Abstract
Objective
The nigral lesion and the resulting contralateral motor signs of Parkinson's disease (PD) are remarkably asymmetric. This study investigated the prevalence of patients with “wrong‐sided” lesions, that is, patients with symptoms on the side ipsilateral to the predominant dopaminergic nigrostriatal deficit.
Methods
The analyzed sample included 434 early unmedicated PD patients from the Parkinson's Progression Markers Initiative database. Asymmetry indices of motor function and putamen [123I]FP‐CIT SPECT were calculated from the screening visit data.
Results
Ipsilateral deficits were unexpectedly common even when only patients with clear motor and dopaminergic asymmetries were included in the analysis (8.1%, n = 24/295). When patients with any asymmetry were included in the analysis, the prevalence of ipsilateral deficits was 15.4% (n = 65/423). Wrong‐sided symptoms were not associated with the PD motor subtype. However, the dataset was heavily biased toward tremor‐dominant patients (85% of patients). Right‐handed PD patients had predominantly right‐sided motor symptoms and left‐sided dopamine defects, whereas the effect was opposite in left‐handed patients (P = 0.005 and 0.028, respectively).
Interpretation
The results indicate that the side of the predominant motor symptoms and the corresponding side of the dopaminergic defects in PD are not random, but are directed by brain lateralization. Importantly, the traditional pathogenetic model of nigral degeneration causing primarily contralateral motor symptoms may be inadequate in many patients.
Introduction
Idiopathic early Parkinson's disease (PD) is a strikingly asymmetric condition compared to other degenerative parkinsonisms, such as multiple system atrophy, progressive supranuclear palsy or Lewy body disease.1 Contralateral asymmetries of motor symptoms in relation to basal ganglia dopaminergic deficits appear to be a fundamental aspect of the disease, although dopamine loss seems to be invariably bilateral,2 and asymmetries become less prominent over the course of the illness.3 The causes and evolution of these characteristic motor asymmetries and the dopaminergic interhemispheric differences in PD are largely unknown.
There are recent intriguing reports of tremor‐dominant PD patients who present symptoms on the “wrong‐side” of the body, that is, on the side ipsilateral to the predominant dopaminergic deficit.4, 5, 6 These patients, if fully characterized, could prove to be highly valuable in our understanding of how tremor and other cardinal symptoms of PD are differentially regulated via basal ganglia and cerebello‐thalamo‐cortical circuits.7
The focus of this study was to explore the relationships between motor and dopaminergic asymmetries in a large sample of PD patients from the Parkinson's Progression Markers Initiative (PPMI) database. The PPMI is an exceptionally large dataset that enables the detailed examination of several clinical and imaging factors in association with asymmetry.
Patients and Methods
Data used in the preparation of this article were obtained from the PPMI database (www.ppmi-info.org/data). For up‐to‐date information on the study, visit www.ppmi-info.org. ClinicalTrials.gov indentifier: NCT01141023. The study was approved in each site by a local institutional review board or an independent ethics committee. A written informed consent was obtained from all subjects participating in the study. The original dataset consisted of 447 unmedicated PD patients with 395 right‐handed, 39 left‐handed, 12 ambidextrous patients, and one patient with unknown handedness. For this analysis, the results of 395 right‐handed and 39 left‐handed PD patients were used. The main demographic and clinical characteristics of the studied samples according to handedness are presented in Table 1.
Table 1.
