Key points
Occlusion of one eye of kittens (monocular deprivation) results in a severe and permanent loss of visual acuity in that eye, which parallels closely the vision loss characteristic of human amblyopia.
We extended earlier work to demonstrate that amblyopic vision loss can be either blocked or erased very fast by a 10 day period of total darkness following a period of monocular deprivation that begins near birth and extends to at least 8 weeks of age.
The parameters of darkness were strict because no visual recovery was observed after 5 days of darkness. In addition, short periods of light introduced each day during an otherwise 10 day period of darkness obliterated the benefits.
Despite recovery of normal visual acuity, only one‐quarter of the animals showed evidence of having attained normal stereoscopic vision.
A period of total darkness may catalyse and improve treatment outcomes in amblyopic children.
Abstract
A 10 day period of total darkness has been shown to either block or erase the severe effects on vision of a prior short period of monocular deprivation (MD) in kittens depending on whether darkness is contiguous or is delayed with respect to the period of MD. We have extended these earlier findings from kittens for which the period of MD began at 1 month and lasted for 1 week to more clinically relevant situations where MD began near birth and lasted for ≥6 weeks. Despite the far longer MD and the absence of prior binocular vision, all animals recovered normal visual acuity in the previously deprived eye. As before, when the period of darkness followed immediately after MD, the vision of both eyes was initially very poor but, subsequently, the acuity of each eye increased gradually and equally to attain normal levels in ∼7 weeks. By contrast, when darkness was introduced 8 weeks after MD, the visual acuity of the deprived eye recovered quickly to normal levels in just 1 week without any change in the vision of the fellow (non‐deprived) eye. Short (15 or 30 min) periods of illumination each day during an otherwise 10 day period of darkness obliterated all the benefits for vision, and a 5 day period of darkness was also completely ineffective. Measurements of depth perception indicated that, despite possessing normal visual acuity in both eyes, only about one‐quarter of the animals showed evidence of having attained normal stereoscopic vision.
Key points
Occlusion of one eye of kittens (monocular deprivation) results in a severe and permanent loss of visual acuity in that eye, which parallels closely the vision loss characteristic of human amblyopia.
We extended earlier work to demonstrate that amblyopic vision loss can be either blocked or erased very fast by a 10 day period of total darkness following a period of monocular deprivation that begins near birth and extends to at least 8 weeks of age.
The parameters of darkness were strict because no visual recovery was observed after 5 days of darkness. In addition, short periods of light introduced each day during an otherwise 10 day period of darkness obliterated the benefits.
Despite recovery of normal visual acuity, only one‐quarter of the animals showed evidence of having attained normal stereoscopic vision.
A period of total darkness may catalyse and improve treatment outcomes in amblyopic children.
Abbreviations
- CI
confidence interval
- MD
monocular deprivation
- OD
open door
Introduction
Extensive investigations over the last 50 years have provided a detailed account of the early development of mammalian central visual pathways, as well as the visual capabilities that they support. The initial studies (Hubel & Wiesel, 1963, 1977; Wiesel & Hubel, 1963, 1974) were conducted on cats and monkeys, both of which share many close similarities to humans with respect to their ocular anatomy and the organization of their central visual pathways. Similar to humans, the two eyes face forward so that their visual fields overlap across about two‐thirds of their total extent, which, in addition to semi‐decussation of the two optic nerves at the optic chiasm, provides an anatomical foundation for stereoscopic vision in both species (Bough, 1970; Fox & Blake, 1971; Harwerth & Boltz, 1979; Ptito et al. 1991; Harwerth et al. 1995). Studies on the development of the central visual pathways of cats and monkeys, as well as more recent investigations conducted on ferrets and rodents, suggest that development occurs in two main stages overlapping shortly after birth. The first of these is most evident in prenatal development and proceeds on the basis of processes that include programmes of gene expression modulated by intrinsically‐driven neural activity, as well as by self organizing processes. The second stage begins postnatally and in parallel with the onset of visual input at a time when the development of the visual pathways is influenced by visually‐driven neural activity. The influence of visual experience during this second developmental stage is perhaps best exemplified by the consequences of early experiential manipulations such as monocular deprivation (MD). In their pioneering studies on development of the primary visual pathways, Hubel and Wiesel (2005) demonstrated the capacity of the visual system for immense anatomical and functional modification even after brief periods of MD imposed in early postnatal life. At the level of the visual cortex, the pre‐existing overall balanced excitatory drive from the two eyes was greatly disturbed by MD such that most cortical cells were almost exclusively controlled by the non‐deprived eye. The ability of abnormal visual experience to effect profound modification within the visual system has been confirmed and extended to other forms of selected visual experience by subsequent studies that are summarized in a number of comprehensive reviews (Movshon & Van Sluyters, 1981; Mitchell & Timney, 1984; Sherman & Spear, 1982; Movshon & Kiorpes, 1990; 1991; Sengpiel & Kind, 2002; Daw, 2006).
In their study of the effects of an early period of MD, Wiesel and Hubel (1963) noted that, along with the large shift in cortical ocular dominance in favour of the non‐deprived eye, a profound impairment of vision was evident when the kittens used their deprived eye. Even though some recovery can occur gradually upon restoration of normal visual input, the vision of the deprived eye remains poor and with residual deficits that closely resemble those observed in human amblyopia (Mitchell, 1988; Hess et al. 1981; Gingras et al. 2005), a developmentally acquired impairment of form vision that cannot be attributed to disease, nor to optical errors. Early observations on the effects of MD lead to the use of this and closely allied forms of early selected visual deprivation on animal models as a means of investigating the anatomical and physiological origins of human amblyopia (Kiorpes & McKee, 1999; Hubel & Wiesel, 2005; Mitchell & Duffy, 2014). The use of MD to model deprivation induced human amblyopia was strengthened by the observation that its cortical effects were observed only when such deprivation was imposed within a certain critical period in early postnatal life extending to ∼6 months of age in kittens (Jones et al. 1984; Daw et al. 1992; Daw 2006; Mitchell & Timney, 1984) and to ∼2 years in monkeys (LeVay et al. 1980). The extension of investigations of developmental neural plasticity to rodents has enhanced our understanding of the molecular underpinnings of amblyopia, particularly as they relate to the regulation of the critical period and synaptic plasticity, as well as the prospect of new approaches for treating amblyopia on the basis of pharmacological interventions that restore or enhance plasticity in the visual cortex.
In addition to potential pharmacological interventions, other promising approaches for treating amblyopia have been suggested on the basis of the results of certain experiential manipulations. A case in point is the possible use of a short (10 day) period of complete darkness that was reported to promote substantial recovery from both the electrophysiological and behavioural effects of an early period of MD in adult rats (He et al. 2006; 2007). In kittens made amblyopic in one eye by an early period of MD, a 10 day period of darkness imposed 8 weeks after restoration of normal visual input promoted rapid improvement of the visual acuity of the deprived (amblyopic) eye to normal levels in 1 week or less (Duffy & Mitchell, 2013). On the other hand, a period of darkness imposed immediately after the same early period of MD had a dramatic effect on the vision of the non‐deprived eye such that, initially, the animal appeared to be blind when using either eye alone. However, over the next 7 weeks, the vision of both eyes improved gradually in concert to eventually attain normal visual acuity. At no point during the slow visual recovery was the vision of one eye worse than the other, suggesting that the period of darkness effectively blocked presentation of the usual behavioural signature of an early period of MD. Because the period of MD imposed for these experiments was short (1 week) and occurred at 1 month of age, kittens had received a prior period of normal binocular experience. In the present study, we investigated whether darkness could either erase or block the profound effects on vision of MD imposed at the time of normal eye opening aiming to minimize any prior binocular visual experience, and we also maintained this darkness for longer periods. Measurements of depth perception were also made on animals for which amblyopia had either been erased or blocked by darkness aiming to observe the consequences for stereoscopic vision. Finally, we also examined the robustness of the effects of darkness when interrupted by short (15–30 min) daily periods of light, as well as the effects of a shorter period of darkness (5 days).
Methods
Animal selection
The reasons for the choice of cats to model human deprivation amblyopia have been provided in detail previously as part of a review also examining the special niche provided by rodent models (Mitchell & Duffy, 2014). Consequently, we only provide a summary of the major arguments. Although the major visual defect associated with amblyopia is a loss of visual acuity in one eye, it has long been recognized clinically that the condition is essentially a binocular disorder. Amblyopia is considered to arise from unbalanced visual input to the two eyes in early life, a view refined by the results of the pioneering experimental studies conducted by Hubel and Wiesel on first cats and, later, non‐human primates (Wiesel & Hubel, 1963; Hubel & Wiesel, 1977). These species, along with humans, share a binocular visual system characterized by frontal eyes, with substantial binocular overlap of the visual fields, vergence eye movements and stereoscopic vision. The visual deficits that follow interruption of binocular concordant visual input to the two eyes of kittens in early postnatal life by interventions such as MD or misalignment of the visual axes are very similar in both their visual characteristics and severity to those manifested in human deprivation and strabsmic amblyopia (Giffin & Mitchell, 1978; Hess, France & Tulanay‐Keesey, 1981; Hess & Holliday, 1992; Gingras, Mitchell & Hess, 2005). As summarized in the Results, these manipulations also lead to severe deficits in stereoscopic vision reflecting those observed in human amblyopes. All of these visual deficits, as well as the anatomical and physiological changes induced by early selected visual deprivation in the central visual pathways, occur only when such deprivation occurs during restricted periods of vulnerability, or critical periods, which have been well documented in cats and, to a lesser extent, monkeys (Daw, 2006).
Ethical approval
All animal procedures followed protocols that were approved by the Dalhousie University Committee on Laboratory Animals and that conformed to guidelines of the Canadian Council on Animal Care.
Animals
Twenty‐one kittens (10 male, 11 female; from seven litters) born and housed in a closed breeding colony at Dalhousie University were used in the present study. For most of the animals, the experiential rearing and visual testing occurred during the period from birth to 4 months of age but, for a subset of animals, the assessment of depth perception continued for a further 2–4 months. Animals were housed in seven colony rooms that were illuminated under a 12:12 h light/dark cycle that was changed occasionally to illumination as high as 14:10 h light/dark for breeding purposes. At night, the animals were housed in large interconnected cages and, during the working day, were allowed to run free in the colony rooms. The animals were fed dry cat chow (Loblaws, Toronto, Canada) ad lib. and, each day, received ∼80 g of wet commercial cat food (Loblaws, Toronto, Canada; Nestle Purina, Mississauga, Canada). The behavioural tests that we employed did not require any reduction in the amount or nature of their daily food. Because the experiments were behavioural in nature, there was no requirement for animals to be killed at the end of behavioural testing. Six remain in the colony as breeders and all others were adopted as house pets by members of the university community after being neutered and inspected by the university veterinarian.
