Key points
Recent studies have shown that some of the deleterious effects of a high‐salt (HS) diet are independent of elevated blood pressure and are associated with impaired endothelial function.
Increased generation of cyclo‐oxygenase (COX‐1 and COX‐2)‐derived vasoconstrictor factors and endothelial activation may contribute to impaired vascular relaxation during HS loading.
The present study aimed to assess the regulation of microvascular reactivity and to clarify the role of COX‐1 and COX‐2 in normotensive subjects on a short‐term HS diet.
The present study demonstrates the important role of COX‐1 derived vasoconstrictor metabolites in regulation of microvascular blood flow during a HS diet.
These results help to explain how even short‐term HS diets may impact upon microvascular reactivity without changes in blood pressure and suggest that a vasoconstrictor metabolite of COX‐1 could play a role in this impaired tissue blood flow.
Abstract
The present study aimed to assess the effect of a 1‐week high‐salt (HS) diet on the role of cyclo‐oxygenases (COX‐1 and COX‐2) and the vasoconstrictor prostaglandins, thromboxane A2 (TXA2) and prostaglandin F2α (PGF2α), on skin microcirculatory blood flow, as well as to detect its effect on markers of endothelial activation such as soluble cell adhesion molecules. Young women (n = 54) were assigned to either the HS diet group (N = 30) (∼14 g day–1 NaCl ) or low‐salt (LS) diet group (N = 24) (<2.3 g day–1 NaCl ) for 7 days. Post‐occlusive reactive hyperaemia (PORH) in the skin microcirculation was assessed by laser Doppler flowmetry. Plasma renin activity, plasma aldosterone, plasma and 24 h urine sodium and potassium, plasma concentrations of TXB2 (stable TXA2 metabolite) and PGF2α, soluble cell adhesion molecules and blood pressure were measured before and after the diet protocols. One HS diet group subset received 100 mg of indomethacin (non‐selective COX‐1 and COX‐2 inhibitor), and another HS group subset received 200 mg of celecoxib (selective COX‐2 inhibitor) before repeating laser Doppler flowmetry measurements. Blood pressure was unchanged after the HS diet, although it significantly reduced after the LS diet. Twenty‐four hour urinary sodium was increased, and plasma renin activity and plasma aldosterone levels were decreased after the HS diet. The HS diet significantly impaired PORH and increased TXA2 but did not change PGF2α levels. Indomethacin restored microcirculatory blood flow and reduced TXA2. By contrast, celecoxib decreased TXA2 levels but had no significant effects on blood flow. Restoration of of PORH by indomethacin during a HS diet suggests an important role of COX‐1 derived vasoconstrictor metabolites in the regulation of microvascular blood flow during HS intake.
Key points
Recent studies have shown that some of the deleterious effects of a high‐salt (HS) diet are independent of elevated blood pressure and are associated with impaired endothelial function.
Increased generation of cyclo‐oxygenase (COX‐1 and COX‐2)‐derived vasoconstrictor factors and endothelial activation may contribute to impaired vascular relaxation during HS loading.
The present study aimed to assess the regulation of microvascular reactivity and to clarify the role of COX‐1 and COX‐2 in normotensive subjects on a short‐term HS diet.
The present study demonstrates the important role of COX‐1 derived vasoconstrictor metabolites in regulation of microvascular blood flow during a HS diet.
These results help to explain how even short‐term HS diets may impact upon microvascular reactivity without changes in blood pressure and suggest that a vasoconstrictor metabolite of COX‐1 could play a role in this impaired tissue blood flow.
Abbreviations
- BMI
body mass index
- BP
blood pressure
- COX
cyclo‐oxygenase
- DBP
diastolic blood pressure
- EDCF
endothelium‐derived contracting factors
- HR
heart rate
- HS
high‐salt
- ICAM‐1
intercellular adhesion molecule‐1
- LDF
laser Doppler flowmetry
- LS diet
low‐salt diet
- MAP
mean arterial pressure
- PGF2α
prostaglandin F2α
- PGH2
prostaglandin H2
- PORH
post‐occlusive reactive hyperaemia
- PRA
plasma renin activity
- ΔR‐O
difference between the quotients of flow change during reperfusion and occlusion in relation to baseline
- sCAM
soluble cell adhesion molecule
- SBP
systolic blood pressure
- sE‐selectin
soluble E‐selectin
- sICAM
soluble intercellular adhesion molecule
- sVCAM
soluble VCAM
- TXA2
thromboxane A2
- VCAM‐1
vascular cell adhesion molecule‐1
- WHR
waist to hip ratio
Introduction
It is well accepted that high‐salt (HS) intake is an essential risk factor in development and progression of hypertension (Bragulat & de la Sierra, 2002; Drenjancevic Peric et al. 2011), whereas a reduction in dietary sodium decreases blood pressure (BP) in many patients with essential hypertension (Cutler et al. 1997; Sacks et al. 2001). Furthermore, numerous studies on experimental animals have shown that changes in salt intake significantly alter vascular reactivity to different physiological stimuli, in conduit vessels and resistance arteries, as well as in the microcirculation (Drenjancevic Peric et al. 2003; Phillips et al. 2004; Zhu et al. 2006), even in normotensive animals. Some of the deleterious effects of a HS diet are independent of elevated BP and may occur in normotensive individuals (Weinberger, 2002) and are associated with impaired endothelial function (Drenjancevic Peric et al. 2011).
However, the effects of acute salt loading on endothelial function and vascular reactivity in young healthy individuals are still scarce and inconsistent. Some studies have demonstrated that high dietary salt intake in young healthy persons was associated with reduced vascular NO bioactivity (Tzemos et al. 2008; Greaney et al. 2012; Liu et al. 2012), whereas others failed to demonstrate such a relationship (Stein et al. 1995; Higashi et al. 2001; Dishy et al. 2003). Therefore, the mechanisms and the time course by which a HS diet impacts upon the endothelium are still unclear. Furthermore, in previous studies, the focus was on conductance and resistance arteries as a surrogate for the endpoint of coronary artery disease (Tzemos et al. 2008; Greaney et al. 2012; Liu et al. 2012). Because the earliest changes and final organ damage in various pathological conditions occur in the microcirculation, it is essential to evaluate blood flow changes specifically in the microcirculation. Animal studies have shown that even a short‐term HS diet significantly impairs vascular function and alters the responses to both vasoconstrictor and vasodilator stimuli in different vascular beds in rats (Drenjancevic Peric et al. 2003; Phillips et al. 2004; Zhu et al. 2006). This is associated with overproduction of vasoconstrictor factors, such as cyclo‐oxygenase (COX)‐derived thromboxane A2 (TXA2) and/or prostaglandin F2α (PGF2α), which may contribute to impaired vascular relaxation (Zhu et al. 2006).
