Low red/far-red light ratios increase cold tolerance in tomato via the activation of phytochrome A and ABA-dependent JA signaling.
Abstract
Light signaling and phytohormones both influence plant growth, development, and stress responses; however, cross talk between these two signaling pathways in response to cold remains underexplored. Here, we report that far-red light (FR) and red light (R) perceived by phytochrome A (phyA) and phyB positively and negatively regulated cold tolerance, respectively, in tomato (Solanum lycopersicum), which were associated with the regulation of levels of phytohormones such as abscisic acid (ABA) and jasmonic acid (JA) and transcript levels of ABA- and JA-related genes and the C-REPEAT BINDING FACTOR (CBF) stress signaling pathway genes. A reduction in the R/FR ratio did not alter cold tolerance, ABA and JA accumulation, and transcript levels of ABA- and JA-related genes and the CBF pathway genes in phyA mutant plants; however, those were significantly increased in wild-type and phyB plants with the reduction in the R/FR ratio. Even though low R/FR treatments did not confer cold tolerance in ABA-deficient (notabilis [not]) and JA-deficient (prosystemin-mediated responses2 [spr2]) mutants, it up-regulated ABA accumulation and signaling in the spr2 mutant, with no effect on JA levels and signaling in the not mutant. Foliar application of ABA and JA further confirmed that JA functioned downstream of ABA to activate the CBF pathway in light quality-mediated cold tolerance. It is concluded that phyA and phyB function antagonistically to regulate cold tolerance that essentially involves FR light-induced activation of phyA to induce ABA signaling and, subsequently, JA signaling, leading to an activation of the CBF pathway and a cold response in tomato plants.
Plants have evolved a set of sophisticated photoreceptors such as phytochromes, cryptochromes, phototropins, UV-B light photoreceptor, and LOV/F-box/Kelch-domain proteins to perceive light with a broad range of wavelengths, from UV light to red light (R)/far-red light (FR; Möglich et al., 2010). The photoreceptors enable a plant to monitor and direct the adaptive responses and developmental transitions to the changing light environment and specifically activate precise downstream signaling pathways (Jiao et al., 2007). Accordingly, light plays a key role in regulating many aspects of plant growth and development, such as seed germination, photosynthesis, pigment biosynthesis, internode elongation, leaf expansion, flowering, and senescence, as well as stress response against both abiotic and biotic factors (Franklin and Whitelam, 2004; Schepens et al., 2004; Mathews, 2006). Light conditions change with season, day, and vegetation canopy. For example, R/FR is reduced naturally at dawn and dusk, and vertically from the top to the bottom within a vegetation canopy (Smith, 1982; Chambers and Spence, 1984; Ballaré et al., 1990). It remains largely unknown how plants integrate these environmental signals, especially R/FR ratio in cold adaptation. It is speculated that the cool temperatures and prolonged twilight reductions in low R/FR-experienced plants grown at northern latitudes may confer some seasonal protection against subsequent freezing snaps (Franklin and Whitelam, 2007).
Increasing studies have demonstrated a complex cross talk between light and temperature signals that is partly mediated by hormone signaling cascades in the regulation of germination, plant architecture, flowering, and plant growth (Franklin, 2009; Franklin et al., 2014). Multiple hormonal pathways are recruited in the diverse light-regulated growth and developmental processes to coordinate the light-dependent adaptive changes (Neff et al., 2006). For instance, GA and abscisic acid (ABA) play important but antagonistic roles in phytochrome-mediated seed germination, while a down-regulation of the jasmonic acid (JA) response has been documented under low-R/FR conditions (Moreno et al., 2009; Lau and Deng, 2010; Cerrudo et al., 2012; de Wit et al., 2013; Kegge et al., 2013; Chico et al., 2014).
In plants, FR and R are perceived by phytochrome A (phyA) and phyB, respectively (Franklin and Quail, 2010). Accumulating studies have shown that phytochromes play a critical role in regulating the expression of a large number of light-responsive genes involved in photomorphogenesis as well as defense and stress responses in plants (Quail, 2002a, 2002b; Franklin and Quail, 2010). The R/FR-mediated shade-avoidance responses are largely transduced by phyB, which changes the expression of a variety of genes associated with hormone biosynthesis and signaling as well as bud development (Kebrom et al., 2006, 2010; Finlayson et al., 2010; Su et al., 2011). ABA regulates bud outgrowth and thus extends the known hormonal pathways associated with the regulation of branching in the shade-avoidance response (Reddy et al., 2013). Previous studies also demonstrated that low R/FR induced spatial accumulation of JA in the stem (Cagnola et al., 2012). Herbivory by canopy arthropods and infection by a range of pathogens are reduced in plants grown in full sunlight, typically with a high R/FR ratio, compared with those grown in shade, with a low R/FR ratio (Roberts and Paul, 2006). In addition to biotic stresses, phytochromes are also involved in the responses to abiotic stresses. For example, genes in the INDUCER OF CBF EXPRESSION (ICE)-C-REPEAT BINDING FACTOR/DRE BINDING FACTOR1 (CBF/DREB1) transcriptional pathway, which plays a critical role in modulating cold stress responses for the induction of COLD-RESPONSIVE (COR) genes (Hsieh et al., 2002; Miura et al., 2007; Thomashow, 2010), are regulated by the circadian clock and specific light spectra (Fowler et al., 2005; Franklin and Whitelam, 2007; Lee and Thomashow, 2012). Especially, phyB and phyD could regulate cold tolerance by suppressing the transcription of genes in the CBF pathway (Franklin and Whitelam, 2007). Although phyA has been implicated in the cold response (Gururani et al., 2015), the relationship between phyA and light quality in the cold response has not yet been substantiated. As an antagonism generally exists between R and FR in the response to many environmental stimuli, it is highly plausible that phyA plays a critical role in the cold response by sensing the changes in light conditions, especially FR intensity.