The original experiments (Duffy & Mitchell, 2013) employed a 7 day period of MD starting at postnatal day (P)30, which is the peak time of vulnerability of the kitten visual cortex to shifts of ocular dominance (Hubel & Wiesel, 1970; Olson & Freeman, 1980). The age and duration of MD also matched the rearing conditions employed in prior anatomical studies (Kutcher & Duffy, 2007; O'Leary et al. 2012), demonstrating that the anatomical consequences at the level of the lateral geniculate nucleus were very substantial and as severe as those that followed periods of MD lasting three times as long. All kittens received a period of MD by eyelid suture that, for twelve animals, began at P30 and, for the remaining nine animals, began at P7 (at around the time of natural eye opening). The age of imposition of MD and its duration, together with the age and duration of the subsequent period of darkness, are listed for all animals in Table 1.
Table 1.
Period of MD and Darkness (with modifications) for each animal
| Cat | Period of MD (postnatal days) | Period of darkness (postnatal days) | Modifications |
|---|---|---|---|
| C150, C153, C156 | 30–37 | 37–47 | |
| C151, C152, C157 | 30–37 | 93–103 | |
| C304 | 30–37 | 94–104 | |
| C301 | 30–37 | 94–104 | 1/4 h L/D |
| C300 | 30–37 | 94–104 | 1/2 h L/D |
| C303 | 30–37 | 94–99 | |
| C155 | 30–37 | 71–81 | |
| C169 | 30–37 | 92–97 | |
| C192, C166 | 7–37 | 37–47 | |
| C190 | 7–37 | 37–47 | 1/2 h L/D |
| C191 | 7–37 | 37–42 | |
| C165 | 7–37 | 93–103 | |
| C220, C222 | 7–43 | 43–53 | |
| C219 | 7–43 | 92–102 | |
| C221 | 7–56 | 113–123 |
L/D, light/dark.
MD
MD was achieved by eyelid suture of the left eye by use of procedures developed to both secure eyelid closure and allow fast recovery of a normal palpebral aperture subsequent to reopening, thus facilitating behavioural assessment of the vision in this eye (Murphy & Mitchell, 1987). The surgery was performed under gaseous isoflurane anesthesia (2–3% in oxygen) and, once anaesthetized, animals received a s.c. injection of ketoprofen (10 mg ml−1) for post‐procedure analgesia. The surgery occurred in two stages: first, the palpebral conjunctivae from the upper and lower eyelids were carefully dissected free from the eyelids and sutured together with 6‐0 vicryl suture material. A broad‐spectrum topical antibiotic (chloromycetin 1%) was applied to the sutured conjunctivae before the second stage of the procedure during which the exposed tissue on the underside of the eyelids was opposed and sutured with 6‐0 or 5‐0 silk. Because the eyelids of kittens open naturally and asynchronously (Blakemore & Cummings, 1975) at between P3 and P12 with a mean of 1 week (P7.7), we initiated the period of MD at P7 before the eyelids of most animals in the present study had opened. Where the eyelids of one or both eyes had not opened, they were gently separated prior to eyelid suture.
Dark rearing
During the 10 day period of complete darkness, kittens were housed within a large cage (1.5 × 0.7 × 0.9 m with a ledge 24 cm in width and 44 cm from the floor at both ends) within a large darkroom (3.8 × 3.5 m) that was part of a dark‐rearing facility previously described in detail (Mitchell, 2013). Because the kittens placed in darkness for 10 days at P37 were not weaned, they were housed in the large cage in the darkroom with their mother and littermates. The mother was removed from the darkroom to an illuminated colony room for ∼1 h each day. Those kittens placed in darkness later in life after they had been weaned were housed individually in regular cat cages or else with a littermate in the large cage. The dark facility consisted of two adjacent large darkrooms that were accessed through a set of smaller anterooms and doors ensuring that the two darkrooms remained light‐tight. Extensive efforts were made to ensure that the darkness was complete and never compromised. The adjacent main darkrooms allowed the cages to be changed and the rooms cleaned each day. A radio that was automatically turned on and off at times that corresponded to the lighting cycle of the regular colony rooms entrained an activity cycle matched closely to the working day of nearby humans.
Behavioural measurement of vision
Visual acuity
Measurements of visual acuity were made by use of a jumping stand, as well as procedures developed and refined in this laboratory over the last three decades (Mitchell et al. 1977; Murphy & Mitchell, 1987; Mitchell, 2013). The method builds upon a kitten's natural tendency to jump from an elevated surface and shapes it to leap for a food and social reward onto one of two adjacent large stimuli that, for measurement of visual acuity, consist of two gratings of identical periods but different orientations. The positive (rewarded) stimulus is a vertical grating, whereas the adjacent unrewarded grating is horizontal. The platform from which the kitten jumps is supported by two yoked laboratory jacks to enable small and continuous changes in its distance to the grating stimuli in a manner commensurate with the kitten's ability to jump at any age. Detailed descriptions of the training that begins at ∼4–5 weeks of age, as well as the testing procedure, are provided in a Mitchell (2013). To summarize, thresholds were measured by a descending method of limits with decrements in stimulus magnitudes (in this case, spatial frequency) that were very small and equated on a logarithmic scale with as many as 12 steps to an octave. For low spatial frequencies many octaves from threshold, the spatial frequency is increased after only one or two successful trials but, when closer to threshold, the minimum number of trials in increased to five. After an error, the animal is denied a food reward (wet kitten or cat food that is sometimes mixed with chicken liver) and is required to repeat the trial. Once an error had been successfully corrected, the animal had to make five consecutively correct responses or else make seven correct responses out of a maximum of 10 trials at any spatial frequency before proceeding to the next higher spatial frequency. The threshold was defined as the highest spatial frequency for which this criterion performance level was achieved. It was typical for animals to perform flawlessly until near threshold where, in addition to errors, they would exhibit general signs of difficulty with its choice including increased latency, vocalizations and variation of its patterns of gaze from the stimuli to other objects in the room. For longitudinal measurements of acuity where it is essential to document the speed of the changes in acuity, we have found it helpful to conduct testing with two people located on each side of the jumping stand, with one providing the reward at the same time as the other prepares the stimuli for the next trial and records behavioural responses.
Depth perception
Both normal humans and cats are better able to detect small differences in the distance of two adjacent objects with two eyes than with one, especially when the head is stationary and where visual stimuli are meagre. The superiority of binocular performance is attributed to stereoscopic vision and the utilization of the depth cue of retinal disparity provided by small differences in the position of the images in the two eyes. The method employed to test depth perception allowed estimates of the accuracy of depth judgments to be made over a wide range of capabilities, including the possible absence of stereoscopic vision (stereoblindness). The ability to discriminate differences in the distance of two stimuli was measured by use of the depth jumping stand, which was designed specifically for this purpose over 30 years ago (Mitchell, Kaye & Timney, 1979). With the depth jumping stand, two stimuli were viewed through a plate of transparent glass (5 mm thick) that formed the top of a box onto which the kittens jumped. Kittens were required to jump toward the closer of the two stimuli that consisted of separate clear plastic plates, transilluminated from below, upon which black opaque circles of three different sizes (5, 14 and 20 mm in diameter) were placed with a distribution density of 30% in a quasi‐random fashion. Opaque rectangular masks (19 × 14.5 cm) placed on the glass surface of the jumping stand blocked view of the edges of the two stimulus plates and restricted their angular dimensions to 15.8 × 12.2 deg at the usual distance of the cat from the landing surface. From the cat's perspective, stimuli appeared as two floating clear surfaces on which black circles of different sizes were distributed in random fashion. Because the distribution of the circles was determined independently for the two stimuli, they appeared to be quite different and thereby limited the ability to employ monocular depth cues such as size and density for depth judgments. Stimuli were attached to two beams that created a see‐saw that allowed the stimulus plates to rest against either of two adjustable stops so that the side of the nearer of the two stimuli could be altered. Diagrams of the depth jumping stand, as well as a photograph of the stimuli, are provided in a recent study (Mitchell et al. 2009).
Measurements of depth perception were initiated between one day (C166) and 98 days (C151 and C152) after completion of the measurements of acuity with an average delay of 6 weeks. Animals were trained initially to jump to the nearer of two surfaces on which circles of different sizes were painted and placed on the top of two adjacent boxes of different heights.
For initial training, three different box heights were used (5, 16.5 and 29 cm) in different combinations. The task was learned very fast because the kittens jumped naturally to the nearest surface without many trials. After 10 successful jumps with the smallest (5 cm) height difference, training began on the depth jumping stand with the largest separation of the stimulus plates in depth (23.7 cm) and with the closest plate always located 2 cm below the surface of the glass top of the jumping stand. After 10 consecutively correct jumps, the depth interval was reduced by 2 cm and the cat's performance was assessed on another block of at least five trials. The same performance criteria were followed for progression to a smaller depth interval as for the conventional jumping stand, namely five consecutively correct trials or a minimum of seven correct out of a maximum of 10 trials. A preliminary assessment of the binocular depth threshold was made on this first session with blocks of trials (a minimum of five) with progressively smaller depth intervals that were changed in 2 cm steps. A few animals performed very well with thresholds equivalent to those of normal cats on this first attempt. Binocular measurements were repeated the next day and continued on subsequent days until identical binocular thresholds were obtained on two consecutive days. Immediately after the second such threshold, a monocular threshold measurement was made with an opaque contact lens placed in one eye following administration of a drop of a local ophthalmic anesthetic proparacaine hydrochloride (0.5%). On the next nine daily consecutive testing sessions, a binocular threshold preceded measurement of a monocular threshold with the occluded eye alternated on successive days. This strategy permitted a fast determination of the magnitude of the difference between binocular and monocular performance in a manner that reduced the opportunity for the animal to learn and utilize unwanted monocular depth cues such as motion parallax. The thresholds over the 10 consecutive test days were expressed as the smallest depth interval (ΔD) for which criterion performance was achieved expressed as a percentage of the distance, D, to the more distant of the two stimuli. Performance usually went from flawless to the level of chance over only two depth intervals.
