Abstract
Relationships between non-avian theropod dinosaurs and extant and fossil birds are a major focus of current paleobiological research. Despite extensive phylogenetic and morphological support, behavioural evidence is mostly ambiguous and does not usually fossilize. Thus, inferences that dinosaurs, especially theropods displayed behaviour analogous to modern birds are intriguing but speculative. Here we present extensive and geographically widespread physical evidence of substrate scraping behavior by large theropods considered as compelling evidence of “display arenas” or leks, and consistent with “nest scrape display” behaviour among many extant ground-nesting birds. Large scrapes, up to 2 m in diameter, occur abundantly at several Cretaceous sites in Colorado. They constitute a previously unknown category of large dinosaurian trace fossil, inferred to fill gaps in our understanding of early phases in the breeding cycle of theropods. The trace makers were probably lekking species that were seasonally active at large display arena sites. Such scrapes indicate stereotypical avian behaviour hitherto unknown among Cretaceous theropods, and most likely associated with terrirorial activity in the breeding season. The scrapes most probably occur near nesting colonies, as yet unknown or no longer preserved in the immediate study areas. Thus, they provide clues to paleoenvironments where such nesting sites occurred.
The close relationships between non-avian and avian theropod dinosaurs (birds) have been extensively studied and debated in recent decades1,2,3. Physical evidence for close relationships between these groups mostly comes from suites of osteological characters subjected to repeated cladistic analysis and refinement1,2,3. Feathered non-avian theropods also provide physical evidence of avian affinities4,5. However, inferences about phylogenetic relationships based on behaviour are generally more speculative. Troodontid dinosaurs buried in in avian-like sleeping postures provide rare physical evidence of stereotypical avian behaviour6,7. While other trace fossils, including nests, footprints, and bite marks also constitute behavioural evidence, they are evidently unrelated to display rituals.
While nests and eggs are clear evidence of later stages in the reproductive cycles of avian and non-avian theropods, until now it has proved impossible to demonstrate direct physical evidence of mating display behaviour in extinct non avian theropods, even though such behaviour is well-known in extant birds8,9,10,11,12,13,14. This means literature on dinosaur mating display behaviour has invariably been speculative15,16,17. For example, while one may infer that some non-avian theropods exhibited sexual dimorphism, presumably expressed in variable display behaviours, there is no physical evidence to prove such suppositions. As a result conclusive physical “evidence for sexual dimorphism in non-avian dinosaurs has been elusive”18 even though paleontologists infer it existed. The popular assumption that strong sexual dimorphism, in such features as crests, indicates well-developed display capacities leading to inter-or intra-sexual selection, has been challenged as simplistic by showing the importance of mutual sexual selection19. Nevertheless, subtle differences in oviraptosaur tail morphology are cited as evidence of sexual dimorphism, indicating tail display functions “likely employed in courtship rituals”16. Although potentially true, this fails to provide direct physical evidence for display behaviour. Likewise, the speculative notion that theropod courtship display behaviour led to powered avian flight15 has few adherents.
Here we report four sites, from a single Cretaceous rock unit in Colorado20 (Supplementary Information S1 and Figure S1) with extensive physical evidence of large scale scrapes, made by the left and right feet of theropod dinosaurs. We show that these scrapes likely resulted from behaviour referred to in the ornithological literature as “nest scrape display,” “nest scrape advertisement display,” “scrape ceremonies,” “pseudo nest-building,” or more generally as courtship or mating display, which is stereotypical in diverse groups of extant birds8,9,10,11,12,13,14. It is also reasoned that these scrapes are not actual nests, or the result of digging for other purposes such as searching for food, water or shelter. Thus, these scrapes represent a hitherto unrecognised category of physical trace fossil evidence ostensibly associated with early behavioural phases in the breeding cycle of non-avian theropod dinosaurs. By implication, these traces, here named Ostenichnus bilobatus, (“bilobed display trace”) were made in the breeding season, probably springtime. Moreover, such lek-like, “display arena”21,22 evidence links two ubiquitous classes of trace fossil: locomotion traces (repichnia) and nest sites (calichnia). In short, the display arena interpretation provides the missing link in a threefold plexus of traces that can be arranged in a coherent time sequence tied to the breeding season: trackmaking-courtship display-nesting. Although such trace-making activities do not necessarily take place at the same locations, given the stereotypical cycle of breeding behaviours among many extant avians, (congregation, display, copulation, nesting, incubation, etc.), it can be confidently inferred that abundant scrapes indicate that nest sites were established nearby. Thus, scrapes are signatures of paleogeographical significance pointing to preferred paleoenvironmental nesting sites, even as shown here where no other direct physical evidence of nesting is preserved.
