A protein S-acyltransferase is involved in leaf senescence via modulating salicylic acid metabolism and perception.
Abstract
The Asp-His-His-Cys-Cys-rich domain-containing Protein S-Acyl Transferases (PATs) are multipass transmembrane proteins that catalyze S-acylation (commonly known as S-palmitoylation), the reversible posttranslational lipid modification of proteins. Palmitoylation enhances the hydrophobicity of proteins, contributes to their membrane association, and plays roles in protein trafficking and signaling. In Arabidopsis (Arabidopsis thaliana), there are at least 24 PATs; previous studies on two PATs established important roles in growth, development, and stress responses. In this study, we identified a, to our knowledge, novel PAT, AtPAT14, in Arabidopsis. Complementation studies in yeast (Saccharomyces cerevisiae) and Arabidopsis demonstrate that AtPAT14 possesses PAT enzyme activity. Disruption of AtPAT14 by T-DNA insertion resulted in an accelerated senescence phenotype. This coincided with increased transcript levels of some senescence-specific and pathogen-resistant marker genes. We show that early senescence of pat14 does not involve the signaling molecules jasmonic acid and abscisic acid, or autophagy, but associates with salicylic acid homeostasis and signaling. This strongly suggests that AtPAT14 plays a pivotal role in regulating senescence via salicylic acid pathways. Senescence is a complex process required for normal plant growth and development and requires the coordination of many genes and signaling pathways. However, precocious senescence results in loss of biomass and seed production. The negative regulation of leaf senescence by AtPAT14 in Arabidopsis highlights, to our knowledge for the first time, a specific role for palmitoylation in leaf senescence.
Protein S-Acyl Transferases (PATs), which were initially identified from yeast (Saccharomyces cerevisiae; Lobo et al., 2002; Roth et al., 2002), catalyze protein S-acylation. Protein S-acylation, commonly known as S-palmitoylation, is a posttranslational lipid modification in which long chain fatty acids, usually palmitate, attach reversibly to cysteines (Resh, 2006; Greaves and Chamberlain, 2011). S-acylation, often coupled with myristoylation or prenylation, is important for cellular protein sorting, vesicle trafficking, activation state control, protein stability, microdomain partitioning of proteins, and protein complex assembly (Greaves and Chamberlain, 2007; Baekkeskov and Kanaani, 2009; Charollais and Van Der Goot, 2009; Hemsley, 2009; Fukata and Fukata, 2010; Hemsley et al., 2013). Recent large-scale proteomics studies indicate that many proteins are palmitoylated in eukaryotic organisms, with at least 50 occurring in yeast (Roth et al., 2006), more than 700 in mammals (Martin and Cravatt, 2009), and more than 500 in Arabidopsis (Arabidopsis thaliana; Hemsley et al., 2013).
PATs are eukaryote-specific proteins with a highly conserved “signature” catalytic Asp-His-His-Cys (DHHC)-Cys-rich domain (CRD) of approximately 50 amino acids. PATs are transmembrane proteins containing 4-6 membrane spanning domains and possess highly variable N- and C-terminal cytosolic domains that are essential for their substrate specificity (Huang et al., 2009; Greaves et al., 2010; González Montoro et al., 2011; Howie et al., 2014). Different numbers of DHHC-CRD PATs have been found in various species, such as seven in yeast (Putilina et al., 1999), 23 in mammals (Fukata et al., 2006), and 24 in Arabidopsis (Hemsley and Grierson, 2008; Batistic, 2012).
To date, research on plant PATs has been very limited compared to humans and yeast. Analysis of the 24 Arabidopsis PATs shows that most of them have a broad and constant expression pattern at different developmental stages (Batistic, 2012). Transient expression of AtPAT-GFP in tobacco (Nicotiana benthamiana) showed that Arabidopsis PATs have very complex cellular membrane localization patterns where half of them reside in the plasma membrane and others in the endoplasmic reticulum, Golgi, vesicles, and tonoplast (Batistic, 2012), indicating that the plasma membrane is the main palmitoylation site in higher plants. This contrasts with mammals where the Golgi is the main site for the palmitoylation machinery (Ohno et al., 2006).
At present, the biological functions of only two Arabidopsis PATs, TIP1/AtPAT24 (Hemsley et al., 2005) and AtPAT10 (Qi et al., 2013; Zhou et al., 2013), have been studied in any detail. AtPAT24 was confirmed as an S-acyltransferase based on rescue of both morphological and temperature sensitive defects in the yeast PAT knockout mutant akr1. The loss-of-function mutants exhibited defects in control of cell size, pollen tube and root hair growth, and cell polarity (Hemsley et al., 2005). Transient expression in tobacco localized AtPAT24 to the Golgi (Batistic, 2012). Functional characterization of AtPAT10 confirmed enzyme activity in vitro and in vivo (Qi et al., 2013). AtPAT10 expression appears ubiquitous, being detectable in all tissues tested throughout growth and development, with the protein predominately localizing to the Golgi and tonoplast (Batistic, 2012; Qi et al., 2013; Zhou et al., 2013). Three T-DNA insertion mutant alleles for AtPAT10 were identified and all exhibited very similar pleiotropic phenotypes, including defects in cell expansion and cell division, vascular patterning, fertility, and also hypersensitivity to salt stress (Qi et al., 2013; Zhou et al., 2013).
Here, we report the identification and characterization of a further PAT, AtPAT14, in Arabidopsis. Two independent transcriptionally null T-DNA insertion lines of AtPAT14 were found to exhibit dramatically reduced stature and early senescence. Leaf senescence is a highly integrated and indispensable feature of the plant life cycle. During senescence, cells undergo orderly changes that mobilize and export valuable nutrient resources to the plant body before the leaf dies or is shed. Senescence is a complex process involving the expression of thousands of genes and multiple signaling pathways (Buchanan-Wollaston et al., 2005; van der Graaff et al., 2006). Many external or internal cues, such as drought, salinity, nutrient deficiency, pathogen attack, imbalance in phytohormones, and defects in autophagy, can trigger precocious leaf senescence, resulting in reduced yield and crop quality. However, the precise mechanisms regulating leaf senescence remain unclear.
We found that the early senescence phenotype displayed by AtPAT14 loss-of-function mutants is not related to abscisic acid (ABA), jasmonic acid (JA), or autophagy, but is associated with a role for PAT14 in the regulation of leaf senescence operating through salicylic acid (SA). Our results provide new insights into the mechanism of leaf senescence highlighting protein palmitoylation as a prominent regulatory component in this pathway.
RESULTS
Arabidopsis PAT14 Shares Homology with Other Known DHHC-PATs
The AtPAT14 (At3g60800) gene is 2,420 nucleotide bp long, consisting of seven exons and six introns. It contains an open reading frame of 924 nucleotides, encoding a putative protein of 307 amino acids, which has a predicted Mr of 34.7 kD. AtPAT14 is most similar to AtPAT13, sharing 66% amino acid identity (Batistic, 2012; data not shown). Protein sequence alignments with other DHHC-PATs from Arabidopsis, yeast, and humans show that AtPAT14 shares high identity only within the DHHC-CRD domain (Supplemental Fig. S1). The TMHMM2.0 online software predicts that AtPAT14 has four transmembrane domains between the 21st to 43rd, 58th to 80th, 173rd to 195th, and 210th to 232nd amino acids, respectively. The conserved DHHC-CRD domain lies between the third and fourth transmembrane domains and is essential for S-acyltransferase activity in other PATs. This domain and the N and C termini are predicted to be cytosolic (Supplemental Fig. S1).
AtPAT14 Is Expressed Ubiquitously Throughout Growth and Development
Reverse transcription (RT)-PCR on total RNA isolated from seedlings and various parts of the mature plant revealed AtPAT14 expression at high levels in all tissues (Supplemental Fig. S2). This is in agreement with publicly available gene expression data analyzed using Genevestigator software and previous reports (Batistic, 2012; data not shown).
Identification of AtPAT14 T-DNA Insertion Knockout Lines
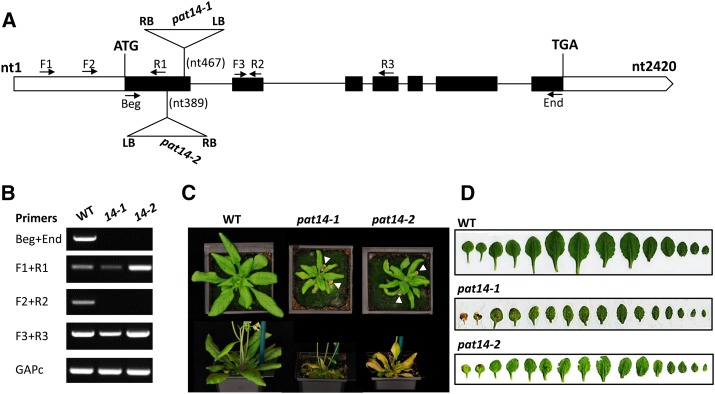
Previous studies on two of the 24 members of the Arabidopsis PAT family revealed their diverse and important functions (Hemsley et al., 2005; Qi et al., 2013; Zhou et al., 2013). To expand our understanding of S-acylation in plants, we searched the available Arabidopsis mutant collections and identified two Arabidopsis T-DNA insertion lines, SALK_026159 and GK-153A10, for AtPAT14. Sequencing of the PCR products amplified from the junction of the T-DNA insertion sites confirmed the position of the T-DNA at nt467 and nt389 near the end of the first exon of AtPAT14 for SALK_026521 and GK-153A10, respectively (Fig. 1A). No transcript was detected for the full coding region and the region across the T-DNA insertion sites in both lines of the homozygous mutant plants, although truncated transcripts were detected upstream and downstream of the T-DNA insertion sites (Fig. 1B), indicating they are most likely transcriptionally null. They were hence named atpat14-1 for SALK_026159 and atpat14-2 for GK-153A10, respectively. Both lines were back-crossed to wild type for two generations, and the mutants recovered were used for subsequent analysis.
Figure 1.
Molecular characterization of the atpat14-1 (SALK_026159) and atpat14-2 (GK-153A10) alleles. A, Schematic presentation of the AtPAT14 gene (solid boxes, exons; empty boxes, untranslated regions; lines, introns) and the positions of the T-DNA in atpat14-1 and atpat14-2. Arrows, Primers used for RT-PCR; RB, right border; LB, left border. B, Amplification of different regions of the AtPAT14 transcript in wild-type, atpat14-1, and atpat14-2 plants using primers according to A. The GAPc transcript served as a control. C, Four- (top) and 6-week-old (bottom) plants of wild-type Columbia-0, atpat14-1, and atpat14-2. White arrowheads highlight leaf senescence in mutants. D, Leaf line-ups of 5-week-old wild-type Columbia-0, atpat14-1, and atpat14-2 plants.