Endothelial dysfunction precedes and underlies the process of cardiovascular disease by promoting the early and late mechanisms of atherosclerosis (Ross, 1999; Verma & Anderson, 2002). Different sets of cell adhesion molecules (CAMs), such as selectins (E‐selectin) and immunoglobin superfamily members [intercellular adhesion molecule‐1 (ICAM‐1) and vascular cell adhesion molecule‐1 (VCAM‐1)], play important role in endothelial activation. Their soluble forms (sCAMs) are detectable in serum and are considered to be markers of endothelial cell activity or injury (Ross, 1999; Verma & Anderson, 2002; Tadzic et al. 2013).
Although most studies have been conducted in males, some evidence suggests that the classical risk factors for cardiovascular disease may not apply to women, as they do in the men, particularly in regard to coronary calcification, diastolic heart failure and microvascular disease (Miller, 2010). Thus, a population of healthy young women was included in present study to clarify the physiology and pathophysiology of the female vascular system.
The present study aimed to determine the possible role of COX enzymes (COX‐1 and/or COX‐2) in the regulation of skin microvascular blood flow after acute HS loading. In addition, we sought to determine plasma concentrations of endothelium derived molecules soluble ICAM (sICAM)‐1, soluble VCAM (sVCAM)‐1 and E‐selectin in young healthy women as potential biomarkers of endothelial activation during high dietary salt intake.
Methods
Study population
Fifty‐four young healthy female medical students recruited by advertisement at the Faculty of Medicine University Osijek participated in the present study. Exclusion criteria included a history of hypertension, coronary artery disease, diabetes, hyperlipidaemia, renal impairment, cerebrovascular and peripheral artery disease. None of the subjects were taking oral contraceptives or drugs that could affect the endothelium. Written informed consent was obtained from each subject. The study protocol and procedures conformed with the standards set by the latest revision of the Declaration of Helsinki and were approved by the Ethical Committee of the Faculty of Medicine, University of Osijek.
Study protocol
This was a randomized, one‐side blinded and placebo‐controlled study. During the study, all subjects were instructed to maintain a low‐sodium diet, with an intake of less than 2.3 g of salt per day (DASH eating plan; US Department of Health and Human Services, 2006) for 7 days. Thirty participants comprising the HS diet group were following a HS diet (intake of ∼14 g of NaCl every day) for 7 days. Salt supplement was given in a form of a NaCl tablet containing 5.85 g of NaCl, once in the morning and once in the evening. The low‐salt (LS) diet group consisted of 24 participants given a placebo.
A venous blood sample was taken after 30 min resting in supine position and a 24 h urine sample was collected before and after each diet protocol. BP and heart rate (HR) were measured at the beginning of each visit after a 15 min rest in a seated position using a semi‐automatic oscillometric monitor (OMRON, Osaka, Japan). The final values of BP and HR were the mean of three repeated measurements. Body mass index (BMI) and waist‐to‐hip ratio (WHR) were measured at each visit.
Assessment of skin microcirculatory blood flow
Microcirculatory blood flow was assessed by laser Doppler flowmetry (LDF) (MoorVMS‐LDF, Axminster, UK), a non‐invasive test that indicates changes in blood flow during post‐occlusive reactive hyperaemia (PORH) following release of an occlusion of blood flow (Stewart et al. 2004; Crakowski et al. 2006; Wright et al. 2006; De Backer et al. 2007) as an indicator of endothelial function. Measurements were performed in a warm room (mean ± SD temperature = 23.5 ± 0.5°C). Data collection started after 30 min of acclimatization to avoid temperature‐related changes in blood flow in subjects resting in supine position and the probe and wire were secured on place. The laser probe was attached to subject's volar forearm skin 13–15 cm from the wrist. After the 5 min baseline measurement, vascular occlusion was induced by inflating a pneumatic cuff on the upper arm to 30–50 mmHg above the systolic blood pressure (SBP). Measurements were taken before, during, and after release of 1 min occlusion. Figure 1 shows a representative plot of the data obtained by LDF measurement (Fig. 1).
Figure 1. LDF measurement of skin microvascular blood flow .
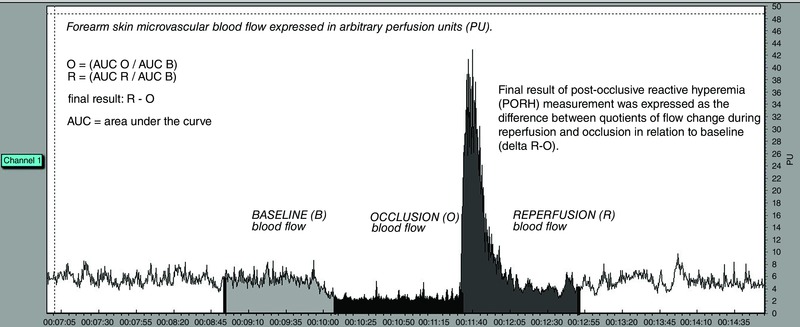
Microcirculatory blood flow in a given time was expressed in arbitrary perfusion units and determined by software calculating the area under the curve (AUC) during baseline flow, occlusion and reperfusion (AUC is denoted by the shaded portions of the trace). Because the flow does not reach the value of zero even when perfusion is absent, flow values are expressed as a quotient of a standard comparator – baseline flow. The final result was expressed as the difference between the quotients of flow change during reperfusion and occlusion in relation to baseline (ΔR‐O). Source: original trace, Laboratory for Vascular Physiology, Department of Physiology and Immunology, Faculty of Medicine, Josip Juraj Strossmayer University of Osijek).
The role of COX‐1 and COX‐2 in modulating tissue blood flow during dietary salt protocols
To investigate the role of the COX enzymes (COX‐1 and COX‐2) in regulating tissue blood flow after dietary salt manipulation, 100 mg of indomethacin (a non‐selective inhibitor of COX‐1 and COX‐2) was given to thirteen participants from the HS diet group over 90 min (Ivancev et al. 2009) and 200 mg of celecoxib (a selective inhibitor of COX‐2) was given to another ten participants from the HS diet group over 180 min (Widlansky et al. 2003) prior to repeated LDF measurement before and after the HS diet protocol. Both indomethacin and celecoxib were administered by oral pill.
Measurement of plasma COX‐dependent vasoconstrictor TXA2 and PGF2α levels
Because TXA2 has a half‐life of only 37 s under physiological conditions, the production of TXA2 in vivo is typically monitored by measurement of TXB2 and 2,3‐dinor TXB2. TXB2 is produced by the non‐enzymatic hydration of TXA2, and was shown to be stable (Stewart et al. 2004). The plasma concentration of the stable TXA2 metabolite, TXB2, was evaluated with a comercially available enzyme immunoassay kit (11‐dehydro‐TXB2 ELISA Kit; ABNOVA Corp., Taipei City, Taiwan).
The plasma concentration of PGF2α was evaluated using a comercially available enzyme immunoassay kit (Prostaglandin F2α ELISA Kit; ABNOVA Corp.).
