Abstract
Objective
Widespread implementation of cerebrospinal fluid (CSF) biomarkers of Alzheimer's disease (AD) in clinical settings requires improved accuracy for diagnosis of prodromal disease and for distinguishing AD from non‐AD dementias. Novel and promising CSF biomarkers include neurogranin, a marker of synaptic degeneration, and YKL‐40, a marker of neuroinflammation.
Methods
CSF neurogranin and YKL‐40 were measured in a cohort of 338 individuals including cognitively healthy controls and patients with stable mild cognitive impairment (sMCI), MCI who later developed AD (MCI‐AD), AD dementia, Parkinson's disease dementia (PDD), dementia with Lewy bodies (DLB), vascular dementia (VaD), and frontotemporal dementia (FTD). The diagnostic accuracy of neurogranin and YKL‐40 were compared with the core AD biomarkers, β‐amyloid (Aβ42 and Aβ40) and tau.
Results
Neurogranin levels were increased in AD and decreased in non‐AD dementia compared with healthy controls. As a result, AD patients showed considerably higher CSF levels of neurogranin than DLB/PDD, VaD and FTD patients. CSF YKL‐40 levels were increased in AD compared with DLB/PDD but not with VaD or FTD. Neither CSF neurogranin nor YKL‐40 levels differed significantly between sMCI patients and MCI‐AD patients. Both biomarkers correlated positively with CSF Aβ40 and tau. CSF neurogranin and YKL‐40 could separate AD dementia from non‐AD dementias (neurogranin, area under the curve [AUC] = 0.761; YKL‐40, AUC = 0.604; Aβ42/neurogranin, AUC = 0.849; Aβ42/YKL‐40, AUC = 0.785), but the diagnostic accuracy was not better compared to CSF Aβ and tau (Aβ42, AUC = 0.755; tau AUC = 0.858; Aβ42/tau, AUC = 0.895; Aβ42/Aβ40, AUC = 0.881). Similar results were obtained when separating sMCI from MCI‐AD cases.
Interpretation
CSF neurogranin and YKL‐40 do not improve the diagnostic accuracy of either prodromal AD or AD dementia when compared to the core CSF AD biomarkers. Nevertheless, the CSF level of neurogranin is selectively increased in AD dementia, whereas YKL‐40 is increased in both AD and FTD suggesting that synaptic degeneration and glial activation may be important in these neurodegenerative conditions.
Introduction
Amyloid‐β (Aβ) containing neuritic plaques and neurofibrillary tangles composed mainly of hyperphosphorylated tau are the neuropathological hallmarks of Alzheimer's disease (AD). According to the amyloid cascade hypothesis that has dominated the field of AD research for the last two decades, abnormal accumulation of Aβ in the brain is the primary initiator of the disease‐associated pathophysiological processes.1 Disappointingly, however, phase III clinical trials in patients with moderate to mild disease have failed to show clinical benefit of drugs reducing Aβ plaque burden.2 The failure has been partly attributed to the fact that new therapies were initiated in the late stages of the disease and to the relatively high rate of misdiagnosis in patients included in the trials thus highlighting the need for early and accurate disease biomarkers.3
Cerebrospinal fluid (CSF) Aβ and tau are at present the most specific fluid biomarkers of AD reflecting amyloid plaque load and severity of neurodegeneration, respectively.4 Decreased CSF levels of Aβ42 (or the Aβ42/Aβ40 ratio) in combination with elevated tau (total or phosphorylated [p‐tau]) levels predict with high accuracy future development of AD in patients with mild cognitive impairment (MCI) and also have some diagnostic value for differentiating AD from non‐AD dementias.5 Nonetheless, further improvement of diagnostic accuracy, especially to differentiate AD from other dementias, would be of value in the clinic. In this context, there is a growing interest in developing novel biomarkers that would monitor other aspects of AD pathology such as for example synaptic dysfunction and neuroinflammation3 and two such biomarkers, neurogranin and YKL‐40, have recently emerged. Neurogranin is calmodulin‐binding postsynaptic protein regulating synaptic plasticity and learning.6, 7 Several studies demonstrated that neurogranin levels are reduced in the brain8, 9 but increased in CSF of AD patients.10, 11 Interestingly, high CSF levels of neurogranin were reported in MCI patients progressing to AD compared with cognitively stable MCI (sMCI) patients and control individuals.10, 11, 12, 13 YKL‐40 (chitinase‐3 like‐1, cartilage glycoprotein‐39) is a secreted glycoprotein considered as a potential marker of ongoing inflammations in a variety of human diseases.14, 15, 16, 17 CSF levels of YKL‐40 appear to be elevated in AD, vascular dementia (VaD) and frontotemporal dementia (FTD)18, 19 but not in Parkinson's disease (PD) or dementia with Lewy bodies (DLB).20 CSF YKL‐40 is also increased in normal aging and in individuals with preclinical AD.21, 22
Despite these encouraging findings it remains to be established if neurogranin and YKL‐40 could provide greater diagnostic accuracy for prediction and differential diagnosis of AD than the core AD CSF biomarkers, Aβ and tau. To this end we compared the diagnostic performance of CSF neurogranin, YKL‐40, Aβ42, Aβ40 and tau in a cohort of 338 individuals including cognitively healthy controls and patients with sMCI, MCI who later developed AD, AD dementia, Parkinson's disease dementia (PDD), DLB, VaD and FTD.