The Physical Trace Fossil Evidence
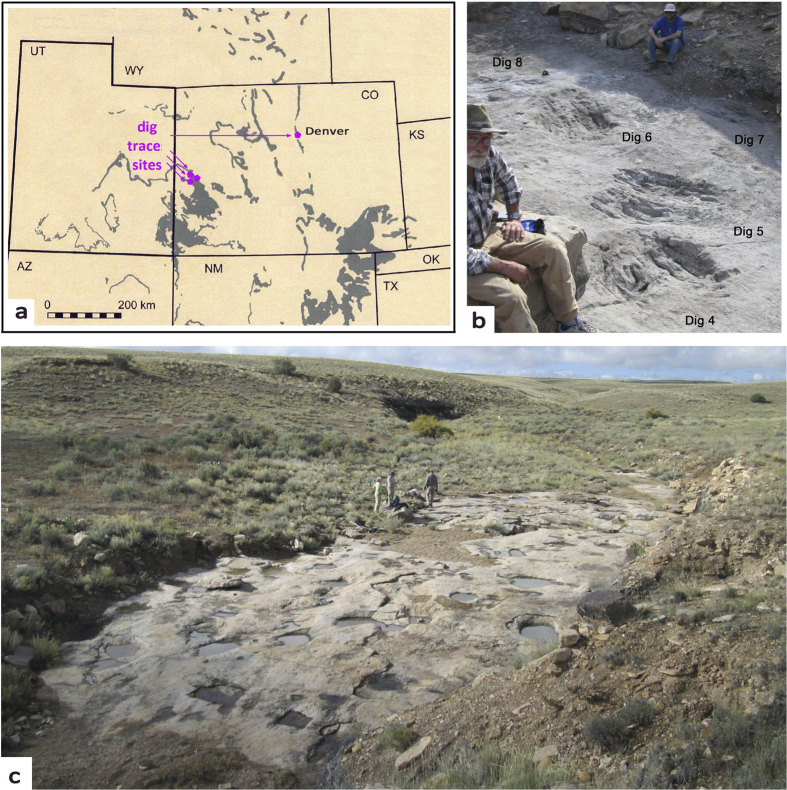
Four sites with large nest scrape display traces have been identified in the Cretaceous (late Albian-Cenomanian), Dakota Sandstone of Colorado, three in the west, and one in the east of the state. The two largest sites are visually spectacular (Fig. 1). The Dakota Sandstone, representing a mosaic of coastal plain wetland, fluvial, lacustrine and coal swamp paleoenvironments (Supplementary Information S1), has yielded ~80 tetrapod tracksites mostly from eastern Colorado. The Dakota Group is also track-rich in the west where an additional ~40 sites have been documented20. Despite this abundance of footprints, display scrapes represent a previously-unrecognised, entirely new category of vertebrate trace fossil. To record this novel physical evidence all sites and representative traces have been subjected to thorough mapping and photogrammetic analyses (Figs 2 and 3: see Supplementary Information S2).
Figure 1.
(a). Locality map, showing outcrops of Dakota Sandstone in western USA, extensively modified in Photoshop CS5 from part of a map by Carpenter (Supplementary Information S1) (b). General view of the Roubideau Creek site (photograph by senior author, M. Lockley, with coauthors KC (foreground) and JM (background) with permission. Note conspicuous scrape marks and mid-line ridge in traces 4–6, c. general view of the Club Gulch site.
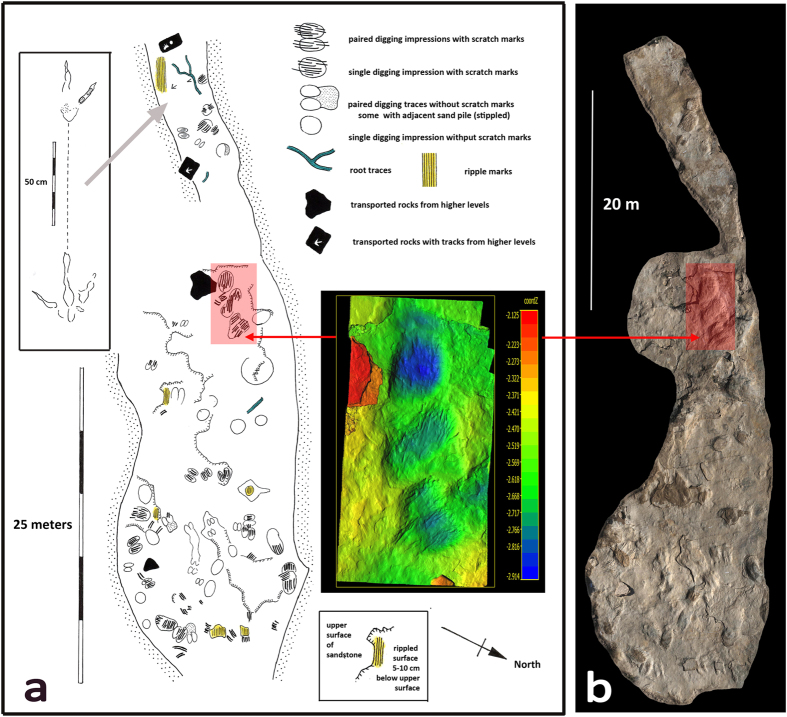
Figure 2.
Map of Club Gulch site (a) prepared in Photoshop CS5 by MGL, with natural color photogrammetic image (b) at same scale by RTM and LGB. Coloured image (inset in a) shows three large scrapes, together covering 5 m. Digging traces are classified as paired (bilobed) or single, with or without scratch marks and adjacent sand aprons.
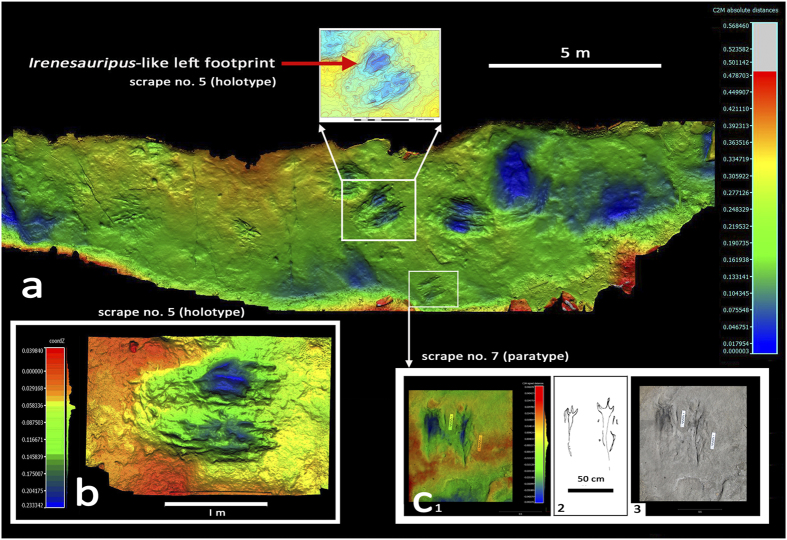
Figure 3.