Characterization of AtPAT14 Loss-of-Function Mutants
The most striking phenotype of both mutant lines is that they exhibited early senescence. At 4 weeks after sowing, when all the rosette leaves of wild type were still green and healthy, the first pairs of true leaves of both mutants were already yellow (Fig. 1, C and D). atpat14-1 aged earlier than atpat14-2. The yellowing of the leaves progressed rapidly in the mutants, and the majority of them become completely yellow at 5 weeks. At this stage, wild-type plants only just started to show signs of aging with the first pair of true leaves turning yellow.
Both atpat14-1 and atpat14-2 plants are much smaller (atpat14-1 is even smaller than atpat14-2) than wild type with smaller leaves, shorter and fewer branches in the primary inflorescence, and fewer siliques on the main inflorescence stem (Fig. 1; Supplemental Table S1). The appearance of atpat14 flowers is similar to wild type, although the petals are smaller. The siliques are shorter with fewer seeds in both atpat14 mutant lines (Supplemental Fig. S3). The mutants have smaller cells in the leaves and petals than wild type (Supplemental Fig. S4; Supplemental Table S1). However, the cell number in the petals is similar between the wild type and the mutants (Supplemental Table S1), indicating that the smaller stature of atpat14 is due to smaller cells.
AtPAT14 Is an S-Acyltransferase
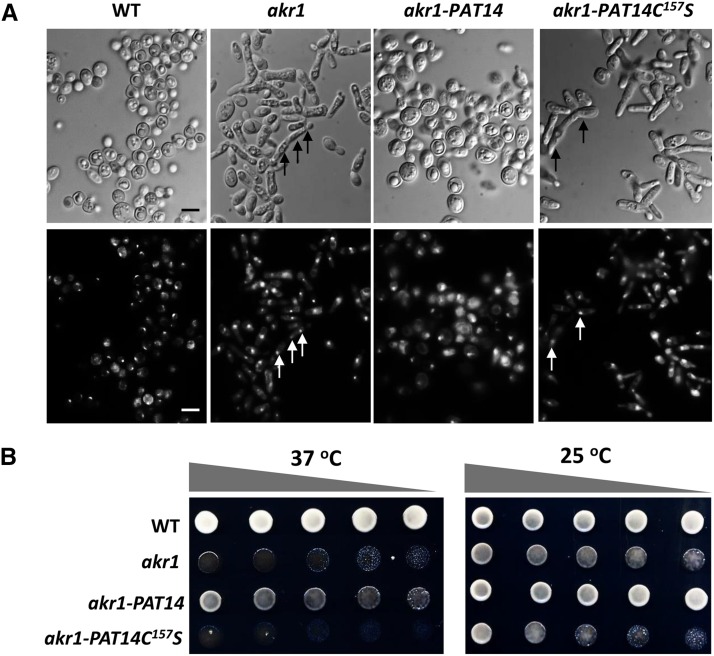
To confirm whether AtPAT14 is an S-acyltransferase and whether DHHC is the catalytic domain, we expressed AtPAT14 and a version carrying a point mutation in the DHHC domain (AtPAT14DHHC157S) in the yeast PAT knockout mutant akr1. Previous studies show that akr1 is sensitive to high temperature (37°C) and grows poorly, having elongated multinucleate cells (Feng and Davis, 2000). Our observations showed that the majority of akr1-AtPAT14 transgenic cells were similar to wild type being round with one nucleus, although they were typically much larger. However, akr1 cells carrying AtPAT14DHHC157S were elongated with multiple nuclei (arrows in bottom right panel, Fig. 2A) and resembled akr1. The growth assay revealed that AtPAT14 can partially rescue the growth defect of akr1 as more growth (though less than wild type) was observed when it was expressed in akr1 compared to akr1 alone. However, transgenic akr1 expressing AtPAT14DHHC157S failed to grow (Fig. 2B).
Figure 2.
Arabidopsis AtPAT14 has S-acyltransferase activity. AtPAT14, but not AtPAT14C157S, can partially rescue growth defects of the yeast temperature sensitive akr1 mutant that lacks the DHHC-PAT AKR1. A, DIC light (top) and UV microscopy of DAPI (1 µg/mL)-stained cells (bottom) of all four genotypes grown at 37°C. Arrows indicate multiple nuclei. B, Growth test. At nonpermissive temperature 37°C (left), wild-type (WT) yeast grew well but akr1 did not. This growth defect was less obvious at 25°C because all genotypes grew well (right). Expressing AtPAT14 in akr1 (PAT14/akr1) largely restored the growth inhibition by 37°C, but expressing AtPAT14C157S (PAT14C157S/akr1) did not. Five microliters of 10-fold serial dilutions from 1 OD600 cells were spotted on solid medium supplemented with 2% Gal and grown at 25°C or 37°C for 3 d. Bars, 10 µm. Cells were transformed with empty vector pYES2 (wild type and akr1) or with AtPAT14 and AtPAT14C157S (PAT14/akr1, PAT14C157S/akr1).
A recent study has shown that the restoration of temperature sensitivity of akr1 requires both the ankyrin repeats and S-acyltransferase function of the AKR1 protein (Hemsley and Grierson, 2011). PAT14 does not have ankyrin repeats and, as such, cannot complement the ankyrin repeat mediated phenotypes. To see if the incomplete complementation of akr1 by PAT14 was due to the lack of ankyrin repeats at its N terminus, we cotransformed PAT14 with a construct containing the S-acyl transferase null form of AKR1 (AKR1C-S), thereby providing the AKR1 ankyrin repeat function (Hemsley and Grierson, 2011). As demonstrated in Supplemental Figure S6, more growth was observed compared to akr1 cells expressing AtPAT14 alone (PAT14/akr1). However, the growth was still less than that found for wild type. A small and comparable amount of growth was also found when expressing AKR1C-S (ANK), or coexpressing AtPAT14C157S and AKR1C-S (ANK+PAT14C157S) in akr1 cells, indicating that this growth was due to the ankrin repeats of AKR1 as there was no rescue at all when expressing AtPAT14C157S alone in akr1 cells. Therefore, this further confirms that the partial rescue of akr1 at 37°C by AtPAT14 was largely dependent on its S-acyltransferase activity.
In summary, this yeast complementation data clearly demonstrates that AtPAT14 can partially complement the function of AKR1 and that this requires the Cys residue in the DHHC-CRD domain, which is essential for S-acyltransferase activity.
AtPAT14 Is Auto-Acylated in Vitro
One of the important characteristics of all PATs is that they can bind palmitic acid to form an intermediate complex, i.e. they are auto-acylated (Roth et al., 2002). To see if AtPAT14 also has this property, we carried out the Acyl-RAC (for resin-assisted capture) assay (Forrester et al., 2011), to capture on thiopropyl Sepharose beads all S-acylated proteins from transgenic akr1 yeast cells. Because AtPAT14 and AtPAT14DHHC157S are fused to the V5 epitope tag at their C termini, their presence on the beads can be detected via western blot using an anti-V5 antibody. As shown in Figure 3, AtPAT14 could be detected among the captured S-acylated proteins, but AtPAT14DHHC157S could not, indicating that AtPAT14 is auto-acylated while AtPAT14DHHC157S is not. This further proves that AtPAT14 is an S-acyltransferase and its activity relies on the Cys residue in the DHHC domain.
Figure 3.
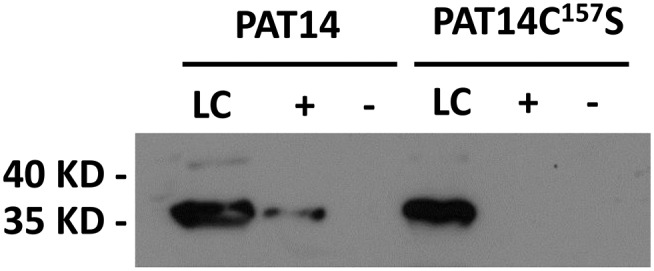
Arabidopsis AtPAT14 is auto-acylated. AtPAT14 (PAT14) and AtPAT14C157S (PAT14C157S) were detected by western blotting with an anti-V5 antibody using the ECL method. The molecular weight of AtPAT14 and AtPAT14C157S is approximately 35 kD. A band corresponding to AtPAT14-V5 was detected in the +NH2OH treated sample, indicating that it is bound to an acyl group via a labile thioester linkage confirming that it is autoacylated. However, no signal was detected for AtPAT14C157S, indicating that it is not autoacyated. LC, Loading control; lane +, NH2OH is present; lane −, no NH2OH.
AtPAT14 Predominantly Localizes to Golgi
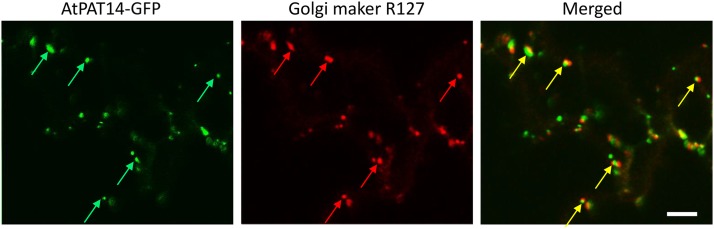
To see where AtPAT14 is localized, we stably transformed 35S:AtPAT14-GFP into atpat14-1 and observed the cotyledons and primary roots of 10-d-old plate-grown seedlings of the complemented plants. We found that AtPAT14-GFP was present as many discrete fluorescent foci in cotyledons and root cells in the elongation and growth terminating zones (Fig. 4, left; Supplemental Fig. S8). To determine the identity of these structures, we crossed the AtPAT14-GFP line with mCherry-labeled marker Wavelines that carry different endomembrane markers (Geldner et al., 2009). Strong colocalization was visualized in the progeny of crosses with the R127 line, which contains a cis-Golgi marker (MEMB12, At5g50440; Uemura et al., 2004; Fig. 4A, middle and right). The highly motile structures of AtPAT14-GFP were seen moving together with this Golgi marker (Supplemental Movie S1). The colocalization was not a complete “overlap,” but instead, the GFP and mCherry appeared next to each other. High magnification views of the PAT14-GFP labeled structures in cotyledon epidermal cells revealed a doughnut-shaped Golgi apparatus that is very similar to the pattern observed by Boevink et al. (1998) using a trans-Golgi marker (STtmd-GFP) in tobacco leaf cells (Supplemental Fig. S7). This indicates that AtPAT14 is localized to the trans face of the Golgi stack. Due to very weak signals from other marker lines, we were unable to determine in which other compartment(s) AtPAT14 may reside. Nevertheless, our data indicate that a large proportion of AtPAT14, if not all, is localized in the trans-Golgi. This is similar to the observation made for transiently expressed AtPAT14-GFP in tobacco leaves (Batistic, 2012).