Materials and Methods
Subjects and methods
This study was performed at the Memory Clinic of Skåne University Hospital in Malmö, Sweden. Seventy‐four patients with AD, 47 patients with DLB/PDD, 34 patients with VaD, 33 patients with FTD and 53 healthy controls were included in this study. We also included 97 individuals with a baseline diagnosis of MCI of which 35 had converted to AD (MCI‐AD), while 62 remained cognitively stable (sMCI). The median clinical follow‐up period for sMCI group was 5.8 years (3.0–9.6). All subjects were assessed by medical doctors with extensive experience in cognitive disorders. All patients with a clinical syndrome of dementia met the DSM‐IIIR criteria for dementia23 combined with the NINCDS‐ADRDA criteria for AD,24 the NINDS‐AIREN criteria for VaD,25 criteria of probable DLB according to the 2005 consensus criteria26 or the 1998 consensus criteria for FTD.27 All the individuals in the FTD group were diagnosed with behavioral variant FTD except for one patient who had semantic dementia. Patients with MCI at baseline had to fulfill the criteria advocated by Petersen.28 The control population consisted of healthy elderly volunteers, who were recruited in the city of Malmö, Sweden. Inclusion criteria were (1) absence of memory complaints or any other cognitive symptoms; (2) preservation of general cognitive functioning; and (3) no active or previous significant neurological or psychiatric disease. The characteristics of the study cohort are given in Table 1.
Table 1.
Demographic data, clinical characteristics and CSF levels of neurogranin, and YKL‐40
| Control (n = 53) | sMCI (n = 62) | MCI‐AD (n = 35) | AD (n = 74) | DLB/PDD (n = 47) | VaD (n = 34) | FTD (n = 33) | |
|---|---|---|---|---|---|---|---|
| Age | 75.3 (6.4) | 69.2 (7.5)a | 75.0 (7.6)b | 76.4 (7.4)b | 74.5 (6.3)b | 75.7 (7.8)b | 71.7 (6.7)c , d , e |
| Sex (% female) | 70% | 56% | 66% | 68% | 40%d , f , g | 47%c , h | 51% |
| MMSE | 28.6 (1.8) | 28.2 (1.2) | 26.4 (1.7)a , b | 19.4 (3.3)a , b , i | 21.9 (5.1)a , b , d , i | 21.5 (4.4)a , b , d , i | 21.8 (6.6)a , b , i |
| APOE 1 or 2 ε4 alleles | 31% | 53% | 80%a , j | 65%a , k | 54%c , g | 24%i , k , l , m | 27%1 |
| Neurogranin, pg/mL | 557 (328) | 542 (279) | 652 (348) | 711 (404)c | 480 (312)d , g | 313 (150)a , b , i , l , m | 370 (194)f , i , j , l |
| YKL‐40, ng/mL | 200 (64) | 184 (69) | 219 (59) | 248 (70)a , b | 217 (65)h | 221 (69) | 222 (59)f , j , n |
| Aβ42, pg/mL | 668 (287) | 486 (200)a | 314 (79)a , b | 260 (106)a , b | 340 (173)a , b , h | 397 (187)a , d , k | 676 (289)b , i , l , o , q |
| Aβ40, pg/mL | 5136 (1531) | 3821 (1377)a | 4219 (1327)f | 3892 (1383)a | 3170 (1137)a , h , j , r | 3209 (1277)a , h , k , r | 4470 (1550)k , h , o , q |
| Tau, pg/mL | 467 (191) | 437 (175) | 643 (224)a , b | 768 (267)a , b , r | 472 (171)i . l | 436 (191)i . l | 382 (205)i . l |
| Aβ42/neurogranin | 1.57 (0.99) | 1.10 (0.60)f | 0.65 (0.46)a , k | 0.50 (0.40)a , b | 0.91 (0.55)a , h | 1.62 (1.20)j , i . l , o | 2.40 (2.03)a , b , i . l , o |
| Aβ42/YKL‐40 | 3.82 (2.23) | 2.83 (1.18)a | 1.57 (0.76)a , b | 1.10 (0.47)a , b | 1.69 (0.90)a , b , h | 1.93 (0.95)a , d , k | 3.08 (1.24)f , i , l , o , p |
| Aβ42/Aβ40 | 0.13 (0.04) | 0.13 (0.04) | 0.08 (0.02)a , b | 0.07 (0.02)a , b | 0.11 (0.04)a , i , k , l | 0.13 (0.04)i , l | 0.15 (0.04)e , i , j , l , o |
| Aβ42/tau | 1.66 (0.82) | 1.25 (0.56)c | 0.54 (0.26)a , k | 0.38 (0.23)a , b | 0.82 (0.50)a | 1.02 (0.54)c , h | 2.57 (3.45)b , f , i , l , o , q |
Data are shown as mean (SD) unless otherwise specified. CSF, cerebrospinal fluid; sMCI, stable mild cognitive impairment; MCI‐AD, mild cognitive impairment that subsequently converted to AD; AD, Alzheimer's disease; DLB/PDD, dementia with Lewy bodies or Parkinson's diseases dementia; VaD, vascular dementia; FTD, frontotemporal dementia; MMSE, Mini Mental State Examination.