Coloured photogrammetric image of Roubideau Creek site (a) with location and details of holotype Ostenichnus bilobatus scrape (dig 5 in Fig. 1) both showing whole scrape (b) and contour relief detail of Irenesauripus-like track (A: top center). Detail of paratype scrape 7 (c 1-3) also shows theropod track morphology.
The largest site reveals ~60 scrapes on a single sandstone surface exposure up to ~50 m long and ~15 m wide (Figs 1 and 2). This area (~750 m2), represents a minimum estimate, for a visible surface that likely extended much further. A second site (Fig. 3) with eight well preserved scrapes occurs on a single sandstone surface ~20 × ~5 m (~100 m2). The size, depth and distribution of these scrapes is variable. However, most typically consist of parallel double troughs, comprised of multiple scrapes separated by a raised central ridge (see formal description). A few show complete outlines of three-toed theropod tracks, and some show thin aprons of excavated sediment aligned with the long axis of the scrapes. Two additional sites with bi- and multi-lobed Ostenichnus bilobatus scrapes have been found elsewhere in the Dakota Group, one in western Colorado, the other in eastern Colorado (Figs 1 and 4).
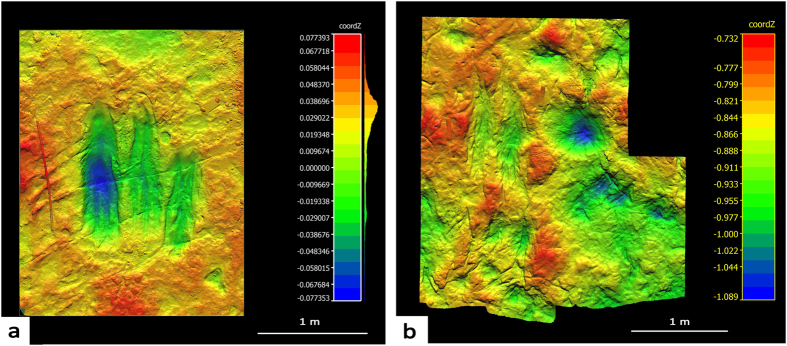
Figure 4.
Theropod display traces from the Dakota Sandstone at Duncan Road in western Colorado (a) and at Dinosaur Ridge in eastern Colorado (b) Colorado. See Supplementary Information SI on geology and the stratigraphy of the Dakota Sandstone and Dinosaur Ridge site.
Trace fossil research recognises that traces represent “fossil behaviour”23 registered by living animals. General trace categories include repichnia for directed locomotion traces, cubichia for resting traces, domichnia for dwelling traces, praedichnia for predation traces and calichnia for breeding traces24. Thus, display scrapes represent a previously unrecognised type of calichnia trace, associated with courtship behaviour.
Some rock units, including the Dakota Sandstone20 are virtually devoid of tetrapod body fossil remains, yet very rich in tetrapod traces25,26. The body-fossil-poor Dakota Sandstone, has yielded tracks of avian and non-avian theropods, ornithopods, ankylosaurs, pterosaurs, crocodilians and turtles as well as diverse invertebrate traces20. Thus, digging traces or scrapes could potentially have been created by any of the several large tetrapods. However, the Dakota Sandstone evidence for theropod scrape-makers is compelling due to configurations indicating a bipedal trace maker with narrow acuminate claw traces. Where scrapes contain clear theropod tracks this evidence is conclusive. As no such traces have previously been reported they require the following formal systematic treatment.
Systematics
Ostendichnus ichnogen nov. Fig. 3
Diagnosis: large, up to 2-meter-long, bilaterally-symmetrical, bilobed to oval impressions with multiple well-defined digital scratch marks aligned parallel or sub parallel to long axis of the whole trace. Up to 10–15% as deep as long. Traces mostly with a single raised central ridge, separating left and right troughs, which may include complete or partial diagnostic tridactyl theropod tracks.
Type material: holotype Denver Museum of Nature and Science (DMNH) EPV.69705 latex mold and fiberglass replica of large digging trace, within which a diagnostic theropod track occurs. Paratypes DMNH EPV. 69703, EPV. 69704, EPV. 69706 and EPV. 69707 latex molds and fiberglass replicas of large digging traces (Fig. 3 and Supplementary Information 2).
Type horizon and locality: lower part of the Cretaceous Dakota Sandstone, Roubideau Creek, Delta County, Colorado. Information on file with DMNS, CU and BLM.
Derivation of name: from ostendo (Latin) meaning “to show”, or “to display”, and ichnos (Latin) meaning “a trace.”
Ostendichnus bilobatus ichnosp nov., Fig. 3
Type material: as for ichnogenus: holotype DMNH EPV.69705 latex mold and fiberglass replica of digging trace number 5.
Type horizon and locality: as for ichnogenus.
Derivation of ichnospecies name: bilobatus meaning two lobes.
Diagnosis: as for ichnogenus.