Statistical analysis
We examined whether the beneficial effects of darkness could be influenced by early binocular vision by testing three hypotheses. The first hypothesis was that, for the Immediate Darkness condition, the time for the visual acuity of the deprived eye to recover to normal (≥6.49 cycles deg–1) would be longer when MD was initiated at 7 days of age than at 30 days of age. The second hypothesis was that, for the Delayed Darkness condition, the acuity of the deprived eye before entering the dark would be lower when MD was initiated at 7 days than when MD was initiated at 30 days. The third hypothesis was that, for the Delayed Darkness condition, the time for the visual acuity of the deprived eye to recover to normal (≥6.49 cycles deg–1) would be longer when MD was initiated at 7 days rather than at 30 days. Each hypothesis was evaluated with a permutation test for unpaired differences as a result of the impracticalities of assignment of large numbers of animals to each group. The tests were one‐tailed because longer durations of deprivation are associated with worse visual performance (Giffin & Mitchell, 1978; Mitchell, 1988). Because the neural changes initiated by MD are probably somewhat similar across groups, and because the quantitative variables that were examined are probably related, we used the TCH procedure to correct for multiplicity of statistical tests (Tukey et al. 1985; Sankoh et al. 1997). The critical P value was 0.029.
For measures of binocular depth perception, we were interested in whether dark reared cats regained normal binocular stereoacuity. Therefore, we compared the bootstrapped 95% confidence intervals (CIs) for binocular performance of the cats in the present study with the binocular performance of normal cats collated from previous investigations that employed the same apparatus and procedures (Mitchell et al. 1979; Kaye et al., 1981, 1982). Demonstration of the exact superiority of binocular over monocular performance was less meaningful and also impractical given that many animals failed to perform the easiest task monocularly with the largest available depth interval. All statistical analysis was performed in MatLab (MathWorks, Natick, MA, USA).
Results
The effects of darkness on kittens for which extended MD began at eye opening (P7)
In the earlier study (Duffy & Mitchell, 2013), the amblyogenic period of MD began at P30, at an age when the eyelids had been open for 3 weeks, the optical media had been clear for 10 days (Bonds & Freeman, 1978), and when visual cortical neurons are maximally susceptible to monocular eyelid suture (Hubel & Wiesel, 1970; Olson & Freeman, 1980). It is possible that the beneficial effects for the vision of the deprived eye observed following a subsequent period of darkness (Duffy & Mitchell, 2013) may have derived in part from the prior existence of a near mature binocular neural template established during the 3 week period of normal binocular visual input that preceded the period of MD. If this is the case, the effects of darkness might be reduced in animals for which the period of MD was initiated much earlier, at around the time of natural eye opening, and without any binocular visual experience prior to the period of MD. It is also possible that darkness might be less effective with longer lengths of MD. These two possibilities were examined in seven kittens for which the period of MD began on P7 and extended to either P37 (n = 3), P43 (n = 3) or P56 (n = 1). For four of the seven kittens, darkness was imposed immediately after the period of MD (Immediate Dark group), whereas, for the remaining three kittens, it occurred 8 weeks later (Delayed Dark group). The results of longitudinal measurements of the visual acuity of the two eyes following the period of darkness for the four animals from the Immediate Dark group are shown in Fig. 1.
Figure 1. Recovery of visual acuity of both eyes in four kittens that received a period of monocular deprivation from eye opening (P7) to either 5 or 6 weeks of age followed immediately by 10 days of complete darkness .
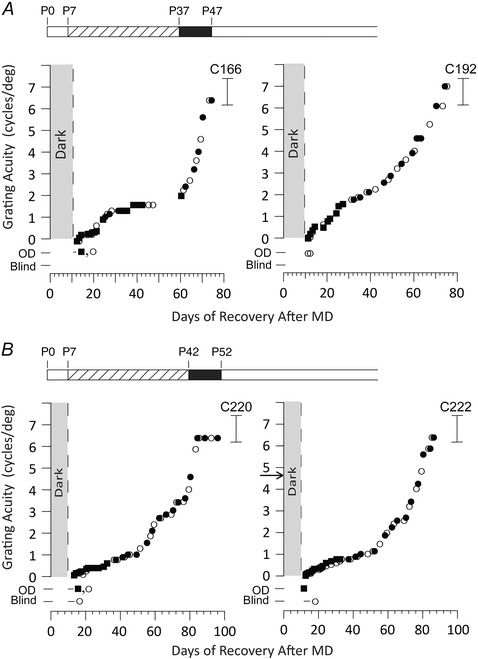
After the period of darkness, animals exhibited extremely poor vision in each eye followed by slow recovery of visual acuity of both eyes that eventually reached normal levels over the course of the next 2 months. At no point in time was the visual acuity of the deprived eye lower than that of its fellow eye. Data indicate the visual acuity for gratings of, respectively, the deprived (open circles) and non‐deprived eye (filled circles). Black square symbols display the acuity with both eyes open and reflect the acuity of the best (non‐deprived) eye. Kittens were designated as ‘Blind’ if they were unable to locate a closed door on the jumping stand by vision alone. Kittens that could pass this task (Open Door or OD) but could not discriminate a vertical from a horizontal grating at stepping height were probably capable only of the ability to make a luminance discrimination. The brackets to the right shows the range of acuities exhibited by normal kittens of an equivalent age and tested in the same manner. The 10 day break in the data for C166 was the result of a holiday and ice storm. The rearing history of animals is displayed in schematic form above the individual data; monocular deprivation (hatched bar) from P7 to P37 days (A) or from P7 to P42 (B) followed by complete darkness for 10 days (black bar). The arrow in the graph for C222 represents the acuity of the non‐deprived eye prior to the onset of darkness.
Despite the much earlier onset of MD and its longer duration, the results were remarkably similar to those reported earlier (Duffy & Mitchell, 2013). As with the animals of the prior study, all four animals in the Immediate Dark condition exhibited extremely poor vision with either eye immediately following the period of darkness. On emergence from the darkroom, the animals froze in position and appeared to be unaware of other kittens moving around them or large objects presented without noise in their close proximity. On the basis of these observations, they behaved as if they were blind in both eyes. Formal tests made on the jumping stand revealed that, with both eyes open, all but one of the kittens were capable only of the ability to make luminance discriminations on the basis of their ability to distinguish an open from a closed door. The remaining kitten (C192) possessed rudimentary form vision with both eyes open but, with its deprived eye, possessed just the ability to make luminance discriminations. Form vision sufficient to allow discrimination of a vertical from a horizontal grating, and hence allow measurement of visual acuity, returned in a few days, although the subsequent recovery of acuity was very gradual. As with the animals of the earlier study, at no point did the visual acuity of one eye appear to be superior to that of the other and, eventually, both eyes achieved visual acuities comparable to normal kittens of an equivalent age. In all major respects, namely the severe reduction of the vision of both eyes following the period of darkness, the gradual but identical rate of the subsequent recovery of the visual acuity of each eye, as well as the eventual attainment of normal acuity, were similar to those reported earlier (Duffy & Mitchell, 2013). However, the recovery time to normal acuity in the four animals for which the period of MD began at P7 was significantly longer than the three animals deprived at P30 (P = 0.0286, one‐tailed unpaired permutation test). This point is made evident from the data shown in Table 2, which indicate the time for the acuity of the deprived eye of each animal, including those from the earlier study (Duffy & Mitchell, 2013) to reach normal levels (6.5 cycles deg–1). Although the three animals reported earlier (C150, C153, C156) attained this value in a median of 47 days (interquartile range = 44–50.75 days), the animals for which the period of MD began at P7 took a median of 69.5 days (interquartile range = 64–75 days) to attain the same acuity.
Table 2.
Periods of MD and darkness for the two groups of animals together with the time required for the visual acuity of the deprived eye to attain normal levels, as well as the the acuity of the deprived eye of the animals in the Delayed Dark group immediately prior to the period of darkness
| Period of MD | Deprived eye | Dark interval | Recovery time | |
|---|---|---|---|---|
| Cat | (postnatal days) | acuity (cycles deg–1) | (postnatal days) | (days to 6.5 cycles deg–1) |
| Immediate Dark group | ||||
| C150 | 30–37 | NA | 37–47 | 43 |
| C153 | 30–37 | NA | 37–47 | 52 |
| C156 | 30–37 | NA | 37–47 | 47 |
| C166 | 7–37 | NA | 37–47 | 63 |
| C192 | 7–37 | NA | 37–47 | 65 |
| C220 | 7–43 | NA | 43–53 | 76 |
| C222 | 7–43 | NA | 43–53 | 74 |
| Delayed Dark group | ||||
| C151 | 30–37 | 2.51 | 37–47 | 9 |
| C152 | 30–37 | 2.66 | 37–42 | 5 |
| C157 | 30–37 | 2.97 | 93–103 | 5 |
| C304 | 30–37 | 0.63 | 43–53 | 10 |
| C155 | 30–37 | 2.66 | 92–102 | 4 |
| C165 | 7–37 | 1.98 | 113–123 | 6 |
| C219 | 7–43 | 1.88 | 113–123 | 7 |
| C221 | 7–56 | 0.79 | 113–123 | 7 |
NA, not available.
A similar concordance with the earlier results was observed for the three animals from the Delayed Dark condition for which the period of MD extended from P7 to P37 or longer followed by the period of darkness started 8 weeks later, at which time the visual acuity of the deprived (amblyopic) eye had been stable for at least 3 weeks. The three animals for which the period of MD began at P7 had a median deprived eye acuity of 1.88 cycles deg–1 (interquartile range = 1.06–1.96), whereas the five animals for which the period of MD began at P30 had a median acuity of 2.66 cycles deg–1 (interquartile range = 2.04–2.74). However, our statistical analysis did not suggest that animals deprived earlier had a deeper level of amblyopia (P = 0.1429, one‐tailed unpaired permutation test). In agreement with results from animals in the Delayed Dark group of the earlier study (Duffy & Mitchell, 2013), the visual acuity of the deprived eye improved very rapidly to normal levels (6.5 cycles deg–1) in ∼1 week without any change in the visual acuity of the non‐deprived eye. The results from the three individual animals shown in Fig. 2 indicate that recovery of the visual acuity of the deprived eye was very fast irrespective of the length of the prior period of MD, a point exemplified by the data from C221 (Fig. 2 C) for which the period of MD was longest. Despite the longest period of MD (from P7 to 8 weeks of age), the latest imposition of darkness (at P112 days) and the deepest amblyopia in the deprived eye, the speed of the recovery of the visual acuity of this eye to normal levels was as fast (7 days) as that observed in the other animals. The close similarity of the speed of recovery of the deprived eye of the three animals in the present study compared to the four kittens from the earlier study for which darkness was delayed is apparent from Table 2. Included in Table 2 are the data from C304 (Fig. 3) that was reared in an identical manner to the animals from the previous study (Duffy & Mitchell, 2013). The median recovery time in the five animals for which the period of MD began at P30 was 5 days (interquartile range = 4.75–9.25 days). By comparison, the vision of the deprived eye for the three animals for which the period of MD occurred at P7 recovered to normal levels in a median of 7 days (interquartile range = 6.25–7 days). The above data do not suggest a difference in recovery rates between the two rearing conditions (P = 0.5, one‐tailed unpaired permutation test).