Differences between right‐handed and left‐handed PD patients and between patients with contralateral (correct‐sided) and ipsilateral (wrong‐sided) dopaminergic deficit
| Variable | Right‐handed | Left‐handed | P‐valuea | Correct‐sided | Wrong‐sided | P‐valuea |
|---|---|---|---|---|---|---|
| n | 395 | 39 | – | 271 | 24 | – |
| Age (years) | 62.9 (9.81) | 63.0 (10.5) | 0.94 | 60.9 (10.0) | 65.1 (10.4) | 0.049 |
| Gender (m/f) | 251/144 | 25/14 | 0.95 | 164/107 | 16/8 | 0.67 |
| Predominant side of motor symptoms (right/left) | 153/114 | 8/20 | 0.005 | 151/120 | 10/14 | 0.21 |
| Predominant side of DAT binding defect (right/left) | 112/155 | 18/10 | 0.028 | 120/151 | 10/14 | 0.83 |
| Motor symptom severity (MDS‐UPDRS Part III score) | 20.2 (8.95) | 22.7 (10.0) | 0.10 | 18.4 (7.4) | 17.8 (8.0) | 0.75 |
| Mean DAT binding (SBR) | 0.833 (0.308) | 0.851 (0.325) | 0.74 | 0.866 (0.289) | 0.759 (0.268) | 0.082 |
| DAT binding asymmetry (%)b | 29.7 (17.5) | 29.7 (15.9) | 1.0 | 36.8 (13.9) | 22.3 (10.7) | <0.0001 |
| Motor symptom asymmetry (%)c | 66.0 (31.4) | 58.8 (26.2) | 0.17 | 76.6 (23.4) | 69.6 (24.0) | 0.17 |
| MDS‐UPDRS Part III tremor score | 5.64 (3.74) | 5.79 (3.23) | 0.80 | 5.35 (3.35) | 6.33 (4.05) | 0.18 |
| MDS‐UPDRS Part III without tremord | 14.6 (8.08) | 16.9 (9.12) | 0.09 | 13.0 (6.84) | 11.5 (6.32) | 0.30 |
| PD motor subtype (TD/PIGD/intermediate) | 335/36/24 | 34/2/3 | 0.67 | 230/21/20 | 21/2/1 | 0.84 |
| Tremor asymmetry index | 0.066 (0.779) | −0.067 (0.774) | 0.31 | 0.030 (0.837) | 0.035 (0.748) | 0.88 |
| Bradykinesia‐rigidity asymmetry index | 0.096 (0.747) | −0.135 (0.660) | 0.064 | 0.100 (0.808) | −0.192 (0.784) | 0.090 |
| Symptoms on correct/wrong body side | 245/22 | 26/2 | 1.0 | – | – | – |
| Handedness (r/l) | – | – | – | 245/26 | 22/2 | 1.0 |
Two‐hundred and ninety‐five PD patients with clearly lateralized tracer uptake and clearly lateralized motor symptoms were included in the analysis of corrected‐sided and wrong‐sided symptoms. Values are means (SD) or n. PD, Parkinson's disease; DAT, dopamine transporter; SBR, specific binding ratio; TD, tremor‐dominant subtype; PIGD, postural instability and gait disorder subtype.
Independent samples t‐test or chi‐square test.
([higher − lower putamen SBR]/higher putamen SBR) × 100.
([worse − better side MDS‐UPDRS]/better side MDS‐UPDRS) × 100.
MDS‐UPDRS Part III total score − MDS‐UPDRS Part III tremor score (11 items).
Only the PPMI screening visit data were used, including baseline [123I]FP‐CIT SPECT imaging (patients with available clinical and imaging data included). Medications that might have interfered with dopamine transporter (DAT) SPECT imaging were restricted 6 months prior to imaging. Central SPECT core laboratory of the PPMI (Institute for Neurodegenerative Disorders, New Haven, CT) generated striatal specific binding ratios (SBRs) using a previously described process8 that included iterative reconstruction from raw projection data, site‐specific attenuation correction using phantom data, spatial normalization of images to Montreal Neurological Institute (MNI) space and region‐of‐interest (ROI) analyses using a fixed template (right and left caudate, right and left putamen, occipital cortex) for SBR calculations ([striatal region count density/occipital count density] − 1). For ROI analyses, eight striatal planes were averaged into a single slice image. Structural brain MRI scans were also performed at baseline. For this study, left and right putamen SBRs were used for calculations. Several factors possibly associated with asymmetries were derived from the clinical and imaging data (Table 2). Significant motor asymmetry was defined as: 0.3 < asymmetry index < −0.30 (asymmetry index = MDS‐UPDRS Part III right − left score/right + left score). Significant DAT asymmetry was defined as: 0.05 < asymmetry index < −0.05 (asymmetry index = right − left putamen SBR/right + left putamen SBR).
Table 2.