1 APOE data was only available from 11 FTD patients.
Demographic factors and clinical characteristics were compared using Students t‐test, one‐way ANOVA and chi‐square tests. CSF biomarkers were analyzed with univariate general linear models controlling for age and gender; acompared with controls, P < 0.001; bcompared with sMCI, P < 0.001; ccompared with controls, P < 0.05; dcompared with AD, P < 0.01; ecompared with VaD, P < 0.05; fcompared with controls, P < 0.01; gcompared with MCI‐AD, P < 0.05; hcompared with AD, P < 0.05; icompared with MCI‐AD, P < 0.001; jcompared with sMCI, P < 0.01; kcompared with sMCI, P < 0.05; lcompared with AD, P < 0.001; mcompared with DLB/PDD, P < 0.01; ncompared with DLB/PDD, P < 0.05; ocompared with DLB/PDD, P < 0.001; pcompared with VaD, P < 0.01; qcompared with VaD, P < 0.001; rcompared with MCI‐AD, P < 0.01.
The design of this study has been approved by the Local Ethics Committee of Lund University, Sweden and the study procedure was conducted in accordance with the Helsinki Declaration. All study participants gave their informed consent to research.
CSF sampling and biological assays
For all patients and controls, blood plasma and CSF samples were drawn at some point between 8 am and 12 am. The procedure and analysis of the CSF followed the Alzheimer's Association Flow Chart for CSF biomarkers.29
CSF neurogranin was measured using an in‐house sandwich enzyme‐linked immunosorbent (ELISA) assay, as described previously.11 CSF levels of YKL‐40 were measured using a commercial available ELISA kit (R&D Systems, Minneapolis, MN). CSF Aβ42, Aβ40 and tau were analyzed using Euroimmun immunoassay (EUROIMMUN AG, Lübeck, Germany). All measurements were performed by board‐certified laboratory technicians who were blinded to clinical data.
Statistical analysis
SPSS (IBM, Armonk, NY) and R version 3.1.230 were used for statistical analysis. Neurogranin and YKL‐40 levels were not normally distributed and therefore ln‐transformed before analysis. Neurogranin levels were below the detection limit of the assay for 9 cases (3%), which were assigned concentration of 120 pg/mL, equal to the lower detection limit of the assay. YKL‐40 levels were above the detection limit of the assay for nine cases (3%), which were assigned concentration of 400 ng/mL, equal to the higher detection limit of the assay.
We used Students t‐test, one‐way analysis of variance (ANOVA) and chi‐square tests to compare demographic factors and clinical characteristics (age, gender, Mini Mental State Examination [MMSE], APOE ε4). There were significant differences in age and gender between the diagnostic groups (Table 1). Therefore, for group‐wise comparisons of neurogranin and YKL‐40, we used univariate general linear models controlling for age and gender. sMCI patients, MCI patients who later progressed to AD (MCI‐AD) and AD dementia patient were included in all the analysis as separate diagnostic categories. The diagnostic accuracy of CSF biomarkers was assessed with the receiving operating characteristic (ROC) curve analysis. Differences in the area under the ROC curve (AUC) of two ROC curves were compared using bootstrap method.31 P ≤ 0 .05 was considered statistically significant.
Results
The demographics are given in Table 1. CSF levels of YKL‐40 correlated positively with age in controls (r = 0.382, P = 0.005) as well as in AD patients (r = 0.309, P = 0.007). In the AD group, women showed slightly higher neurogranin levels than men (t (72) = 2.18, P = 0.033), but this was not the case in the controls. We did not find any differences in either neurogranin or YKL‐40 concentrations between APOE ε4 allele carriers and non‐carriers (data not shown).
CSF levels of neurogranin and YKL‐40 in different diagnostic groups
The CSF levels of neurogranin were increased in patients with AD dementia (P = 0.027) and at the same time decreased in patients with VaD (P < 0.001) and FTD (P = 0.006) compared to cognitively healthy controls (Fig. 1A). Neurogranin levels were higher in AD dementia than in non‐AD dementias, that is, DLB/PDD (P = 0.002), VaD (P < 0.001) and FTD (P < 0.001).
Figure 1.