Laboratory testing
Blood samples were analysed for plasma electrolytes (sodium and potassium), as well as urea and creatinine levels. Twenty‐four hour urine sample analyses for sodium, potassium, urea, creatinine and albumin levels were performed at the Department of Clinical Laboratory Diagnostics, University Hospital Osijek. Plasma renin activity (PRA) and plasma aldosterone concentrations were measured at the Clinical Institute of Nuclear Medicine and Radiation Protection, University Hospital Osijek, using standard radioimmunoassay techniques.
Concentrations of the serum sCAMs ICAM‐1, VCAM‐1 and E‐selectin
As endothelial activation markers, the serum concentrations of sICAM‐1, sVCAM‐1 and soluble E‐selectin (sE‐selectin) were assessed using commercially available ELISA kits (Anti‐Human CD54 ICAM‐1; Anti‐Human CD106 VCAM‐1; Anti‐Human CD62E E‐selectin; eBioscience, Carlsbad, CA, USA). All measurements were performed at the Laboratory for Molecular and Clinical Immunology, Department of Physiology and Immnunology, Faculty of Medicine University of Osijek.
Statistical analysis
All results are reported as the mean ± SD. Clinical characteristics before and after specific diet protocols were compared using a paired t test. The normality of data distribution was assessed by the Kolmogorov–Smirnov normality test. The Wilcoxon rank‐sum test was used when variables were not normally distributed. Student's t test was used to compare parameters between experimental protocols. When variables were not normally distributed, the Mann–Whitney rank sum test was used. When more than two repeated measures were analysed, a one‐way repeated measures ANOVA test was used. For post test multiple comparison analyses, the Holm–Sidak method was used. P < 0.05 was considered statistically significant. SigmaPlot, version 11.2 (Systat Software, Inc., Chicago, IL, USA) was used for statistical analysis.
Results
Subject profiles
Fifty‐four female subjects (mean ± SD age = 20 ± 2 years) participated in the present study. There was no difference in BMI (kg m–2) (LS diet 22.9 ± 3.6 vs. HS diet 22.1 ± 2.6, P = 0.729) or WHR (WHR LS diet 0.73 ± 0.03 vs. HS diet 0.73 ± 0.04, P = 0.732) between the two study groups. All participants were normotensive before the diet period. Systolic BP, diastolic blood pressure (DBP) and mean arterial pressure (MAP) were similar and did not change during the HS diet period, although they decreased significantly after the LS diet protocol. HR was significantly decreased after the HS diet but was not changed after the LS diet protocol (Table 1).
Table 1.
Arterial BP and HR of the study population
| Before HS | After HS | Before LS | After LS | |
|---|---|---|---|---|
| Variable | diet | diet | diet | diet |
| SBP | 105 ± 9 | 105 ± 10 | 105 ± 10 | 100 ± 9* |
| DBP | 71 ± 8 | 71 ± 7 | 69 ± 9 | 66 ± 7* |
| MAP | 82 ± 8 | 82 ± 8 | 81 ± 9 | 77 ± 7* |
| HR | 76 ± 10 | 71 ± 9* | 74 ± 13 | 74 ± 14 |
Results are expressed as the mean ± SD.
*P < 0.05 before vs. after HS diet (n = 30) or before vs. after LS diet (n = 24).
Biochemical parameters
The plasma sodium level increased during the HS diet protocol. The plasma creatinine level increased after the LS diet. There were no other statistically significant differences in baseline plasma electrolytes (potassium) or in urea and creatinine concentrations before and after either the LS or HS diet protocols. As expected, PRA and plasma aldosterone levels increased after the LS diet and decreased after the HS diet. Urinary sodium excretion rose significantly after the HS diet, and fell as expected after the LS diet. There was no statistically significant difference in 24 h urine urea and creatinine concentrations before and after either diet protocol. The 24 h urine albumin concentration was decreased after the LS diet and was unchanged after the HS diet (Table 2).
Table 2.
Biochemical parameters
| Variable | Before HS diet | After HS diet | Before LS diet | After LS diet |
|---|---|---|---|---|
| 24 h urine volume (mL) | 1221 ± 796 | 1389 ± 980 | 1146 ± 593 | 1327 ± 580 |
| 24 h urine sodium (mmol dU–1) | 126.8 ± 45.2 | 232.0 ± 91.4* | 118.7 ± 56 | 82.6 ± 43.1* |
| 24 h urine potassium (mmol dU–1) | 43.8 ± 13.8 | 43.8 ± 15.5 | 42.8 ± 16.3 | 39.8 ± 16.7 |
| 24 h urine urea (mmol dU–1) | 10191 ± 2663 | 9675 ± 2871 | 10076 ± 2922 | 9713 ± 3443 |
| 24 h urine cretinine (μmol dU–1) | 220 ± 58 | 224 ± 87 | 219 ± 77 | 196 ± 67 |
| 24 h urine albumine (dU–1) | 4.6 ± 2.4 | 5.0 ± 3.7 | 8.4 ± 8.1 | 5.6 ± 6.3* |
| Plasma sodium (mmol L–1) | 137 ± 1* | 138 ± 1 | 137 ± 2 | 137 ± 2 |
| Serum potassium (mmol L–1) | 4.6 ± 1.1 | 4.4 ± 0.5 | 4.7 ± 1.6 | 4.3 ± 0.4 |
| Serum urea (mmol L–1) | 4.1 ± 0.8 | 3.9 ± 0.9 | 3.9 ± 1.2 | 3.8 ± 1.1 |
| Serum creatinine (μmol dU–1) | 66 ± 9 | 64 ± 8 | 63 ± 9 | 68 ± 10* |
| PRA (ng mL–1 h–1) | 1.43 ± 1.63 | 0.87 ± 0.86* | 1.23 ± 1.53 | 1.99 ± 1.7* |
| Plasma aldosterone (pmol L–1) | 849 ± 462 | 553 ± 394* | 668 ± 418 | 1176 ± 816* |
Results are expressed as the mean ± SD.
*P < 0.05 before vs. after HS diet (n = 30) or before vs. after LS diet (n = 24).
LDF measurement of skin microcirculatory blood flow
PORH was significantly reduced in the HS diet group compared to the control PORH values (ΔR‐O control 1.4 ± 0.4 vs. HS diet 1.1 ± 0.3, P < 0.001) (Fig. 2 A). PORH was not significantly different in the LS diet protocol compared to control PORH values before the LS salt diet (ΔR‐O control 1.2 ± 0.3 vs. LS diet 1.3 ± 0.3, P = 0.562) (Fig. 2 B).
Figure 2. Changes of PORH .

Relative changes of PORH before and after HS, A and LS diet, B, protocols.