Figure 4.
AtPAT14 is predominantly localized to the Golgi in Arabidopsis. Confocal microscopy observation of atpat14-1 cotyledon of 10-d-old seedlings complemented with the 35S:ATPAT14-GFP construct and crossed with the Golgi marker line R127. Green arrows, AtPAT14-GFP; red arrows, R127; yellow arrows, colocalization. Bar, 5 µm.
To see if mutation of the Cys within the DHHC motif has an effect on AtPAT14 localization, we observed transgenic Arabidopsis that carried the AtPAT14DHHC157S-GFP construct. Similar florescent structures were seen in these plants as for AtPAT14-GFP, indicating that the mutation has no observable effect on the localization of the protein (Supplemental Fig. S8). Observation of AtPAT10 (Qi et al., 2013) and the mammalian DHHC2 (Greaves et al., 2011) resulted in the same conclusion that mutation of the Cys in the DHHC domain to an Ala residue blocks autopalmitoylation but does not have a gross effect on protein localization. This suggests that intramolecular autopalmitoylation of PATs is not having a major impact on targeting of the protein.
Expression of Senescence-Associated Genes and Defense-Related Genes Is Accelerated in pat14 Mutants
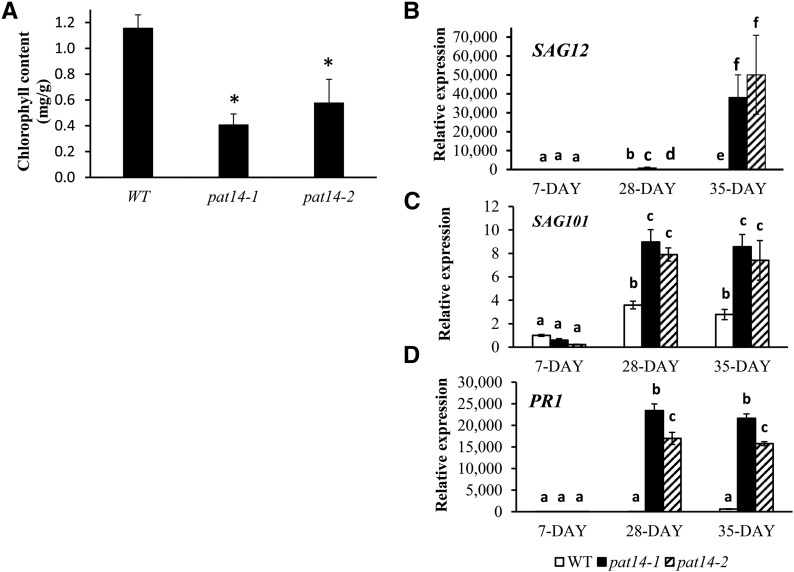
Senescence involves degradation of leaf chlorophyll, resulting in yellow or brown leaves. Measurement of the chlorophyll content of the first pair of true leaves of 29-d-old plants showed a 3- and 2-fold reduction in pat14-1 and pat14-2, respectively, compared to wild type (Fig. 5A). The pat14 null mutation is responsible for this early senescence phenotype as a construct carrying AtPAT14 restored both pat14-1 and pat14-2 to wild type (Supplemental Fig. S5; data not shown).
Figure 5.
AtPAT14 is involved in early senescence. A, Chlorophyll content analysis. Left, The first pair of true leaves from 28-d plants were used to measure the chlorophyll content. B, Transcript level of SAG12. C, Transcript level of SAG101. D, Transcript level of the pathogen related gene 1, PR1. Seven-day-old plate-grown seedlings and the sixth and seventh rosette leaves of 28-d- and 35-d-old soil-grown plants were collected and used for RNA extraction followed by RT and real-time PCR. The gene expression level in the seedlings of 7-d-old wild type (WT) is regarded as 1, and the relative expression level of all four genes was calculated. *P < 0.05 in Student’s t test in A, and the different letters above the columns in B to D indicate statistically different values analyzed by one-way ANOVA.
Next, using real-time PCR, we compared the expression levels of two commonly used senescence marker genes, senescence association genes 12 (SAG12) and 101 (SAG101; Vogelmann et al., 2012), in wild-type and mutant plants in the early and later developmental stages. A similar low-level expression was found in 7-d-old seedlings in wild type and pat14. However, at 28 and 35 d, their expression levels were significantly upregulated in mutant lines compared to wild type (Fig. 5, B and C). Although the transcript level of SAG12 was increased by 7- and 14-fold in the 28- and 35-d-old wild-type plants compared to 7-d-old seedlings, it was increased by 1,594- and 70,594-fold in pat14-1, and 251- and 67,676-fold in pat14-2 for the same aged plants, respectively (Fig. 5B). Similarly, SAG101 was increased by 3.6 fold in wild type, while 15- and 36-fold increases were found in pat14-1 and pat14-2 mutant leaves of 28-d-old plants (Fig. 5C). For Pathogenesis-related gene 1 (PR1), which is also regarded as an SA signaling gene and is readily inducible by SA (Zhang et al., 2013), a massive increase in transcript level of 212,815- and 121,357-fold was found in 28-d-old pat14-1 and pat14-2 plants compared to only a 14-fold increase in wild type (Fig. 5D). Likewise, the transcript levels of SAG101 and PR1 were much higher in 35-d-old mutant plants compared to wild type. This clearly demonstrates that AtPAT14 regulates the expression of genes related to senescence and defense.
The Early Senescence Phenotype in pat14 Is Not Caused by Defects in Autophagy
The importance of autophagy in the regulation of leaf senescence in Arabidopsis has been demonstrated by the characterization of many autophagy genes (ATG for short), where their loss-of-function mutants, such as atg2, atg5, atg7, atg9, and atg11, all exhibited early senescence (Hanaoka et al., 2002; Thompson et al., 2005; Farmer et al., 2013; Avila-Ospina et al., 2014; Li and Vierstra, 2014). To see if early senescence in pat14 mutants is caused by defects in autophagy, we carried out carbon starvation assays on plate-grown seedlings. Seedlings of both atg5-1 and atg9-2 became yellow after this treatment, whereas wild-type, pat14-1, and pat14-2 mutant seedlings remained green (Supplemental Fig. S9A). Seedlings of pat14 contained a similar amount of chlorophyll as wild type, whereas atg9-2 had much less chlorophyll (Supplemental Fig. S9B)—therefore, pat14 mutants do not share the same phenotype as atg5 and atg9 under these conditions.
To confirm this notion, we utilized the autophagy molecular marker ATG8-GFP to determine if autophagic bodies were absent in the pat14 mutant background. ATG8 proteins are useful molecular markers of autophagosomes in plants and can be used to define atg mutants defective in the autophagic processes (Yoshimoto et al., 2004; Contento et al., 2005; Thompson et al., 2005; Chung et al., 2010; Li and Vierstra, 2014). As shown in Supplemental Figure S10, many fluorescent foci were seen in primary root tips when ATG8-GFP was expressed in wild-type plants, while virtually no such structures were seen in the transgenic atg9-2 root, indicating autophagy bodies are lacking in atg9-2 as reported by Li and Vierstra (2014). However, in the roots of pat14-1 and pat14-2 seedlings expressing ATG8-GFP, the number and size of fluorescent foci were similar to those in the wild-type background, indicating that autophagy is normal in pat14 mutants. Therefore, early senescence in AtPAT14 loss-of-function mutants is very unlikely due to defect in autophagy, i.e. AtPAT14 is not involved in autophagy-mediated senescence.
pat14 Mutants Are Hypersensitive to SA but Insensitive to ABA and JA Treatment
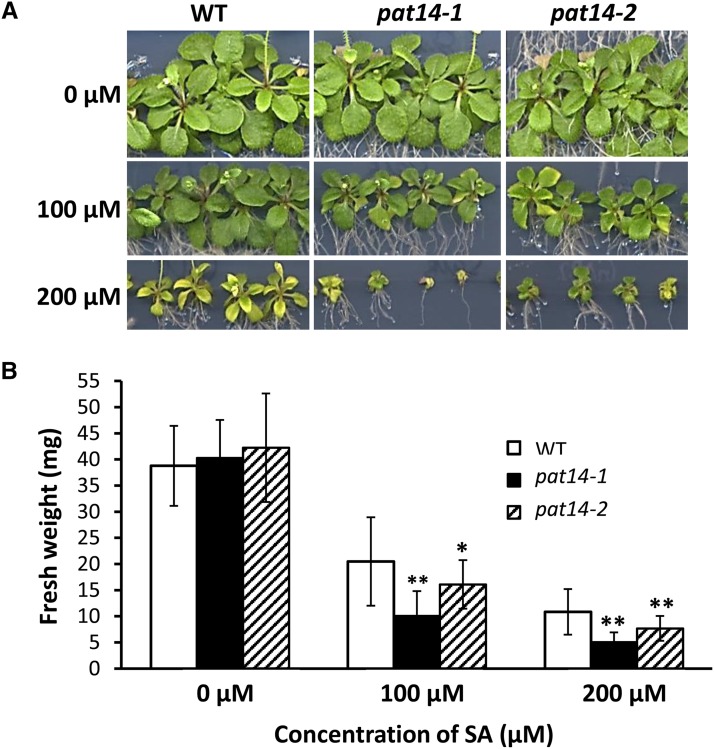
High levels of phytohormones, such as SA, ABA, and JA, can cause senescence in plants (Schippers et al., 2007). To see the effect of these phytohormones on pat14 mutants, we first treated seedlings of wild-type, pat14-1, and pat14-2 with SA. The seedlings of pat14 were indistinguishable from wild type when grown on media lacking SA (Fig. 6A, top). However, when 100 μm of SA was added, a significant reduction in the growth of mutant lines was observed compared to wild type (Fig. 6A, middle). The shoot fresh weight was 52.8%, 25.1%, and 38.2% of untreated seedlings for wild type, pat14-1, and pat14-2, respectively (Fig. 6B). A similar effect was found for seedlings treated with 200 μM of SA (Fig. 6A, bottom; Fig. 6B). We next tested the effect of ABA and JA on seedling growth using the same method. pat14 and wild-type seedlings treated with 0.5 and 1 µm ABA had a similar phenotype and reduction in shoot fresh weight (Supplemental Fig. S11, A and B). Likewise, no observable differences were found between pat14 and wild-type seedlings treated with 15 and 30 µm of JA (Supplemental Fig. S12, A and B).
Figure 6.