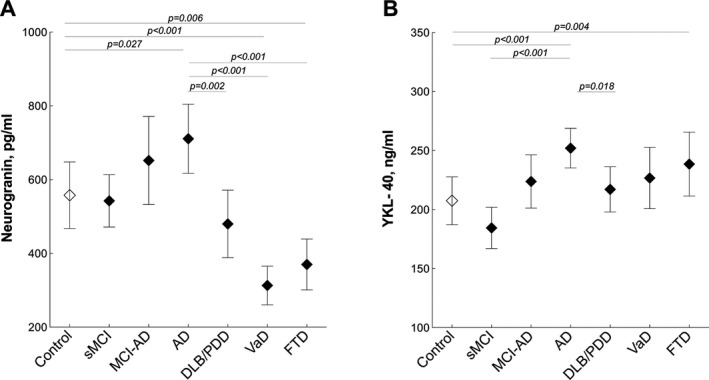
Cerebrospinal fluid (CSF) levels of neurogranin and YKL‐40. Neurogranin (A) and YKL‐40 (B) were measured in the CSF of patients with Alzheimer's disease (AD), stable mild cognitive impairment (sMCI), MCI that progressed to AD (MCI‐AD), dementia with Lewy bodies or Parkinson's disease dementia (DLB/PDD), vascular dementia (VaD), frontotemporal dementia (FTD) and cognitively healthy controls. Data are presented as mean ± 95% confidence interval; P values are from univariate general linear models controlling for age and gender.
When compared with cognitively healthy controls, the CSF levels of YKL‐40 were increased in patients with AD dementia (P < 0.001) and FTD (P = 0.004). CSF YKL‐40 levels were also increased in patients with AD dementia compared with cognitively stable cases with MCI (P < 0.001) and DLB/PDD (P = 0.018). The patients with MCI, who subsequently developed AD dementia (MCI‐AD), did not have higher levels of YKL‐40 when compared to the cognitively stable patients with MCI (P = 0.085) or AD patients (P = 0.146) (Fig. 1B).
Associations with CSF Aβ and tau
In order to establish if changes in CSF levels of neurogranin and YKL‐40 are related to amyloid and/or tau pathology in AD, we examined associations between these biomarkers and CSF Aβ and tau in cognitively healthy controls and in patients with AD dementia or MCI who later developed AD (MCI‐AD). Both neurogranin and YKL‐40 correlated with tau as well as with Aβ40 in all studied diagnostic groups (Table 2). While we also found that neurogranin and YKL‐40 positively correlated with Aβ42 in AD patients, this was not the case in any other diagnostic groups (Table 2). Finally, there were significant negative associations between the Aβ42/Aβ40 ratio and both neurogranin and YKL‐40 in MCI‐AD patients whereas in control and AD groups the ratio only correlated with neurogranin.
Table 2.
Associations between CSF neurogranin, YKL‐40 and the core AD biomarkers
| Tau | Aβ42 | Aβ40 | Aβ42/Aβ40 | |||||
|---|---|---|---|---|---|---|---|---|
| Neurogranin | YKL‐40 | Neurogranin | YKL‐40 | Neurogranin | YKL‐40 | Neurogranin | YKL‐40 | |
| Controls | 0.706 *** | 0.358 ** | 0.197 | 0.053 | 0.590 *** | 0.308 * | −0.343 * | −0.251 |
| MCI‐AD | 0.708 *** | 0.592 *** | 0.242 | 0.012 | 0.646 *** | 0.509 ** | −0.530 ** | −0.630 *** |
| AD | 0.719 *** | 0.554 *** | 0.257 * | 0.246 * | 0.625 *** | 0.446 *** | −0.365 *** | −0.182 |
Data are derived from linear regression models adjusting age and gender. CSF, cerebrospinal fluid; AD, Alzheimer's disease; MCI‐AD, mild cognitive impairment that subsequently converted to AD.
Significant results are shown in bold; *P ≤ 0.5; **P ≤ 0.01; and ***P ≤ 0.001.
CSF neurogranin and YKL‐40 as clinical biomarkers of AD dementia
We next sought to determine whether CSF neurogranin and YKL‐40 could improve the differential diagnosis of AD dementia when compared to the standard AD biomarkers, CSF Aβ and tau. Given that the ratios of Aβ42/Aβ40 or Aβ42/tau perform better than Aβ42 or tau alone,32 we also assessed the ability of Aβ42/neurogranin and Aβ42/YKL‐40 ratios to distinguish different dementia groups.
The results of the ROC analysis are summarized in Table 3. For all examined diagnostic groups, the Aβ42/neurogranin and Aβ42/YKL‐40 ratios showed improved accuracy (larger AUC) in comparison with neurogranin and YKL‐40, respectively. When comparing individual AUC, we found that the Aβ42/neurogranin ratio was not significantly different from the Aβ42/Aβ40 ratio and performed poorer than the Aβ42/tau ratio when separating patients with AD dementia from patients with non‐AD dementias (Table 3). The results were similar for differentiating patients with AD dementia from MCI patients, who later developed AD dementia (MCI‐AD).
Table 3.