Cold stress induces a number of ABA and JA biosynthetic and signaling genes that ultimately trigger the accumulation of ABA and JA (Knight et al., 2004; Gusta et al., 2005; Hu et al., 2013). JA could also increase cold tolerance by regulating the ICE-CBF/DREB1 cascade, whereas ABA biosynthesis and signaling components are important for the expression of COR genes in the cold response (Gilmour and Thomashow, 1991; Mantyla et al., 1995; Hu et al., 2013). Until now, the roles of light signaling and phytohormones in the cold stress response have been investigated independently, and it was observed that both regulatory systems share some common regulatory and response genes. This raises the possibility that light-induced activation of phytochromes might modify cold tolerance via the action of phytohormones such as ABA and JA.
To test the above hypothesis, this study examined the responses to cold under different light quality conditions and analyzed the changes in the biosynthesis of ABA and JA, and transcripts of their signaling-related genes, in tomato (Solanum lycopersicum), a cold-sensitive species. This study demonstrates that phyA and phyB function antagonistically to confer cold tolerance in tomato. Activation of phyA by FR induced ABA signaling that, in turn, triggered JA signaling, leading to an activation of the CBF pathway and cold response in tomato plants. These findings offer a clue to the elaborate regulatory interplay between light and hormone signaling in response to cold stress.
RESULTS
CBFs Mediate R- and FR-Regulated Cold Tolerance
To assess the potential role of R and FR in processes that contribute to cold tolerance, tomato plants were exposed to monochromatic R and FR at 4°C for 7 d followed by assessment of cold tolerance. It was found that R increased, but FR decreased, relative electrolyte leakage from the leaf cells, resulting in decreased and increased plant survival rates, respectively, compared with plants grown in the dark (Fig. 1A). We also observed that R exposure caused a reduction in CBF1 transcript levels in the cold-treated leaves, while FR had the reverse effect, which was independent of the applied light intensities (Fig. 1A). Meanwhile, exposure to cold temperature under dark conditions also resulted in an increased accumulation of ABA and JA, and this increase was further enhanced by exposure of plants to FR (Fig. 1B). In contrast, the increase in ABA accumulation was attenuated and JA accumulation was completely abolished by R treatment. To elucidate the potential involvement of photosynthesis-induced oxidative stress under R in the cold response, we also compared the cold tolerance of plants grown in the dark and in R and blue light conditions. At 25°C, no significant differences were found in net CO2 assimilation rate (Pn) in plants grown under R and blue light conditions. R decreased the cold tolerance and the transcript of CBF1; however, the cold tolerance and the transcript of CBF1 for plants grown under blue light conditions were not significantly altered compared with those grown in the dark (Supplemental Fig. S1). These results suggest that R and FR had opposite roles in cold tolerance that may be mediated by phytochromes but not by photosynthesis.
Figure 1.
R and FR function antagonistically in the regulation of cold tolerance. A, Plant survival rates, relative electrolyte leakage, and CBF1 transcript levels after exposure to R, FR, and dark conditions at 4°C. B, R and FR at 40 μmol m−2 s−1 induced changes in endogenous ABA and JA accumulation. The relative electrolyte leakage was determined after 7 d of cold stress; survival rates were measured by recovery at 25°C for 6 d after the chilling treatment (n = 4); each replicate had 16 plants, and CBF1 transcript levels were analyzed 6 h after chilling treatment. Data are presented as means of four biological replicates ± sd. Different letters indicate significant differences (P < 0.05) according to Tukey’s test. FW, Fresh weight.
To determine whether R- and FR-regulated cold tolerance was mediated by a CBF-dependent pathway, we generated CBF1-silenced (pTRV-CBF1) plants as well as CBF1/2/3-cosilenced (pTRV-CBF1/2/3) plants using a virus-induced gene silencing (VIGS) strategy (Supplemental Fig. S2A). Transcript analysis of the leaflets in the middle of the fourth fully expanded leaves revealed that the transcripts for these genes were suppressed by 65% to 80% in the respective silenced plants (Supplemental Fig. S2B). We next compared the cold tolerance of these two silenced plants grown under high red light/far-red light ratio (H-R/FR; R/FR = 72:28) and low red light/far-red light ratio (L-R/FR; R/FR = 33:67) conditions (Fig. 2; Supplemental Fig. S2C). Both silenced plants showed an increased sensitivity to cold compared with empty vector-transformed control plants (pTRV), as evidenced by decreases in the Fv/Fm and Pn and an increase in relative electrolyte leakage, with more obvious effects in the pTRV-CBF1/2/3 plants (Fig. 2, A, B, and D). Moreover, levels of oxidized protein (proteins with carbonyl groups), detected as a marker for oxidative damage in vivo, were significantly increased by cold and were much higher in the pTRV-CBF1 and pTRV-CBF1/2/3 plants than in the pTRV plants (Fig. 2C). While the L-R/FR treatment increased Fv/Fm and Pn reduced cellular injury and protein oxidation in the pTRV plants, it had little effect on the pTRV-CBF1/2/3 plants. Although L-R/FR exposure decreased relative electrolyte leakage from the leaf cells of the pTRV-CBF1 plants, the effects were less significant compared with the pTRV plants. Therefore, we conclude that the positive influence of FR and the negative influence of R on cold tolerance are mediated by CBFs and that this is accompanied by changes in ABA and JA biosynthesis.
Figure 2.