Effect of the non‐selective (COX‐1 and COX‐2) and selective (COX‐2) inhibitors on skin microcirculatory blood flow before and after the HS diet
Both indomethacin (ΔR‐O control 1.4 ± 0.4 vs. control + indomethacin 1.3 ± 0.3, P = 0.545) and celecoxib (ΔR‐O control 1.5 ± 0.3 vs. control + celecoxib 1.4 ± 0.3, P = 0.578) had no significant effect on PORH before the HS diet (Fig. 3 A and B). The non‐selective COX‐1 and COX‐2 inhibitor indomethacin eliminated the reduction in PORH after 1 min of occlusion in the HS diet group compared to the PORH value before indomethacin administration (ΔR‐O HS diet 1.0 ± 0.2 vs. HS diet + indomethacin 1.3 ± 0.5, P < 0.05) (Fig. 3 A). However, after the selective COX2 inhibitor celecoxib, PORH remained impaired in the HS diet group (ΔR‐O HS diet 1.1 ± 0.4 vs. HS diet + celecoxib 1.1 ± 0.3, P = 0.552) compared to baseline (Fig. 3 B).
Figure 3. Changes of peripheral blood flow .

Relative changes of peripheral blood flow between occlusion and reperfusion before and after the HS diet protocol with indomethacin, A or celecoxib, B, administration.
Effect of the HS diet on vasoconstrictor prostaglandins TXB2 and PGF2α plasma levels
Plasma levels of TXB2 increased significantly after 1 week of the HS diet compared to control levels before the HS diet protocol (cTXB2 pg mL–1 control before the HS diet 108 ± 39 vs. after the HS diet 160 ± 31, P = 0.033) (Fig. 4 A). On the other hand, plasma PGF2α levels were not changed after the HS diet protocol compared to control levels before the diet (cPGF2α pg mL–1 control before the HS diet 59 ± 33 vs. after the HS diet 50 ± 40, P = 0.209) (Fig. 4 B).
Figure 4. Effect of the HS diet on vasoconstrictor prostaglandins TXB2 and PGF2α plasma levels .
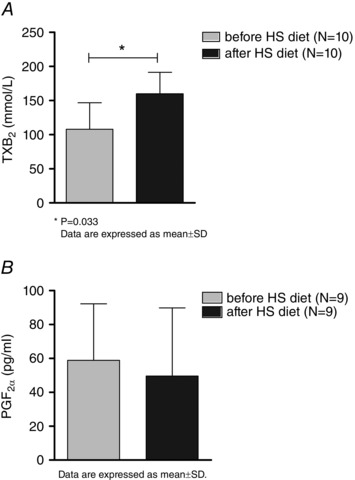
Significant increase of TXB2, A, with no changes in PGF2α, B, 1 week after HS dietary intake compared to control levels.
Effect of the HS diet and the non‐selective COX‐1 and COX‐2 inhibitor indomethacin and the selective COX‐2 inhibitor celecoxib on TXB2 plasma levels
Both indomethacin (cTXB2 pg mL–1 after the HS diet 150 ± 100 vs. HS diet + indomethacin 50 ± 18, P = 0.005) (Fig. 5 A) and celecoxib administration (cTXB2 pg mL–1 after the HS diet 160 ± 20 vs. HS diet + celecoxib 83 ± 31, P = 0.008) (Fig. 5 B) significantly decreased TXB2 levels after the HS diet protocol.
Figure 5. Effect of the HS diet and the non‐selective COX‐1 and COX–2 inhibitor indomethacin and the selective COX–2 inhibitor celecoxib on TXB2 plasma levels .
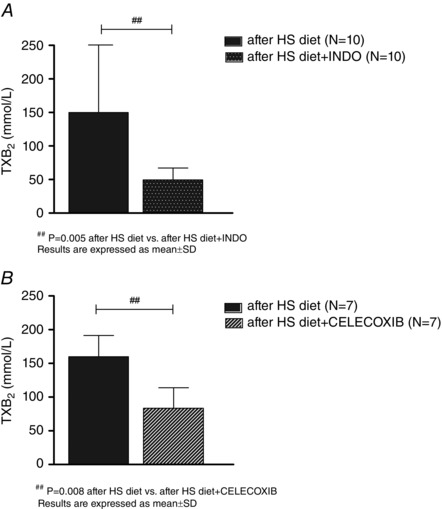
Significant decrease of TXB2 plasma levels in HS diet groups after indomethacin, A and celecoxib, B, administration.
Concentrations of the sCAMs ICAM‐1, VCAM‐1 and E‐selectin
sCAM concentrations did not change after either the HS or LS diet protocols (Table 3).
Table 3.
sCAMs
| Variable | Before HS diet | After HS diet | Before LS diet | After LS diet |
| sICAM‐1 (ng mL–1) | 235.59 ± 60.66 | 224.71 ± 65.05 | 255.42 ± 108.29 | 256.84 ± 99.7 |
| sVCAM‐1 (ng mL–1) | 2511 ± 2880 | 2385 ± 4427 | 2429 ± 2300 | 2071 ± 2582 |
| sE‐selectin (ng mL–1) | 38.45 ± 26.79 | 53.31 ± 63.35 | 37.62 ± 21.91 | 57.99 ± 41.17 |
Results are expressed as the mean ± SD.
sICAM‐1 HS diet (n = 22), sVCAM‐1 HS diet (n = 10), sE‐selectin HS diet (n = 10).
sICAM‐1 LS diet (n = 22), sVCAM‐1 LS diet (n = 13), sE‐selectin LS diet (n = 13).
Discussion
The salient finding of the present study is that 1 week of the HS diet impaired the microvascular endothelial function (Fig. 2 A), which was prevented by inhibiting COX enzymes with indomethacin but not with celecoxib (Fig. 3). The HS diet increased plasma concentrations of TXA2 (Fig. 4 A) but did not change PGF2α levels (Fig. 4 B). Plasma levels of TXA2 were significantly reduced after both indomethacin and celecoxib administration (Figs 4 and 5), suggesting an important role of other COX‐1 derived vasoconstrictor metabolites in the regulation of microvascular blood flow during the HS diet. It is generally accepted that a high dietary salt intake represents an independent risk factor in the development and progression of hypertension (Bragulat & de la Sierra, 2002; Mohan & Campbell, 2009; Drenjancevic Peric et al. 2011). In addition, the DASH study (the source of the LS diet plan used in the present study) demonstrated dose‐related effects of salt reduction on systemic BP values (Sacks et al. 2001). Although the mechanisms by which changes in dietary salt intake impact upon BP and microvascular function are not well understood, the HS diet can promote endothelial dysfunction prior to and/or independently of any increases in BP (Bragulat & de la Sierra, 2002; Weinberger, 2002; Drenjancevic Peric et al. 2011).