Explanations and equations of the investigated clinical and imaging factors
| Variable | Explanation/equation |
|---|---|
| Mean DAT binding | (higher putamen SBR + lower putamen SBR)/2 |
| DAT binding asymmetry index | (right − left putamen SBR)/(right + left putamen SBR) |
| Motor symptom severity | Total score of the MDS‐UPDRS motor part (part III) |
| PD motor subtype (TD/PIGD/intermediate) | The ratio of 11 tremor and 5 PIGD items in MDS‐UPDRS. Ratio of ≥1.15 was classified as tremor‐dominant, ratio ≤0.90 was classified as PIGD and 0.90 < ratio < 1.15 was classified as intermediate15 |
| Motor symptom asymmetry index |
(right − left side score)/(right + left side score) Score of lateralized items in MDS‐UPDRS Part III was calculated separately for the left and right side (rigidity in upper extremity + rigidity in lower extremity + finger tapping + hand movement + pronation/supination + toe tapping + leg agility + hand postural tremor + hand kinetic tremor + hand rest tremor + foot rest tremor) |
| Predominant side of motor symptoms (right/left/symmetric) |
Right = (MDS‐UPDRS Part III right − left side score/right + left side score) > 0.3 Left = (MDS‐UPDRS Part III right − left side score/right + left side score) < −0.3 Symmetric = −0.3 ≤ (MDS‐UPDRS Part III right − left side score/right + left side score) ≤0.3 |
| Predominant side of DAT binding defect |
Right = (right − left putamen SBR/right + left putamen SBR) > 0.05 Left = (right − left putamen SBR/right + left putamen SBR) < −0.05 |
| Tremor asymmetry index |
(right − left side score)/(right + left side score) Score of lateralized tremor items in MDS‐UPDRS Part III was calculated separately for the left and right side (hand postural tremor + hand kinetic tremor + hand rest tremor + foot rest tremor) |
| Bradykinesia‐rigidity asymmetry index |
(right − left side score)/(right + left side score) Score of lateralized bradykinesia or rigidity items in MDS‐UPDRS Part III was calculated separately for the left and right side (rigidity in upper extremity + rigidity in lower extremity + finger tapping + hand movement + pronation/supination + toe tapping + leg agility) |
DAT, dopamine transporter; SBR, specific binding ratio; TD, tremor‐dominant subtype; PIGD, postural instability and gait disorder subtype.
Chi‐squared tests were used to investigate categorical variables, and independent samples t‐tests were used to investigate continuous variables between handedness groups and between patients with contralateral and ipsilateral dopaminergic deficits. The level of two‐tailed statistical significance was set at P < 0.05.
Results
Of 434 patients, 353 (81.3%) patients were classified as having clear motor asymmetry (0.30 < asymmetry index < −0.30). In these patients, the mean (SD, range) side‐to‐side difference in MDS‐UPDRS Part III was 8.7 points (3.4, 2–20). Similarly, 341 (78.6%) patients were classified as having clear dopaminergic asymmetry (0.05 < asymmetry index < −0.05). In these patients, the mean (SD, range) relative side‐to‐side difference in putamen SBR was 34.9% (14.4, 10.7–80.3). Of 434 patients, 295 (68.0%) patients were classified as having both clear motor and dopaminergic asymmetry.
There was a discrepancy in laterality in 24/295 (8.1%) patients (lower putamen uptake ipsilateral to the predominant symptoms) (Fig. 1A and B). Ipsilateral dopaminergic defects were associated with smaller DAT binding asymmetries (t 293 = −4.98, P < 0.0001) and older age (t 293 = 1.98, P = 0.049) but not with handedness, motor subtype, predominant side of motor symptoms or other investigated factors (Table 1). The data broken down by handedness, predominant side of motor symptoms, and motor subtype are presented in Table 3. Ipsilateral deficits were observed over a wide range of motor and dopaminergic asymmetries (Fig. 1A and B). The prevalence of ipsilateral deficits was similar when only tremor scores (8.5%) or bradykinesia/rigidity scores (7.9%) of the MDS‐UPDRS Part III were used (Fig. 1C and D). When all patients with any asymmetry in MDS‐UPDRS and DAT binding were included in the analysis (n = 423), the prevalence of ipsilateral deficits was 15.4% (n = 65/423).