PAT14 knockout mutants are hypersensitive to exogenous SA treatment during early seedling growth. A, Images of seedlings. B, Fresh weight of seedlings. Seeds were geminated and grown on half-strength MS plus 1% Suc medium for 4 d. They were then transferred to the same fresh medium supplemented with 0 (control), 100, and 200 µm SA and grown for a further 15 d before being scanned, and the shoot weight of each seedling was recorded (n = 20). *P < 0.05 and **P < 0.01 in Student’s t test.
Therefore, AtPAT14 loss-of-function mutants are hypersensitive to SA, but respond to ABA and JA in a similar manner as wild type.
SA Pathway Related Genes Are Upregulated in pat14 Mutants
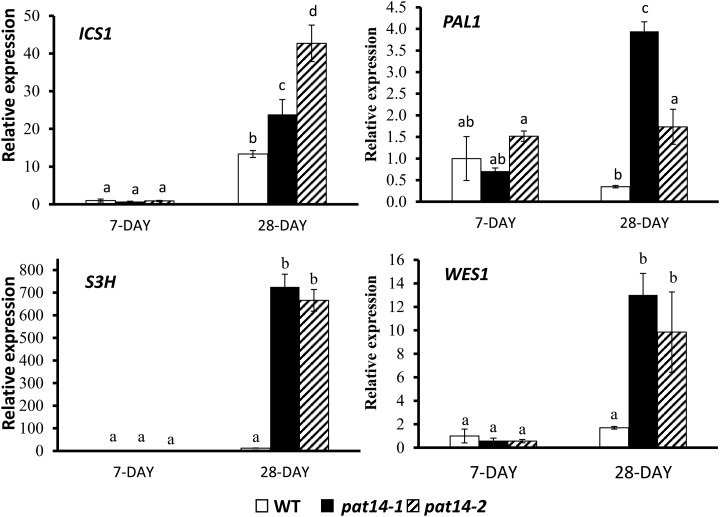
To further confirm the involvement of the SA pathway in early senescence in the pat14 mutants, we carried out real-time PCR to compare the expression levels of some SA metabolic and signaling genes in wild type and pat14 mutants. SA is synthesized by two major pathways, with 90% being derived from the isochorismate synthase (ICS) pathway, and 10% from the Phe ammonia-lyase (PAL) pathway (Dempsey et al., 2011). As shown in Figure 7, low levels of ICS1 transcript was present in both mutants and wild type in the 7-d-old seedlings. However, ICS1 transcript increased significantly in the fifth and sixth leaves of 28-d-old soil-grown plants for wild type and pat14 mutants. Strikingly, much higher transcript levels were found in the mutants than wild type: 32- and 47-fold for pat14-1 and pat14-2, compared to a 13-fold increase for wild type (Fig. 7). For PAL1, the transcript level in wild type was unchanged between the 7-d-old seedlings and mature plants, whereas in the mutant lines it was upregulated in mature plants with 10- and 3-fold increases for pat14-1 and pat14-2, respectively (Fig. 7). Therefore, at maturity, the SA biosynthetic genes are much more active in pat14 mutants than wild-type plants.
Figure 7.
Transcript levels of several SA metabolic genes monitored by real-time PCR. Seven-day-old plate-grown seedlings and the sixth and seventh rosette leaves of 28-d-old soil-grown plants were collected for RNA extraction and reverse transcription followed by real-time PCR. The gene expression level in the 7-d-old wild-type (WT) seedlings is regarded as 1, and the relative expression level in 28-d-old leaves was calculated. The experiments were repeated three times. Each bar represents the mean ± SE (n = 3). Different letters above the columns indicate statistically different values analyzed by one-way ANOVA.
Accumulation of SA to high levels in plants can induce the expression of SA modification genes, which can inactivate SA to maintain homeostasis in the cell. Arabidopsis plants lacking two of the most important SA modifying genes, SA 3-hydroxylase (S3H) and GH3 acyl adenylase family member 3.5 (GH3.5/WES1), show an early senescence phenotype due to the accumulation of high levels of SA (Dempsey et al., 2011; Zhang et al., 2013). Our results showed that the S3H transcript increased 12-fold in the 28-d-old wild-type leaves compared to 7-d-old seedlings. However, a surge to 1,728- and 925-fold increases of S3H transcript was found in mature pat14-1 and pat14-2 plants (Fig. 7). Similarly, the transcript level of WES1 in mature pat14-1 and pat14-2 plants was much higher than wild type, although to a lesser extent (2-, 21-, and 17-fold increase for wild type, pat14-1, and pat14-2, respectively; Fig. 7). This demonstrates that both SA modification genes, S3H and WES1, are significantly upregulated in the pat14 mutants compared to wild-type mature plants.
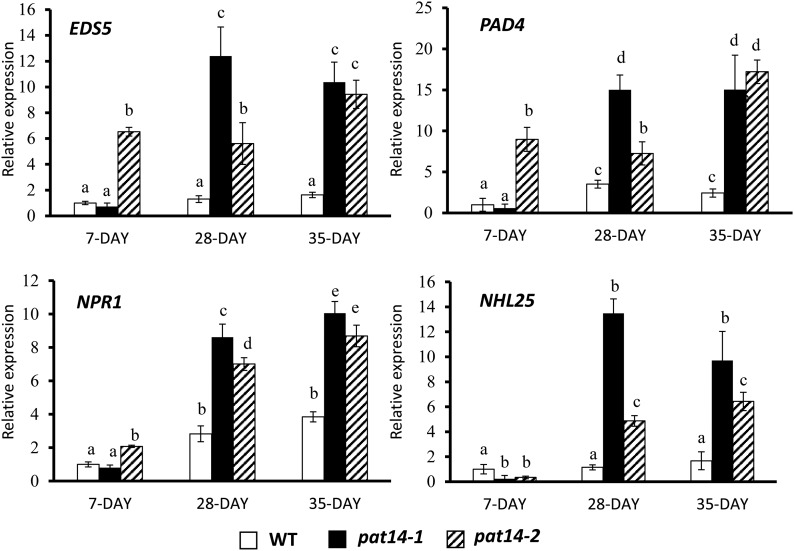
We next measured the transcript levels of SA signaling genes, such as enhanced disease susceptibility 5 (EDS5), phytoalexin deficient 4 (PAD4), Nonexpresser of Pathogenesis-Related genes 1 (NPR1), and NDR1/HIN1-LIKE 25 (NHL25; van der Graaff et al., 2006). Consistent with the SA metabolic genes, these signaling genes also showed higher expression levels in the mature pat14 mutants than in wild type. Interestingly, higher expression levels of EDS5, PAD4, and NPR1, but not NHL25, were also found in the 7-d-old seedlings of pat14-2, but not pat14-1, compared to wild type (7-, 9-, and 2-fold more than wild type). All the signaling genes showed greater upregulation in 28- and 35-d-old plants of both pat14-1 and pat14-2 compared to wild type (10- and 9-fold compared to 2-fold for EDS5; 15- and 17-fold compared to 2-fold for PAD4; 10- and 9-fold compared to 4-fold for NPR1 in 35-d-old plants; Fig. 8). For NHL25, very low-level expression was found in the 7-d-old seedlings in wild type and both mutant lines. However, in the 35-d-old plants, while NHL25 accumulation in wild type was comparable to the seedlings, in the pat14-1 and pat14-2 lines, expression was increased by 10- and 6-fold. These data clearly show that the SA signaling genes were upregulated to a significantly greater extent in the mutants than in wild-type mature plants.
Figure 8.
Transcript levels of SA signaling genes monitored by real-time PCR. The gene expression level in the 7-d-old wild-type (WT) seedlings is regarded as 1, and their relative expression levels in 28-d-old leaves were calculated. The experiments were repeated three times. Each bar represents the mean ± SE (n = 3). Different letters above the columns indicate statistically different values analyzed by one-way ANOVA.
Therefore, the combined quantitative PCR data suggests that AtPAT14 is involved in leaf senescence via the regulation of genes involved in SA metabolism and signaling.
Early Senescence of pat14 Is Partially Rescued by Preventing SA Signaling
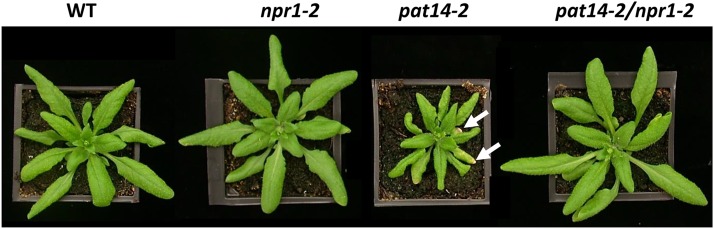
NPR1 has been shown to be a receptor for SA and it functions as a central activator of SA responses (Wu et al., 2012). Mutants defective in NPR1 (npr1) exhibit altered SAG expression patterns and delayed senescence (Morris et al., 2000). To further confirm the involvement of SA in early senescence of pat14 plants, we crossed both pat14-1 and pat14-2 with npr1-2 (Cao et al., 1997). The double mutant pat14/npr1 plants, which largely resemble the wild type, had much delayed senescence compared to the pat14 single mutants (Fig. 9; Supplemental Fig. S13). Therefore, this clearly demonstrates that the early senescence of Arabidopsis PAT14 loss-of-function mutants is caused by a defect in SA signaling pathway.
Figure 9.
Blocking SA signaling in pat14 can rescue its early senescence phenotype. Four-week-old plants of wild type (WT), npr1-2, pat14-2, and pat14-2/npr1-2 double mutants are shown. Arrows indicate yellow leaf tips in pat14-2. No such phenotype was observed in wild type, npr1-2, and the double mutant pat14-2/npr1-2.
SA, OPDA, ABA, and JA Levels during Leaf Senescence
The data described above strongly supports the hypothesis that altered SA levels in the pat14 mutants may be contributing to the enhanced senescence phenotype. To confirm this, we measured SA levels in seedlings and in the seventh and eighth green healthy leaves of 30-d-old wild type and pat14 mutant lines. As shown in Table I, the total amount of SA in 7-d-old seedlings is very low and similar between pat14 and wild type. These data are consistent with the low levels of the key SA biosynthetic gene transcripts, ICS1 and PAL1, detected in seedlings (Fig. 7). However, in the leaves of 30-d-old plants, the SA content increased by 5- and 6-fold in pat14-1 and pat14-2, respectively, while it remained unchanged in wild type. In addition, the SA glycoside content increased by 84- and 120-fold in the 30-d-old pat14-1 and pat14-2 plants, compared to only 20-fold in wild type, representing a 4- and 6-fold difference between the 30-d-old wild-type and pat14-1 and pat14-2 plants, broadly similar to the SA ratios at 30 d (Table I).