Diet protocol
Excessive salt intake is a major problem in almost all parts of the world; for example, the average salt intake for adults in Croatia is 13.3 ± 4.3 g day–1 (223.6 ± 74.0 mmol Na per 24 h urine) for men and 10.2 ± 4.2 g day–1 (177.3 ± 69.1 mmol Na per 24 h urine) for women (Premuzic et al. 2010). The fifty‐four participants in the present study had an average salt intake of 7.7 ± 3.1 g day–1 before the diet protocol (as calculated by the amount of sodium in the 24 h urine sample, 129.9 ± 51.6 mmol dU–1), which is below the Croatian average. Salt intake in the HS group was 14 g day–1 and the range of sodium excretion in the present study is not uncommon in the everyday life of many individuals. Decreased urinary sodium levels in the LS diet subjects and elevated urinary sodium levels, as well as decereased PRA and plasma aldosterone levels in subjects on the HS diet, confirmed that experimental protocol was conducted consistently, and also that the subjects conformed with the diet guidelines (Table 1).
BP
Many epidemiological and interventional studies have demonstrated a clear relationship between salt intake and hypertension (Bragulat & de la Sierra, 2002; Drenjancevic Peric et al. 2011). However, BP sensitivity to changes in sodium intake in young healthy individuals and the duration of HS consumption needed to increase BP in salt‐sensitive individuals remain undefined. Tzemos et al. (2008) reported that 7 days of acute salt loading significantly increased levels of brachial SBP but with no changes in DBP in young healthy individuals. By contrast, Starmans‐Kool et al. (2010) demonstrated that 14 days of the HS diet provoked minimal changes in brachial BP, although with a strong association between dietary salt manipulation and carotid BP. In the present study, there was no significant change in SBP, DBP and MAP after 1 week of the HS diet. However, we found a significant decrease in brachial SBP, DBP and MAP after 7 days of the LS diet protocol (Table 1). These results are consistent with the results of studies examining the impact of the DASH diet on BP in normotensive individuals (Sacks et al. 2001). However, the reduced BP in our LS diet group is unlikely to indicate different salt‐sensitivity of the individuals because, in the DASH‐Sodium trial collaborative research group study, SBP responses to changes in sodium intake over time were found to be inconsistent and non‐reproducible (Vollmer et al. 2001). Regardless, the results of the present study support current recommendations for a lower salt intake directed at the general public rather than ‘susceptible’ individuals (Vollmer et al. 2001; Obarzanek et al. 2003).
LDF studies
In the literature, we have only identified one study that focused on the impact of HS diet on microvascular regulation in humans (Greaney et al. 2012). LDF provides important clinical data in a non‐invasive fashion to evaluate microcirculatory impairment in various pathological conditions (Stewart et al. 2004; Crakowski et al. 2006; De Backer et al. 2007), including salt loading. However, there is still controversy in the literature regarding what mediators contribute to PORH. The results of the present study provide important new information contributing to our understanding of the precise mechanisms underlying the increase in post occlusion skin blood flow. Many mediators are known to contribute to PORH (Roustit & Crakowski, 2012). Sensory nerves are partially involved through an axon reflex response (Larkin & Williams, 1993; Lorenzo & Minson, 2007). Regarding the endothelium, endothelium‐derived hyperpolarizing factor is involved because it appears that large‐conductance calcium activated potassium channels play a major role (Lorenzo & Minson, 2007), whereas the results are conflicting concerning the implication of prostaglandins (Binggeli et al. 2003; Dalle‐Ave et al. 2004; Medow et al. 2007). The inhibition of NO synthesis does not alter PORH on the forearm (Wong et al. 2003), although recent work suggests that COX inhibition unmasks the NO dependence of reactive hyperaemia in the human cutaneous circulation (Medow et al. 2007). The results of the present study show that COX‐derived metabolites are not involved in PORH in healthy subjects, which is consistent with some previous studies (Hellmann et al. 2015) but, for the first time, we have shown that COX‐derived metabolites have an important role in mediating PORH with respect to the HS diet condition. Future studies are needed to clarify what other mediators are involved in PORH in both physiological and pathological conditions, as well as the precise mechanisms that lead to this ‘switch’ in PORH mediators after the HS diet.
The results of recent studies evaluating the influence of HS diet on endothelial dysfunction in young healthy individuals are inconsistent. Tzemos et al. (2008) demonstrated impaired endothelial function after 7 days of HS intake, which was evaluated by measuring forearm blood flow by stain‐gauge venous occlusion plethysmography. Liu et al. (2012) observed significantly lower flow‐mediated dilatation of the brachial artery after 1 week of HS loading. Du Pont et al. (2013) reported that an excess of salt intake in humans impairs flow‐mediated dilatation of the brachial artery independently of changes in BP. Dickinson et al. (2011) reported impaired postprandial endothelial function as assessed by flow‐mediated dilatation of the brachial artery after only one HS meal in healthy individuals. By contrast, Stein et al. (1995) found no effect of salt intake in methacholine‐induced vasodilatation in a group of healthy subjects. By contrast to these studies, the advantage of the present study is the utilization of the LDF method for direct, non‐invasive assessment of the microcirculation. One of the only studies that involved a HS diet and microvascular function reported that, in salt‐resistant adults, NO‐mediated cutaneous vasodilatation in response to local heating was attenuated during a HS diet and was subsequently improved by the local infusion of ascorbic acid. These data are consistent with those obtained in the present study, suggesting that dietary sodium‐induced declines in microvascular function are independent of BP and that oxidative stress contributes to this impairment (Greaney et al. 2012).
The present study showed a significant impairment in PORH in the young healthy women on the HS diet protocol for 7 days, indicating that even a short exposure to HS diet can lead to the development of endothelial dysfunction in microvessels. Although Greaney et al. (2012) reported that a high‐sodium diet altered NO‐mediated dilatation in the microvasculature possibly by provoking oxidative stress, the results of the present study shed new light on the idea that COX‐derived vasoconstrictors may have an important role in this microvascular impairment. By contrast to the HS diet, we did not observe any significant changes in the peripheral blood flow after 1 week of the LS diet compared to the pre‐LS diet control.
Effects of COX‐1 and COX‐2 inhibition on hyperaemic blood flow during the HS diet
The present study has shown that 1 week of the HS diet significantly increased plasma concentrations of TXB2, a stable metabolite of TXA2, and a potent vasoconstrictor produced by thromboxane A‐synthase that converts the arachidonic acid derivate prostaglandin H2 (synthesized by COX) to TXA2. Moreover, indomethacin treatment restored blood flow, whereas celecoxib did not induce any change in the impaired hyperaemic blood flow, after 1 week of the HS diet (Fig. 3). However, increased plasma TXA2 levels after the HS diet were significantly reduced after administration of both indomethacin and celecoxib (Figs 4 and 5). This suggests that some other vasoconstrictor dominantly derived by COX‐1 may play a role in impaired microvascular reactivity in subjects on the HS diet.