Figure 2. Rapid recovery of the visual acuity of the deprived (amblyopic) eye of three kittens that received a period of MD from eye opening (P7) to either 5, 6 or 8 weeks of age and then placed in complete darkness 8 weeks later .
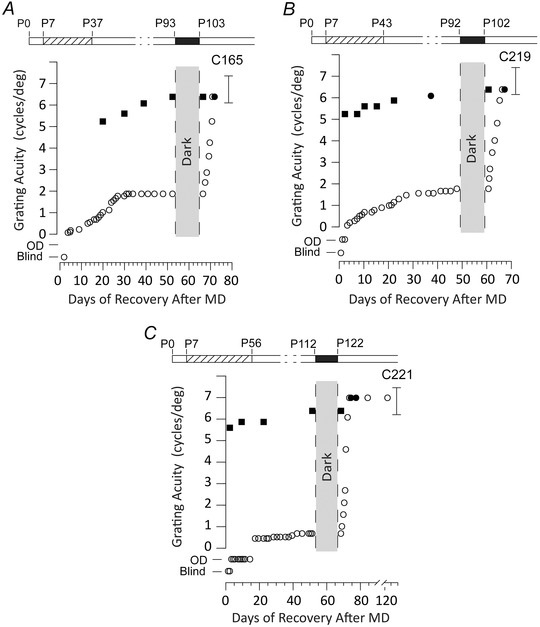
The acuity of the deprived eye (open circles) improved rapidly to normal levels in 5–7 days following the period of darkness without any decrement of the visual acuity of the non‐deprived eye (filled squares, measurements of binocular visual acuity; filled circles, measurements of the acuity of the non‐deprived eye). The brackets to the right of each graph show the range of acuities exhibited by normal kittens of an equivalent age and tested in the same manner. The rearing history of the animals is displayed in schematic form above the individual data; monocular deprivation (hatched bars) from P7 to P37 days (A) or from P7 to P43 (B) or from P7 to P56 (C) followed by complete darkness for 10 days (black bars). The designation of vision as Blind or Open Door are as described in the legend to Fig. 1.
Figure 3. Five days of total darkness is insufficient to promote recovery of the deprived eye in monocularly deprived kittens .
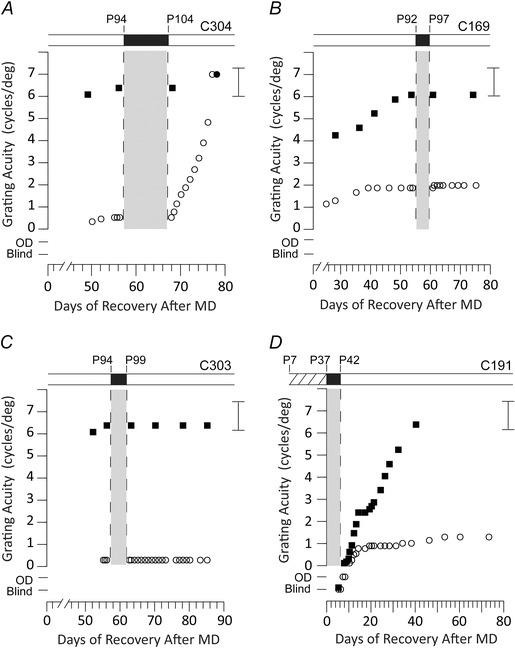
Data from four kittens that received a period of MD from either P30 to P37 (A–C) or from P7 to P37 (D) and which subsequently either received a period of darkness immediately after the period of monocular deprivation (D) or 8 weeks later (A–C). The partial rearing history of the four animals is displayed in schematic form above the individual data with the period of darkness indicated by a black bar and the period of MD (D) by hatching. The individual graphs display the results of longitudinal measurements of the grating acuity of either the deprived eye (open circle symbols), the non‐deprived eye (filled circle symbols) or the binocular acuity (filled square symbols) as a function of the time after termination of the period of MD. A, the rapid recovery of the acuity of the deprived eye of an animal that received 10 days of darkness at P94. B and C, two animals that displayed no recovery in the acuity of the deprived eye following 5 days of darkness imposed at P92 or P94. D, results for an animal placed in darkness for 5 days immediately after a period of MD that ended at P37. Although the short period of darkness resulted in a severe reduction in the vision of both eyes, only the vision of the non‐deprived eye recovered to normal levels. Brackets to the right of each graph display the range of acuities measured in normal kittens of an equivalent age and tested in the same manner. The designation of vision as Blind or Open Door (OD) are as described in the legend to Fig 1.
As in the previous study (Duffy & Mitchell, 2013), the immediate effects of darkness on the vision of the non‐deprived eye for the animals in the two groups were very different. Although the vision of the non‐deprived eye was reduced drastically for the animals of Fig. 1 where the periods of MD and darkness were contiguous, for animals where darkness was imposed 8 weeks after the period of MD (Fig. 2), the vision of this eye was unaffected. An explanation for the divergent outcomes with respect to the vision of the non‐deprived eye for the animals from the two treatment groups emerged from a recent study (Mitchell et al. 2015) conducted on normal animals. Animals placed in darkness for 10 days early in life exhibited a drastic loss of vision in both eyes, although the effects on vision declined rapidly with the age of imposition of darkness, such that animals exposed at 10 weeks experienced no loss of vision at all. Thus, the lack of any effect on the vision of the non‐deprived eye for the animals for which darkness was delayed (Fig. 2) probably reflects the fact that it was imposed well beyond the termination of this short critical period for the effects of darkness on the vision of this eye.
The effects of 5 days of darkness
The fast and extensive recovery of visual acuity of the amblyopic eye promoted by 10 days of darkness invites investigation of the minimum length of darkness required to achieve this outcome. As a first step, we investigated the consequences of 5 days of darkness imposed on three amblyopic kittens either immediately after the period of MD (C191) or 8 weeks later (C169 and C303). The results for these three animals, as well as those for a littermate (C304) that received 10 days of darkness at the same time as its littermate (C303), are shown in Fig. 3. In comparison with the rapid improvement of the visual acuity of the deprived eye of C304 after 10 days of darkness (Fig. 3 A), the acuity of the deprived eye of C169 (Fig. 3 B) and of C303 (Fig. 3 C) remained unchanged in the weeks following 5 days of darkness. Apparently, 5 days of darkness was unable to effect any improvement in the vision of the amblyopic eye when imposed several weeks after the period of MD.
By contrast to the complete lack of any visual improvement in the situation where darkness was delayed with respect to the period of MD, immediate imposition of 5 days of darkness on one animal (C191) (Fig. 3 D) resulted in alterations of the vision of the two eyes, albeit more so for the non‐deprived eye. As is apparent from the data obtained for this animal shown in Fig. 3 D, the immediate effects of 5 days of darkness on the vision of each eye were as severe as those that followed 10 days darkness because, initially, the animal appeared to be blind in both eyes. Subsequently, however, the visual acuity of the non‐deprived eye improved at a faster rate than that of the fellow eye that stalled after ∼10 days at a low level from which it showed little further improvement. Meanwhile, the visual acuity of the non‐deprived eye improved rapidly to attain normal levels in 35 days, several weeks faster than the time required for the non‐deprived eye to attain normal acuity after 10 days of darkness (Fig. 1). Although the initial consequences of 5 days of darkness for the vision of the non‐deprived eye were as severe as those observed after the longer dark exposure, subsequent improvement of the acuity of this eye to normal levels was not accompanied by any appreciable restoration of the vision of the deprived (amblyopic) eye. Thus, with respect to the vision of the amblyopic eye, 5 days of darkness conferred no benefit irrespective of its timing with respect to the prior amblyogenic period of MD.
The effects of brief exposure to light during a 10 day period of darkness
The design of the darkroom facility and the strict protocols that were followed ensured that the kittens received no exposure to light during the 10 days when they were housed in darkness. However, from many perspectives, including possible translation to treatment of human amblyopia, it was important to test the strictness of the requirements for darkness. To explore this issue, three kittens were removed from the darkroom and placed in an illuminated room for either 15 (n = 2) or 30 min (n = 1) at 09.00 h each morning of their 10 days in the darkroom. For one kitten (C190), the period of darkness occurred immediately after the period of MD but, for the other two, it was delayed. The results from three animals (Fig. 4) show the drastic negative consequences of even the shortest daily light exposure for recovery of the deprived eye. For the two kittens that received delayed dark exposure (Fig. 4 B and C), the beneficial effects of darkness for the visual acuity of the deprived eye were eliminated completely by as little as 15 min of daily light exposure. Although a similar overall outcome was observed (Fig. 4 A) for the kitten (C190) that was placed in the darkroom immediately after the period of MD and received 30 min of daily light exposure, there are still indications for an effect of darkness on the vision of the non‐deprived eye. The immediate consequences of the period of interrupted darkness for the vision of the non‐deprived eye were much reduced compared to that observed with complete darkness because this eye was not temporarily rendered blind and recovered quickly to normal levels. However, there was little evidence of any beneficial effects of the period of darkness for the acuity of the deprived eye. As with the other animals, 30 min of light each day obliterated the benefits of 10 uninterrupted days in darkness.
Figure 4. Short daily periods of light eliminated the beneficial effects of darkness .