Description: large, bilaterally-symmetrical, bilobed to oval impressions or scrapes 0.75 to 2.00 m long and 0.50 to 1.25 m wide; depth variable, 5 to 25 cm. Multiple well-defined digital scratch marks align with the whole trace, as does a raised medial ridge defining the long axis of the trace. Some scratch marks have sharp anterior terminations, indistinguishable from typical theropod digit traces. Together with sand crescents, where sediment was pushed back by posterior motion of digits, or thrown posteriorly as a thin apron, the left and right sides of scrapes are defined. In some scrapes complete or partial theropod tracks are recognisable components of the scrapes.
Interpretation of Ostednichnus bilobatus
The sharply-terminated scratch marks found in association with diagnostic theropod tracks represent active theropod scraping or scratching. The most complete theropod tracks include a large Irenesauripus-like27,28 left theropod track on the left side of the holotype scrape (Fig. 3a) and a smaller right theropod track associated with the right side of a shallow paratype scrape (Fig. 3c). The variable size and depth of the scrapes, indicate different levels of activity and persistence by different sized theropods, which based on footprint length had hip heights between ~1.0 and ~2.0 m29 and full body lengths between ~2.5 and ~5.0 m. This implies either two or more different species, or co-occurrence of conspecific adults and sub-adults of quite different sizes. Elapsed time between scraping episodes cannot be estimated accurately, but was likely short given the similar, good preservation of all scrapes.
Interpretation of nest scrape displays
Interpretation of these scrapes as evidence of mating display arenas or courtship ritual sites requires elimination of other possible digging behaviour interpretations unrelated to mating display. We can then demonstrate whether or not the behaviour, and resultant trace fossils, are consistent with behaviours of other similar or related species, in this case extant birds.
Possible explanations for the scrapes reported from the Dakota Formation sites in Colorado, are that 1) they are actual nest sites or colonies, 2) they represent evidence of dinosaurs digging for food, water or shelter, 3) they are territory-marking scrapes, and 4) they are nuptial display arenas or scrape ceremony sites (Table 1). The nest site explanation is unconvincing because there is no evidence of eggs, eggshells, hatching remains, or the type of well-defined nest rims30 recorded at many nest sites (Fig. 5). Even if eggshell and hatchling remains were removed by parents or taphonomic process, the variable shapes, depths and distribution of scrapes do not conform to the typical shapes and regular-spacing configuration of nests in known dinosaur nest colonies30 or those of extant avians such as gannets or flamingos. It is also difficult to conceive of dinosaurs nesting, incubating and rearing young in scrapes of variable size and depth without obliterating the clear scratch marks and prominent median ridges seen in most examples. Among extant ground nesting colonial birds, such as gannets or flamingoes, nest spacing is highly regular. Nest materials are built into large mounds with preservation potential, and there are no signs of irregularly configured scrape marks. Postulating a “failed nest site” is also unconvincing because a site where theropods congregated to scrape, before moving elsewhere, leaves trace evidence essentially indistinguishable from a display arena (interpretation 4). Moreover, it requires that we assume failed nest digging attempts that are not purposeful or integral display phases in the breeding cycle. Such non-display activity would presumably waste energy, and might only be explained, tentatively, as bouts of stereotypical scrape behavior for territorial or other purposes such as testing unsuitable substrates that were later abandoned. In this regard nest scrape display is a type of territorial behavior which is better-known, documented and widespread than any scrape behavior unrelated to display.
Table 1. Comparison of digging or scrape trace fossil evidence with the four working hypotheses considered here. √ indicates consistency with one or more hypotheses. x indicates lack of consistency with one or more hypotheses, ? indicates uncertain degree of consistency with one or more hypotheses.
| Trace evidence | Working hypotheses (I-IV) |
|||
|---|---|---|---|---|
| Details of trace morphology | I: Nest colony hypothesis | II: Digging for food or water | III: Territorial marking | IV: Breeding / mating display |
| Diagnostic theropod tracks | √ | √ | √ | √ |
| Abundant digit and claw traces | x | x | √ | √ |
| Variable size of traces | x | √ | √ | √ |
| Variable depth of traces | x | √ | √ | √ |
| Variable spacing of traces | x | ? | ? | √ |
| High density of traces | √ | ? | x | √ |
| Frequent overlap of traces | x | ? | ? | √ |
| Isolated traces | x | ? | √ | √ |
| No clear tracks between traces | √ | x | ? | √ |
| Few deposits of excavated sediment | ? | ? | ? | √ |
| No diagnostic nest rims or morphology | x | √ | √ | √ |