Numerous studies have shown the relevance of COX‐derived metabolites in vascular reactivity regulation: prostaglandins and thromboxane are critical modulators of vascular tone in both physiological and pathophysiological conditions (Davidge, 2001). Endothelial cells contain up to 20 times more COX than smooth muscle cells, indicating that the endothelium is the primary source of increased COX activity (Phillips et al. 2004). COX converts arachidonic acid into endoperoxides (PGH2), the intermediate of the prostanoid biosynthesis, which can either act as an endothelium‐derived contracting factors (EDCF) per se, or be further transformed into prostacyclin, TXA2 and various other prostaglandins, including prostaglandin D2, prostaglandin E2 and PGF2α by their respective synthases (Wong & Vanhoutte, 2010). Under normal physiological conditions, eicosanoids (primarily prostacyclin) generally induce vasorelaxation. However, in vascular pathogenesis, there may be an imbalance where COX‐dependent vasoconstriction becomes more dominant (Wong & Vanhoutte, 2010). In addition, the involvement of individual prostanoids in EDCF‐mediated responses varies depending on the species, the blood vessels studied, the endothelium‐dependent agonist used, and the age and disease state of the donor, which makes things even more complicated (Schuligoi et al. 2009; Wong & Vanhoutte, 2010; Eskildsen et al. 2014). Numerous human studies, conducted in the presence of clinical conditions characterized by endothelial dysfunction, have shown that endothelial cells, in response to different agonists and physical stimuli, became a source of EDCFs, mainly COX‐derived prostanoids. Moreover, in a number of vascular complications, common cardiovascular risk factors, such as oxidative stress and dyslipidaemia, may modulate COX‐dependent function leading to the impairment of vascular function (Davidge, 2001; Pitt et al. 2002). Many human pathological conditions characterized by a decline in endothelial function are associated with a progressive decrease in NO bioavailability and an increase in the production of EDCFs (Versari et al. 2009). Furthermore, previous studies have reported that COX‐1 is the preferential constitutive isoform of COX mediating endothelium‐dependent contractions in the large arteries of rats and mice. However, with ageing or disease, COX‐2 can be induced and then subsequently contributes to EDCF‐mediated responses (Versari et al. 2009). Nonetheless, the number of studies in humans dealing with the possible role of changes in salt intake on vascular COX activity is limited. The findings of the present study are consistent with those of Lombard et al. (2002), who reported that the vasoconstrictor COX metabolites TXA2 and PGH2 play a role in mediating paradoxical constriction in response to reduced in the middle cerebral arteries of rats fed a HS diet. Matrougi et al. (2001) showed that the impairment of flow‐induced dilatation in normotensive rats fed HS diet was accompanied by the induced expression of COX‐2. However, to our knowledge, the present study is the first to investigate this issue in young healthy women. Our findings suggest that short‐term exposure to a HS diet leads to significant alterations in arachidonic acid metabolism. In the present study, we demonstrated that COX enzymes (more specifically COX‐1) play an important role in the development of microvascular (endothelial) dysfunction after the HS diet in young healthy individuals. In light of the above findings, it is important to emphasize two major novelties of the present study: (1) for the first time, it is demonstrated that COX‐derived vasoconstriction takes part in the decline of microvascular reactivity after 1 week of the HS diet and (2) COX‐1 is the preferential constitutive isoform of COX that mediates endothelium‐dependent contractions in the human microvasculature after the HS diet. However, to demonstrate the role of each COX‐derived prostanoid in microvascular reactivity impairment after HS loading, as well as the mechanisms that regulate the balance between NO and EDCFs and the processes transforming the endothelium from a protective organ to a source of vasoconstrictor, pro‐aggregatory and promitogenic mediators remain to be determined in future studies.
Nonetheless, the specific roles of COX‐1 and COX‐2 in regulating vascular function during HS loading in young healthy humans are still growing in number and remain worthy of further investigation.
Endothelium activation markers and dietary salt manipulation
CAMs such as ICAM‐1, VCAM‐1 and E‐selectin have recently emerged as an important marker of endothelial activation preceding the adhesion of the activated leukocytes and initiating atherosclerotic lesions (Verma & Anderson, 2002; Tadzic et al. 2013). One goal of present study was to determine changes of serum sCAMs levels with dietary salt intake change, as a marker of endothelial activation and susceptibility to atherosclerosis. At present, 1 week of LS diet does not change the serum concentrations of sCAMs. This result was expected because the LS diet protocol did not affect microvascular reactivity, indicating preserved endothelial function. On the other hand, even though PORH was significantly impaired in the group that was on the HS diet protocol, the concentrations of serum sCAMs were unaffected by the HS diet protocol. To our knowledge, this is the first study to investigate the effects of the HS diet on serum sCAM levels in young healthy women because most of the existing studies have been conducted in hypertensive patients. For example, Ferri et al. (1998) showed that plasma sE‐selectin but not sICAM‐1 and sVCAM‐1 levels increased with 2 weeks of the HS diet in salt‐sensitive patients (Ferri et al. 1998). Tadzic et al. (2013) have shown that, in hypertension, there is high concentration of sICAM‐1 and sVCAM‐1, with low sE‐selectin levels, as markers of endothelial cell activation. These differences between the present study and the previously cited studies may suggest that increases in sCAMs levels are the result of elevated BP, rather than exposure to HS diet alone. It is possible that, in the present study, 1 week of the HS diet and/or the degree of salt loading was not sufficient to provoke endothelial activation and/or injury, and only affected the mechanisms responsible for vascular reactivity.
Study limitations
Although there are known and accepted limitations in the use of LDF measurements as an indicator of tissue blood flow, the present study employed well‐established parameters to describe relative changes during hyperaemic blood flow. To further evaluate the reproducibility of our measurements, control tests were performed in which repeated LDF measurements of three individuals were evaluated. In these tests, variability was significantly smaller compared to the SD of the actual LDF measurements (data not shown).
In conclusion, the present study is the first to investigate the effect of acute dietary salt manipulation on microcirculatory blood flow in young healthy female subjects. One week of the HS diet reduced post‐occlusion blood flow (an indicator of endothelial function) and increased serum thromboxane levels. Microcirculatory reactivity could be restored with indomethacin in the HS diet group. This is the first study to: (1) confirm the deleterious effect of acute salt loading on microvascular reactivity in women; (2) demonstrate significant effect of vasoconstrictor metabolites of COX enzymes, especially COX‐1, in the development of impaired endothelial function after acute salt loading in humans; and (3) establish LDF as a non‐invasive reliable method of assessing microcirculatory blood flow in humans. The present findings provide the foundation for further investigations into the exact role of the specific COX isoforms and their metabolites that influence vascular endothelial function in the microcirculation during HS diets.
Additional information
Competing interests
The authors have no competing interests to declare.