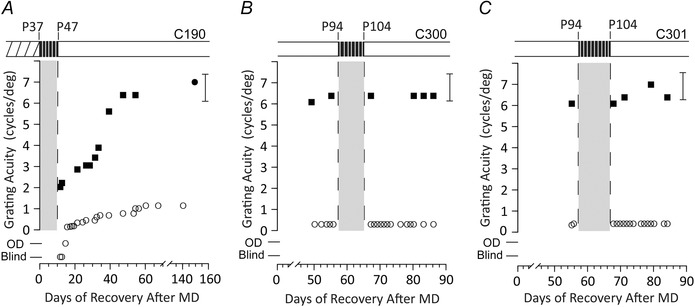
Results of longitudinal measurements of the visual acuity of one or both eyes of three kittens that had received a period of MD from either P7 to P37 (A) or from P30 to P37 (B and C) and which subsequently received a period of interrupted darkness either immediately after the period of MD (A) or 8 weeks later (B and C). The rearing history of the four animals is displayed in schematic form above the individual data with the period of interrupted darkness indicated by a black bar (and grey shading below) and the period of MD by hatching. The individual graphs display the results of longitudinal measurements of the grating acuity of either the deprived eye (open circle symbols), the non‐deprived eye (filled circle symbols) or the binocular acuity (filled square symbols) as a function of the time after termination of the period of MD. A, data for C190 that was placed in darkness immediately after the end of the period of MD. During the period of darkness, it was moved to an illuminated room for 30 min each day. B and C, data for C300 and C301 for which the period of darkness occurred 8 weeks after termination of the period of MD. During the period of darkness, they were removed to an illuminated room for, respectively, 30 and 15 min each day. The brackets to the right of each graph display the range of acuities measured in normal kittens of an equivalent age and tested in the same manner. The designation of vision as Blind or Open Door (OD) are as described in the legend to Figs 1 and 2.
Monocular and binocular depth perception
It has long been recognized from animal studies that manipulations such as MD and strabismus, which interfere with concordant early visual input to the two eyes, have dire consequences for stereoscopic vision (Blake & Hirsch, 1975; Packwood & Gordon, 1975; Crawford et al. 1983; Crawford et al. 1984). These findings echo longstanding clinical opinion that procedures for alleviation of human amblyopia only rarely allow for recovery of good stereoscopic vision (Levi et al. 2015; Birch, 2013). With these considerations in mind, we examined whether the recovery of visual acuity in the deprived eye promoted by darkness was accompanied by the acquisition of stereopsis. This issue was examined using 11 kittens exposed to 10 days of darkness and included the six animals from an earlier study (Duffy & Mitchell, 2013). Because of potentially different outcomes for kittens in the Immediate Dark as opposed to the Delayed Dark conditions, the results from these two conditions are shown separately in Figs 5 and 6.
Figure 5. Depth discrimination thresholds for five animals placed in darkness immediately after a period of MD reveal only two showed evidence of stereopsis despite the absence of amblyopia .
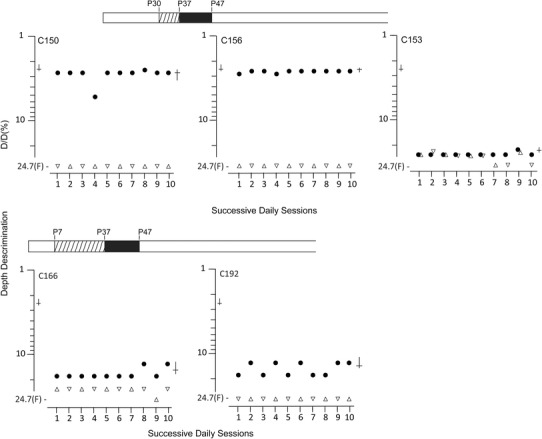
Results of measurements of depth discrimination on ten successive daily testing sessions in which binocular thresholds (filled circle symbols) were measured immediately prior to a monocular threshold that was alternated between the right (upright triangle symbols) and left (inverted triangle symbols) eye each day. Depth discrimination thresholds represent the smallest detectable difference in depth (ΔD) between two adjacent surfaces seen through the masked glass top of the jumping stand expressed as a percentage of the distance of the cat from the more distant (D) of the two surfaces. Occasions for which a cat failed to detect the nearest surface when separated by the maximum amount (24.7%) are denoted by the letter ‘F’. Where variation existed in the daily thresholds the means and bootstrapped 95% CIs are shown to the right, respectively, by a horizontal and an intersecting vertical line. Corresponding values for normal animals from previous studies are shown to the left.
Figure 6. Depth discrimination thresholds for five animals placed in darkness 8 weeks after a period of MD reveal that despite the absence of amblyopia, only one showed evidence that it possessed stereopsis .
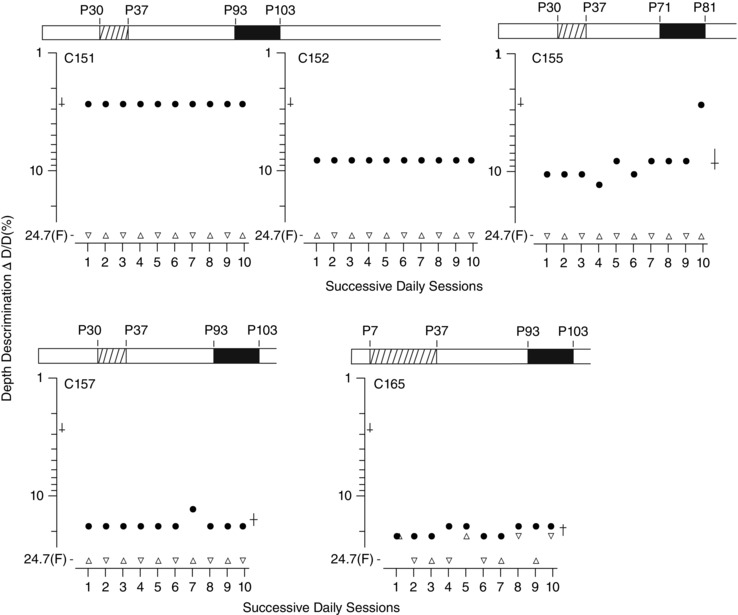
Results of measurements of depth discrimination on ten successive daily testing sessions in which binocular thresholds (filled circle symbols) were measured immediately prior to a monocular threshold that was alternated between the right (upright triangle symbols) and left (inverted triangle symbols) eye each day. Depth discrimination thresholds represent the smallest detectable difference in depth (ΔD) between two adjacent surfaces seen through the masked glass top of the jumping stand expressed as a percentage of the distance of the cat from the more distant (D) of the two surfaces. Occasions for which a cat was unable to detect the nearest surface when separated by the maximum amount (24.7%) are denoted by the letter ‘F’. Where variation existed in the daily thresholds the means and bootstrapped 95% CIs are shown to the right, respectively, by a horizontal and an intersecting vertical line. Corresponding values for normal animals from previous studies are shown to the left.
At the time that measures of depth perception were gathered, all 11 animals had normal visual acuity in both eyes and all appeared to possess normal eye alignment. Once the animals were trained and their binocular depth discrimination thresholds showed no improvement over two successive sessions, formal comparisons of monocular and binocular depth discrimination thresholds were made on 10 successive daily testing sessions. Each session began with measurement of a binocular threshold followed immediately by a second threshold measurement made with one eye occluded with an opaque hard contact lens. The occluded eye was alternated between the right and left eyes on successive sessions to provide 10 binocular and five monocular thresholds for each eye. The immediate adjacency of the binocular and monocular measurements each day highlighted differences in the ability of the animals to make depth judgments in the two testing situations that were often reflected by changes in the animal's general behaviour (e.g. latency to jump, vocalizations, balking), as well as by the thresholds themselves.
By contrast to the uniformity of the consequences of darkness for visual acuity where all 11 kittens achieved normal and equal visual acuity in the two eyes, the outcome of the measurements of depth perception were quite heterogeneous irrespective of the timing of darkness with respect to the prior period of MD. The results from each of the five cats placed in darkness immediately after the period of MD (Immediate Dark group) (Fig. 5) were remarkably consistent over the 10 consecutive testing sessions, as reflected by the means and bootstrapped 95% CIs. The results fell into two distinct categories with respect to their binocular thresholds, as also reflected by the magnitude of the differences between these and their monocular thresholds. Although two animals exhibited good binocular thresholds that were substantially superior to their monocular performance and similar to thresholds obtained in normal cats, the other three kittens performed poorly even with both eyes open. For the two animals (C150, C156) that performed well binocularly, the period of MD began at P30. With both eyes open, these two animals could discriminate a 1.8 cm depth difference from 70 cm (C150) or 75 cm(C156) corresponding to binocular thresholds of, respectively, 2.82% (95% CI = 2.56–3.56) and 2.54% (95% CI = 2.5–2.62), which are values that overlap extensively with the performance of four normal cats (mean 2.58%; 95% CI = 2.32–2.67) tested on the same apparatus by use of similar procedures in earlier studies (Mitchell et al. 1979; Kaye et al. 1981; 1982). Also, similar to normal animals, their performance when using either eye alone (monocular testing) was more than one order of magnitude worse compared to that at the level of chance with the maximum depth interval (22.4 cm) equivalent to a depth threshold of 24.7%. On the basis of the large binocular superiority, and for simple stimuli as employed in the present study, these two animals probably possess normal stereoscopic vision. By conspicuous contrast, the binocular thresholds for the remaining three animals were from 5.8 (C192) to 9.5 (C153) times worse compared to normal values (2.58%) and either about the same as the monocular values (C153) or only slightly better (C166 and C 192). At best, the small binocular superiority shown by C166 and C192 is evidence of only very crude stereoscopic vision. Conservatively, it can be concluded that only two of the five animals in this group showed evidence of useful stereoscopic vision. Although, for both these animals, the period of MD began at P30, the other animal so reared (C153) did not appear to acquire stereoscopic vision.
The results from animals in the Delayed Dark group (Fig. 6) were more diverse than those from the group of animals for which the periods of MD and darkness were contiguous. One animal (C151) performed binocularly at normal levels but so poorly with each eye alone that it was unable to perform the task monocularly. This large binocular superiority is consistent with the presence of normal stereoscopic vision. Binocular thresholds for two others, C152 and C155, were about three times poorer than those for C151 but still substantially better than their monocular performance, which was at the level of chance with the largest depth interval. This obvious binocular superiority suggests that these two cats may have developed crude stereoscopic vision. On the other hand, the equally poor binocular and monocular performance of C157 and C165 implies that they were stereoblind.