| % of consistent evidence for hypotheses I-IV | 27% | ~36% | ~55% | 100% |
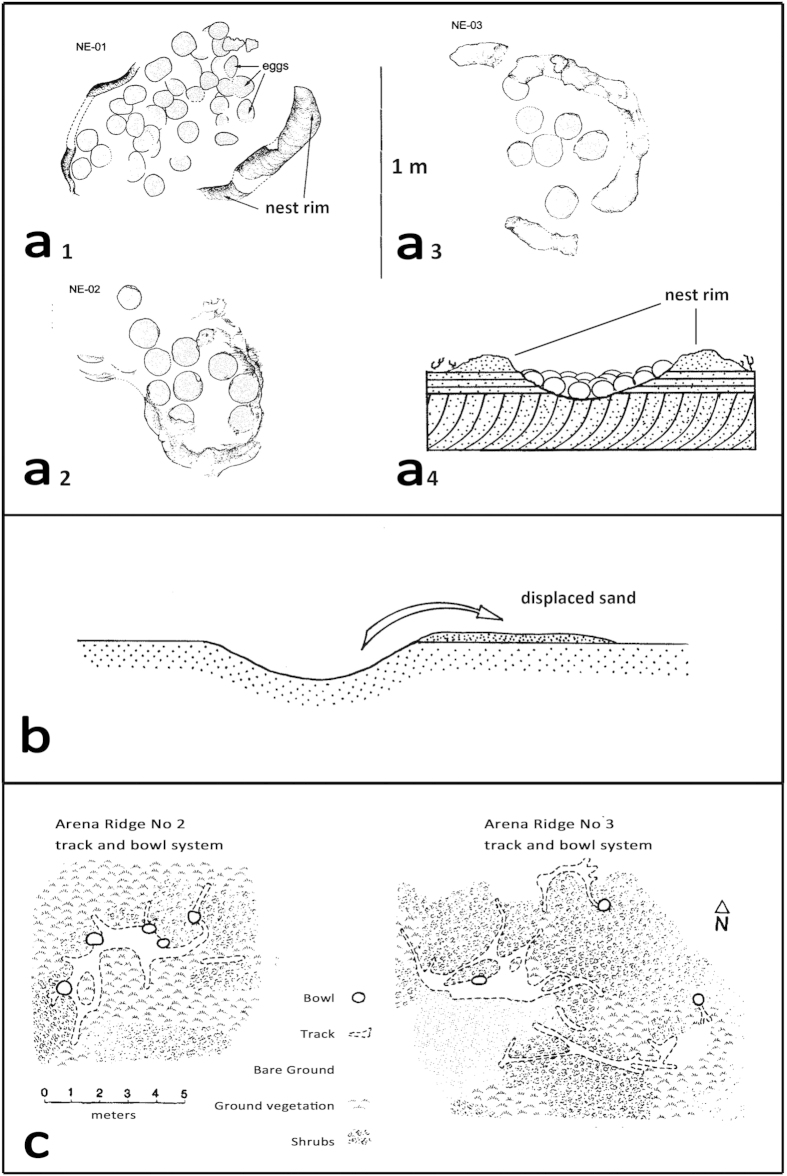
Figure 5. a1-a3: maps of Cretaceous sauropod nests from Argentina with cross, section (a4), after30.
Note the nest rim in all cases. (b) cross section of Club Gulch dig trace with thin apron of displaced sand. Note lack of sediment rim. (a,b) drawn to approximately the same scale. c: map showing distribution of Kakapo nest scrape bowls, (modified after12).
Explanations based on digging for food or water also suffer from a lack of consistent and compelling evidence. It is known that elephants and other tetrapods dig for water31,32 or food. However, any successful attempt to dig to the level of the water table would produce pooling that would wash out scrape marks in sandy sediment. Some authors have speculated about the “scratch digging” potential of sauropods33,34. Such notions can be dismissed here because the scratch morphology fits theropods, not herbivorous sauropods. Moreover, despite ~120 known tracksites in the Dakota Sandstone20 the unit was deposited during the sauropod hiatus in North America35 when no sauropod tracks are known. Digging for prey is a plausible theropod activity supported by one diagnostic deinonychosaurid claw trace that penetrated a bioturbated zone of burrows attributed to small tetrapods, possibly mammals36. However, these traces bear no morphological resemblance to the large surface scrapes here described as Ostenichnus bilobatus, and there is also no evidence of burrows, or even buried carrion, at any of the scrape sites. Dinosaur burrows are small and very rare. A report of a ~30-cm-diameter, sub-cylindrical burrow containing an adult and two juvenile ornithopod dinosaurs37 could be a case of “denning,” but there is no definitive proof that a dinosaur (presumably the adult) dug the burrow in which it died. The diggers of other possible dinosaur burrows38 have only been inferred.
Could the scrapes be territorial markings ? Mammalian carnivores, notably cats, dig scrapes in unconsolidated sediment along game trails and mark them with urine39. Moreover, these traces have even been labelled “scrapes.” However, they are almost always isolated, and often along upland game trails were preservation potential is very low. Unlike ureotelic mammals, reptiles and birds are uricothelic, excreting uric acid as a final product of nitrogenous metabolism. Scent marking of territory is a mammalian trait, not known in water-conserving uricothelic reptiles and birds. This is another reason that searching for water by theropod dinosaurs is an unconvincing explanation for scrapes. In the case of the Dakota Sandstone, representing a coastal plain system with abundant evidence of saturated substrates, surface water was abundant.
Turning to the fourth hypothesis, that the scrapes represent display arenas containing evidence of ceremonial scrapes, we argue that the evidence (Table 1) consistently supports such interpretations. Ornithological literature provides many reports of scrape ceremonies and nest scrape display activity, separate from actual nest site selection and occupation. Supplementary Information 3. A compelling parallel with the Cretaceous scrapes from Colorado was reported for the Atlantic puffin (Fratercula arctica) in the run up to breeding, which thanks to “repeated scratching and kicking produces two parallel furrows on the floor of the burrow [with a] characteristic ridge between [that] becomes worn down and less obvious during the breeding season”8. The puffin is an active digger, although it often occupies and enlarges existing burrows. Unlike a large non-avian theropod it can stand more or less erect in a burrow. The traces produced by digging or scraping: i.e. two parallel furrows, with a ridge between, are strikingly similar to the theropod traces Ostenichnus bilobatus. The ostrich, the largest living bird, much closer in size to the Cretaceous theropod scrape-makers, produces shallow scrapes similar in size (~2–3 meters diameter) to those described here40 but has not been reported to engage in “nest scrape display” away from its chosen nest site, in which incubation obliterates any preservable scrape traces. These two very different birds are among the few whose scrapes have been described or illustrated, even briefly. For most of the many birds whose nest-scrape display behaviour has been documented in detail, the scrape morphology is considered an incidental behavioural bi-product, whereas the ritual ceremonies (movements) have been analysed and classified in detail8,9,10,11,12,13,14, often with intriguing video footage (Supplementary Information 3). At least one vertebrate ichnologist has speculated that theoretically one might find traces made by dinosaurs engaging in display behaviour, possibly even in the act of copulation38. Such conjectures are not new41,42 but they have been, until now, entirely speculative based only on the assumption that coition, and pre-copulation courtship, occurs in all tetrapods. However, the point is well-taken as it is the physical trace evidence, mostly ignored by modern ornithologists, that is potentially most important in paleontology38.