ROC analysis of the CSF biomarkers
| AUC, 95% CI | AUC difference versus Aβ42/Aβ40 (P‐value) | AUC difference versus Aβ42/tau (P‐value) | |
|---|---|---|---|
| AD versus non‐AD dementias | |||
| Neurogranin | 0.761, 0.688–0.834 | ||
| YKL‐40 | 0.604, 0.521–0.687 | ||
| Aβ42/neurogranin | 0.849, 0.792–0.906 | −0.32 (0.130) | −0.046 (0.023) |
| Aβ42/YKL‐40 | 0.785, 0.721–0.848 | −0.096 (<0.001) | −0.110 (<0.001) |
| Aβ42 | 0.755 0.686–0.824 | ||
| Tau | 0.858, 0.805–0.912 | ||
| Aβ42/Aβ40 | 0.881, 0.833–0.930 | ||
| Aβ42/tau | 0.895, 0.848–0.942 | ||
| AD versus MCI‐AD | |||
| Neurogranin | 0.538, 0.423–0.652 | ||
| YKL‐40 | 0.609, 0.500–0.719 | ||
| Aβ42/neurogranin | 0.642, 0.532–0.752 | −0.001 (0.980) | −0.128 (0.001) |
| Aβ42/YKL‐40 | 0.725 0.628–0.824 | 0.082 (0.136) | −0.045 (0.224) |
| Aβ42 | 0.720, 0.620–0.821 | ||
| Tau | 0.650, 0.543–0.757 | ||
| Aβ42/Aβ40 | 0.643, 0.533–0.753 | ||
| Aβ42/tau | 0.770, 0.676–0.864 | ||
| sMCI versus MCI‐AD | |||
| Neurogranin | 0.593, 0.471–0.715 | ||
| YKL‐40 | 0.689, 0.579–0.799 | ||
| Aβ42/neurogranin | 0.746, 0.643–0.848 | −0.099 (0.008) | −0.101 (0.003) |
| Aβ42/YKL‐40 | 0.823, 0.737–0.909 | −0.022 (0.492) | −0.024 (0.363) |
| Aβ42 | 0.774, 0.682–0.866 | ||
| Tau | 0.791, 0.698–0.883 | ||
| Aβ42/Aβ40 | 0.845, 0.767–0.923 | ||
| Aβ42/tau | 0.847, 0.767–0.927 | ||
Significant results are shown in bold. ROC, receiver operating characteristic; CSF, cerebrospinal fluid; AUC, area under the curve; AD, Alzheimer's disease; MCI‐AD, mild cognitive impairment that subsequently converted to AD; sMCI, stable mild cognitive impairment.
The diagnostic accuracy of the Aβ42/YKL‐40 was not improved compared with the Aβ42/Aβ40 and Aβ42/tau ratios when differentiating patients with AD dementia from those with non‐AD dementias. Furthermore, the Aβ42/YKL‐40 ratio was not significantly different from either the Aβ42/Aβ40 ratio or the Aβ42/tau ratio in distinguishing AD from MCI‐AD.
CSF neurogranin and YKL‐40 as clinical biomarkers of AD during the MCI stage
Finally, we studied whether neurogranin or YKL‐40 could improve the prediction of future development of AD in patients with MCI. The Aβ42/neurogranin ratio performed poorer than either the Aβ42/Aβ40 or the Aβ42/tau ratio in distinguishing patients with sMCI from MCI patients who later developed AD. The accuracy of the Aβ42/YKL‐40 ratio was not significantly different from either the Aβ42/Aβ40 ratio or the Aβ42/tau when differentiating sMCI from MCI‐AD (Table 3).
Discussion
Patient care and drug development in AD are in critical need of accurate early disease biomarkers. These biomarkers will reduce the costs and failure rate of clinical trials by guiding the selection of patients who will benefit from a given treatments and by effectively evaluating patient response to new drugs. The complexity of AD provides a strong rationale for use of multiple biomarkers that monitor different pathophysiological pathways driving the disease. In this study, we assessed the diagnostic accuracy of neurogranin and YKL‐40, which are considered promising biomarkers of synaptic dysfunction and neuroinflammation, two pathogenic mechanisms implicated in AD.33, 34 The study included patients with AD, VaD, DLB/PDD, FTD and sMCI as well as MCI patients who later progressed to AD and healthy controls. This allowed us for the first time simultaneous measurements of CSF levels neurogranin and YKL‐40 in prodromal AD, AD dementia and most non‐AD dementias in a relatively large sample, thus reducing the bias associated with analytical variability. We initially compared CSF level of neurogranin and YKL‐40 in different diagnostic groups. In agreement with existing data, we observed increased CSF levels of neurogranin and YKL‐40 in AD patients compared with healthy controls.19, 35, 36 However, in the present study, neurogranin levels were not significantly increased in MCI patients who subsequently developed AD dementia (MCI‐AD), which contrasts several previous papers that found increased CSF neurogranin also in this early phase of AD.10, 13, 37 This discrepancy in the results could be due to the relatively small number of patients in the MCI‐AD group in our study. However, it could be noted that even though the MCI‐AD group was not very large in the present study, the levels of both Aβ42 and tau were significantly different between sMCI and MCI‐AD, which was not the case for neurogranin. At the same time, we found that neurogranin and YKL‐40 were increased in AD compared with non‐AD dementias including DLB/PDD and VaD. These results suggested that neurogranin and YKL‐40 might improve differentiation between AD dementia and other non‐AD dementias. However, to be considered for