Discussion
The decisive outcomes of the four studies described above bolster and extend previous results (Duffy & Mitchell, 2013) showing that a 10 day period of complete darkness could either block or erase the effects on vision of a prior 7 day period of MD depending on the temporal relationship between the two manipulations. First, the benefits of 10 days of darkness for the vision of the deprived (amblyopic) eye were shown to extend to situations where the period of MD began near the time of natural eye opening and lasted until 8 weeks of age, indicating that the dark‐induced recovery does not require prior normal visual input. Second, a shorter 5 day period of darkness neither prompted improvement in the visual acuity of the amblyopic eye when imposed 8 weeks after a period of MD, nor did it block the development of amblyopia when periods of MD and darkness were contiguous. Third, even brief (15 or 30 min) daily periods of light exposure during 10 days of darkness prevented all the beneficial effects of the latter for the vision of the amblyopic eye. Finally, only a small minority of animals that recovered normal visual acuity in the deprived eye following a period of darkness showed evidence of having acquired normal stereoscopic vision. The implications of these four main findings are discussed not only in relation to the implications for the underlying neural and molecular mechanisms, but also with respect to possible translational use of darkness for clinical treatment of certain forms of human amblyopia.
Darkness blocks or erases amblyopia following extended periods of MD initiated at P7
In an earlier study (Duffy & Mitchell, 2013), MD was imposed at P30 at the peak of the critical period for ocular dominance plasticity (Olson & Freeman, 1980) and lasted for only 1 week. The first issue to be addressed was the robustness of the visual recovery induced by darkness to changes in both the length and onset of the prior amblyogenic period of MD. To preclude the possibility that the benefits may apply only to situations where there was a period of normal visual experience prior to the period of MD, we investigated the speed and extent of visual recovery from periods of MD initiated at P7, at the mean time of natural eye opening. In addition, the duration of the period of MD was extended to terminate as late as 8 weeks of age. The early start and long duration of the period of MD was also considered to resemble more closely the timing of the experiential events that precede the development of human amblyopia. The close similarity of the results from the animals investigated in the present study (Figs 1 and 2) compared to those reported earlier (Duffy & Mitchell, 2013) indicates that the visual recovery promoted by darkness did not require an earlier period of normal visual experience. Although the pace of recovery in the Immediate Dark group was slightly slower when MD was initiated at P7, all animals attained normal acuity. At P7, the eyelids of one or both eyes of some of the kittens may have been open for only several hours or up to a day but, because the optical media of the eye at this age are very cloudy (Bonds & Freeman, 1978), any visual experience would have been very brief and compromised by poor image quality. In addition to the early start of the period of MD, the duration of MD lasted from 30 to 49 days longer than the 7 days employed in the previous study (Duffy & Mitchell, 2013). Despite the substantial differences in the parameters of the period of MD, the speed and magnitude of visual recovery promoted by darkness was remarkably similar to those reported earlier.
Although the extent of visual recovery appeared to be just as substantial in animals for which MD extended to 8 weeks of age as it was for animals deprived to only 5 or 6 weeks of age, it is important to note that darkness was imposed at either the same age (P93) or else at the same time with respect to the prior period of MD, namely 8 weeks later, as was the case in the previous study. For the animal deprived to 8 weeks (C221), darkness was imposed 8 weeks later at P112 but still at an age well within the critical period of ocular dominance plasticity in the kitten visual cortex that extends to between 6 and 8 months of age (Olson & Freeman, 1980; Daw et al. 1992; Jones et al. 1984). It remains to be seen whether the speed and/or extent of the visual recovery promoted by darkness declines at ages beyond P112. However, the unchanged speed and extent of visual recovery observed as the age of imposition of darkness increased from 93 to 112 days suggests that any critical period for the effects of darkness may be long. Studies conducted in rats suggest that 10 days of darkness can reverse the physiological effects of MD in the visual cortex, as well as promote substantial but incomplete visual recovery in adult rats well outside the critical period of ocular dominance plasticity (He et al., 2006, 2007). However, as argued elsewhere (Mitchell & Duffy, 2014), there are reasons to question whether these results may transfer from rats to species such as cats and monkeys that have frontally located eyes and semi‐decussated visual pathways and also possess stereoscopic vision.
Effects of shorter periods of darkness or introduction of intermittent light exposure
The original choice of a 10 day period of darkness was made on the basis of its effect on levels of neurofilament in the visual cortex (O'Leary et al. 2012). In a subsequent study, 10 or 15 days of darkness reduced neurofilament to levels equivalent to those at an earlier stage of development, whereas 5 days of darkness had no such effect (Duffy & Mitchell, 2013). On the other hand, the magnitude and speed of visual recovery in and of itself raises the question of whether shorter periods of darkness may still exert some benefits. The results, as shown in Fig. 3 were unequivocal in demonstrating that 5 days of darkness provided no visual benefits because it neither blocked the effects of MD when imposed immediately afterward, nor did it promote any measurable improvement of the acuity of the amblyopic eye when imposed 8 weeks later. The only effect of 5 days of darkness was negative because it reduced the visual acuity of the non‐deprived eye when imposed immediately after MD (C191) (Fig. 3 D). Although this effect was only temporary, darkness did not reduce in any obvious way the depth of amblyopia that developed eventually in the deprived eye. The lack of any benefits for vision of 5 days of darkness may reflect the minimal effects of darkness on levels of neurofilament or of other braking molecules in the visual cortex and lateral geniculate nucleus.
Short (15 or 30 min) daily periods of light exposure also obliterated any benefits of a 10 day period otherwise spent in complete darkness. Moreover, as with 5 days of darkness, the only remaining influence of the latter was observed in the situation (C190) (Fig. 4 A) where darkness immediately followed the period of MD. Again, the remaining influence of the period of darkness was negative because it temporarily reduced the vision of the non‐deprived eye without any reduction in the depth of amblyopia in the other eye. The strictness of the parameters of the period of darkness holds important implications with respect to its potential translation to treatment of amblyopia in humans. Both a shorter period of darkness and the ability to introduce daily periods of light to facilitate indispensable daily activities such as eating and ablution would be attractive from the point of view of acceptance of darkness as a therapy, as well as compliance with it once initiated. However, a number of lighting parameters still remain to be explored such as low (scotopic) light levels or very brief or temporally modulated light.
Depth perception
Both recovery conditions with respect to the relative timing of the periods of MD and darkness might be considered favourable for the acquisition of stereoscopic vision. The situation where periods of MD and darkness were contiguous (Immediate Dark) could be considered auspicious for the development of good stereopsis because at no time after the period of darkness was the visual acuity of one eye handicapped with respect to the other. In the other condition (Delayed Dark), darkness was imposed after an 8 week period of binocular vision and at a time when the visual acuity of the non‐deprived eye was at age‐matched normal levels. Thus, it is possible that, following the period of darkness, pre‐existing mature functional connections with the non‐deprived eye may guide or instruct the establishment of neural connections with the deprived eye.
Although darkness promoted recovery of visual acuity in the amblyopic eye to normal levels in all eleven animals for which measurements of depth perception were made, only three (C150, C151 and C156) showed evidence that they possessed stereoscopic vision equivalent to that achieved by normal cats when tested in the same manner. With both eyes open, all three animals were able to discriminate small depth differences of 1.8 cm at observation distances of 68–73 cm. For an average interocular distance of 3.2 cm at the time of testing, this performance corresponds to retinal disparities of 3.6–4.1 min arc. On the other hand, with either eye alone they were unable to discriminate the largest depth difference on the jumping stand that was over 12 times larger (22.4 cm). The binocular performance of these three animals not only matched that of normal cats (Mitchell et al. 1979; Kaye et al., 1981, 1982), but also showed a similar superiority to their monocular abilities, which, together, was consistent with them possessing normal stereopsis. Methods that permit independent control of the stimuli presented to the two eyes would permit both unique presentation of retinal disparity cues to depth and also allow evaluation of the presence or absence of different types of stereopsis. Although the simple displays as used in the present study provide evidence of the presence of local stereopsis, more complex displays are required to probe for global stereopsis where identification of the stimulus requires simultaneous detection of the relative disparities of many local elements. Indeed, in a past study of the visual recovery promoted by certain occlusion regimens to alleviate amblyopia following an early period of MD, it was shown that, although such procedures could allow recovery of local stereopsis as assessed in the present study, the same animals failed tests of global stereopsis (Mitchell, Ptito & Lepore, 1994). Conservatively, it could be stated that only three animals of the present study exhibited normal local stereopsis. The remaining eight cats either showed evidence of poor local stereopsis (n = 3) or behaved as if stereoblind (n = 5). The outcome of darkness as applied the present study echoes clinical opinion that restorative procedures alleviating amblyopia by themselves seldom produce improvement of stereoscopic vision (Birch, 2013).
The outcome with respect to depth perception appeared to be similar no matter whether the period of darkness followed immediately after the period of MD or was delayed. Because darkness blocked the development of amblyopia in the first situation, such that the deprived eye was never at a disadvantage with respect to the fellow eye, it might be considered that the conditions were favourable for the development of stereoscopic vision. In the second (Delayed Dark) condition, darkness occurred at a time when the visual acuity of the non‐deprived eye was near normal levels. Thus, it is possible that the pre‐existing mature functional connections with the non‐deprived eye may guide or instruct the establishment of neural connections with the deprived eye following darkness. Functional evidence of such a role for prior experience has been reported in relation to the restoration of orientation maps in the kitten visual cortex following reverse occlusion (Kim & Bonhoeffer, 1994). A structural trace of prior experience has also been reported in terms of changes in spine density on layer 5 pyramidal cells in the mouse visual cortex after repeated episodes of MD (Hofer et al. 2009). The mature connections established by the non‐deprived eye in the visual cortex prior to late imposition of darkness could provide a scaffold for the rapid recovery of functional synaptic connections with the deprived eye and for the fast and extensive recovery of the visual acuity of this eye observed (Fig. 2). One factor that may contribute to the outcome with respect to stereoscopic vision is the length of the period of binoular vision prior to the period of MD that would favour those animals for which MD occurred at P30 as opposed to P7. Indeed, two of the three animals in the immediate dark condition (C150 and C156) (Fig. 5) acquired stereopsis, whereas neither animal for which MD began at P7 did so. The only animal from the delayed dark condition (C151) (Fig. 6) that acquired stereopsis received its period of MD at P30. The three other animals that had the same visual history, as well as the animal for which the period of MD began at P7, did not develop stereoscopic vision, suggesting that the 8 week period of binocular vision following the period of MD conferred no benefit.