Figure 1.
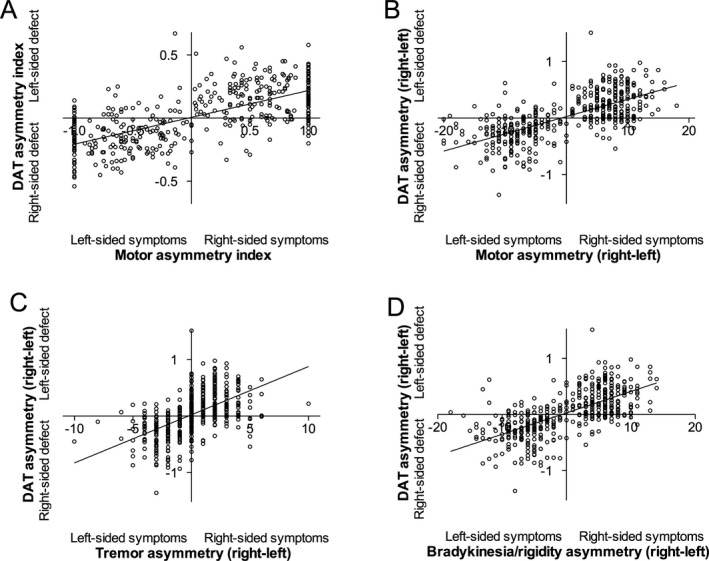
The relationship between motor and dopaminergic asymmetry in patients with early Parkinson's disease. (A) Correlation between motor and dopaminergic asymmetry indices ([right − left]/[right + left]). Values −1.0 or 1.0 denote complete motor asymmetry (Hoehn and Yahr stage 1). (B) Correlation between motor and dopaminergic absolute asymmetry scores (right–left). (C) Correlation between lateralized tremor scores and dopaminergic asymmetry (right–left). (D) Correlation between lateralized bradykinesia/rigidity scores and dopaminergic asymmetry (right–left).
Table 3.
Patients with clearly asymmetric motor symptoms and clearly asymmetric tracer uptake (n = 295) broken down by handedness, predominant side of motor symptoms and motor subtype
| Right‐handed Right‐sided | Right‐handed Left‐sided | Left‐handed Right‐sided | Left‐handed Left‐sided | |||||
|---|---|---|---|---|---|---|---|---|
| TD | PIGD/IM | TD | PIGD/IM | TD | PIGD/IM | TD | PIGD/IM | |
| n (total) | 124 | 29 | 104 | 10 | 7 | 1 | 16 | 4 |
| n (symptoms on wrong body side) | 8 | 2 | 11 | 1 | 0 | 0 | 2 | 0 |
| Prevalence of ipsilateral deficits % | 6.5 | 6.9 | 10.6 | 10.0 | 0 | 0 | 12.5 | 0 |
TD, tremor‐dominant; PIGD/IM, PIGD or intermediate.
There were more right‐handed PD patients with predominantly right‐sided motor symptoms compared to the number of right‐handed patients with left‐sided motor symptoms, and the effect was opposite in left‐handed PD patients (P = 0.005, Table 1). Similarly, there were more right‐handed PD patients with predominantly left‐sided DAT decreases compared to the number of right‐handed patients with right‐sided DAT decreases, and the effect was opposite in left‐handed PD patients (P = 0.028, Table 1).
Discussion
The primary results indicate the following: (1) ipsilateral dopaminergic deficits in PD are unexpectedly common in early PD with a prevalence of 8.1% in patients with clear asymmetries, and (2) the side of the predominant motor symptoms and the corresponding side of the dopaminergic defects in PD are not random but are directed by brain lateralization.