clinical applications a new CSF AD biomarker should show better diagnostic performance than CSF Aβ42 and tau, the two biomarkers that already have been incorporated in the diagnostic framework of AD proposed by the International Working Group (IWG) for New Research Criteria for the Diagnosis of AD and by the US National Institute on Aging–Alzheimer's Association (NIA‐AA).38 Recent evidence suggests that the ratios of CSF Aβ42 to Aβ40 or tau have a greater diagnostic accuracy in AD39, 40 and show improved concordance with amyloid positron emission tomography (PET) imaging.41 Therefore, we next evaluated neurogranin and YKL‐40 as biomarkers of AD in comparison with the Aβ42/Aβ40 and Aβ42/tau ratios. Using ROC curve analysis we found that similar to Aβ and tau, the Aβ42/neurogranin and Aβ42/YKL‐40 ratio performed better than neurogranin and YKL‐40 alone. However, neither the Aβ42/neurogranin ratio nor the Aβ42/YKL‐40 ratio was more accurate than the Aβ42/Aβ40 and Aβ42/tau ratios when differentiating AD from non‐AD dementias or cognitively sMCI from MCI that converted to AD. This is in agreement with two earlier reports showing that the YKL‐40/Aβ42 ratio is comparable to but not better than the tau/Aβ42 ratio for predicting cognitive decline in healthy individuals and conversion of MCI to AD.19, 42
One potential explanation for our findings could be that changes in CSF levels of neurogranin and YKL‐40 are closely related to amyloid and/or tau pathology. In fact, our study as well as several other studies demonstrated that CSF neurogranin and YKL‐40 correlate strongly with tau levels in patients with AD dementia, prodromal AD and control subjects.10, 42, 43 On the other hand, CSF total tau and phosphorylated tau also correlate tightly in AD and control populations, see for example, Blennow et al.,44 but not in patients with stroke45 or Creutzfeld‐Jakob disease46 who show a very marked increase in CSF total tau while phosphorylated tau does not change, indicating that correlations between CSF biomarkers within AD populations do not rule out that they reflect different pathogenic processes. Additionally, we have recently reported that in AD there is a positive association between CSF levels of neurogranin and Aβ40 and a negative association between neurogranin and the Aβ42/Aβ40 ratio.10 Remarkably, in the present study we found that not only neurogranin but also YKL‐40 is positively associated with Aβ40 and the Aβ42/Aβ40 ratio. These findings together with previously reported changes in γ‐secretase activity in the brain of AD and MCI patients47 suggest that in AD neuroinflammation and synaptic dysfunction might be associated with neurodegeneration and with dysregulation of amyloid precursor protein pathway.
In conclusion, our study demonstrates that CSF neurogranin and YKL‐40 do not provide any added clinical diagnostic value to already existing AD biomarkers during prodromal and dementia stages. However, longitudinal studies with repeated CSF measurements over time are warranted to determine the usefulness of the different biomarkers to measure the disease progression during different stages of AD. It is very likely that neurogranin will be a marker that can monitor the effects of new disease‐modifying therapies on synaptic integrity, while YKL‐40 might be used to investigate the effects of novel drugs affecting neuroinflammation.
Author Contributions
O. H. designed the project. O. H., H. Z. and K. B. supervised the study. S. J., J. H., and O. H. performed acquisition, analysis and interpretation of data. S. J. and O. H. drafted the paper. A. S. and M. L. W. acquired the FTD data. All authors critically revised the manuscript for intellectual content.
Conflict of Interest
Drs Janelidze, Hertze, Landqvist Waldö, Santillo, Hansson report no disclosures. Drs Blennow and Zetterberg are co‐founders of Brain Biomarker Solutions in Gothenburg AB, a GU Ventures‐based platform company at the University of Gothenburg. KB has served at Advisory Boards for IBL International, Roche Diagnostics, Eli Lilly and Amgen, and as a consultant for Novartis and Alzheon.
Acknowledgments
The authors would like to thank the collaborators of this study including Christer Nilsson and Karin Nilsson for recruitment and clinical evaluations of FTD patients. Work in the authors' laboratory was supported by the European Research Council, the Swedish Research Council, the Strategic Research Area MultiPark (Multidisciplinary Research in Parkinson's disease) at Lund University, the Crafoord Foundation, the Swedish Brain Foundation, the Torsten Söderberg Foundation, the Knut and Alice Wallenberg Foundation and the Swedish federal government under the ALF agreement.