The generally poor outcome with respect to recovery of stereoscopic vision, no matter when darkness was imposed in relation to the period of MD, suggests that the requirements for the development of stereopsis are more strict than those required for recovery of good visual acuity in the amblyopic eye. The importance of the length of the period of binocular vision prior to the amblyogenic event (in this case MD) raises the possibility of a shorter critical period for the benefits of darkness with respect to recovery of stereoscopioc vision. Alternatively, the critical periods for the benefits of darkness for visual acuity and for stereopsis may be similar, although either longer periods of darkness or additional measures designed to refine binocular neural connectivity may be necessary to achieve good stereopsis.
Underlying mechanisms
The earlier study on kittens (Duffy & Mitchell, 2013) was conducted within the context of current opinion (Hensch, 2004, 2005) linking the decline of plasticity during critical periods to the combined action of molecules with slow postnatal developmental profiles serving as ‘brakes’ on plasticity as their levels increase. A primary motive for the earlier study was the suggestion that darkness might slow or reverse the development of these molecules and thereby effectively reset the developing visual cortex to a structurally more plastic state. In addition to the evidence provided by the behavioural findings, measurements of the developmental profile of neurofilament protein, a putative braking molecule in the visual cortex, was shown to be slow and, furthermore, levels were reduced following a short period of total darkness. In a subsequent study, the developmental profiles of the individual neurofilament proteins in the visual cortex were all shown to be slow and complementary to the profile of the sensitive period, a pattern that was also confirmed in human visual cortex (Song et al. 2015). Although the effects of brief periods of complete darkness on levels of other putative braking molecules such as various chondroitin sulphate proteoglycans (Kind et al. 2013; Pizzorusso et al. 2002) or myelin‐related proteins (McGee et al. 2005) have not yet been examined, our working hypothesis is that the behavioural effects observed in the present study represent the combined effects of darkness on many braking molecules and plasticity accelerators. It is also possible that 10 days of darkness promotes a reduction in the levels of these and other as yet unidentified molecules, either simultaneously or in a beneficial sequence. The required constellation of modifications that promote plasticity and recovery following 10 days in darkness is evidently not replicated when darkness is reduced in duration to 5 days or when it is interrupted with brief daily light exposure.
Although immersion in total darkness effectively eliminates all visually driven neural activity, comparable outcomes might result from pharmacological interventions that temporally block neural activity of retinal ganglion cells or their axons in the optic nerve. Moreover, it is possible that pharmacological or visual manipulations producing correlated neural activity from the two eyes, such as binocular exposure to a Ganzfeld, may also be beneficial. Because these potential alternative interventions present their own substantial barriers to implementation, a period of total darkness currently provides the most accessible means for investigating the mechanisms that underlie the amazing visual recovery promoted by elimination of visually driven neural activity.
The eight‐ to 10‐fold difference in the speed of recovery of the deprived eye when darkness was imposed immediately as opposed to 8 weeks after the period of MD implies that different neural processes may be engaged in the these two situations. The period of darkness in the Immediate Dark condition occurred as early as P37, or ∼8 weeks before it was implemented in the Delayed Dark condition. The slow speed of the subsequent recovery of vision of the deprived eye could be attributed to either the early age at which darkness was imposed or to an interaction between the neural events precipitated during the period of MD and the adjacent period of darkness, or both (Freeman & Olson, 1979). With respect to the former, 10 days of darkness imposed very early in normal kittens has been shown to have drastic effects on the vision of both eyes but only when imposed before ∼10 weeks of age (Mitchell et al. 2015). For all the kittens studied to date where darkness followed immediately after the period of MD, the former was imposed well within this critical period, such that the vision of both the deprived and non‐deprived eyes was severely impacted. As suggested above, the subsequent slow but equal recovery of the acuity of both eyes presumably reflects similar underlying processes by which each eye re‐establishes effective synaptic connections within the visual cortex. Remarkably, the period of darkness apparently erased any advantage held by the non‐deprived eye during the prior period of MD.
By contrast, for kittens where the dark period was delayed, darkness occurred several weeks beyond the critical period for the deleterious effects of darkness on vision that terminates at 10 weeks (Mitchell et al. 2015), such that there was no impact on the visual acuity of the non‐deprived eye. As suggested above, neural connections with this eye could act as a foundation for the establishment of more potent connections with the amblyopic eye. The existence of a ‘blueprint’ provided by established synaptic connections with the non‐deprived eye might account for the fast recovery of vision after the period of darkness. It is also possible that this fast recovery may depend on the presence of a long (8 week) period of binocular vision following the period of MD. The substantial changes in vision associated with short periods of darkness either alone or following MD point to a complex interplay between visual input and genetic programmes of development in the central visual pathway in early postnatal life.
Although the discussion of the findings up to this point have emphasized the effects of darkness on the expression of various putative braking molecules, the results are also consistent with a theoretical account of synaptic plasticity whereby alteration of cellular or synaptic activities, such as those that accompany darkness, induces a change to the capacity for synaptic plasticity referred to as metaplasticity (Abraham & Bear, 1996; Cooper & Bear, 2012). A prediction derived from this theory is that the 10 day period of darkness should provoke an alteration of the ratio of NR2A to NR2B subunits of NMDA receptors within regions of the primary visual pathway.
Clinical implications
The ability of darkness to block or erase the effects on vision of a prior period of MD in kittens has obvious clinical implications for treatment of human amblyopia. A particularly attractive feature of darkness as a potential therapy for amblyopia is its effectiveness in early life, thereby potentially removing significant barriers to career and lifestyle choices posed by either or both the reduced acuity or stereoscopic vision (Levi et al. 2015). Despite the speed and extent of the recovery observed in kittens, the suggestion that darkness should be used as a therapy for amblyopia in humans would probably meet with skepticism. The level of acceptance of darkness as a therapy will depend to a considerable extent on the manner of its implementation, especially with respect to addressing some of the obvious obstacles to the performance of basic bodily needs and social interactions in a totally dark environment. The recent finding reported by Duffy et al. (2014) indicating that the effects of darkness are not replicated by binocular eyelid suture suggests that a transluscent binocular bandage may not provide a simple alternative to the use of a darkroom facility. Additional work on animal models is necessary to establish key parameters with respect to the efficacy of darkness, including the possible optimum age for its benefits and whether it can be interrupted for very brief intervals by short periods of restricted or special illumination. Despite the need for this additional information, it is already apparent that darkness (or a close variant) should not be considered in isolation but rather as part of a treatment programme because it must follow removal of any barrier(s) to concordant binocular visual input or otherwise the period of darkness may exacerbate the pre‐existing amblyopia. In addition, indications that the outcome for stereoscopic vision may be more heterogeneous than for visual acuity suggest that darkness should be followed closely by additional therapies such as the use of binocular contrast matching (Li et al. 2013) and video games (Vedamurthy et al. 2015) designed to strengthen and refine binocular cortical neural connections.
Additional information
Competing interests
The authors declare that they have no competing interests.
Author contributions
DEM and KRD designed the research. DEM, KRD, KM and KH performed the experiments and collected research data. NAC and DEM analysed data. DEM, KRD and NAC wrote the manuscript. All experiments were performed at Dalhousie University. All authors have approved the final version of the manuscript and agree to be accountable for all aspects of the work. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Funding
The research was supported by the Canadian Institutes of Health Research (grant number MOP102653).
Acknowledgements
We thank Jan Kennie, Matthew Smithen and Dahlia Bukhamseen for their care of the animals and for assistance with the training of some of the animals.
