and physical performance. Accordingly, three-point bending tests on tibiae of Irisin-treated mice showed that bending strength and energy to fracture were increased by +65% and +9.5%, respectively.19
Conversely, Irisin did not induce any change in trabecular bone, and this result was somewhat expected considering that young mice, used for this study, have a healthy trabecular pattern. However, this is also in line with previous literature data reporting that cortical bone is more sensitive to myokines or other anabolic factors released by muscle.25,26,27 Notably, change in the expression myostatin, also released from skeletal muscle, undergoes opposite direction to Irisin release following exercise, as it has been shown that myostatin signaling is inhibited by acute resistance exercise.28 In addition, myostatin knock out mice displayed BAT expansion, an effect mediated by the AMPK-PGC1α-FNDC5 pathway in skeletal muscle.29 As well recognized, myostatin, besides being a critical autocrine/paracrine inhibitor of skeletal muscle growth, negatively regulates bone metabolism.27 Accordingly, it is plausible that there may be an inverse relationship between Irisin and myostatin synthesis.
The action of irisin on bone-forming cells
The action of Irisin on osteoblast differentiation is receptor mediated, as Erk phosphorylation is stimulated within 5 min of Irisin application. The increased differentiation and activity of bone-forming cells was proved by upregulation of activating transcription factor 4 (Atf4) and, consequently, by enhancement of a number of alkaline phosphatase (ALP)-positive colonies and nodules of mineralized matrix. Consistently, ALP and collagen I mRNA expression were upregulated upon Irisin treatment in vitro.19
In vivo data showed higher Atf4 expression in bone marrow of Irisin-treated mice, suggesting a substantial commitment of osteoblast precursor toward osteogenesis.19 Accordingly, the expression of osteopontin (OPN), one of the most abundant bone matrix protein, was found higher in tibiae of mice treated with Irisin.19 In several studies, OPN expression was shown to be modulated in response to mechanical stimulation30,31 and, therefore, it has been defined as a mechanically responsive gene.32 Consistently with the loading-mimetic function of Irisin, this myokine might be the mediator of loading-induced increase in OPN expression.
Interestingly, the expression of sclerostin, one of the Wnt/β-catenin pathway inhibitors,33,34,35 was found strongly downregulated in tibiae of Irisin-treated mice.19 In agreement with these results, sclerostin is known as one of the key proteins involved in mechanical loading, as demonstrated by Robling et al.,36 who showed that Sost expression was decreased by loading, resulting in increased bone formation. Although these data suggested a powerful effect of Irisin on Sost inhibition, thus confirming that this myokine exerts loading-mimetic functions, further studies will be required to understand whether Irisin also exerts direct influence on osteocytes, which are the mechano-receptors of bone and are considered the primary source of Sclerostin.
A possible combined direct and indirect effect of irisin on the skeleton
The firstly described role for Irisin was its ability to induce the so-called ‘browning response' in white fat adipose tissue.10 During the same time, it has been reported that this inducible form of brown adipose (iBAT) is anabolic for the skeleton.37 Studies on transgenic mice overexpressing FoxC2 in the adipose tissue, a well-established model for BAT induction, showed that these mice displayed a higher trabecular bone mass, as compared with age-matched WT animals, due to increased bone formation, triggered by Wnt10b and IGFBP2, two pivotal bone anabolic molecules released from BAT.37
Another work by Zhang et al.38 demonstrated that therapeutic treatment of normal and obese mice with r-Irisin at a dose of 3500 μg kg−1 per week strongly induced the browning expansion in white fat depots. In our experiments, we used a lower dose of r-Irisin (100 μg−1 kg per week) that was effective on bone but not sufficient to induce the browning response, ruling out an indirect action of Irisin on bone mass via iBAT.19 However, our findings did not exclude the possibility that Irisin, if used at higher dose, could exert a synergic effect by directly stimulating new bone synthesis through osteoblasts and through BAT expansion.