Table I. Phytohormone analysis of 7-d-old seedlings and 30-d-old leaves of wild-type and pat14 mutants.
7 d, Shoots of 7-d-old plate grown seedlings; 30 d, the seventh and eighth leaves of 30-d-old soil-grown plants. SA, ABA, and JA are expressed as µg per g of dry weight, while SA-glycoside and OPDA are peak areas. Values are means ± sd of three replicates.
| SA | SA-Glycoside | OPDA | ABA | JA | |
|---|---|---|---|---|---|
| Wild type | |||||
| 7 d | 4.10 ± 0.51 | 455.9 ± 41.5 | 235.9 ± 71.8 | 0.23 ± 0.05 | 0.17 ± 0.04 |
| 30 d | 4.49 ± 1.11 | 9,348.5 ± 1,386.5 | 4,759.6 ± 1,204.3 | 0.39 ± 0.11 | 0.13 ± 0.02 |
| pat14-1 | |||||
| 7 d | 3.87 ± 0.23 | 546.7 ± 88.5 | 226.0 ± 125.8 | 0.19 ± 0.01 | 0.12 ± 0.02 |
| 30 d | 19.49 ± 1.05 | 45,788.0 ± 4,289.3 | 11,856.0 ± 1,710.7 | 0.34 ± 0.07 | 0.40 ± 0.02 |
| pat14-2 | |||||
| 7 d | 4.22 ± 0.21 | 369.4 ± 9.2 | 182.1 ± 46.8 | 0.35 ± 0.10 | 0.15 ± 0.02 |
| 30 d | 25.58 ± 1.59 | 44,581.4 ± 2,997.5 | 11,711.9 ± 3,384.2 | 0.29 ± 0.11 | 0.11 ± 0.02 |
Consistent with the ABA treatment studies, ABA did not show significant differences between wild type and the pat14 mutants (Table I). We also monitored changes in the phytohormones JA and the related jasmonate metabolite cis-(+; cis)-12-oxo-phytodienoic acid (OPDA). In wild type, OPDA levels increased from 7-d-old seedlings to 30-d-old leaves. Strikingly, OPDA levels were 3-fold greater in both mutants compared to wild type, at 30 d (representing increases in OPDA levels from days 7-30 of 50-60 fold in the mutants compared 20-fold for wild type). Interestingly, these differences between mutant and wild type were not reflected in JA abundances (OPDA is a precursor of JA). All samples, with the exception of pat14-1 had similar JA levels. pat14-1 had twice the level of JA, and this may be a consequence of its more severe growth defects (Fig. 1).
DISCUSSION
Loss of Enzyme Activity of AtPAT14 Causes Growth Defects
In this study, we identified, to our knowledge, a novel protein S-acyltransferase, AtPAT14, from Arabidopsis. To study its biological function, we first isolated two T-DNA knockout mutant alleles, atpat14-1 and atpat14-2 (Fig. 1). The mutant plants exhibited altered patterns of growth and development, with smaller stature and early leaf senescence being the most obvious. Interestingly, atpat14-1 showed more severe growth defects than atpat14-2 throughout. atpat14-1 and atpat14-2 lines expressing 35S:AtPAT14 show a wild-type phenotype, clearly demonstrating that the phenotypes observed in the mutants were caused by AtPAT14 being rendered nonfunctional (Supplemental Fig. S5).
Although AtPAT14 contains the characteristic DHHC-CRD domain (Supplemental Fig. S1), in order to confirm it is indeed an S-acyl transferase, we first carried out in vitro and in vivo studies on its enzyme activity in yeast and Arabidopsis. The fact that AtPAT14 can partially rescue the morphological and growth defects of yeast akr1 mutant lacking the PAT AKR1, that it undergoes auto-palmitoylation, demonstrates that AtPAT14 can indeed function as an S-acyltransferase (Figs. 2 and 3). Importantly, the Cys to Ser mutation in the DHHC catalytic domain of AtPAT14 failed to restore the growth defect of yeast akr1 lines carrying this mutant polypeptide and no AtPAT14DHHC157S auto-acylation was detected. Further, 35S:AtPAT14DHHC157S is unable to rescue the growth defects of Arabidopsis pat14 mutant lines. Collectively, these data confirm that, as for other characterized PATs, AtPAT14 functions as a PAT through its enzymatic catalytic DHHC domain.
Early Senescence in AtPAT14 Loss-of-Function Mutants Is Not Caused by Defects in ABA and JA
Disruption of the AtPAT14 gene results in early senescence in Arabidopsis (Fig. 1). This coincides with a loss of more than half of the chlorophyll (Fig. 5A) and massive upregulation of senescence marker genes SAG12, SAG101, and PR1 (Fig. 5, B–D). Senescence results in activation of signaling pathways involving the stress-related plant hormones SA, ABA, and JA (Weaver et al., 1998; Morris et al., 2000; He et al., 2002). However, the absence of obvious phenotypic differences between ABA-treated seedlings of pat14 and wild type suggests that early senescence was not due to a defect in ABA signaling (Supplemental Fig. S9). Consistent with this conclusion, no differences in ABA accumulation were observed between mutant and wild-type seedlings or mature leaves (Table I). Application of JA had a similar effect on the growth behavior of wild-type and mutant seedlings (Supplemental Fig. S10). JA accumulation was identical between mutant and wild-type seedlings and leaves. Only pat14-1, with the more severe growth phenotype, showed twice the JA content (Table I). Therefore, the early senescence phenotype in AtPAT14 loss-of-function mutants is unlikely to be caused by defective ABA and JA signaling pathways.
Early Senescence in AtPAT14 Loss-of-Function Mutants Is Not Caused by Defects in Autophagy
Senescence involves nutrient recycling and remobilization, which is believed to be partially carried out by autophagy (Chung et al., 2010). Indeed, in higher plants, defects in autophagy lead to inefficient recycling of carbon and nitrogen, which is manifested in an enhanced senescence phenotype (Thompson et al., 2005; Bassham et al., 2006; Yoshimoto et al., 2009; Chung et al., 2010; Li and Vierstra, 2014). However, we found that early senescence of atpat14 is not caused by defects in autophagy. We conclude this as (1) under carbon starvation and dark treatment, pat14 seedlings were as green as wild type, while the two autophagy defective mutants, atg5-1 (Thompson et al., 2005; Chung et al., 2010) and atg9-2 (Li and Vierstra, 2014), became chlorotic (Supplemental Fig. S8). Thus, loss-of-function of AtPAT14 does not block autophagy-mediated nutrient remobilization under these conditions; (2) observation of autophagosomes in roots of pat14 mutants shows that a large number of autophagic bodies were labeled by the autophagy molecular marker GFP-ATG8 as was the case in wild type (Supplemental Fig. S8). In contrast, very few fluorescent foci were observed in the atg9 root. This clearly demonstrates that the starvation- and dark-induced senescence in atg5 and atg9 mutants is not found in AtPAT14 loss-of-function mutants, and hence, the autophagy process seems to be normal in pat14.
Taken together, these data clearly demonstrate that there is no direct involvement of ABA, JA, and autophagy in the early senescence phenotype of pat14 mutants, i.e. AtPAT14 is unlikely to be involved in ABA and JA signaling or autophagy-regulated leaf senescence in Arabidopsis.
Early Senescence in AtPAT14 Loss-of-Function Mutants Is Caused by Defects in the SA Signaling Pathway
The plant hormone SA is best known for its role in response to pathogen attacks. The accumulation of SA during different plant resistance responses is the basis of systemic acquired resistance. Plants are hypersensitive to SA produced by pathogen invasion, causing rapid programmed cell death (Kapulnik et al., 1992; Vlot et al., 2009). Leaf senescence is a slow form of programmed cell death that allows plants to mobilize nutrients released from senescing cells to sink and actively growing tissues (Gan and Amasino, 1997; Zhang et al., 2012). Although the role of SA in leaf senescence and its underlying molecular mechanism have been less studied, there is some evidence that both disease defense and leaf senescence share SA signaling and regulatory components (Morris et al., 2000; Love et al., 2008; Rivas-San Vicente and Plasencia, 2011). Senescing leaves of Arabidopsis contain approximately 3-fold more SA than green leaves (Morris et al., 2000). The Arabidopsis mutant npr1 lacking the SA signal transducer NPR1 shows delayed leaf senescence. Likewise, introducing a bacterial salicylate hydroxylase (encoded by NahG) results in very low levels of SA, and these plants display a significant delay in leaf senescence (Morris et al., 2000). These results are corroborated by studies of the recently identified equivalent plant enzyme, a SA 3-hydroxylase (S3H) from Arabidopsis. S3H converts SA to 2,3-dihydroxybenzoic acid, rendering SA inactive. Leaf senescence can be accelerated or delayed by blocking or overexpressing S3H due to the increased or decreased levels of active SA in these plants (Zhang et al., 2013).
To see if the senescence-like phenotype of pat14 plants was related to SA, we monitored the transcript levels of some of the SA synthetic, catabolic, and signaling genes. The results revealed that both the SA synthetic genes ICS1 and PAL1 showed much higher transcript levels in leaves derived from the pat14 mutant than wild type (Fig. 7). This could lead to a hyper-accumulation of SA in the mutants, and indeed, the mutant leaves were found to contain 6 times more SA than wild type (Table I). SA can induce the expression of the SA catabolic gene S3H, and the induced S3H enzyme in turn hydrolyses SA to its inactive form (2,3-dihydroxybenzoic acid) to prevent over accumulation of SA (Zhang et al., 2013). Thus, a positive feed-forward mechanism may account for the higher transcript levels of S3H and WES1 seen in the pat14 mutant leaves (Fig. 7). The over accumulation of SA in the mutants can induce the expression of SA signaling genes that were readily detected by quantitative PCR (Fig. 8).
Consistent with a role for AtPAT14 in the negative regulation of SA during senescence, the transcript levels of the senescence-specific marker genes SAG12 and PR1 are massively upregulated in pat14 mutant leaves (Fig. 6). Studies have shown that the expression of both SAG12 and PR1 rely on the presence of SA, since very low levels of SA were present in the SA signaling defective mutants npr1 and pad4 and in NahG overexpressing plants (Morris et al., 2000). However, increasing SA levels by blocking S3H or by spraying healthy green leaves with SA can induce the expression of PR1 in wild type (Morris et al., 2000; Zhang et al., 2013). The fact that both SAG12 and PR1 were found to be highly expressed in the pat14 mutants suggests that AtPAT14 can regulate SA levels by either blocking SA synthesis, signaling, or both. Loss-of-function pat14 mutants may thus relieve this repression with the concomitant loss of SA homeostasis by overaccumulation of SA and/or heightened perception, resulting in precocious leaf senescence. This scenario is supported by the much higher SA levels detected in mature pat14 leaves compared to wild type (Table I) and delayed leaf senescence in pat14/npr1 double mutant plants (Fig. 9; Supplemental Fig. S13). Taken together, our data suggest that AtPAT14 plays very important roles in leaf senescence via the fine-tuning of SA biosynthesis and/or perception.