Among shorebirds (Charadriidae), nest scrape displays are reported for seven Charadrius species9 including the Wilson’s plover, Charadrius wilsonia9, banded dotterel, Charadrius bicinctus10, the piping plover (Charadrius melodus). Scrape displays occur from the first day a dotterel pair occupies a territory. “A male makes a scrape in sand, shuffling with his breast and kicking backwards. Shallow scrapes are often made that are never used as nests”10. The Knot, Calidris canutus engages in multiple pre-coition “nest scrape displays,” also referred to as “nest scrape advertisement displays”11. The importance of Charadrius scrapes in relation to Cretaceous theropods is that they indicate that extant, ground-nesting shorebirds make multiple scrapes, most of which are never occupied as nests. Such pre-nuptial behaviour is very energetic, expending much more energy than needed to excavate a single nest. However, it is a purposeful part of the mating ritual, not a “failed” nest construction attempt.
The list of substrate scraping birds is long and diverse, even if the morphology of their scrapes is only sporadically recorded (Supplementary Information 3). Studies of the Kakapo Strigops habroptilus, a ground-dwelling, nocturnal New Zealand parrot, demonstrate that it also digs multiple scrapes as part of its courtship rituals12,43. Studies of this species include maps showing how scrapes are irregularly distributed, from 1-5 m apart, within the male’s display area otherwise known as a lek43 (Fig. 5). The lek, or display arena has generated much debate among ornithologists interested in sexual selection. They define both intrasexual leks, where males display to one another, and intersexual leks where females observe male displays. The importance of mutual sexual selection has also been discussed in relation to dinosaurs and pterosaurs19. The ornithological literature includes maps of lek territories for many species44,45, and much importance is attached to how sexual selection drives the evolution of size, dimorphism and other characteristics in lekking species. Thus, we must consider whether the Cretaceous theropod congregation sites described here may have been analogous to certain avian lek or arena display sites44,45 as appears to be the case (Supplementary Information 3). The afore-cited studies8,9,10,11,12,13,14 deal with behaviour that is both territorial and sexual, being an integral part of the breeding cycle: specifically pre-nuptial courtship prior to copulation. It is intriguing to imagine the vocalizations of large theropods during courtship scrape ceremonies and copulation (Fig. 6).
Figure 6. Reconstruction of theropods engaged in scrape ceremony display activity, based on trace fossil evidence from the Dakota Sandstone, Colorado.

Artwork and graphics coordination by Xing Lida.
Discussion
Modern avian nest scrape displays and display arenas are created as an integral part of the breeding cycle. Avian courtship involves many stereotypical behaviours, and as such could be predicted to occur in ancestral non-avian theropods. Arguably, the Colorado scraping evidence could be the result of territorial behavior not directly related to nest scrape ceremonies. However, such an inference is problematic and unparsimonious because it infers the same stereotypical and territorial scraping behaviour used in courtship. It also infers an area of territorial dispute, with scrapes and scrape distributions that would be impossible to differentiate from a display arena. Thus, given the ubiquity of display arenas and leks among extant avians this interpretation best fits the evidence reported here. While some display arena scrape sites may represent leks of ground-dwelling birds, many leks, especially of tree-dwellers, are devoid of scrape evidence.
The Colorado evidence, points to a longevity of behaviour suggesting that theropod leks were integral to the social structure of at least some Cretaceous species. We assert that while most display behaviours (flapping, vocalization etc.,) leave no physical evidence, nest-scape displays do, and that their occurrence in the track record was even speculatively predicted38. Even so, a recent review of “the fossil evidence of bird behaviour”46 revealed little evidence of nests, and nothing about nest scrapes or display behaviour. Likewise the fossil record legacy of most behaviour is scant or ambiguous42. However, when made by large theropods scrapes are correspondingly large, with high preservation potential and distributions evidently extending over quite large areas. In the examples cited here the preservation potential is enhanced by sediment aggradation associated with transgressive sea-level rise47,48. This is in stark contrast to the poor preservation potential for small scrapes made in loose sand or gravel by plover-sized species, or scrapes made by ostriches in dry savannah settings. The Cretaceous evidence conforms to all predicted features of display scrapes including, variable size, depth and distribution (Table 1). Conversely, it is inconsistent with established nest sites, digging for prey or water, burrow construction or as isolated territorial scent markers. More importantly, these scrapes can be interpreted as the missing physical evidence which indicates that non-avian theropods engaged in stereotypical avian courtship and lek-like behaviours, which were previously only a matter of speculation among paleobiologists.
Additional Information
How to cite this article: Lockley, M. G. et al. Theropod courtship: large scale physical evidence of display arenas and avian-like scrape ceremony behaviour by Cretaceous dinosaurs. Sci. Rep. 6, 18952; doi: 10.1038/srep18952 (2016).
Supplementary Material
Acknowledgments
The field work at the three western Colorado sites authorized by Bureau of Land Management (BLM) permits CO C76204 and C C75359. Work at Dinosaur Ridge conducted under History Colorado Permit 2014-69. Joint funding for field research between 2012 and 2014 by the Korean National Research Institute of Cultural Heritage (NRICH) and University of Colorado Denver. Dr. Joseph Sertich, Denver Museum of Nature and Science and Mark Riegner Prescott College, Arizona read earlier versions of this manuscript, and Dr. David W. E. Hone, Queen Mary University of London, provided editorial advice. Walter Thurner (C.F. Maier Composites Inc., Golden, Colorado) made fiberglass replicas of the type series for the Denver Museum of Nature and Science collections. Yujiang Han helped create artwork.