Irisin synthesis beyond the muscle and its autocrine action
In our studies, we also demonstrated that the highest expression of the Irisin precursor, FNDC5, was found in the muscle tissue, although it was also detectable to a considerable extent in brain and bone tissues. Modest levels of Irisin precursor were also found in BAT, inguinal WAT and epididymal WAT, as already demonstrated by others.39 Roca-Rivada et al. defined Irisin as the new adipokine with autocrine and endocrine activity, demonstrating that FNDC5/Irisin has a different pattern of secretion depending on adipose tissue type.39
Besides skeletal muscle and adipose tissue, it has been shown that Irisin might have a role in the central nervous system, as demonstrated by studies that revealed that rat and mice cerebellar Purkinje cells expressed FNDC5,40 which is also required for the adequate neural differentiation of mouse embryonic stem cells41 and regulates hippocampal neurogenesis in a dose-dependent manner.42 The key role of Irisin in neuronal differentiation and function gains particular relevance for neurodegenerative diseases, such as Alzheimer and Parkinson, and it may represent the molecular explanation for the positive correlation between physical activity and healthy brain.
The human ‘sport-hormone'
Although the powerful effect of the newly discovered hormone Irisin has been largely confirmed by results in rodents, whether these effects translate to humans needs to be better elucidated. Throughout the past 2 years, studies in humans have tried to clarify the role of Irisin in physiological conditions and in disease states. With regard to the skeleton, Irisin levels were found inversely correlated with serum sclerostin levels in adults, independent of age or gender.43 Likewise, post-menopausal women showed inverse correlation between Irisin levels and vertebral fragility fractures.44,45 Conversely, Irisin measured in athletes was positively associated with bone density and strength,46 supporting the idea that Irisin could have a protective role on bone health. Unfortunately these studies, as many other human studies in which Irisin levels were measured by ELISA assays, have been debated because of poor antibody specificity that may have given artifacts.47 These authors also claimed that the start codon of the human FNDC5 gene is mutated from the normal ATG to ATA, which would be translated to protein with extremely low efficiency,47 although there are numerous examples of proteins being expressed from unusual start codons.48 Very recently, Jedrychowski et al.49 have developed a quantitative mass spectrometry and unbiased assay for the detection of human Irisin in plasma. In this work, authors showed that human Irisin circulates at ∼3.6 ng ml−1 in sedentary individuals, and its concentration significantly increased to ∼4.3 ng ml−1 after aerobic physical activity. This elegant work provides an accurate state-of-the-art technique, which confirms that human Irisin is nevertheless regularly translated from its non-canonical start codon and unequivocally demonstrates that human Irisin is not a myth, rather this myokine exists and its synthesis is increased by physical activity.
Conclusion
The discovery that the exercise-induced myokine Irisin has profound effects in enhancing bone mass and improving its geometry adds another milestone to the biological evidence, supporting the existence of a tight relationship between the muscle tissue and the skeleton. The new revealed role for Irisin as a mediator of this connection could better clarify the molecular mechanisms underlying the positive effect of physical exercise on bone. Purposefully designed studies in osteoporotic murine models should shed further light on the potential exercise-mimetic action of Irisin in inhibiting or restoring bone loss. Therefore, extension of these findings on human patients could encourage the use of Irisin as a therapeutic strategy for the prevention and treatment of osteoporosis during menopause, aging, immobility, muscle wasting (sarcopenia) and the absence of mechanical load (microgravity).
Acknowledgments
This work was supported in part by ERISTO grant (to MG), by MIUR grant ex60% (to MG) and by SIOMMMS grant (to GC).
Footnotes
MG and GC are named inventors of a pending patent application related to the work described.