SA and OPDA in Early Senescence of pat14
Analysis of the phytohormones in the leaves of 30-d-old plants revealed that both SA and OPDA accumulated to much higher levels in pat14 mutants than wild type (Table I). The accumulation of OPDA in the pat14 mutants may be due to a metabolic feedback mechanism resulting from accumulation of the palmitate C16:3 substrate (Dave and Graham, 2012). OPDA is emerging as a phytohormone in its own right (Dave et al., 2011; Goetz et al., 2012). Unlike the antagonistic effect of JA and SA in plant-microbe interactions, it functions through a synergistic effect with SA, which is independent of the JA signaling cascade (Dave and Graham, 2012; Montillet et al., 2013; Schenk and Schikora, 2014). The idea that OPDA can act as an independent signaling molecule is supported by a transcriptional analysis of Arabidopsis following application of OPDA. More than 150 genes related to detoxification, stress responses, and secondary metabolism were found to respond specifically to OPDA treatment, but not to JA or methyl jasmonate (Taki et al., 2005; Mueller et al., 2008).
Although the role of OPDA in leaf senescence is less understood, there is some evidence to show its involvement in this process. For instance, Arabidopsis leaves undergoing natural or stress-induced senescence accumulate high levels of JA and OPDA (Seltmann et al., 2010). Application of Arabidopside A and OPDA can cause senescence of oat leaves (Hisamatsu et al., 2006). Arabidopsides are OPDA esterified galactolipids that are found in Arabidopsis and all members of the Brassicaceae (Stelmach et al., 2001; Buseman et al., 2006). They are thought to provide a rapidly available source of cis-OPDA. The fact that pat14 leaves accumulated much more OPDA than wild type yet the JA levels were similar suggests that OPDA, but not JA, plays a role in early senescence in pat14. Furthermore, because pat14 leaves also produced more SA, together with OPDA these two phytohormones could act synergistically (as is the case in plant defense) to accelerate senescence in the mutant.
In summary, we have shown for the first time, to our knowledge, that protein palmitoylation is involved in leaf senescence in Arabidopsis. We identify AtPAT14 as a negative regulator of leaf senescence in Arabidopsis that likely acts by altering SA homeostasis and perception in concert with OPDA, in a process that is independent of ABA, JA, and autophagy.
MATERIALS AND METHODS
Plant Material and Growth Conditions
Wild-type Arabidopsis (Arabidopsis thaliana), the two T-DNA insertion lines SALK_026159 (Alonso et al., 2003), and GK-153A10 (Kleinboelting et al., 2012) in the background of Columbia-0 were obtained from the Arabidopsis Biological Resource Center (ABRC; http://www.arabidopsis.org/abrc/). For npr1-2 (N3801), seeds were obtained from the Nottingham Arabidopsis Stock Centre (NASC). Seeds were surface sterilized and germinate, and seedlings grown under long days (LD) as described previously (Qi et al., 2013).
Identification of AtPAT14 T-DNA Insertion Mutants
PCR-based genotyping was carried out to isolate homozygous T-DNA mutants from SALK_026159 and GK-153A10 lines using primers LBb1/SALK026159RP and SALK026159LP/SALK026521RP, and GABILBb1/GABI_153A10LP and GABI_153A10LP/GABI_153A10RP (Supplemental Table S1), respectively. PCR products amplified from the junction of the T-DNA insertion sites were sequenced to confirm their location in both lines as described previously (Qi et al., 2013).
RT-PCR and Real-Time PCR
Total RNA was extracted using Trizol (Invitrogen) according to the manufacturer’s instructions. Oligo(dT)-primed cDNAs were synthesized from 1 μg RNA using the First-Strand cDNA Synthesis Kit (Transgen). To detect transcripts in wild type and both T-DNA homozygous lines, four pairs of gene specific primers were used to amplify the AtPAT14 full coding region (Beg+End), upstream (F1+R1), across (F2+R2), and downstream (F3+R3) of the T-DNA insertion sites. For real-time PCR to detect transcripts of genes in the SA pathway, UltraSYBR PCR Mixture (With ROX; CWBIO) was used and the programs were run on a Stepone Plus real-time PCR system (Applied Biosystems). The relative transcript levels were calculated by the 2−ΔΔt method with the AtEF-1α gene as an internal control (Livak and Schmittgen, 2001). At least three replicates were included in each experiment, and the experiments were repeated three times. The sequences of all primers used are shown in Supplemental Tables S2 and S3.
Generating the pat14/npr1 Double Mutant
The newly isolated pat14-1 and pat14-2 homozygous plants were crossed with npr1-2 (Cao et al., 1997). The F2 progenies were genotyped for pat14 by PCR as above. To identify npr1-2, PCR was carried out with the NPR1For and NPR1Rev primer pair (Supplemental Table S2), and the products were sequenced to detect the point mutation in the NPR1 gene as described by Cao et al. (1997).
Complementation and Auto-Acylation in Yeast
The coding region of AtPAT14 was PCR-amplifed without a stop codon with primers 3g60800Beg/3g60800End (Supplemental Table S2) using KOD high fidelity polymerase (Novagen) from first-strand cDNAs prepared from leaf total RNA. The AtPAT14DHHC157S point mutant was made by PCR-based mutagenesis with primers PAT14DHHC-SFor and PAT14DHHC-SRev (Supplemental Table S2). Both AtPAT14 and AtPAT14DHHC157S were cloned into the Gateway donor vector pDONR/Zeo to generate entry clones pDONR-AtPAT14 and pDONR-AtPAT14DHHC157S following protocols of the manufacturer (Gateway Technology). They were then recombined into the Gateway destination vector pYES-DEST52 (C-terminal V5 tagged). Both pYES-AtPAT14 and pYES-AtPAT14DHHC157S were expressed in wild-type BY4741 and akr1 yeast (Saccharomyces cerevisiae), respectively. AKR1C-S was cloned in the yeast expression vector pESC-Leu (Stratagene) and was a gift from Dr. Pier Hemsley (Hemsley and Grierson, 2011). It was cotransformed with empty pYES2 vector, pYES-AtPAT14, or pYES-AtPAT14DHHC157S in akr1 cells. Growth and observation of the transgenic yeast were according to the method by Qi et al. (2013). For the auto-acylation assay by Acyl-RAC (Forrester et al., 2011), total proteins were extracted from akr1 yeast cells expressing AtPAT14 and AtPAT14DHHC157S. The S-acylated proteins were captured on thiopropyl sepharose beads (Sigma), and AtPAT14 and ATPAT14DHHC157S were detected by western blotting with anti-V5 primary and HRP-conjugated secondary antibodies (Bethyl).
Complementation of pat14 and Subcellular Localization of AtPAT14
For complementation of the pat14-1 and pat14-2, the C-terminal GFP tagged AtPAT14 and AtPAT14DHHC157S were cloned into pEarlyGate103 (Earley et al., 2006). Transgenics were created and selected as described previously (Qi et al., 2004).
For the subcellular localization of AtPAT14 transgenic pat14-1 plants complemented by 35S:AtPAT14-GFP were crossed with mCherry tagged marker Wavelines (Geldner et al., 2009). Primary roots and cotyledons of 10-d-old seedlings from the crossed F1 progeny were observed. Images were taken with a Zeiss LSM 510 inverted confocal microscope using a 488 nm argon ion laser with 505 to 530 nm band pass filter for detection of GFP and a 543 nm HeNe laser with a 560 to 615 nm band pass filter for detection of mCherry.
Chlorophyll Content Measurement
To estimate chlorophyll content, the methods described by Porra et al. (1998) and Katsiarimpa et al. (2013) were followed. Briefly, seedlings (10 mg) or fresh rosette leaves (20 mg) were homogenized in 500 μL of 96% ethanol in preweighed 1.5 mL microfuge tubes. After centrifugation, the supernatant was transferred to a fresh tube, and the OD at 470, 649, and 665 nm was recorded. The chlorophyll content is the sum of chlorophyll a (13.95 × OD665)−(6.88 × OD649) plus chlorophyll b (24.96 × OD649)−(7.32 × OD665), which is expressed as μg/mg fresh weight.
Carbon Deficiency and Phytohormone Treatment
The carbon deficiency assay was carried out according to Hanaoka et al. (2002). Briefly, seeds of wild type, pat14-1 and pat14-2, atg5-1, and atg9-2 were germinated and cultured vertically on half-strength Murashige and Skoog (MS) plus 1% Suc under LD for 7 d. They were then transferred to half-strength MS only and incubated in the dark for a further 7 d. For hormone treatment, seeds were germinated and grown on half-strength MS plus 1% Suc under LD for 4 d. The seedlings were transferred to half-strength MS without or with ABA, JA, and SA and left to grow for up to 3 weeks before being scanned and weighed.
Phytohormone Analysis
Each hormone determination was measured in triplicate using samples derived from 7-d-old seedlings and the seventh and eighth leaves of 30-d-old plants. Hormone extractions were performed on 3 mg of powdered, freeze-dried tissue exactly as detailed in Forcat et al. (2008). OPDA was measured according to Dave et al. (2011) using OPDA (Larodan) as a standard.
Accession Numbers
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers AtPAT14, NM_115944; AKR1, NM_001180572; MEMB12, NM_124426; SAG12, NM_123957; SAG101, NM_121497; PR1, NM_127025; ATG5, NM_121735; ATG9, NM_179833; ATG8, NM_001198362; ICS1, NM_202414; PAL1, NM_129260; WES1, NM_118860; EDS5, NM_120063; PAD4, NM_115103; NPR1, NM_105102; and NHL25, NM_123055.
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Protein sequence alignment and secondary structure prediction of AtPAT14.
Supplemental Figure S2. Expression level of AtPAT14 in seedlings and various tissues of mature Arabidopsis plants.
Supplemental Figure S3. Comparison of Arabidopsis reproductive organs and seed development from wild-type Columbia-0 and AtPAT14 mutant lines atpat14-1 and atpat14-2.
Supplemental Figure S4. The pat14 mutant has smaller cells than wild type.
Supplemental Figure S5. Loss of AtPAT14 PAT activity causes the phenotypic defects of the atpat14 mutant Arabidopsis plant.