Footnotes
Author Contributions M.G.L. and G.D.G. are research permit holders for survey and report compliance at B.L.M. sites in Colorado and Utah. Site management coordination by N.A.M., B.H.B. and G.H. Western Colorado sites found, cleaned/excavated, molded, replicated and mapped by J.M., K.C. and M.G.L. with additional field work by K.J.H., J.D.L., K.S.K. and D.Y.K. Map preparation, photography and photogrammetry by M.G.L., R.T.M., L.G.B., N.A.M. and B.H.B. Stratigraphic sections measured by K.J.H. with assistance from MGL. Manuscript and figure preparation by M.G.L. with assistance from K.J.H., L.G.B., R.T.M., L.X., D.S. and J.D.L. Artwork by L.X. and colleagues.
References
- Xu X., Zhou Z., Dudley R., Mackem S., Chuong C.-M., Erickson G. M. & Varricchio D. J. An integrative approach to understanding bird origins. Science, 346, issue 6215 (2014). [DOI] [PubMed] [Google Scholar]
- Sereno P. C. The origin and evolution of dinosaurs. Ann. Rev Earth Planet Sci. 25, 435–89 (1997). [Google Scholar]
- Forster C. A., Sampson S. D., Chiappe L. M. & Krause D. W. The theropod ancestry of birds: new evidence from the Late Cretaceous of Madagascar. Science, 279, 1915–19 (1998). [DOI] [PubMed] [Google Scholar]
- Clarke J. Feathers before flight. Science, 340, 690–692 (2013). [DOI] [PubMed] [Google Scholar]
- Godefroit P., Cau A., Dong-Yu H., Escuillie F., Wenhao W. & Dyke G. A Jurassic avialian dinosaur from Chinan resolves the early phylogenetic history of birds. Nature. 498, 359–362 (2013). [DOI] [PubMed] [Google Scholar]
- Xu X. & Norell M. A. A new troodontid dinosaur from China with avian-like sleeping posture. Nature 431, 838–841 (2004). [DOI] [PubMed] [Google Scholar]
- Gao C., Morschhauser E. M., Varricchio D. J., Liu J. & Zhao B. A Second Soundly -Sleeping Dragon: New Anatomical Details of the Chinese Troodontid Mei long with Implications for Phylogeny and Taphonomy. PLoS one 7, e45203 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris M. P. The Puffins, [224] T & A. D Poyser, Waterhouses, England (1984). [Google Scholar]
- Bergstrom P. W. Breeding Displays and Vocalizations of Wilson’s Plovers. Wilson Bulletin, 100, 36–49 (1988). [Google Scholar]
- Bomford M. Breeding displays and calls of the banded dotterel (Caradrius bicinctus) Notornis, 33, 219–232 (1986). [Google Scholar]
- Whitfield D. P. & Brade J. J. The breeding behavior of the Knot Calidris canutus. Ibis, 133, 246–255 (1991). [Google Scholar]
- Powesland R. G., Lloyd B. D., Best H. A. & Merton D. V. Breeding biology of the Kakapo Strigops habroptilus on Stewart Island, New Zealand. Ibis. 134, 361–373 (1992). [Google Scholar]
- Cairns W. E. Biology and behavior of breeding Piping Plovers. Wilson Bulletin. 94, 531–545 (1982). [Google Scholar]
- Bertram B. C. R. The Ostrich Communal nesting System. [196] Princeton Univ. Press. (1992). [Google Scholar]
- Cowen R. & Lipps J. H. An adaptive scenario for the origin if birds and of flight in birds. Proc. 3rd North Am. Paleont. Convention. Montreal 109–112 (1982). [Google Scholar]
- Persons S. W. IV, Currie P. J. & Norell M. A. Oviraptorosaur tail forms and functions. Acta Palaeont. Polonica. 59, 553–567, 10.4202/app.2012.0093 (2014). [DOI] [Google Scholar]
- Persons S. W. IV, Funston G. F., Currie P. J. & Norell M. A. A possible instance of sexual dimorphism in the tails of two oviraptorosaur dinosaurs. Sci. Rep. 5, 9472, 10.1038/srep09472 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitta E. T. Evidence for Sexual Dimorphism in the Plated Dinosaur Stegosaurus mjosi (Ornithischia, Stegosauria) from the Morrison Formation. (Upper Jurassic) of Western USA. PloS one 10(4), e0123503 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hone D. W. E., Naish D. & Cuthill I. C. Does Mutual sexual selection explain the evolution of head crests in pterosaurs and dinosaurs. Lethaia, 10.1111/j.1502-3931.2011.00300.x. [DOI] [Google Scholar]
- Lockley M. G., Cart K., Martin J., Prunty R., Houck K., Hups K., Lim J.-D., Kim K.-S., Houck K. & Gierlinski G. A bonanza of new tetrapod tracksites from the Cretaceous Dakota Group, western Colorado: implications for paleoecology. New Mexico Mus. Nat. Hist. Sci. Bull. 62, 393–409 (2014). [Google Scholar]
- Armstrong E. A. Bird display: an introduction to bird psychology. [381] Cambridge University Press (1942) [Google Scholar]
- Payne R. B. Sexual selection, Lek and arena behaviour, and sexual size dimorphism in birds. Ornith. Monogr. 33, 1–52 (1984). [Google Scholar]