References
- Dunstan D. Diabetes: exercise and T2DM-move muscles more often!. Nat Rev Endocrinol 2011; 7: 189–190. [DOI] [PubMed] [Google Scholar]
- Baxter-Jones AD, Kontulainen SA, Faulkner RA, Bailey DA. A longitudinal study of the relationship of physical activity to bone mineral accrual from adolescence to young adulthood. Bone 2008; 43: 1101–1107. [DOI] [PubMed] [Google Scholar]
- Andreoli A, Celi M, Volpe SL, Sorge R, Tarantino U. Long-term effect of exercise on bone mineral density and body composition in post-menopausal ex-elite athletes: a retrospective study. Eur J Clin Nutr 2012; 66: 69–74. [DOI] [PubMed] [Google Scholar]
- Keyak JH, Koyama AK, LeBlanc A, Lu Y, Lang TF. Reduction in proximal femoral strength due to long-duration spaceflight. Bone 2009; 44: 449–453. [DOI] [PubMed] [Google Scholar]
- Oppl B, Michitsch G, Misof B, Kudlacek S, Donis J, Klaushofer K et al. Low bone mineral density and fragility fractures in permanent vegetative state patients. J Bone Miner Res 2014; 29: 1096–1100. [DOI] [PubMed] [Google Scholar]
- Clarke BL, Khosla S. Physiology of bone loss. Radiol Clin North Am 2010; 48: 483–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaji H. Linkage between muscle and bone: common catabolic signals resulting in osteoporosis and sarcopenia. Curr Opin Clin Nutr Metab Care 2013; 16: 272–277. [DOI] [PubMed] [Google Scholar]
- Robling AG, Turner CH. Mechanical signaling for bone modeling and remodeling. Crit Rev Eukaryot Gene Expr 2009; 19: 319–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts LD, Boström P, O'Sullivan JF, Schinzel RT, Lewis GD, Dejam A et al. β-Aminoisobutyric acid induces browning of white fat and hepatic β-oxidation and is inversely correlated with cardiometabolic risk factors. Cell Metab 2014; 19: 96–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boström P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC et al. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 2012; 481: 463–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himms-Hagen J, Melnyk A, Zingaretti MC, Ceresi E, Barbatelli G, Cinti S. Multilocular fat cells in WAT of CL-316243-treated rats derive directly from white adipocytes. Am J Physiol Cell Physiol 2000; 279: C670–C681. [DOI] [PubMed] [Google Scholar]
- Barbatelli G, Murano I, Madsen L, Hao Q, Jimenez M, Kristiansen K et al. The emergence of cold-induced brown adipocytes in mouse white fat depots is determined predominantly by white to brown adipocyte transdifferentiation. Am J Physiol Endocrinol Metab 2010; 298: E1244–E1253. [DOI] [PubMed] [Google Scholar]
- Rosen ED, Spiegelman BM. What we talk about when we talk about fat. Cell 2014; 156: 20–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee P, Linderman JD, Smith S, Brychta RJ, Wang J, Idelson C et al. Irisin and FGF21 are cold-induced endocrine activators of brown fat function in humans. Cell Metab 2014; 19: 352–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cinti S. Transdifferentiation properties of adipocytes in the adipose organ. Am J Physiol Endocrinol Metab 2009; 297: E977–E986. [DOI] [PubMed] [Google Scholar]
- Rosenwald M, Perdikari A, Rulicke T, Wolfrum C. Bi-directional interconversion of brite and white adipocytes. Nat Cell Biol 2013; 15: 659–667. [DOI] [PubMed] [Google Scholar]
- Wang QA, Tao C, Gupta RK, Scherer PE. Tracking adipogenesis during white adipose tissue development, expansion and regeneration. Nat Med 2013; 19: 1338–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldén TB, Hansen IR, Timmons JA, Cannon B, Nedergaard J. Recruited vs. nonrecruited molecular signatures of brown, "brite," and white adipose tissues. Am J Physiol Endocrinol Metab 2012; 302: E19–E31. [DOI] [PubMed] [Google Scholar]