Silencing of the tomato CBF1 and CBF1/2/3 genes abolishes L-R/FR-induced cold tolerance. A and B, Changes in the maximum photochemical efficiency of PSII (Fv/Fm; A) and relative electrolyte leakage (B) in CBF-silenced plants after chilling at 4°C, as influenced by the R/FR ratio. The false color code depicted at the bottom of the image ranges from 0 (black) to 1 (purple). Fv/Fm and relative electrolyte leakage were determined 7 d after the chilling treatment. C, Western-blot detection of oxidized proteins in leaves after 3 d of cold stress under H-R/FR or L-R/FR conditions. The western-blot experiment was performed three times with three independent biological replicates with similar results. D, Leaf Pn recorded after plants had been exposed to 4°C under H-R/FR or L-R/FR conditions for 7 d, followed by recovery at 25°C for 1 d. The R/FR ratios for the H-R/FR and L-R/FR treatments were 72:28 and 33:67, respectively. Data are presented as means of four biological replicates ± sd except for Fv/Fm, which was the mean for 15 leaves from independent plants. Different letters indicate significant differences (P < 0.05) according to Tukey’s test.
Phytochromes Play Important Roles in R- and FR-Regulated Cold Tolerance
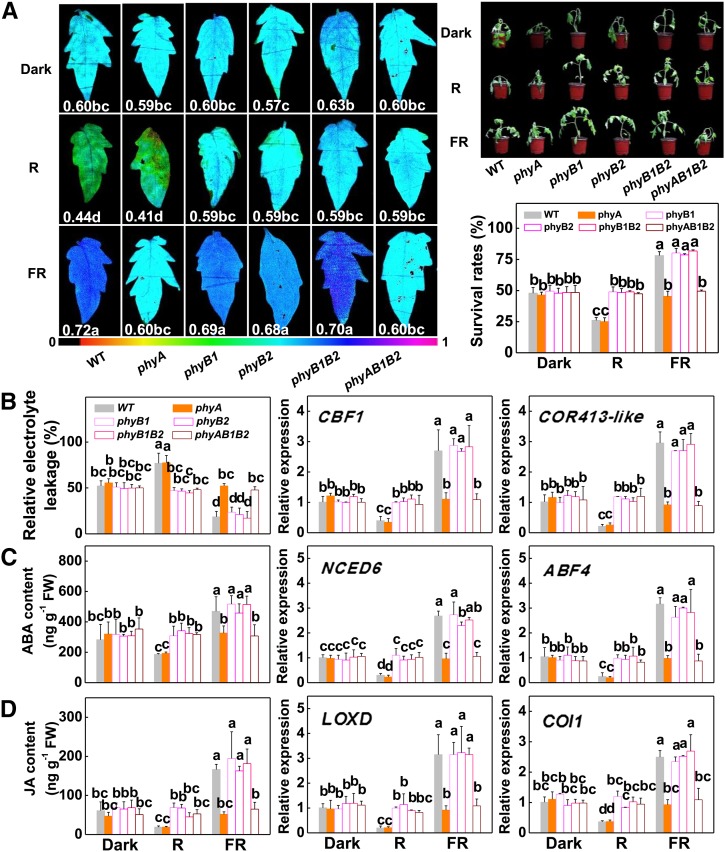
Plants perceive R and FR signals through the action of phyB and phyA photoreceptors, respectively. To determine the role of phytochromes in the FR- or L-R/FR-induced activation of the CBF-dependent pathway, and hence cold tolerance, we used the wild type and five phytochrome mutants, phyA, phyB1, phyB2, phyB1B2, and phyAB1B2, to compare their responses to cold stress under dark, R, and FR conditions. Under dark conditions, exposure to the cold stress did not induce any significant difference in the tolerance, as indicated by the little changes in Fv/Fm, survival rates, and relative electrolyte leakage among the wild type, phyA, phyB1, phyB2, phyB1B2, and phyAB1B2 (Fig. 3, A and B). Compared with plants grown in the dark, the wild-type plants had decreased and increased cold tolerance under R and FR conditions, respectively, as indicated by the changes in Fv/Fm values, survival rates, and relative electrolyte leakage. In comparison, the cold tolerance of phyA and phyAB1B2 plants was not altered under FR conditions as compared with those grown in the dark. Meanwhile, phyA plants had decreased cold tolerance, while that for phyAB1B2 plants was not altered under R conditions. In contrast, the cold tolerance for phyB1, phyB2, and phyB1B2 plants was not altered under R conditions but was significantly increased under FR conditions as compared with those grown in the dark. This suggests that light conditions modified cold tolerance via phyA and phyB and that the phyA plants were more sensitive to cold than wild-type, phyB1, phyB2, and phyB1B2 plants. Similar to the changes in cold tolerance, the transcript levels of genes involved in ABA (NCED6 and ABF4) and JA (LOXD and COI1) biosynthesis and signaling and those of the CBF pathway (CBF1 and COR413-like), as well as ABA and JA accumulation, were down-regulated and up-regulated, respectively, in the wild-type plants under R and FR conditions compared with the dark (Fig. 3, B–D). The transcript levels of these same genes and the accumulation of ABA and JA were consistently reduced in the phyA plants under R conditions but were not significantly altered under FR conditions, respectively, as compared with those grown in the dark. In contrast, the transcript levels of these same genes, and ABA and JA accumulation, were induced by FR but not altered by R in phyB1, phyB2, and phyB1B2 plants. Moreover, lack of both phyA and phyB in phyAB1B2 plants compromised R- and FR-induced changes in the transcript levels of these same genes and the accumulation of ABA and JA.
Figure 3.
Dependency on phytochromes in light quality-regulated cold tolerance. A, Images of the Fv/Fm, chilling phenotypes, and survival rates in wild-type (WT) and phytochrome mutant plants after exposure to 4°C at R, FR, or dark conditions for 7 d. The false color code depicted at the bottom of the image ranges from 0 (black) to 1 (purple). Survival rates were measured by recovery at 25°C for 6 d after the chilling treatment (4°C for 7 d; n = 4), and each replicate had 16 plants. B, Relative electrolyte leakage and transcript levels of genes involved in the CBF pathway at 7 d and 6 h, respectively, after chilling treatment. C and D, ABA (C) and JA (D) levels at 12 h and transcript levels of ABA (C) and JA (D) biosynthesis- and signaling-related genes at 6 h after cold treatment at 4°C in plants grown under different light quality conditions. Data are presented as means of four biological replicates ± sd except for Fv/Fm, which was the mean for 15 leaves from independent plants. Different letters indicate significant differences (P < 0.05) according to Tukey’s test. FW, Fresh weight.