There is recent work to suggest that a proportion of PD patients do not present their primary symptoms on the side of the body contralateral to the predominant dopamine deficit, as would be expected. Aquirregomozcorta and colleagues reported three cases of PD with rest tremor and ipsilateral defects in DAT SPECT and encouraged others to report similar cases.4 Erro et al. further analyzed a dataset of 46 [123I]FP‐CIT scans of PD patients and reported a prevalence of 4.3% of scans that were named SWpIDDs (Scans With predominant Ipsilateral Dopaminergic Deficit).5 Other tremulous SWpIDD cases have also been reported.6 According to the data presented here, ipsilateral deficits are frequent in the DAT SPECT scans of PD patients. Discrepancies appear common even in patients with clear motor and DAT asymmetries. In the present analysis, artifacts due to head tilt can be considered unlikely because all scans were spatially normalized to the same orientation before asymmetry calculations. However, it is important to bear in mind that the prevalence of ipsilateral deficits is dependent on the criteria of significant asymmetry and the scanning was performed in different institutions using different scanners with different resolutions. Slight side‐to‐side differences in DAT SPECT and MDS‐UPDRS may merely be noise that reflects inaccuracies in the methodology. Therefore, the prevalence reported here as the primary result (8.1%) was derived from a subsample of patients that showed very clear motor and DAT asymmetries. Nearly one‐third of patients were excluded from this analysis due to milder asymmetries. The results show that PD patients with wrong‐sided symptoms are older, and, in particular, they have lower degrees of DAT binding asymmetry. From a practical point of view, the current results demonstrate that a discrepancy between motor symptoms and DAT binding in clinical [123I]FP‐CIT SPECT should not be interpreted as suggesting less likely diagnosis of PD if the dopaminergic loss is clear. From a theoretical point of view, they imply that the model of contralateral dopaminergic loss that automatically mirrors lateralized motor symptoms may be insufficient. The degree of midline crossing of nigrostriatal pathways seems to be highly variable in early PD. A fraction of corticospinal neurons is known to descend ipsilaterally and these neurons are engaged during a voluntary motor behavior.9 Although ipsilateral motor pathways have been poorly studied in PD, unilateral deep brain stimulation improves also ipsilateral symptoms in PD10 underlining the clinical importance of the issue.
The present results concerning handedness fit with the theory that the initial insult in early PD, regardless of the pathogenetic mechanism, is not random but is related to hand dominance. A meta‐analysis previously provided evidence of an association between the predominant side of PD symptoms and hand dominance.11 The clinical part of this study is in line with this association by showing that the predominant motor symptom side is related to handedness. As for dopamine function an earlier study by Scherfler and colleagues with 68 right‐handed PD patients reported that right‐handed patients had lower DAT binding in the left striatum, in accordance with the clinical asymmetry.12 This study replicates previous motor and dopaminergic results in right‐handed PD patients and extends the findings to left‐handed patients with reversed asymmetry. The results provide support that the side of the lesion in PD is not due to random choice, but it is directed by hemispheric dominance. Importantly, this explanation is only partial, as there is a considerable proportion of PD patients who do not follow this pattern.
The generalizability of these results is subject to certain limitations. Being limited to DAT binding in the putamen, this study lacks information outside the primary motor‐related dopaminergic pathway, and extrastriatal cortical measurements of monoaminergic function in relation to asymmetries are required. It should be noted that [123I]FP‐CIT is also a serotonergic tracer although serotonin transporter is not a major determinator of binding in striatal regions. Handedness was self‐reported without actual measurements of hand dominance. Although self‐reported handedness appears valid for research purposes,13 it is a crude categorical variable that probably does not sufficiently represent the continuum of motor cerebral lateralization. Finally, a selection bias in the PPMI dataset seems likely as 85% of patients were classified as tremor‐dominant and only 15% were classified as postural instability and gait disorder subtype (PIGD)/intermediate. Included patients in PPMI were expected to remain unmedicated for 6 months, which probably favored patients with the less progressive tremor‐dominant subtype. As a result, the proportion of tremor‐dominant patients is much higher in PPMI compared to previously reported.14 This unbalance could have affected prevalence estimations in different motor phenotypes and the results of this study must be interpreted with caution as they primarily reflect asymmetries in tremor‐dominant patients. Furthermore, due to intrinsic properties in MDS‐UPDRS (possibly different floor or ceiling effects in tremor and nontremor motor scores), it is possible that the postulated differences in asymmetry between tremor‐dominant and PIGD patients can be detected in other samples with advanced PD patients.