Supplemental Figure S6. Restoration of akr1growth defects by PAT14 at the nonpermissive high temperature of 37°C was enhanced by the ankrin repeats of AKR1.
Supplemental Figure S7. AtPAT14 is predominantly localized in Golgi.
Supplemental Figure S8. Mutation in Cys-157 of the DHHC motif has no effect on AtPAT14 localization.
Supplemental Figure S9. Early senescence in atpat14 is not caused by autophagy deficiency.
Supplemental Figure S10. The subcellular localization of ATG8-GFP is not changed in pat14 mutant roots.
Supplemental Figure S11. PAT14 mutants are not sensitive to exogenous ABA treatment during early seedling growth.
Supplemental Figure S12. PAT14 mutants are not sensitive to exogenous JA treatment during early seedling growth.
Supplemental Figure S13. The early senescence phenotype of pat14 is rescued by blocking SA signaling in the mutant.
Supplemental Movie S1. AtPAT14 colocalizes with the Golgi marker R127.
Supplemental Table S1. Phenotypic analysis of wild type, pat14-1, and pat14-2.
Supplemental Table S2. Sequence of primers used for cloning and standard PCR.
Supplemental Table S3. Sequence of primers used for real-time PCR.
Supplementary Material
Acknowledgments
We thank the ABRC and NASC for providing Arabidopsis T-DNA insertion lines for AtPAT14, ATG5 (atg5-1), and npr1-2. Thanks also go to Dr. Taijoon Chung for sharing atg9-2 and the ProUBQ10-GFP-ATG8a construct. We thank Ms. Debbie Salmon for help with hormone analyses. We are very grateful for the kind help of Professor Chris Hawes with confocal scanning microscopy observation. We would also like to thank Dr. Piers Hemsley for providing us with the pESC-AKRC-S construct. Lastly, we would like to thank the editor and the reviewers for their constructive comments.
Glossary
- PAT
Protein S-Acyl Transferase
- DHHC
Asp-His-His-Cys
- CRD
Cys-rich domain
- JA
jasmonic acid
- ABA
abscisic acid
- SA
salicylic acid
- RT
reverse transcription
- OPDA
cis-(+; cis)-12-oxo-phytodienoic acid
- S3H
SA 3-hydroxylase
- LD
long day
- MS
Murashige and Skoog
Footnotes
This work was supported by the National Natural Science Foundation of China (grant no. 31170233 to B.Q.).
This article can be viewed without a subscription.
References
- Alonso JM, Stepanova AN, Leisse TJ, Kim CJ, Chen H, Shinn P, Stevenson DK, Zimmerman J, Barajas P, Cheuk R, Gadrinab C, Heller C, et al. (2003) Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301: 653–657 [DOI] [PubMed] [Google Scholar]
- Avila-Ospina L, Moison M, Yoshimoto K, Masclaux-Daubresse C (2014) Autophagy, plant senescence, and nutrient recycling. J Exp Bot 65: 3799–3811 [DOI] [PubMed] [Google Scholar]
- Baekkeskov S, Kanaani J (2009) Palmitoylation cycles and regulation of protein function (Review). Mol Membr Biol 26: 42–54 [DOI] [PubMed] [Google Scholar]
- Bassham DC, Laporte M, Marty F, Moriyasu Y, Ohsumi Y, Olsen L, Yoshimoto K (2006) Autophagy in development and stress responses of plants. Autophagy 2: 2–11 [DOI] [PubMed] [Google Scholar]
- Batistic O. (2012) Genomics and localization of the Arabidopsis DHHC-cysteine-rich domain S-acyltransferase protein family. Plant Physiol 160: 1597–1612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batistic O, Rehers M, Akerman A, Schlücking K, Steinhorst L, Yalovsky S, Kudla J (2012) S-Acylation-dependent association of the calcium sensor CBL2 with the vacuolar membrane is essential for proper abscisic acid responses. Cell Res 22: 1155–1168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boevink P, Oparka K, Santa Cruz S, Martin B, Betteridge A, Hawes C (1998) Stacks on tracks: the plant Golgi apparatus traffics on an actin/ER network. Plant J 15: 441–447 [DOI] [PubMed] [Google Scholar]
- Buchanan-Wollaston V, Page T, Harrison E, Breeze E, Lim PO, Nam HG, Lin JF, Wu SH, Swidzinski J, Ishizaki K, Leaver CJ (2005) Comparative transcriptome analysis reveals significant differences in gene expression and signalling pathways between developmental and dark/starvation-induced senescence in Arabidopsis. Plant J 42: 567–585 [DOI] [PubMed] [Google Scholar]
- Buseman CM, Tamura P, Sparks AA, Baughman EJ, Maatta S, Zhao J, Roth MR, Esch SW, Shah J, Williams TD, Welti R (2006) Wounding stimulates the accumulation of glycerolipids containing oxophytodienoic acid and dinor-oxophytodienoic acid in Arabidopsis leaves. Plant Physiol 142: 28–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao H, Glazebrook J, Clarke JD, Volko S, Dong X (1997) The Arabidopsis NPR1 gene that controls systemic acquired resistance encodes a novel protein containing ankyrin repeats. Cell 88: 57–63 [DOI] [PubMed] [Google Scholar]
- Charollais J, Van Der Goot FG (2009) Palmitoylation of membrane proteins (Review). Mol Membr Biol 26: 55–66 [DOI] [PubMed] [Google Scholar]
- Chung T, Phillips AR, Vierstra RD (2010) ATG8 lipidation and ATG8-mediated autophagy in Arabidopsis require ATG12 expressed from the differentially controlled ATG12A and ATG12B loci. Plant J 62: 483–493 [DOI] [PubMed] [Google Scholar]
- Contento AL, Xiong Y, Bassham DC (2005) Visualization of autophagy in Arabidopsis using the fluorescent dye monodansylcadaverine and a GFP-AtATG8e fusion protein. Plant J 42: 598–608 [DOI] [PubMed] [Google Scholar]
- Dave A, Graham IA (2012) Oxylipin signaling: a distinct role for the jasmonic acid precursor cis-(+)-12-oxo-phytodienoic acid (cis-OPDA). Front Plant Sci 3: 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dave A, Hernández ML, He Z, Andriotis VME, Vaistij FE, Larson TR, Graham IA (2011) 12-Oxo-phytodienoic acid accumulation during seed development represses seed germination in Arabidopsis. Plant Cell 23: 583–599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempsey DA, Vlot AC, Wildermuth MC, Klessig DF (2011) Salicylic acid biosynthesis and metabolism. Arabidopsis Book 9: e0156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earley KW, Haag JR, Pontes O, Opper K, Juehne T, Song K, Pikaard CS (2006) Gateway-compatible vectors for plant functional genomics and proteomics. Plant J 45: 616–629 [DOI] [PubMed] [Google Scholar]
- Farmer LM, Rinaldi MA, Young PG, Danan CH, Burkhart SE, Bartel B (2013) Disrupting autophagy restores peroxisome function to an Arabidopsis lon2 mutant and reveals a role for the LON2 protease in peroxisomal matrix protein degradation. Plant Cell 25: 4085–4100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y, Davis NG (2000) Akr1p and the type I casein kinases act prior to the ubiquitination step of yeast endocytosis: Akr1p is required for kinase localization to the plasma membrane. Mol Cell Biol 20: 5350–5359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forcat S, Bennett MH, Mansfield JW, Grant MR (2008) A rapid and robust method for simultaneously measuring changes in the phytohormones ABA, JA and SA in plants following biotic and abiotic stress. Plant Methods 4: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrester MT, Hess DT, Thompson JW, Hultman R, Moseley MA, Stamler JS, Casey PJ (2011) Site-specific analysis of protein S-acylation by resin-assisted capture. J Lipid Res 52: 393–398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukata Y, Fukata M (2010) Protein palmitoylation in neuronal development and synaptic plasticity. Nat Rev Neurosci 11: 161–175 [DOI] [PubMed] [Google Scholar]
- Fukata Y, Iwanaga T, Fukata M (2006) Systematic screening for palmitoyl transferase activity of the DHHC protein family in mammalian cells. Methods 40: 177–182 [DOI] [PubMed] [Google Scholar]
- Gan S, Amasino RM (1997) Making sense of senescence (molecular genetic regulation and manipulation of leaf senescence). Plant Physiol 113: 313–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geldner N, Dénervaud-Tendon V, Hyman DL, Mayer U, Stierhof YD, Chory J (2009) Rapid, combinatorial analysis of membrane compartments in intact plants with a multicolor marker set. Plant J 59: 169–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetz S, Hellwege A, Stenzel I, Kutter C, Hauptmann V, Forner S, McCaig B, Hause G, Miersch O, Wasternack C, Hause B (2012) Role of cis-12-oxo-phytodienoic acid in tomato embryo development. Plant Physiol 158: 1715–1727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greaves J, Carmichael JA, Chamberlain LH (2011) The palmitoyl transferase DHHC2 targets a dynamic membrane cycling pathway: regulation by a C-terminal domain. Mol Biol Cell 22: 1887–1895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greaves J, Chamberlain LH (2007) Palmitoylation-dependent protein sorting. J Cell Biol 176: 249–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greaves J, Chamberlain LH (2011) DHHC palmitoyl transferases: substrate interactions and (patho)physiology. Trends Biochem Sci 36: 245–253 [DOI] [PubMed] [Google Scholar]
- Greaves J, Gorleku OA, Salaun C, Chamberlain LH (2010) Palmitoylation of the SNAP25 protein family: specificity and regulation by DHHC palmitoyl transferases. J Biol Chem 285: 24629–24638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanaoka H, Noda T, Shirano Y, Kato T, Hayashi H, Shibata D, Tabata S, Ohsumi Y (2002) Leaf senescence and starvation-induced chlorosis are accelerated by the disruption of an Arabidopsis autophagy gene. Plant Physiol 129: 1181–1193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Fukushige H, Hildebrand DF, Gan S (2002) Evidence supporting a role of jasmonic acid in Arabidopsis leaf senescence. Plant Physiol 128: 876–884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemsley PA. (2009) Protein S-acylation in plants (Review). Mol Membr Biol 26: 114–125 [DOI] [PubMed] [Google Scholar]
- Hemsley PA, Grierson CS (2008) Multiple roles for protein palmitoylation in plants. Trends Plant Sci 13: 295–302 [DOI] [PubMed] [Google Scholar]