Author contributions
ACa, BJ, JHL, SAP, VS, IM and ID conceived and designed the experiments. ACa, Aco, IJ, VS, IM and ID were involved in the collection, assembly and analysis of data. Aca, Aco, IJ, BJ, JHL, SAP, VS, IM and ID were involved in the interpretation of data. ACa, ACo, IJ and ID were involved in drafting the article. ACa, BJ, JHL, SAP and ID were involved in revising the article critically for important intellectual content. All authors have approved the final version of the manuscript and agree to be accountable for all aspects of the work. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed. The study protocol was performed at the Laboratory for Clinical and Sports Physiology, Department of Physiology and Immunology, Faculty of Medicine, Josip Juraj Strossmayer University of Osijek. Serum sCAMs, TXA2 and PGF2α level measurements were performed at the Laboratory for Molecular and Clinical Immunology, Department of Physiology and Immunology, Faculty of Medicine, Josip Juraj Strossmayer University of Osijek. Blood and 24 h urine samples analyses were performed at the Department of Clinical Laboratory Diagnostics, University Hospital Osijek. PRA and plasma aldosterone concentrations were measured at the Clinical Institute of Nuclear Medicine and Radiation Protection, University Hospital Osijek.
Funding
The present study was supported by a grant from the Ministry of Science, Education and Sports, Republic of Croatia, #219‐2160133‐2034 and Cro‐Hu bilateral project: Common pathways for microcirculatory dysfunction in diabetes mellitus and hypertension (2009‐2011).
Acknowledgements
We thank to Lidija Rasic, a student at the Faculty of Medicine, Josip Juraj Strossmayer University of Osijek, for help with the data collection.
References
- Binggeli C, Spieker LE, Corti R, Sudano I, Stojanovic V, Hayoz D, Luscher TF & Noll G (2003). Statins enhance postischemic hyperemia in the skin circulation of hypercholesterolemic patients: a monitoring test of endothelial dysfunction for clinical practice? J Am Coll Cardiol 42, 71–77. [DOI] [PubMed] [Google Scholar]
- Bragulat E & de la Sierra A (2002). Salt intake, endothelial dysfunction, and salt‐sensitive hypertension. J Clin Hypertens 4, 41–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crakowski JL, Minson CT, Salvat‐Melis M & Halliwill JR (2006). Methodological issues in the assessment of skin microvascular endothelial function in humans. Trends Pharmacol Sci 9, 503–509. [DOI] [PubMed] [Google Scholar]
- Cutler JA, Follmann D & Allender PS (1997). Randomized trials of sodium reduction: an overview. Am J Clin Nutr 65 (Suppl 2), 643S–651S. [DOI] [PubMed] [Google Scholar]
- Dalle‐Ave A, Kubli S, Golay S, Delachaux A, Liaudet L, Waeber B & Feihl F (2004). Acetylcholine‐induced vasodilation and reactive hyperemia are not affected by acute cyclo‐oxygenase inhibition in human skin. Microcirculation 11, 327–336. [DOI] [PubMed] [Google Scholar]
- Davidge ST (2001). Prostaglandin H synthase and vascular function. Circ Res 89, 650–660. [DOI] [PubMed] [Google Scholar]
- De Backer D, Hollanberg S, Boerma C, Goedhart, Buchele G, Ospina‐Tascon G, et al (2007). How to evaluate the microcirculation: report of a round table. Crit Care 11, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson KM, Clifton PM & Keogh JB (2011). Endothelial function is impaired after a high‐salt meal in healthy subjects. Am J Clin Nutr 93, 500–505. [DOI] [PubMed] [Google Scholar]
- Dishy V, Sofowora GG, Imamura H, Nishimi Y, Xie HG, Wood AJ & Stein CM (2003). Nitric oxide production decreases after salt loading but is not related to blood pressure changes or nitric oxide‐mediated vascular responses. J Hypertens 21, 153–157. [DOI] [PubMed] [Google Scholar]
- Drenjancevic Peric I, Jelakovic B, Lombard JH, Kunert MP, Kibel A & Gros M (2011). High‐salt diet and hypertension: focus on the renin‐angiotensin system. Kidney Blood Press Res 34, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drenjancevic‐Peric I, Frisbee JC, Lombard JH (2003). Skeletal muscle arteriolar reactivity in SS BN13 consomic rats and Dahl salt‐sensitive rats. Hypertension 41, 1012–1015. [DOI] [PubMed] [Google Scholar]
- Du Pont JJ, Greaney JL, Wenner MM, Lennon‐Edwards SL, Sanders PW, Farquhar WB & Edwards DG (2013). High dietary sodium intake impairs endothelium‐dependent dilation in healthy salt‐resistant humans. J Hypertens 31, 530–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskildsen MP, Hansen PB, Stubbe J, Toft A, Walter S, Marcussen N, Rasmussen LM, Vanhoutte PM & Jensen BL (2014). Prostaglandin I2 and prostaglandin E2 modulate human intrarenal artery contractility through prostaglandin E2‐EP4, prostacyclin‐IP, and thromboxane A2‐TP receptors. Hypertension 64, 551–556. [DOI] [PubMed] [Google Scholar]
- Ferri C, Bellini C, Desideri G, Giuliani E, De Siati L, Cicogna S & Santucci A (1998). Clustering of endothelial markers of vascular damage in human salt‐sensitive hypertensive: influence of dietary sodium load and depletion. Hypertension 32, 862–868. [DOI] [PubMed] [Google Scholar]
- Greaney JL, DuPont JJ, Lennon‐Edwards SL, Sanders PW, Edwards DG & Farquhar WB (2012). Dietary sodium loading impairs microvascular function independent of blood pressure in humans: role of oxidative stress. J Physiol 590, 5519–5528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellmann M, Gaillard‐Bigot F, Roustit M & Cracowski JL (2015). Prostanoids are not involved in postocclusive reactive hyperaemia in human skin. Fundam Clin Pharmacol 29, 510–516. [DOI] [PubMed] [Google Scholar]
- Higashi Y, Sasaki S, Nakagawa K, Kimura M, Noma K, Sasaki S, et al (2001). Sodium chloride loading does not alter endothelium‐dependent vasodilatation of forarm vasculature in either salt‐sensitive or salt‐resistant patients with essential hypertension. Hypertens Res 24, 711–716. [DOI] [PubMed] [Google Scholar]
- Ivancev V, Bakovic D, Obad A, Breskovic T, Palada I, Joyner MJ & Dujic Z (2009). Effects of indomethacin on cerebrovascular response to hypercapnea and hypocapnea in breath‐hold diving and obstructive sleep apnea. Respir Physiol Neurobiol 166, 152–158. [DOI] [PubMed] [Google Scholar]
- Larkin SW & Williams TJ (1993). Evidence for sensory nerve involvement in cutaneous reactive hyperemia in humans. Circ Res 73, 147–154. [DOI] [PubMed] [Google Scholar]