- Seilacher A. Fossil behavior. Sci. Amer. 217, 72–80 (1967). [Google Scholar]
- Bromley R. G. Trace Fossils. [361] Chapman Hall (1996). [Google Scholar]
- Lockley M. G. Tracking Dinosaurs. [238] Cambridge University Press (1991). [Google Scholar]
- Lockley M. G. & Hunt A. P. A Review of Vertebrate Ichnofaunas of the Western Interior United States: Evidence and Implications in Caputo M. V., Peterson J. A. & Franczyk K. J. (eds.) Mesozoic Systems of the Rocky Mountain Region, United States. p. 95–108 (1994). [Google Scholar]
- Sternberg C. M. Dinosaur tracks from Peace River, British Columbia. Bull. Nat. Mus. Canada, 68, 59–85 (1932). [Google Scholar]
- Lockley M. G., Gierlinski G., Martin J. & Cart K. An unusual theropod tracksite in the Cretaceous Dakota Group, western Colorado: implications for ichnodiversity. New Mexico Mus. Nat. Hist. Sci. Bull. 62, 411–415 (2014). [Google Scholar]
- Thulborn R. A. Dinosaur tracks. [410] Chapman Hall (1990) [Google Scholar]
- Chiappe L. M., Schmitt J. G., Jackson F. D., Garrido A., Gingus L. & Grellet-Tinner G. Nest structure for sauropods: sedimentary criteria for recognition of dinosaur nesting traces. Palaios. 19, 89–95 (2004). [Google Scholar]
- Ramey E. M., Ramey R. R., Brown L. M. & Kelley S. T. Desert-dwelling African elephants (Loxodonta africana) in Namibia dig wells to purify drinking water. Pachyderm 53, 66–72 (2013). [Google Scholar]
- Clabby C. Forest Elephant Chronicles. Amer. Sci. 100, 416–417 (2012). [Google Scholar]
- Bonnan M. F. Pes anatomy in sauropod dinosaurs: implications for functional morphology, evolution, and phylogeny. In: Carpenter K., Tidwell V. editors. Thunder-lizards. Indiana University Press, Bloomington. p. 346–380 (2005). [Google Scholar]
- Fowler D. W. & Hall E. L. Scratch digging sauropods revisited. Hist. Biol. 23, 27–40 (2010). [Google Scholar]
- Lucas S. G. & Hunt A. P. Alamosaurus and the sauropod hiatus in the Cretaceous of the North American Western Interior. In Farlow J. O. ed. Paleobiology of the Dinosaurs. Geol. Soc. Am. Special Paper. 238, 75–85 (1989). [Google Scholar]
- Simpson E., Hilbert-Wolf H. L., Michael C., Wizevich M. C., Tindall S. E., Fasinski B. R., Storm L. P. & Needle M. D. Predatory digging behavior by dinosaurs. Geol. 38, 699–702 (2010). [Google Scholar]
- Varricchio D. J., Martin A. J. & Katsura Y. First trace and body fossil evidence of a burrowing, denning dinosaur. Proc. Royal Soc. Ser. B. 10.1098/rspb.2006.0443 (2007). [DOI] [PMC free article] [PubMed]
- Martin A. J. Dinosaurs without Bones. [460] Pegasus Books, London (2014). [Google Scholar]
- Ghoddousi A., Hamidi A. K., Ghadirian T., Ahayeri D., Hamzehpour M., Moshiri H., Zohrabi H. & Julayi L. Territorial marking by Persian Leopard (Panthera pardus saxicolor Pocock, 1927) in Bamu National Park, Iran. Zool. Middle East. 44, 101–103 (2008). [Google Scholar]
- Folch A. Handbook of the Birds of the World, vol. 1, Lynx Editions (1992). [Google Scholar]
- Long J. A. The dawn of the deed. The prehistoric origins of sex. University of Chicago Press (2012) [Google Scholar]
- Isles T. E. The socio-sexual behaviour of extant archosaurs: implications for understanding dinosaur behaviour. Hist. Biol. 21, 139–214 (2009). [Google Scholar]
- Merton D. V. Morris R. B. & Atkinson A. A. E. Lek behaviour in a parrot: the Kakapo Strigops habroptilus of New Zealand. Ibis, 126, 277–283 (1984). [Google Scholar]
- Jiguet F., Arroyo B. & Bretagnolle V. Lek mating systems: a case study in the Little Bustard Tetrax tetrax. Behav. Procsses, 51, 63–82 (2000). [DOI] [PubMed] [Google Scholar]
- Thery M. The evolution of leks through female choice: differential clustering and space utilization in six sympatric manakins. Behav. Ecol. Sociobiol. 30, 227–237 (1992) [Google Scholar]
- Naish D. The fossil record of bird behavior. Jl. Zoology, 10.111/jzo.12113 (2014). [DOI] [Google Scholar]
- Lockley M. G., Holbrook J., Hunt A. P., Matsukawa M. & Meyer C. The Dinosaur Freeway: a Preliminary Report on the Cretaceous Megatracksite, Dakota Group, Rocky Mountain Front Range and Highplains; Colorado, Oklahoma and New Mexico, in Flores R. (ed.), Mesozoic of the Western Interior, SEPM Midyear Meeting Fieldtrip Guidebook, 39–54 (1992). [Google Scholar]
- Lockley M. G., Holbrook J. Kukihara R. & Matsukawa M. 2006. An ankylosaur-dominated dinosaur tracksite in the Cretaceous Dakota Group of Colorado and its paleoenvironmental and sequence stratigraphic context. New Mexico Mus. Nat. Hist. Sci. Bull. 35, 95–104 (2006). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.