We then compared the changes in cold tolerance for wild-type, phyA, and phyB1B2 plants grown at a range of R/FR ratios. Compared with wild-type plants, phyB1B2 plants showed enhanced cold tolerance while phyA plants had reduced cold tolerance at different R/FR ratio conditions (Fig. 4, A–C). As the R/FR ratio decreased, both wild-type and phyB1B2 plants showed decreased relative electrolyte leakage, increased transcripts of CBF1, NCED6, and LOXD, and increased ABA and JA accumulation. However, the phyA mutation compromised R/FR-induced changes in these parameters. Further experiments with H-R/FR (72:28) and L-R/FR (33:67) light conditions revealed that the increased cold tolerance for wild-type and phyB1B2 plants under L-R/FR conditions, as indicated by the increased Fv/Fm values and survival rates and the decreased relative electrolyte leakage, were associated with increased transcripts of genes involved in the CBF pathway (CBF1, COR413-like, SIZ1-like, ICE1, and COR47-like), ABA biosynthesis and signaling (NECD6, ABF4, AREB, AIM1, and RD22-like), and JA biosynthesis and signaling (LOXD, COI1, LOXF, AOS2, and AOC; Supplemental Figs. S3 and S4). Again, the phyA mutation compromised L-R/FR-induced changes in these parameters. Taken together, these results suggest that ABA and JA may be involved in phyA- and phyB-mediated cold tolerance that is promoted by FR and suppressed by R conditions, respectively.
Figure 4.
Effects of R/FR ratio and the irradiance of FR on cold tolerance in wide-type (WT) and phyA and phyB1B2 mutant plants. A, Relative electrolyte leakage and transcript levels of CBF1 gene expression after 4°C treatment for 7 d and 6 h, respectively. B and C, ABA (B) and JA (C) levels at 12 h and transcript levels of ABA (B) and JA (C) biosynthesis-related genes (NCED6 [B] and LOXD [C]) at 6 h after cold treatment at 4°C in plants grown under different R/FR ratios. D, Relative electrolyte leakage of plants after growth under white light (W) or R conditions, supplied with different intensities of FR (0, 50, 100, and 200 μmol m−2 s−1), at 4°C for 7 d. The intensity of white light and R both are 100 μmol m−2 s−1. E, ABA and JA levels at 12 h after cold treatment at 4°C in plants grown under white light conditions with different intensities of FR (0, 50, 100, and 200 μmol m−2 s−1). F, Transcript levels of CBF1, NCED6, and LOXD at 6 h after cold treatment at 4°C in plants grown under white light conditions with different intensities of FR (0, 50, 100, and 200 μmol m−2 s−1). For A, B, and C, the light intensity was 120 μmol m−2 s−1. Data are presented as means of four biological replicates ± sd. Different letters indicate significant differences (P < 0.05) according to Tukey’s test. FW, Fresh weight.
We also compared the changes in cold tolerance for the wild-type plants grown at a range of FR intensities with a given R or white light intensity. As the intensity of FR was increased, Fv/Fm was also increased; however, relative electrolyte leakage was decreased (Fig. 4D; Supplemental Fig. S5). A detailed examination revealed that ABA and JA accumulation and transcripts of genes involved in the CBF pathway (CBF1, COR413-like, SIZ1-like, ICE1, and COR47-like), ABA biosynthesis and signaling (NECD6, ABF4, AREB, AIM1, and RD22-like), and JA biosynthesis and signaling (LOXD, COI1, LOXF, AOS2, and AOC; Fig. 4, E and F; Supplemental Fig. S6) were increased with the increase of FR intensities. All these results suggest that FR plays a critical role in cold tolerance in plants under different light conditions.
Integration of Light Signaling and Cold Stimuli Is Essential for the Induction of Cold Tolerance
To understand the relationship between cold tolerance and R/FR ratio in the growth environment, H-R/FR- and L-R/FR-pretreated or nontreated tomato plants were subjected to a cold treatment for 7 d under H-R/FR and L-R/FR conditions. At optimal growth temperatures, the R/FR ratio had no significant effects on the transcription of genes involved in ABA biosynthesis (NCED6), ABA signaling (ABF4, AREB, AIM1, and RD22-like), JA biosynthesis (LOXD, LOXF, AOS2, and AOC), JA signaling (COI1), and the CBF pathway (CBF1, COR413-like, SIZ1-like, ICE1, and COR47-like; Fig. 5, B–D; Supplemental Fig. S7B). ABA and JA levels were also not significantly altered by the R/FR ratio at the optimal temperature (Fig. 5, C and D). However, plants grown under L-R/FR conditions showed an increased cold tolerance compared with those grown under H-R/FR conditions, as indicated by a lower relative electrolyte leakage and the accumulation of superoxide and hydrogen peroxide in the leaves as well as higher Fv/Fm values (Fig. 5, A and B; Supplemental Fig. S7A). Cold-induced reduction in Fv/Fm was more severe in leaf edge than in the middle of the leaf in most cases. In addition, cold stress induced a more significant increase in the transcription of genes involved in these processes and pathways in plants grown under L-R/FR conditions (Fig. 5, B–D; Supplemental Fig. S7B). We also noticed a greater increase in ABA and JA accumulation in the leaves of plants grown under L-R/FR conditions than in plants grown under H-R/FR conditions after a 4°C treatment for 12 h (Fig. 5, C and D). However, the growth light conditions before cold treatment did not alter the cold tolerance, as pretreatment with L-R/FR and H-R/FR before cold treatment did not significantly alter the changes in Fv/Fm, reactive oxygen species accumulation (superoxide and hydrogen peroxide), ABA and JA accumulation, and transcripts of genes involved in ABA biosynthesis and signaling (NCED6, ABF4, AREB, AIM1, and RD22-like), JA biosynthesis and signaling (LOXD, LOXF, AOS2, AOC, and COI1), and the CBF pathway (CBF1, COR413-like, SIZ1-like, ICE1, and COR47-like; Fig. 5; Supplemental Fig. S7).