References
- 1. Hardy JA, Higgins GA. Alzheimer's disease: the amyloid cascade hypothesis. Science 1992;256:184–185. [DOI] [PubMed] [Google Scholar]
- 2. Karran E, Mercken M, De Strooper B. The amyloid cascade hypothesis for Alzheimer's disease: an appraisal for the development of therapeutics. Nat Rev Drug Discov 2011;10:698–712. [DOI] [PubMed] [Google Scholar]
- 3. Lista S, Zetterberg H, Dubois B, et al. Cerebrospinal fluid analysis in Alzheimer's disease: technical issues and future developments. J Neurol 2014;261:1234–1243. [DOI] [PubMed] [Google Scholar]
- 4. Blennow K, Hampel H, Weiner M, Zetterberg H. Cerebrospinal fluid and plasma biomarkers in Alzheimer disease. Nat Rev Neurol 2010;6:131–144. [DOI] [PubMed] [Google Scholar]
- 5. Blennow K, Dubois B, Fagan AM, et al. Clinical utility of cerebrospinal fluid biomarkers in the diagnosis of early Alzheimer's disease. Alzheimers Dement 2015;11:58–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pak JH, Huang FL, Li J, et al. Involvement of neurogranin in the modulation of calcium/calmodulin‐dependent protein kinase II, synaptic plasticity, and spatial learning: a study with knockout mice. Proc Natl Acad Sci USA 2000;97:11232–11237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhong L, Brown J, Kramer A, et al. Increased prefrontal cortex neurogranin enhances plasticity and extinction learning. J Neurosci 2015;35:7503–7508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Davidsson P, Blennow K. Neurochemical dissection of synaptic pathology in Alzheimer's disease. Int Psychogeriatr 1998;10:11–23. [DOI] [PubMed] [Google Scholar]
- 9. Reddy PH, Mani G, Park BS, et al. Differential loss of synaptic proteins in Alzheimer's disease: implications for synaptic dysfunction. J Alzheimers Dis 2005;7:103–117; discussion 73–80. [DOI] [PubMed] [Google Scholar]
- 10. De Vos A, Jacobs D, Struyfs H, et al. C‐terminal neurogranin is increased in cerebrospinal fluid but unchanged in plasma in Alzheimer's disease. Alzheimers Dement 2015; DOI: 10.1016/j.jalz.2015.05.012. [DOI] [PubMed] [Google Scholar]
- 11. Kvartsberg H, Portelius E, Andreasson U, et al. Characterization of the postsynaptic protein neurogranin in paired cerebrospinal fluid and plasma samples from Alzheimer's disease patients and healthy controls. Alzheimers Res Ther 2015;7:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kester MI, Teunissen CE, Crimmins DL, et al. Neurogranin as a cerebrospinal fluid biomarker for synaptic loss in symptomatic Alzheimer disease. JAMA Neurol 2015; DOI: 10.1001/jamaneurol.2015.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Portelius E, Zetterberg H, Skillback T, et al. Cerebrospinal fluid neurogranin: relation to cognition and neurodegeneration in Alzheimer's disease. Brain 2015;138(Pt 11):3373–3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bonneh‐Barkay D, Bissel SJ, Wang G, et al. YKL‐40, a marker of simian immunodeficiency virus encephalitis, modulates the biological activity of basic fibroblast growth factor. Am J Pathol 2008;173:130–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bonneh‐Barkay D, Zagadailov P, Zou H, et al. YKL‐40 expression in traumatic brain injury: an initial analysis. J Neurotrauma 2010;27:1215–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Comabella M, Fernandez M, Martin R, et al. Cerebrospinal fluid chitinase 3‐like 1 levels are associated with conversion to multiple sclerosis. Brain 2010;133(Pt 4):1082–1093. [DOI] [PubMed] [Google Scholar]
- 17. Kjaergaard AD, Johansen JS, Bojesen SE, Nordestgaard BG. Elevated plasma YKL‐40, lipids and lipoproteins, and ischemic vascular disease in the general population. Stroke 2015;46:329–335. [DOI] [PubMed] [Google Scholar]
- 18. Alcolea D, Carmona‐Iragui M, Suarez‐Calvet M, et al. Relationship between beta‐secretase, inflammation and core cerebrospinal fluid biomarkers for Alzheimer's disease. J Alzheimers Dis 2014;42:157–167. [DOI] [PubMed] [Google Scholar]
- 19. Olsson B, Hertze J, Lautner R, et al. Microglial markers are elevated in the prodromal phase of Alzheimer's disease and vascular dementia. J Alzheimers Dis 2013;33:45–53. [DOI] [PubMed] [Google Scholar]
- 20. Wennstrom M, Surova Y, Hall S, et al. The inflammatory marker YKL‐40 is elevated in cerebrospinal fluid from patients with Alzheimer's but not Parkinson's disease or dementia with Lewy bodies. PLoS One 2015;10:e0135458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Alcolea D, Martinez‐Lage P, Sanchez‐Juan P, et al. Amyloid precursor protein metabolism and inflammation markers in preclinical Alzheimer disease. Neurology 2015;85:626–633. [DOI] [PubMed] [Google Scholar]
- 22. Sutphen CL, Jasielec MS, Shah AR, et al. Longitudinal cerebrospinal fluid biomarker changes in preclinical Alzheimer disease during middle age. JAMA Neurol 2015;72:1029–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. American Psychiatric Association . Work Group to Revise DSM‐III. Diagnostic and statistical manual of mental disorders: DSM‐III‐R. 3rd ed Washington, DC: American Psychiatric Association, 1987. [Google Scholar]