- Colaianni G, Cuscito C, Mongelli T, Pignataro P, Buccoliero C, Liu P et al. The myokine Irisin increases cortical bone mass. PNAS 2015; 112: 12157–12162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colaianni G, Cuscito C, Mongelli T, Oranger A, Mori G, Brunetti G et al. Irisin enhances osteoblast differentiation in vitro. Int J Endocrinol 2014; 2014: 902186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonseca H, Moreira-Gonçalves D, Coriolano HJ, Duarte JA. Bone quality: the determinants of bone strength and fragility. Sports Med 2014; 44: 37–53. [DOI] [PubMed] [Google Scholar]
- Seeman E. An exercise in geometry. J Bone Miner Res 2002; 17: 373–380. [DOI] [PubMed] [Google Scholar]
- Orwoll ES. Toward an expanded understanding of the role of the periosteum in skeletal health. J Bone Miner Res 2003; 18: 949–954. [DOI] [PubMed] [Google Scholar]
- Lieberman DE, Polk JD, Demes B. Predicting long bone loading from cross-sectional geometry. Am J Phys Anthropol 2004; 123: 156–171. [DOI] [PubMed] [Google Scholar]
- Ducher G, Bass SL, Saxon L, Daly RM. Effects of repetitive loading on the growth-induced changes in bone mass and cortical bone geometry: a 12-month study in pre/peri- and postmenarcheal tennis players. J Bone Miner Res 2011; 26: 1321–1329. [DOI] [PubMed] [Google Scholar]
- Johannesdottir F, Aspelund T, Siggeirsdottir K, Jonsson BY, Mogensen B, Sigurdsson S et al. Mid-thigh cortical bone structural parameters, muscle mass and strength, and association with lower limb fractures in older men and women (AGES-Reykjavik Study). Calcif Tissue Int 2012; 90: 354–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkasrawy MN, Hamrick MW. Myostatin (GDF-8) as a key factor linking muscle mass and bone structure. J Musculoskelet Neuronal Interact 2010; 10: 56–63. [PMC free article] [PubMed] [Google Scholar]
- MacKenzie MG, Hamilton DL, Pepin M, Patton A, Baar K. Inhibition of myostatin signaling through Notch activation following acute resistance exercise. PLoS ONE 2013; 8: e68743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan T, Liang X, Bi P, Kuang S. Myostatin knockout drives browning of white adipose tissue through activating the AMPK-PGC1α-Fndc5 pathway in muscle. FASEB J 2013; 27: 1981–1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harter LV, Hruska KA, Duncan RL. Human osteoblast-like cells respond to mechanical strain with increased bone matrix protein production independent of hormonal regulation. Endocrinology 1995; 136: 528–535. [DOI] [PubMed] [Google Scholar]
- Kubota T, Yamauchi M, Onozaki J, Sato S, Suzuki Y, Sodek J. Influence of an intermittent compressive force on matrix protein expression by ROS 17/2.8 cells, with selective stimulation of osteopontin. Arch Oral Biol 1993; 38: 23–30. [DOI] [PubMed] [Google Scholar]
- Toma CD, Ashkar S, Gray ML, Schaffer JL, Gerstenfeld LC. Signal transduction of mechanical stimuli is dependent on microfilament integrity: identification of osteopontin as a mechanically induced gene in osteoblasts. J Bone Miner Res 1997; 12: 1626–1636. [DOI] [PubMed] [Google Scholar]
- Paszty C, Turner CH, Robinson MK. Sclerostin: a gem from the genome leads to bone-building antibodies. J Bone Miner Res 2013; 25: 1897–1904. [DOI] [PubMed] [Google Scholar]
- Lin C, Jiang X, Dai Z, Guo X, Weng T, Wang J et al. Sclerostin mediates bone response to mechanical unloading through antagonizing Wnt/beta-catenin signaling. J Bone Miner Res 2009; 24: 1651–1661. [DOI] [PubMed] [Google Scholar]
- Li X, Ominsky MS, Niu QT, Sun N, Daugherty B, D'Agostin D et al. Targeted deletion of the sclerostin gene in mice results in increased bone formation and bone strength. J Bone Miner Res 2008; 23: 860–869. [DOI] [PubMed] [Google Scholar]