References
- Abraham WC & Bear MF (1996). Metaplasticity: the plasticity of synaptic plasticity. Trends Neurosci 19, 126–130. [DOI] [PubMed] [Google Scholar]
- Birch EE (2013). Amblyopia and binocular vision. Prog Ret Eye Res 33, 67–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore C & Cummings RM (1975). Eye opening in kittens. Vision Res 15, 1417–1418. [DOI] [PubMed] [Google Scholar]
- Blake R & Hirsch, HV (1975). Deficits in binocular depth perception in cats after alternating monocular deprivation. Science 190, 1114–1116. [DOI] [PubMed] [Google Scholar]
- Bonds AB & Freeman RD (1978). Development of the optical quality of the kitten eye. Vision Res 18, 391–398. [DOI] [PubMed] [Google Scholar]
- Bough EW (1970). Stereoscopic vision in the macaque monkey Nature 225, 41–42. [DOI] [PubMed] [Google Scholar]
- Cooper LN & Bear MF (2012). The BVM theory of synapse modification at 30: interaction of theory with experiment. Nature Rev Neurosci 13, 798–810. [DOI] [PubMed] [Google Scholar]
- Crawford MLJ, Smith EL, Harwerth RS & Von Noorden GK (1984). Stereoblind monkeys have few binocular neurons. Invest Ophthalmol Vis Sci 25, 779–781. [PubMed] [Google Scholar]
- Crawford MLJ, von Noorden GK, Meharg LS, Rhodes JW, Harwerth RS, Smith EL & Miller DD (1983). Binocular neurons and binocular function in monkeys and children. Invest Ophthalmol Vis Sci 24, 491–495. [PubMed] [Google Scholar]
- Daw NW (2006). Visual Development, 2nd edn Springer: New York, NY. [Google Scholar]
- Daw NW, Fox KD, Sato H & Czepita D (1992). Critical period for monocular deprivation in the cat visual cortex. J Neurophysiol 67, 197–202. [DOI] [PubMed] [Google Scholar]
- Duffy KR, Bukhamseen DH, Smithen MJ & Mitchell DE (2014). Binocular eyelid closure promotes anatomical but not behavioral recovery from monocular deprivation. Vis Res 114, 151–160. [DOI] [PubMed] [Google Scholar]
- Duffy KR & Mitchell DE (2013). Darkness alters maturation of visual cortex and promotes fast recovery from monocular deprivation. Curr Biol 23, 382–386. [DOI] [PubMed] [Google Scholar]
- Fox R & Blake RR (1971). Stereoscopic vision in the cat. Nature 233, 55–56. [DOI] [PubMed] [Google Scholar]
- Freeman RD & Olson CR (1979). Is there a ‘consolidation’ effect for monocular deprivation? Nature 284, 404–406. [DOI] [PubMed] [Google Scholar]
- Giffin F & Mitchell DE (1978). The rate of recovery of vision after early monocular deprivation in kittens. J Physiol 274, 511–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingras G, Mitchell DE & Hess RF (2005). The spatial localization deficit in visually deprived kittens. Vis Res 45, 975–989. [DOI] [PubMed] [Google Scholar]
- Harwerth RS & Boltz RL (1979). Behavioral measures of stereopsis in monkeys using random‐dot stereograms. Physiol Behav 22, 229–234. [DOI] [PubMed] [Google Scholar]
- Harwerth RS, Smith EL 3rd & Siderov J (1995). Behavioral studies of local stereopsis and disparity vergence in monkeys. Vis Res 35, 1755–1770. [DOI] [PubMed] [Google Scholar]
- He HY, Hodos W & Quinlan EM (2006). Visual deprivation reactivates rapid ocular dominance plasticity in adult visual cortex. J Neurosci 26, 2951–2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He HY, Ray B, Dennis K & Quinlan EM (2007). Experience‐dependent recovery of vision following chronic deprivation amblyopia. Nature Neurosci 10, 1134–1136. [DOI] [PubMed] [Google Scholar]
- Hensch TK (2004). Critical period regulation. Ann Rev Neurosci 27, 549–579. [DOI] [PubMed] [Google Scholar]
- Hensch TK (2005). Critical period plasticity in local cortical circuits. Nature Rev Neurosci 6, 877–888. [DOI] [PubMed] [Google Scholar]
- Hess RF, France T & Tulanay‐Keesey U (1981). Residual vision in humans who have been monocularly deprived of pattern stimulation in early life. Exp Brain Res 44, 295–311. [DOI] [PubMed] [Google Scholar]
- Hess RF & Holliday IA (1992). The spatial localization deficit in amblyopia. Vis Res 32, 1319–1339. [DOI] [PubMed] [Google Scholar]
- Hofer SB, Mrsic‐Floger TD, Bonhoeffer T & Hubener M (2009). Experience leaves a lasting structural trace in cortical circuits. Nature 457, 313–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel DH & Wiesel TN (1963). Receptive fields of cells in striate cortex of very young, visually inexperienced kittens. J Neurophysiol 26, 994–1002. [DOI] [PubMed] [Google Scholar]
- Hubel DH & Wiesel TN (1970). The period of susceptibility to the physiological effects of unilateral eye closure in kittens. J Physiol 206, 419–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel DH & Wiesel TN (1977). Plasticity of ocular dominance columns in monkey striate cortex. Phil Trans Roy Soc Lond B 278, 377–409. [DOI] [PubMed] [Google Scholar]
- Hubel DH & Wiesel TN (2005). Brain and Visual Perception: The Story of a 25 year Collaboration. Oxford University Press: New York, NY. [Google Scholar]
- Jones KR, Spear PD & Tong L (1984). Critical periods for effects of monocular deprivation: differences between striate and extrastriate cortex. J Neurosci 4, 2543–2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaye M, Mitchell DE & Cynader M (1981). Selective loss of binocular depth perception following ablation of cat visual cortex. Nature 293, 60–62. [DOI] [PubMed] [Google Scholar]
- Kaye M, Mitchell DE & Cynader, M (1982). Depth perception, eye alignment and cortical ocular dominance of dark‐reared cats. Dev Brain Res 2, 37–53. [DOI] [PubMed] [Google Scholar]
- Kim DS & Bonhoeffer T (1994). Reverse occlusion leads to a precise restoration of orientation preference maps in visual cortex. Nature 370, 370–372. [DOI] [PubMed] [Google Scholar]
- Kind PC, Sengpiel F, Beaver CJ, Crocker‐Buque A, Kelly GM, Matthews RT & Mitchell DE (2013). The development and activity‐dependent expression of aggrecan in the cat visual cortex. Cereb Cortex 23, 349–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiorpes L & McKee SP (1999). Neural mechanisms underlying amblyopia. Curr Op Neurobiol 9, 480–486. [DOI] [PubMed] [Google Scholar]
- Kutcher MR & Duffy KR (2007). Cytoskeleton alteration correlates with gross structural plasticity in the catb lateral geniculate nucleus. Vis Neurosci 24, 775–785. [DOI] [PubMed] [Google Scholar]
- LeVay S, Wiesel TN & Hubel DH (1980). The development of ocular dominance columns in normal and visually deprived monkeys. J Comp Neurol 191, 1–51. [DOI] [PubMed] [Google Scholar]
- Levi DM, Knill DC & Bavalier D (2015). Stereopsis and amblyopia: a mini‐review Vis Res 114, 17–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Thompson B, Deng D, Chan LY, Yu M & Hess RF (2013). Dichoptic training enables the adult amblyopic brain to learn. Curr Biol 23, R308–R309. [DOI] [PubMed] [Google Scholar]
- McGee AW, Yang Y, Fischer QS, Daw NW & Strittmatter SM (2005). Experience‐driven plasticity of visual cortex limited by myelin and Nogo receptor. Science 305, 2222–2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell DE (1988). The extent of visual recovery from early monocular or binocular visual deprivation in kittens. J Physiol 395, 639–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell DE (2013). A shot in the dark: the use of darkness to investigate visual development and as a therapy for amblyopia. Clin Exp Optom 96, 363–372. [DOI] [PubMed] [Google Scholar]
- Mitchell DE. Crowder NA, Holman K, Smithen M & Duffy KR (2015). Ten days of darkness causes temporary blindness during an early critical period in felines. Proc Roy Soc Lond Ser B 282, 20142756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell DE & Duffy KR (2014). The case from animal studies for balanced binocular treatment strategies for human amblyopia. Ophthal Physiol Optics 34, 129–145. [DOI] [PubMed] [Google Scholar]
- Mitchell DE, Giffin F & Timney B (1977). A behavioral technique for the rapid assessment of the visual capabilities of kittens. Percep 6, 181–193. [DOI] [PubMed] [Google Scholar]
- Mitchell DE, Kaye M & Timney B (1979). Assessment of depth perception in cats. Percep 8, 389–396. [DOI] [PubMed] [Google Scholar]
- Mitchell DE, Kennie J, Schwarzkopf & Sengpiel F (2009). Daily mixed visual experience that prevents amblyopia in cats does not always allow the development of good binocular depth perception. J Vis 9, 22. [DOI] [PubMed] [Google Scholar]
- Mitchell DE, Ptito M & Lepore F (1994). Depth perception in monocularly deprived cats following part‐time reverse occlusion. Eur J Neurosci 6, 967–972. [DOI] [PubMed] [Google Scholar]
- Mitchell DE & Timney B (1984). Postnatal development of function in the mammalian visual system In Handbook of Physiology Section I: The Nervous System, Vol. 3, Part I Sensory Processes, ed. Darian‐Smith I, pp. 507–555. American Physiological Society, Bethesda, MD. [Google Scholar]
- Movshon JA & Kiorpes L (1990). The role of experience in visual development In Development of Sensory Systems in Mammals, ed. Coleman JR, pp. 155–202. Wiley: New York, NY. [Google Scholar]
- Movshon JA & Van Sluyters RC (1981). Visual neuronal development. Ann Rev Psychol 32, 477–522. [DOI] [PubMed] [Google Scholar]
- Murphy KM & Mitchell DE (1987). Reduced visual acuity in both eyes of monocularly deprived kittens following a short or a long period of reverse occlusion. J Neurosci 7, 1526–1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Leary TP, Kutcher MR, Mitchell DE & Duffy KR (2012). Recovery of neurofilament following early monocular deprivation. Front Syst Neurosci 6, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson CR & Freeman RD (1980). Profile of the sensitive period for monocular deprivation in kittens. Exp Brain Res 39, 17–21. [DOI] [PubMed] [Google Scholar]
- Packwood J & Gordon B (1975). Stereopsuis in normal domestic cat, Siamese cat, and cat raised with alternating monocular occlusion. J Neurophysiol 38, 1485–1499. [DOI] [PubMed] [Google Scholar]
- Pizzorusso T, Medini P, Berardi N, Chierzi S, Fawcett JW & Maffei L (2002). Reactivation of ocular dominance plasticity in the adult visual cortex. Science 298, 1248–1251. [DOI] [PubMed] [Google Scholar]
- Ptito M, Lepore F & Guillemot JP (1991). Stereopsis in the cat: behavioral demonstration and underlying mechanisms. Neuropsychologia 29, 443–464. [DOI] [PubMed] [Google Scholar]
- Rauschecker JP (1991). Mechanisms of visual plasticity: Hebb synapses, NMDA receptors and beyond. Physiol Rev 71, 587–615. [DOI] [PubMed] [Google Scholar]
- Sankoh AJ, Huquw MF & Dubey SD (1997). Some comments on frequently used multiple endpoint adjustment methods in clinical trials. Stats Med 16, 2529–2542. [DOI] [PubMed] [Google Scholar]
- Sengpiel F & Kind PC (2002). The role of activity in development of the visual system. Curr Biol 12, R818–826. [DOI] [PubMed] [Google Scholar]
- Sherman SM & Spear PD (1982). Organization of visual pathways in normal and visually deprived cats. Physiol Rev 62, 738–855. [DOI] [PubMed] [Google Scholar]
- Song S, Mitchell DE, Crowder NA & Duffy KR (2015). Postnatal accumulation of intermediate filaments in the cat and human primary visual cortex. J Comp Neurol 523, 2111–2126. [DOI] [PubMed] [Google Scholar]
- Tukey JW, Ciminera JL & Heyse JF (1985). Testing the statistical certainty of a response to increasing doses of a drug. Biometrics 41, 295–301. [PubMed] [Google Scholar]
- Vedamurthy I, Nahum M, Bavalier D & Levi DM (2015). Mechanisms of recovery of visual function in adult amblyopia through a tailored action video game. Sci Rep 5, 8482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesel TN & Hubel DH (1963). Single‐cell responses in striate cortex of kittens deprived of vision in one eye. J Neurophysiol 26, 1003–1017. [DOI] [PubMed] [Google Scholar]
- Wiesel TN & Hubel DH (1974). Ordered arrangement of orientation columns in monkeys lacking visual experience. J Comp Neurol 158, 307–318. [DOI] [PubMed] [Google Scholar]