- 24. McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer's disease: report of the NINCDS‐ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology 1984;34:939–944. [DOI] [PubMed] [Google Scholar]
- 25. Roman GC, Tatemichi TK, Erkinjuntti T, et al. Vascular dementia: diagnostic criteria for research studies. Report of the NINDS‐AIREN International Workshop. Neurology 1993;43:250–260. [DOI] [PubMed] [Google Scholar]
- 26. Geser F, Wenning GK, Poewe W, McKeith I. How to diagnose dementia with Lewy bodies: state of the art. Mov Disord 2005;20(Suppl 12):S11–S20. [DOI] [PubMed] [Google Scholar]
- 27. Neary D, Snowden JS, Gustafson L, et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology 1998;51:1546–1554. [DOI] [PubMed] [Google Scholar]
- 28. Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med 2004;256:183–194. [DOI] [PubMed] [Google Scholar]
- 29. Ewers M, Mattsson N, Minthon L, et al. CSF biomarkers for the differential diagnosis of Alzheimer's disease. A large‐scale international multicenter study. Alzheimers Dement 2015; DOI: 10.1016/j.jalz.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 30. R Core Team . R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing, 2014. Available at: http://www.R-project.org/ (accessed October 2014). [Google Scholar]
- 31. Robin X, Turck N, Hainard A, et al. pROC: an open‐source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics 2011;12:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hampel H, Frank R, Broich K, et al. Biomarkers for Alzheimer's disease: academic, industry and regulatory perspectives. Nat Rev Drug Discov 2010;9:560–574. [DOI] [PubMed] [Google Scholar]
- 33. Heppner FL, Ransohoff RM, Becher B. Immune attack: the role of inflammation in Alzheimer disease. Nat Rev Neurosci 2015;16:358–372. [DOI] [PubMed] [Google Scholar]
- 34. Selkoe DJ. Alzheimer's disease is a synaptic failure. Science 2002;298:789–791. [DOI] [PubMed] [Google Scholar]
- 35. Perrin RJ, Craig‐Schapiro R, Malone JP, et al. Identification and validation of novel cerebrospinal fluid biomarkers for staging early Alzheimer's disease. PLoS One 2011;6:e16032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Thorsell A, Bjerke M, Gobom J, et al. Neurogranin in cerebrospinal fluid as a marker of synaptic degeneration in Alzheimer's disease. Brain Res 2010;1362:13–22. [DOI] [PubMed] [Google Scholar]
- 37. Kvartsberg H, Duits FH, Ingelsson M, et al. Cerebrospinal fluid levels of the synaptic protein neurogranin correlates with cognitive decline in prodromal Alzheimer's disease. Alzheimers Dement 2014;11(10):1180–1190. [DOI] [PubMed] [Google Scholar]
- 38. Dubois B, Feldman HH, Jacova C, et al. Advancing research diagnostic criteria for Alzheimer's disease: the IWG‐2 criteria. Lancet Neurol 2014;13:614–629. [DOI] [PubMed] [Google Scholar]
- 39. Blennow K, Mattsson N, Scholl M, et al. Amyloid biomarkers in Alzheimer's disease. Trends Pharmacol Sci 2015;36:297–309. [DOI] [PubMed] [Google Scholar]
- 40. van Rossum IA, Vos S, Handels R, Visser PJ. Biomarkers as predictors for conversion from mild cognitive impairment to Alzheimer‐type dementia: implications for trial design. J Alzheimers Dis 2010;20:881–891. [DOI] [PubMed] [Google Scholar]
- 41. Fagan AM, Shaw LM, Xiong C, et al. Comparison of analytical platforms for cerebrospinal fluid measures of beta‐amyloid 1‐42, total tau, and p‐tau181 for identifying Alzheimer disease amyloid plaque pathology. Arch Neurol 2011;68:1137–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Craig‐Schapiro R, Perrin RJ, Roe CM, et al. YKL‐40: a novel prognostic fluid biomarker for preclinical Alzheimer's disease. Biol Psychiatry 2010;68:903–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Antonell A, Mansilla A, Rami L, et al. Cerebrospinal fluid level of YKL‐40 protein in preclinical and prodromal Alzheimer's disease. J Alzheimers Dis 2014;42:901–908. [DOI] [PubMed] [Google Scholar]
- 44. Blennow K, Wallin A, Agren H, et al. Tau protein in cerebrospinal fluid: a biochemical marker for axonal degeneration in Alzheimer disease? Mol Chem Neuropathol 1995;26:231–245. [DOI] [PubMed] [Google Scholar]
- 45. Hesse C, Rosengren L, Andreasen N, et al. Transient increase in total tau but not phospho‐tau in human cerebrospinal fluid after acute stroke. Neurosci Lett 2001;297:187–190. [DOI] [PubMed] [Google Scholar]
- 46. Riemenschneider M, Wagenpfeil S, Vanderstichele H, et al. Phospho‐tau/total tau ratio in cerebrospinal fluid discriminates Creutzfeldt‐Jakob disease from other dementias. Mol Psychiatry 2003;8:343–347. [DOI] [PubMed] [Google Scholar]
- 47. Kakuda N, Shoji M, Arai H, et al. Altered gamma‐secretase activity in mild cognitive impairment and Alzheimer's disease. EMBO Mol Med 2012;4:344–352. [DOI] [PMC free article] [PubMed] [Google Scholar]