- Robling AG, Niziolek PJ, Baldridge LA, Condon KW, Allen MR, Alam I et al. Mechanical stimulation of bone in vivo reduces osteocyte expression of Sost/sclerostin. J Biol Chem 2008; 283: 5866–5875. [DOI] [PubMed] [Google Scholar]
- Rahman S, Lu Y, Czernik PJ, Rosen CJ, Enerback S, Lecka-Czernik B. Inducible brown adipose tissue, or beige fat, is anabolic for the skeleton. Endocrinology 2013; 154: 2687–2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Li R, Meng Y, Li S, Donelan W, Zhao Y et al. Irisin stimulates browning of white adipocytes through mitogen-activated protein kinase p38 MAP kinase and ERK MAP kinase signaling. Diabetes 2014; 63: 514–525. [DOI] [PubMed] [Google Scholar]
- Roca-Rivada A, Castelao C, Senin LL, Landrove MO, Baltar J, Crujeiras AB et al. FNDC5/Irisin is not only a myokine but also an adipokine. PLoS ONE 2013; 11 8: e60563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dun SL, Lyu RM, Chen YH, Chang JK, Luo JJ, Dun NJ. Irisin-immunoreactivity in neural and non-neural cells of the rodent. Neuroscience 2013; 240: 155–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashemi MS, Ghaedi K, Salamian A, Karbalaie K, Emadi-Baygi M, Tanhaei S et al. Fndc5 knockdown significantly decreased neural differentiation rate of mouse embryonic stem cells. Neuroscience 2013; 231: 296–304. [DOI] [PubMed] [Google Scholar]
- Moon HS, Dincer F, Mantzoros CS. Pharmacological concentrations of Irisin increase cell proliferation without influencing markers of neurite outgrowth and synaptogenesis in mouse H19-7 hippocampal cell lines. Metabolism 2013; 62: 1131–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klangjareonchai T, Nimitphong H, Saetung S, Bhirommuang N, Samittarucksa R, Chanprasertyothin S et al. Circulating sclerostin and Irisin are related and interact with gender to influence adiposity in adults with prediabetes. Int J Endocrinol 2014; 2014: 261545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palermo A, Strollo R, Maddaloni E, Tuccinardi D, D'Onofrio L, Briganti S et al. Irisin is associated with osteoporotic fractures independently of bone mineral density, body composition or daily physical activity. Clin Endocrinol (Oxf) 2015; 82: 615–619. [DOI] [PubMed] [Google Scholar]
- Anastasilakis AD, Polyzos SA, Makras P, Gkiomisi A, Bisbinas I, Katsarou A et al. Circulating Irisin is associated with osteoporotic fractures in postmenopausal women with low bone mass but is not affected by either teriparatide or denosumab treatment for 3 months. Osteoporos Int 2014; 25: 1633–1642. [DOI] [PubMed] [Google Scholar]
- Singhal V, Lawson EA, Ackerman KE, Fazeli PK, Clarke H, Lee H et al. Irisin levels are lower in young amenorrheic athletes compared with eumenorrheic athletes and non-athletes and are associated with bone density and strength estimates. PLoS ONE 2014; 9: e100218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrecht E, Norheim F, Thiede B, Holen T, Ohashi T, Schering L et al. Irisin - a myth rather than an exercise-inducible myokine. Sci Rep 2015; 5: 8889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov IP, Firth AE, Michel AM, Atkins JF, Baranov PV. Identification of evolutionarily conserved non-AUG-initiated N-terminal extensions in human coding sequences. Nucleic Acids Res 2011; 39: 4220–4234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jedrychowski MP, Wrann CD, Paulo JA, Gerber KK, Szpyt J, Robinson MM et al. Detection and Quantitation of Circulating Human Irisin by Tandem Mass Spectrometry. Cell Metab 2015; 22: 734–740. [DOI] [PMC free article] [PubMed] [Google Scholar]