Figure 5.
Cold tolerance is regulated by the R/FR ratio during the cold treatment but not by pretreatment. A, Images of the Fv/Fm and the accumulation of hydrogen peroxide (3,3′-diaminobenzidine [DAB] staining) and superoxide (nitroblue tetrazolium [NBT] staining) in tomato leaves. Plants were grown under white light (W), H-R/FR (H), or L-R/FR (L) at 25°C for 4 d as pretreatments before cold stress and then exposed to H-R/FR or L-R/FR at 25°C or 4°C for 7 d. The false color code depicted at the bottom of the image ranges from 0 (black) to 1 (purple). B, Relative electrolyte leakage and transcript levels of genes involved in the CBF pathway in tomato leaves at different R/FR ratio conditions after 25°C or 4°C treatment for 7 d and 6 h, respectively. C and D, ABA (C) and JA (D) production at 12 h and transcript levels of ABA (C) and JA (D) biosynthesis- and signaling-related genes at 6 h after 25°C or 4°C treatment under different R/FR regimes, respectively. The R/FR ratios for the H-R/FR, L-R/FR, and white light treatments were 72:28, 33:67, and 67:33, respectively. The arrows indicate transfer between growth light conditions. Data are presented as means of four biological replicates ± sd except for Fv/Fm, which was the mean for 15 leaves from independent plants. Different letters indicate significant differences (P < 0.05) according to Tukey’s test. FW, Fresh weight.
ABA and JA Are Essential for R- and FR-Regulated Cold Tolerance
Since FR and L-R/FR conditions induced the transcription of genes involved in ABA and JA biosynthesis and the accumulation of ABA and JA, we next investigated whether the increased accumulation of ABA and JA was essential for the FR- and L-R/FR-regulated cold tolerance. The tomato notabilis (not) mutant, which is deficient in ABA (Burbidge et al., 1999), and the suppressor of prosystemin-mediated responses2 (spr2) mutant, which is deficient in JA (Li et al., 2003), both showed increased cold sensitivity, as indicated by decreased Fv/Fm values, survival rates, and Pn values as well as increased relative electrolyte leakage from the leaf cells, following exposure to cold conditions for 7 d (Fig. 6; Supplemental Fig. S8A). Although L-R/FR increased cold tolerance in wild-type plants, it failed to reverse the effects of cold stress in the not and spr2 mutants. Finally, we assessed cold tolerance after foliar application of exogenous ABA and methyl jasmonate (MeJA). From dose trials using a range of ABA (0–250 μm) and MeJA (0–200 μm) concentrations, we observed that 50 μm was the most effective concentration for both compounds that enhanced cold tolerance in the wild type, as evidenced by higher Fv/Fm and Pn and a lower relative electrolyte leakage after exposure to 4°C for 3 d (Supplemental Fig. S8, B and C). These results indicate that ABA and JA both play essential roles in the L-R/FR-mediated cold tolerance.
Figure 6.
Cold tolerance as influenced by ABA and JA biosynthesis under L-R/FR and H-R/FR conditions. A, Images of the Fv/Fm in the ABA-deficient mutant not and the JA-deficient mutant spr2 after exposure to 4°C under different R/FR ratios for 7 d. The false color code depicted at the bottom of the image ranges from 0 (black) to 1 (purple). B and C, Relative electrolyte leakage at 7 d after treatment. JA (B) and ABA (C) levels at 12 h after treatment and transcript levels of CBF-related genes and JA (B) and ABA (C) biosynthesis- and signaling-related genes at 6 h after chilling at 4°C at different R/FR ratios in wild-type (WT), spr2, and not plants. The R/FRs ratios for the H-R/FR and L-R/FR treatments were 72:28 and 33:67, respectively. Data are presented as means of four biological replicates ± sd except for Fv/Fm, which was the mean for 15 leaves from independent plants. Different letters indicate significant differences (P < 0.05) according to Tukey’s test. FW, Fresh weight.
ABA Is Essential for the Induction of JA Signaling in Response to Cold Stress under L-R/FR Growth Conditions
Transcript analysis revealed that the expression of JA biosynthesis- and signal transduction-related genes such as LOXD, COI1, AOS2, and AOC was down-regulated in the not plants (Fig. 6B; Supplemental Fig. S9A). Furthermore, transcripts of these genes and the accumulation of JA were not changed with the differences in the R/FR ratio in the not plants subjected to the cold stress. In contrast, transcript levels of NCED6, ABF4, AREB, and AIM1 genes remained unaltered in spr2 plants compared with wild-type plants, while the transcript abundance of these ABA-related genes and the concentration of ABA were both significantly higher in both spr2 and wild-type plants grown under L-R/FR conditions (Fig. 6C; Supplemental Fig. S9B). We also found that both not and spr2 plants had lower transcript levels of CBF pathway genes (CBF1, COR413-like, SIZ1-like, ICE1, and COR47-like) than wild-type plants following the cold treatment, and expression was not induced by the L-R/FR conditions (Fig. 6, B and C; Supplemental Fig. S9).
To establish whether the action of ABA and JA in the cold response occurs in a linear sequence, we analyzed the changes in cold tolerance after foliar application of exogenous ABA and MeJA in the spr2 and not mutants. We observed that 50 μm MeJA significantly improved cold tolerance, with increased Fv/Fm values and CBF1 transcript levels as well as decreased relative electrolyte leakage in the not and wild-type plants (Fig. 7, A and C). However, application of 50 μm ABA did not induce cold tolerance in the spr2 plants (Fig. 7, B and D). Consistent with this, ABA application induced an increase in JA accumulation and transcript levels of the genes associated with JA biosynthesis in the wild-type plants, while MeJA application did not up-regulate ABA accumulation and the transcription of genes in the ABA signaling pathway (Fig. 7, E and F). This set of data thus suggests that ABA functions upstream of JA in the cold response in tomato plants.