- Hemsley PA, Grierson CS (2011) The ankyrin repeats and DHHC S-acyl transferase domain of AKR1 act independently to regulate switching from vegetative to mating states in yeast. PLoS One 6: e28799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemsley PA, Kemp AC, Grierson CS (2005) The TIP GROWTH DEFECTIVE1 S-acyl transferase regulates plant cell growth in Arabidopsis. Plant Cell 17: 2554–2563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemsley PA, Weimar T, Lilley KS, Dupree P, Grierson CS (2013) A proteomic approach identifies many novel palmitoylated proteins in Arabidopsis. New Phytol 197: 805–814 [DOI] [PubMed] [Google Scholar]
- Hisamatsu Y, Goto N, Hasegawa K, Shigemori H (2006) Senescence-promoting effect of arabidopside A. Z Naturforsch C Biosci 61: 363–366 [DOI] [PubMed] [Google Scholar]
- Howie J, Reilly L, Fraser NJ, Vlachaki Walker JM, Wypijewski KJ, Ashford ML, Calaghan SC, McClafferty H, Tian L, Shipston MJ, Boguslavskyi A, Shattock MJ, et al. (2014) Substrate recognition by the cell surface palmitoyl transferase DHHC5. Proc Natl Acad Sci USA 111: 17534–17539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang K, Sanders S, Singaraja R, Orban P, Cijsouw T, Arstikaitis P, Yanai A, Hayden MR, El-Husseini A (2009) Neuronal palmitoyl acyl transferases exhibit distinct substrate specificity. FASEB J 23: 2605–2615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapulnik Y, Yalpani N, Raskin I (1992) Salicylic acid induces cyanide-resistant respiration in tobacco cell-suspension cultures. Plant Physiol 100: 1921–1926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsiarimpa A, Kalinowska K, Anzenberger F, Weis C, Ostertag M, Tsutsumi C, Schwechheimer C, Brunner F, Hückelhoven R, Isono E (2013) The deubiquitinating enzyme AMSH1 and the ESCRT-III subunit VPS2.1 are required for autophagic degradation in Arabidopsis. Plant Cell 25: 2236–2252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinboelting N, Huep G, Kloetgen A, Viehoever P, Weisshaar B (2012) GABI-Kat SimpleSearch: new features of the Arabidopsis thaliana T-DNA mutant database. Nucleic Acids Res 40: D1211–D1215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Vierstra RD (2014) Arabidopsis ATG11, a scaffold that links the ATG1-ATG13 kinase complex to general autophagy and selective mitophagy. Autophagy 10: 1466–1467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo S, Greentree WK, Linder ME, Deschenes RJ (2002) Identification of a Ras palmitoyltransferase in Saccharomyces cerevisiae. J Biol Chem 277: 41268–41273 [DOI] [PubMed] [Google Scholar]
- Love A, Geri C, Laird J, Yuri B, Loake G, Sadanandom A, Milner J (2008) An effector protein encoded by cauliflower mosaic virus inhibits SA-dependent defence responses in Arabidopsis via an NPR1-dependent mechanism. Ann Meet Soc Exp Biol Marseille 150: S193–S193 [Google Scholar]
- Martin BR, Cravatt BF (2009) Large-scale profiling of protein palmitoylation in mammalian cells. Nat Methods 6: 135–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montillet JL, Leonhardt N, Mondy S, Tranchimand S, Rumeau D, Boudsocq M, Garcia AV, Douki T, Bigeard J, Laurière C, Chevalier A, Castresana C, et al. (2013) An abscisic acid-independent oxylipin pathway controls stomatal closure and immune defense in Arabidopsis. PLoS Biol 11: e1001513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González Montoro A, Chumpen Ramirez S, Quiroga R, Valdez Taubas J (2011) Specificity of transmembrane protein palmitoylation in yeast. PLoS One 6: e16969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris K, MacKerness SAH, Page T, John CF, Murphy AM, Carr JP, Buchanan-Wollaston V (2000) Salicylic acid has a role in regulating gene expression during leaf senescence. Plant J 23: 677–685 [DOI] [PubMed] [Google Scholar]
- Mueller S, Hilbert B, Dueckershoff K, Roitsch T, Krischke M, Mueller MJ, Berger S (2008) General detoxification and stress responses are mediated by oxidized lipids through TGA transcription factors in Arabidopsis. Plant Cell 20: 768–785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno Y, Kihara A, Sano T, Igarashi Y (2006) Intracellular localization and tissue-specific distribution of human and yeast DHHC cysteine-rich domain-containing proteins. Biochim Biophys Acta 1761: 474–483 [DOI] [PubMed] [Google Scholar]
- Porra RJ, Urzinger M, Winkler J, Bubenzer C, Scheer H (1998) Biosynthesis of the 3-acetyl and 13(1)-oxo groups of bacteriochlorophyll a in the facultative aerobic bacterium, Rhodovulum sulfidophilum--the presence of both oxygenase and hydratase pathways for isocyclic ring formation. Eur J Biochem 257: 185–191 [DOI] [PubMed] [Google Scholar]
- Putilina T, Wong P, Gentleman S (1999) The DHHC domain: a new highly conserved cysteine-rich motif. Mol Cell Biochem 195: 219–226 [DOI] [PubMed] [Google Scholar]
- Qi B, Doughty J, Hooley R (2013) A Golgi and tonoplast localized S-acyl transferase is involved in cell expansion, cell division, vascular patterning and fertility in Arabidopsis. New Phytol 200: 444–456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi B, Fraser T, Mugford S, Dobson G, Sayanova O, Butler J, Napier JA, Stobart AK, Lazarus CM (2004) Production of very long chain polyunsaturated omega-3 and omega-6 fatty acids in plants. Nat Biotechnol 22: 739–745 [DOI] [PubMed] [Google Scholar]
- Resh MD. (2006) Palmitoylation of ligands, receptors, and intracellular signaling molecules. Science STKE 2006: re14. [DOI] [PubMed] [Google Scholar]
- Rivas-San Vicente M, Plasencia J (2011) Salicylic acid beyond defence: its role in plant growth and development. J Exp Bot 62: 3321–3338 [DOI] [PubMed] [Google Scholar]
- Roth AF, Feng Y, Chen L, Davis NG (2002) The yeast DHHC cysteine-rich domain protein Akr1p is a palmitoyl transferase. J Cell Biol 159: 23–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth AF, Wan J, Bailey AO, Sun B, Kuchar JA, Green WN, Phinney BS, Yates JR III, Davis NG (2006) Global analysis of protein palmitoylation in yeast. Cell 125: 1003–1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schippers JHM, Jing H, Hille J, Dijkwel PP (2007) Developmental and hormonal control of leaf senescence. Ann Plant Rev 26: 145–170 [Google Scholar]
- Seltmann MA, Stingl NE, Lautenschlaeger JK, Krischke M, Mueller MJ, Berger S (2010) Differential impact of lipoxygenase 2 and jasmonates on natural and stress-induced senescence in Arabidopsis. Plant Physiol 152: 1940–1950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stelmach BA, Müller A, Hennig P, Gebhardt S, Schubert-Zsilavecz M, Weiler EW (2001) A novel class of oxylipins, sn1-O-(12-oxophytodienoyl)-sn2-O-(hexadecatrienoyl)-monogalactosyl Diglyceride, from Arabidopsis thaliana. J Biol Chem 276: 12832–12838 [DOI] [PubMed] [Google Scholar]
- Taki N, Sasaki-Sekimoto Y, Obayashi T, Kikuta A, Kobayashi K, Ainai T, Yagi K, Sakurai N, Suzuki H, Masuda T, Takamiya K, Shibata D, et al. (2005) 12-Oxo-phytodienoic acid triggers expression of a distinct set of genes and plays a role in wound-induced gene expression in Arabidopsis. Plant Physiol 139: 1268–1283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson AR, Doelling JH, Suttangkakul A, Vierstra RD (2005) Autophagic nutrient recycling in Arabidopsis directed by the ATG8 and ATG12 conjugation pathways. Plant Physiol 138: 2097–2110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uemura T, Ueda T, Ohniwa RL, Nakano A, Takeyasu K, Sato MH (2004) Systematic analysis of SNARE molecules in Arabidopsis: dissection of the post-Golgi network in plant cells. Cell Struct Funct 29: 49–65 [DOI] [PubMed] [Google Scholar]
- van der Graaff E, Schwacke R, Schneider A, Desimone M, Flügge UI, Kunze R (2006) Transcription analysis of arabidopsis membrane transporters and hormone pathways during developmental and induced leaf senescence. Plant Physiol 141: 776–792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlot AC, Dempsey DA, Klessig DF (2009) Salicylic acid, a multifaceted hormone to combat disease. Annu Rev Phytopathol 47: 177–206 [DOI] [PubMed] [Google Scholar]
- Vogelmann K, Drechsel G, Bergler J, Subert C, Philippar K, Soll J, Engelmann JC, Engelsdorf T, Voll LM, Hoth S (2012) Early senescence and cell death in Arabidopsis saul1 mutants involves the PAD4-dependent salicylic acid pathway. Plant Physiol 159: 1477–1487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver LM, Gan S, Quirino B, Amasino RM (1998) A comparison of the expression patterns of several senescence-associated genes in response to stress and hormone treatment. Plant Mol Biol 37: 455–469 [DOI] [PubMed] [Google Scholar]
- Wu Y, Zhang D, Chu JY, Boyle P, Wang Y, Brindle ID, De Luca V, Després C (2012) The Arabidopsis NPR1 protein is a receptor for the plant defense hormone salicylic acid. Cell Reports 1: 639–647 [DOI] [PubMed] [Google Scholar]
- Yoshimoto K, Hanaoka H, Sato S, Kato T, Tabata S, Noda T, Ohsumi Y (2004) Processing of ATG8s, ubiquitin-like proteins, and their deconjugation by ATG4s are essential for plant autophagy. Plant Cell 16: 2967–2983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimoto K, Jikumaru Y, Kamiya Y, Kusano M, Consonni C, Panstruga R, Ohsumi Y, Shirasu K (2009) Autophagy negatively regulates cell death by controlling NPR1-dependent salicylic acid signaling during senescence and the innate immune response in Arabidopsis. Plant Cell 21: 2914–2927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Halitschke R, Yin C, Liu CJ, Gan SS (2013) Salicylic acid 3-hydroxylase regulates Arabidopsis leaf longevity by mediating salicylic acid catabolism. Proc Natl Acad Sci USA 110: 14807–14812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Xia X, Zhang Y, Gan SS (2012) An ABA-regulated and Golgi-localized protein phosphatase controls water loss during leaf senescence in Arabidopsis. Plant J 69: 667–678 [DOI] [PubMed] [Google Scholar]
- Zhou L, Li S, Feng Q, Zhang Y, Zhao X, Zeng Y, Wang H, Jiang L, Zhang Y (2013) Protein S-ACYL Transferase10 is critical for development and salt tolerance in Arabidopsis. Plant Cell 25: 1093–1107 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.