- Liu FQ, Mu JJ, Liu DC, Shi DC, Huang Q, Yuan ZY et al (2012). Endothelial dysfunction in normotensive salt‐sensitive subjects. J Hum Hypertens 26, 247–252. [DOI] [PubMed] [Google Scholar]
- Lombard JH, Sylvester FA, Phillips SA & Frisbee JC (2002). High‐salt diet impairs vascular relaxation mechanisms in rat middle cerebral arteries. AJP 284, H1124–H1133. [DOI] [PubMed] [Google Scholar]
- Lorenzo S & Minson CT (2007). Human cutaneous reactive hyperaemia: role of BKCa channels and sensory nerves. J Physiol 585, 295–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matrougi K, Loufrani L, Veby BI & Henrion D (2001). High NaCl intake decreases both flow‐induced dilation and pressure‐induced myogenic tone in resistance arteries from normotensive rats: involvement of cyclooxygenase‐2. Pharmacol Toxicol 89, 183–187. [DOI] [PubMed] [Google Scholar]
- Medow MS, Taneja I & Stewart JM (2007). Cyclooxygenase and nitric oxide synthase dependence of cutaneous reactive hyperemia in humans. Am J Physiol Heart Circ Physiol 293, H425–H432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller VM (2010). Sex‐based differences in vascular function. Women's Health 6, 737–752. [DOI] [PubMed] [Google Scholar]
- Mohan S & Campbell NR (2009). Salt and high blood pressure. Clin Sci (Lond) 117, 1–11. [DOI] [PubMed] [Google Scholar]
- Obarzanek E, Proschan MA, Vollmer WM, et al (2003). Individual blood pressure responses to changes in salt intake. Results from the DASH‐Sodium Trial. Hypertension 42, 459–467. [DOI] [PubMed] [Google Scholar]
- Phillips SA, Drenjancevic‐Peric I, Frisbee JC & Lombard JH (2004). Chronic AT(1) receptor blockade alters mechanisms mediating responses to hypoxia in rat skeletal muscle resistance arteries. Am J Physiol Heart Circ Physiol 287, H545–H552. [DOI] [PubMed] [Google Scholar]
- Pitt B, Pepine C & Willerson JT (2002). Cyclooxygenase‐2 inhibition and cardiovascular events. Circulation 106, 167–169. [DOI] [PubMed] [Google Scholar]
- Premuzic V, Erceg I, Jovanovic A, Reiner Z & Jelakovic B (2010). Unos soli u odrasloj populaciji. Hrvatski casopis za javno zdravstvo 6, 21. [Google Scholar]
- Ross R (1999). Atherosclerosis – an inflammatory disease. N Engl J Med 340, 115–126. [DOI] [PubMed] [Google Scholar]
- Roustit M & Cracowski JL (2012). Non‐invasive assessment of skin microvascular function in humans: an insight into methods. Microcirculation 19, 47–64. [DOI] [PubMed] [Google Scholar]
- Sacks F, Svetkey L, Vollmer W, Appel L, Bray G, Harsha D, et al (2001). Effects on blood pressure of reduced dietary sodium and the dietary approaches to stop hypertension (DASH) diet. N Engl J Med 344, 3–10. [DOI] [PubMed] [Google Scholar]
- Schuligoi R, Sedej M, Waldhoer M, Vukoja A, Sturm EM, Lippe IT, Peskar BA & Heinemann A (2009). Prostaglandin H2 induces the migration of human eosinophils through the chemoattractant receptor homologous molecule of Th2 cells, CRTH2. J Leukoc Biol 85, 136–145. [DOI] [PubMed] [Google Scholar]
- Starmans‐Kool MJ, Stanton AV, Xu, YY , McG Thom SA, Parker KH & Hughes AD (2010). High dietary salt intake increases carotid blood pressure and wave reflection in normotensive healthy young men. J Appl Physiol 110, 468–471. [DOI] [PubMed] [Google Scholar]
- Stein CM, Nelson R, Brown M, Wood M & Wood AJJ (1995). Dietary sodium intake modulates vasodilation mediated by nitroprusside but not by methacholine in the human forearm. Hypertension 25, 1220–1223. [DOI] [PubMed] [Google Scholar]
- Stewart J, Kohen A, Brouder D, Rahim F, Adler S, Garrick R, et al (2004). Noninvasive interrogation of microvasculature for signs of endothelial dysfunction in patients with chronic renal failure. AJP 287, H2687–H2696. [DOI] [PubMed] [Google Scholar]
- Tadzic R, Mihalj M, Vcev A, Ennen J, Tadzic A & Drenjancevic I (2013). The Effects of arterial blood pressure reduction on endocan and soluble adhesion molecules (CAMs) and CAMs ligands expression in hypertensive patients on ca‐channel blocker therapy. Kidney Blood Press Res 37, 1–9. [DOI] [PubMed] [Google Scholar]
- Tzemos N, Lim PO, Wong S, Struthers AD & MacDonald TM (2008). Adverse cardiovascular effects of acute salt loading in young normotensive individuals. Hypertension 51, 1525–1530. [DOI] [PubMed] [Google Scholar]
- Weinberger MH (2002). Salt sensitivity is associated with an increased mortality in both normal and hypertensive humans. J Clin Hypertens (Greenwich) 4, 274–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma S & Anderson TJ (2002). Fundamentals of endothelial function for the clinical cardiologist. Circulation 105, 546–549. [DOI] [PubMed] [Google Scholar]
- Versari D, Daghini E, Virdis A, Ghiadoni L & Taddei S (2009). Endothelium‐dependent contractions and endothelial dysfunction in human hypertension. Br J Pharmacol 157, 527–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widlansky ME, Price DT, Gokce N, Eberhardt RT, Duffy SJ, Holbrook M, Maxwell C, Palmisano J, Keaney JF Jr, Morrow JD & Vita JA (2003). Short‐ and long‐term COX‐2 inhibition reverses endothelial dysfunction in patients with hypertension. Hypertension 42, 310–315. [DOI] [PubMed] [Google Scholar]
- Vollmer WM, Sacks FM, Ard J, et al (2001). DASH‐Sodium Collaborative Research Group: effects of diet and sodium intake on blood pressure: subgroup analysis of the DASH‐Sodium trial. Ann Intern Med 135, 1019–1028. [DOI] [PubMed] [Google Scholar]
- Wong BJ, Wilkins BW, Holowatz LA & Minson CT (2003). Nitric oxide synthase inhibition does not alter the reactive hyperemic response in the cutaneous circulation. J Appl Physiol 95, 504–510. [DOI] [PubMed] [Google Scholar]
- Wong MS & Vanhoutte PM (2010). COX‐mediated endothelium‐dependent contractions: from the past to recent discoveries. Acta Pharmacol Sin 31, 1095–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright CI, Kroner CI & Draijer R (2006). Non‐invasive methods and stimuli for evaluating the skin's microcirculation. J Pharmacol Toxicol Methods 54, 1–25. [DOI] [PubMed] [Google Scholar]
- Zhu J, Drenjancevic‐Peric I, McEwen S, Friesema J, Schulta D, Yu M et al (2006). Role of superoxide and angiotensin II suppression in salt‐induced changes in endothelial Ca2+ signalling and NO production in rat aorta. Am J Physiol Heart Circ Physiol 291, H929–H939. [DOI] [PubMed] [Google Scholar]