In summary, the current data highlight the high number of PD patients who have motor symptoms on the wrong side of the body in relation to their dopaminergic degeneration. The data do not suggest a specific tremor‐related SWpIDD phenotype but confirmatory studies are required. Further studies are also needed to investigate the role of ipsilateral corticospinal pathways in the flipped asymmetry at the early stages of the disease.
Conflict of Interest
Dr. Kaasinen reports no relevant conflicts of interest.
References
- 1. Djaldetti R, Ziv I, Melamed E. The mystery of motor asymmetry in Parkinson's disease. Lancet Neurol 2006;5:796–802. [DOI] [PubMed] [Google Scholar]
- 2. Yagi S, Yoshikawa E, Futatsubashi M, et al. Progression from unilateral to bilateral parkinsonism in early Parkinson disease: implication of mesocortical dopamine dysfunction by PET. J Nucl Med 2010;51:1250–1257. [DOI] [PubMed] [Google Scholar]
- 3. Nandhagopal R, Kuramoto L, Schulzer M, et al. Longitudinal progression of sporadic Parkinson's disease: a multi‐tracer positron emission tomography study. Brain 2009;132(Pt 11):2970–2979. [DOI] [PubMed] [Google Scholar]
- 4. Aguirregomozcorta M, Stamelou M, Antonini A, et al. Patients with rest‐tremor and scans with ipsilateral dopaminergic deficit. J Neurol 2013;260:1132–1135. [DOI] [PubMed] [Google Scholar]
- 5. Erro R, Barone P, Vicidomini C, et al. Patients with Parkinson's disease and scans with (predominant) ipsilateral dopaminergic deficit. J Neurol 2013;260:2405–2406. [DOI] [PubMed] [Google Scholar]
- 6. Hoshiyama E, Kadowaki T, Nakamura A, et al. The decreasing of dopamine‐transporter uptake on the right ipsilateral side of tremor in a patient with Parkinson's disease [abstract]. Mov Disord 2015;30(Suppl 1):996.25778823 [Google Scholar]
- 7. Helmich RC, Hallett M, Deuschl G, et al. Cerebral causes and consequences of parkinsonian resting tremor: a tale of two circuits? Brain 2012;135(Pt 11):3206–3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Prashanth R, Dutta Roy S, Mandal PK, Ghosh S. Automatic classification and prediction models for early Parkinson's disease diagnosis from SPECT imaging. Expert Syst Appl 2014;41:3333–3342. [Google Scholar]
- 9. Tazoe T, Perez MA. Selective activation of ipsilateral motor pathways in intact humans. J Neurosci 2014;34:13924–13934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shemisa K, Hass CJ, Foote KD, et al. Unilateral deep brain stimulation surgery in Parkinson's disease improves ipsilateral symptoms regardless of laterality. Parkinsonism Relat Disord 2011;17:745–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. van der Hoorn A, Burger H, Leenders KL, de Jong BM. Handedness correlates with the dominant Parkinson side: a systematic review and meta‐analysis. Mov Disord 2012;27:206–210. [DOI] [PubMed] [Google Scholar]
- 12. Scherfler C, Seppi K, Mair KJ, et al. Left hemispheric predominance of nigrostriatal dysfunction in Parkinson's disease. Brain 2012;135(Pt 11):3348–3354. [DOI] [PubMed] [Google Scholar]
- 13. Reiss M, Reiss G, Freye HA. Some aspects of self‐reported hand preference. Percept Mot Skills 1998;86(3 Pt 1):953–954. [DOI] [PubMed] [Google Scholar]
- 14. Von Coelln FR, Barr E, Gruber‐Baldini A, et al. Motor subtypes of Parkinson disease are unstable over time. Neurology 2015;84:S48.002. [Google Scholar]
- 15. Stebbins GT, Goetz CG, Burn DJ, et al. How to identify tremor dominant and postural instability/gait difficulty groups with the Movement Disorder Society Unified Parkinson's Disease Rating Scale: comparison with the Unified Parkinson's Disease Rating Scale. Mov Disord 2013;28:668–670. [DOI] [PubMed] [Google Scholar]