Figure 7.
ABA acts upstream of JA in the cold response. A and B, Images of the Fv/Fm in the wild type (WT) and the ABA-deficient mutant not (A) or the JA-deficient mutant spr2 (B) as influenced by foliar application of MeJA or ABA. The false color code depicted at the bottom of the image ranges from 0 (black) to 1 (purple). C and D, Relative electrolyte leakage and transcript levels of CBF1 in wild-type and not (C) or spr2 (D) mutant plants as influenced by foliar application of MeJA or ABA. E and F, ABA (E) and JA (F) levels, ABA biosynthesis gene (NCED6; E), and JA biosynthesis gene (LOXD; F) relative expression following foliar application of MeJA or ABA in wild-type plants. Fifty micromolar MeJA or ABA was applied 12 h prior to exposure to cold conditions at 4°C. Samples for the determination of relative electrolyte leakage, gene transcript levels, and phytohormone contents were collected 3 d, 6 h, and 12 h after chilling at 4°C. Data are presented as means of four biological replicates ± sd except for Fv/Fm, which was the mean for 15 leaves from independent plants. Different letters indicate significant differences (P < 0.05) according to Tukey’s test. FW, Fresh weight.
DISCUSSION
Light quality is known to modulate plant responses to biotic stresses. For example, plants grown in L-R/FR environments in dense canopies are more susceptible to pests, in particular herbivores, than those that grow in H-R/FR (Griebel and Zeier, 2008; Moreno et al., 2009; Cerrudo et al., 2012). In this study, we demonstrate that light quality also influences responses to abiotic stress and, specifically, that FR and L-R/FR have a positive effect on cold tolerance, which contrasts with its negative effect on biotic stress resistance (Figs. 1 and 5; Supplemental Fig. S7). The CBF pathway is well documented as being a key factor in cold or freezing tolerance in Arabidopsis (Arabidopsis thaliana) and other plants (Hsieh et al., 2002; Kasuga et al., 2004; Oh et al., 2005), and we established in this study that FR and R up-regulated and down-regulated, respectively, the transcription of CBF1 (Fig. 1A). Moreover, silencing of CBF1 or cosilencing of CBF1/2/3 expression compromised this L-R/FR-induced tolerance to cold (Fig. 2; Supplemental Fig. S2), suggesting an antagonistic effect of R and FR in the regulation of cold tolerance via the CBF pathway. It is worth noting that R/FR conditions before the cold treatment did not alter the tolerance, transcript levels of genes involved in the CBF pathway, and ABA and JA biosynthesis and signaling, suggesting an essentiality for the integration of light signaling and cold stimuli (Fig. 5; Supplemental Fig. S7).
Phytochromes perceive light signals from the environment and play important roles in stress responses, and studies have shown that ectopic expression of an oat (Avena spp.) phyA gene in hybrid aspen (Populus tremula × Populus tremuloides; clone T89) can prevent cold acclimation (Olsen et al., 1997), whereas phyB and phyD have been reported to negatively regulate cold tolerance by repressing the expression of CBF and its downstream gene COR (Franklin and Whitelam, 2007). However, phyB is considered as an important photoreceptor linking light signaling to the cold stress signaling pathway (Kim et al., 2002). Here, we observed that a lack of phyA resulted in reductions in the cold tolerance and transcript levels of CBF pathway genes under FR conditions, while a lack of phyB1, phyB2, or phyB1B2 improved the cold tolerance and increased the transcript levels of CBF pathway genes under R conditions (Fig. 3). Furthermore, decreasing the R/FR ratio in the growth environment led to increased cold tolerance and transcript levels of CBF pathway genes in wild-type and phyB1B2 plants but had negligible effects in phyA plants (Fig. 4, A–C; Supplemental Figs. S3 and S4). It is apparent that phyA and phyB positively and negatively regulate the CBF pathway and the response to cold by perceiving FR and R, respectively. This conclusion is also supported by the R dependency for the role of FR in cold tolerance, as monochromatic FR conditions were more effective for the induction of cold tolerance over R and white light conditions (Figs. 1, 3, and 4). Our results also revealed that phyA and phyB function antagonistically in the regulation of cold tolerance, with the role of phyA being more significant, as indicated by the consistent lower tolerance for the phyA plants under different R/FR ratios (Fig. 4, A–C).
ABA and JA are known to play important roles in responses to various environmental stresses (Lee and Luan, 2012; Santino et al., 2013). In our study, increased transcript levels of genes involved in ABA and JA biosynthesis and their signaling, as well as the accumulation of these hormones, were induced by FR and L-R/FR in wild-type, phyB1, phyB2, and phyB1B2 plants but not in phyA plants (Figs. 3, C and D, and 4, B and C; Supplemental Figs. S3B and S4). In contrast, phyB1, phyB2, and phyB1B2 plants exhibited increased transcript levels of these genes and the accumulation of ABA and JA over wild-type and phyA plants under R conditions (Figs. 3, C and D, and 4, B and C). These results suggest that ABA and JA are involved in phyA- and phyB-mediated cold tolerance that is promoted by FR and suppressed by R conditions, respectively. This is consistent with earlier observations that phytochromes are involved in the regulation of phytohormones in germinating seeds, young seedlings, and shade responses (Sawada et al., 2008; Dubois et al., 2010; Robson et al., 2010; Chen et al., 2014; Hsieh and Okamoto, 2014). Although it is well known that ABA activates a cascade of downstream signaling events in response to cold exposure (Knight et al., 2004), the role of JA in abiotic stress responses remains elusive. Like ABA application, exogenous JA alleviated the negative effects of cold stress, likely via an up-regulation of the CBF pathway (CBF1; Fig. 7; Supplemental Fig. S8). Recently, Hu et al. (2013) demonstrated that JA functions as an upstream signal in the ICE-CBF/DREB1 pathway and promotes freezing tolerance in Arabidopsis. Light-induced JA action may involve phytochrome-mediated changes in JASMONATE ZIM-DOMAIN (JAZ) gene expression or JAZ protein stability (Ballaré, 2014). Here, we also found that cold induced increases in ABA and JA levels and the expression of their biosynthesis- and signaling-related genes as well as an activation of the CBF pathway (Fig. 5, C and D; Supplemental Fig. S7B). ABA- or JA-deficient plants showed decreased expression of CBF pathway-related genes and were more sensitive to cold than wild-type plants (Figs. 6 and 7; Supplemental Figs. S8A and S9), further suggesting that the activation of CBF gene expression is ABA and JA dependent.
JA and ABA function synergistically or antagonistically to regulate protective responses in plants to biotic and abiotic stresses (Melan et al., 1993; Peña-Cortés et al., 1996; Moons et al., 1997; Anderson et al., 2004; Adie et al., 2007). An antagonistic interaction has been described between the ABA and JA signaling pathways that modulates the expression of defense and disease resistance genes in Arabidopsis (Moons et al., 1997; Anderson et al., 2004). However, in another study using tomato and potato (Solanum tuberosum) plants, it was shown that JA could act downstream of ABA in inducing the expression of PROTEINASE INHIBITOR II in response to wounding (Peña-Cortés et al., 1996), while the expression of LOX1, an important gene in the JA biosynthesis pathway, was induced by exogenous application of either ABA and MeJA (Melan et al., 1993). Additionally, ABA was reported to be required for JA biosynthesis in wounding responses to Pythium irregulare in Arabidopsis (Adie et al., 2007). Consistent with the findings of these studies, we found that ABA was required for the induction of JA signaling in response to cold under different R/FR ratio growth regimes. This conclusion is based on the following evidence: (1) ABA-deficient not plants had significantly reduced JA accumulation compared with wild-type plants, and the L-R/FR-induced increase in JA accumulation was compromised in not plants, whereas L-R/FR could still induce ABA accumulation in the spr2 mutant (Fig. 6, B and C); (2) the not mutant had decreased transcript levels of genes involved in JA biosynthesis and signaling, while L-R/FR-induced changes in the transcript levels of genes involved in ABA biosynthesis and signaling were not compromised in JA-deficient spr2 plants (Fig. 6, B and C; Supplemental Fig. S9); and (3) foliar application of MeJA partially compensated the deficiency of ABA accumulation in not plants in response to cold, while foliar application of ABA failed to rescue the changes in CBF1 transcript and cold tolerance in JA-deficient spr2 plants (Fig. 7).
To date, the mechanisms underlying light quality-induced cold tolerance are largely unknown; however, we have shown here that FR and R perceived by phyA and phyB have positive and negative effects on cold tolerance, respectively, where phyA plays a predominant role in tomato. This effect was mediated, at least in part, by synergistic interactions of ABA and JA. In response to cold stress, the activation of phyA by FR increased ABA and JA accumulation, thus promoting CBF gene expression and subsequent cold tolerance. This study elucidates a mechanism by which phyA and phyB antagonistically regulate ABA and JA signaling and CBF-mediated cold tolerance. As, under field conditions, the decrease in temperature is associated with longer twilight periods during autumn months at some northern areas, which is characterized by the reduction in R/FR, this study unveils novel mechanisms that emphasize the biological significance of light quality in nature. Meanwhile, the above part of a vegetation canopy usually shows increased sensitivity to cold. It is highly plausible that plants use phytochromes such as phyA and phyB to integrate these environmental stimuli in response to cold stress.
MATERIALS AND METHODS
Plant Material and VIGS
Seeds of wild-type tomato (Solanum lycopersicum ‘Ailsa Craig’) and the ABA biosynthesis mutant not in the cv Ailsa Craig background, the wild-type cv Moneymaker and the phyA, phyB1, phyB2, phyB1B2, and phyAB1B2 mutants in the cv Moneymaker background, as well as the wild-type cv Castlemart were obtained from the Tomato Genetics Resource Center (http://tgrc.ucdavis.edu). The JA biosythesis mutant spr2 in the cv Castlemart background was obtained from Chuanyou Li (Chinese Academy of Sciences). Seedlings were grown in pots with a mixture of three parts peat to one part vermiculite, receiving Hoagland nutrient solution. The growth conditions were as follows: 12-h photoperiod, temperature of 25°C/20°C (day/night), and photosynthetic photon flux density of 600 µmol m−2 s−1.
The Tobacco rattle virus (TRV)-based vectors (pTRV1/2) were used for the VIGS of tomato genes (Liu et al., 2002). The CBF1 and CBF1/2/3 complementary DNA fragments were PCR amplified using the gene-specific primers listed in Supplemental Table S1. The amplified CBF1 and CBF1/2/3 fragments were digested with EcoRI/XbaI and BamHI/XbaI, respectively, and ligated into the corresponding sites of the pTRV2 vector. Empty pTRV2 vector was used as a control. All constructs were confirmed by sequencing and subsequently transformed into Agrobacterium tumefaciens strain GV3101. VIGS was performed by infiltration into the fully expanded cotyledons of 15-d-old tomato seedlings with A. tumefaciens harboring a mixture of pTRV1 and pTRV2-target gene in a 1:1 ratio. Plants were grown at 21°C in a growth chamber with a 12-h daylength for 30 d until control pTRV-PDS plants (silencing of the gene encoding phytoene desaturase) showed strong bleaching (Ekengren et al., 2003). Quantitative reverse transcription (qRT)-PCR was performed to determine the gene-silencing efficiency (Livak and Schmittgen, 2001).
Cold and Light Treatments
Plants at the four-leaf stage were used for all experiments, which were carried out in controlled environment growth chambers (ConvironE15; Conviron). Plants were grown under dark or monochromatic R, FR, and blue light (40 µmol m−2 s−1), different intensities of R and FR (20, 40, and 60 µmol m−2 s−1), or different R/FR ratios (100:0, 72:28, 33:67, 20:80, and 0:100) with an aerial temperature of 25°C, or 4°C for the cold treatment. For the different R/FR ratio treatments, plants were kept at a 12/12-h light cycle under 120 µmol m−2 s−1 and 85% humidity unless indicated otherwise. R light-emitting diode (LED) lamps (λmax = 660 nm; Philips), FR LED lamps (λmax = 735 nm; Philips), and blue LED lamps (λmax = 450 nm; Philips) were used for the different light quality treatments. The R/FR ratio was calculated as the quantum flux density from 655 to 665 nm divided by the quantum flux density from 730 to 740 nm. The cold treatment lasted 7 d for all experiments, unless stated otherwise.
ABA and JA Treatment
To determine the effective concentration of ABA and JA in cold tolerance, wild-type plants were pretreated with 0, 10, 50, 100, and 250 μm ABA or 0, 25, 50, 100, and 200 μm MeJA at 12 h prior to cold treatment at 4°C for 3 d. To unveil the relation between ABA and JA in cold tolerance, 50 μm MeJA or ABA was applied onto not plants and spr2 plants, respectively, 12 h prior to exposure to cold conditions at 4°C for 3 d. The plant hormones ABA (Sigma-Aldrich) and MeJA (Sigma-Aldrich) solutions were prepared by dissolving the solutes in ethanol followed by dilution with distilled water (ethanol:water [v/v] = 1:10,000), respectively. Twenty milliliters of solution was applied onto each plant. The control plants received the same amount of water, which contained the same amount of ethanol.
Cold Tolerance Assays
The percentage of plants that were viable 6 d after recovery at the optimum growth conditions was recorded. The Fv/Fm was measured using an Imaging-PAM Chlorophyll Fluorometer equipped with a computer-operated PAM-control unit (IMAG-MAXI; Heinz Walz), as described previously (Zhou et al., 2012). The Pn was determined with an infrared gas analyzer-based portable photosynthesis system (LI-6400; LI-COR). The air temperature, relative humidity, CO2 concentration, and photosynthetic photon flux density were maintained at 25°C, 85%, 380 μmol mol−1, and 600 μmol m−2 s−1, respectively. Relative electrolyte leakage was determined as described previously (Cao et al., 2007). The accumulation of superoxide and hydrogen peroxide in the leaves was detected using nitroblue tetrazolium and 3,3′-diaminobenzidine staining (Xia et al., 2009). Levels of oxidized proteins in the leaves were assayed by immunoblot detection using an OxyBlot protein oxidation detection kit (Chemicon International), according to the manufacturer’s instructions.
Measurement of ABA and JA Levels
Phytohormone extraction and analysis from tomato leaves were performed using previously reported procedures with minor modifications (Durgbanshi et al., 2005; Wu et al., 2007; Alba et al., 2015). Briefly, 100 mg of frozen leaf material was homogenized in 1 mL of ethyl acetate, which had been spiked with D6-ABA and D5-JA (OlChemIm) as internal standards to a final concentration of 100 ng mL−1. The homogenate was shaken for 12 h in the dark at 4°C before centrifugation at 18,000g for 10 min at 4°C. The supernatant (the ethyl acetate phase) was collected, and the pellet was reextracted with 1 mL of ethyl acetate and centrifuged for 10 min at 4°C at 18,000g. The supernatants were combined and evaporated to dryness under N2 gas. The residue was resuspended in 0.5 mL of 70% (v/v) methanol and centrifuged at 18,000g for 2 min at 4°C, and the supernatants were analyzed by HPLC-mass spectrometry on an Agilent 1290 infinity HPLC system (including a vacuum degasser, a binary pump, a column oven, and an autosampler) coupled to a Agilent 6460 Triple Quad liquid chromatography-mass spectrometry device (Agilent Technologies). HPLC analysis was performed using an Agilent Zorbax XDB C18 column (150 mm × 2.1 mm, 3.5 μm). The mobile phase consisted of a mixture of solvent A (0.1% formic acid in water; E. Merck) and solvent B (methanol; E. Merck) at a flow rate of 0.3 mL min−1 with the following gradient: 0 to 1.5 min, A:B at 60:40; followed by 6.5 min of solvent A:B at 0:100; subsequently returning to solvent A:B at 60:40 for 5 min until the end of the run. The column temperature was kept at 40°C, and the injection volume was 20 μL. A negative electrospray ionization mode was used for detection. The parent ions, daughter ions, and collision energies used in these analyses are listed in Supplemental Table S2. Phytohormone accumulation was expressed as nanograms per gram of fresh mass leaf material.
qRT-PCR Analysis
Total RNA was extracted from tomato leaves using the RNAprep Pure Plant Kit (Tiangen Biotech) according to the manufacturer’s instructions. Residual DNA was removed with the RNase Mini Kit (Qiagen). One microgram of total RNA was reverse transcribed using a ReverTra Ace qPCR RT Kit (Toyobo), following the manufacturer’s recommendations. The gene-specific primer pairs, as shown in Supplemental Table S3, were designed on the basis of EST sequences and used for amplification. qRT-PCR was performed using a Roche LightCycler 480 real-time PCR machine. The PCR was run at 95°C for 3 min, followed by 40 cycles of 30 s at 95°C, 30 s at 58°C, and 1 min at 72°C. The tomato ACTIN gene was used as an internal control. The relative gene expression was calculated as described previously (Livak and Schmittgen, 2001).
Statistical Analysis
The experimental design was a completely randomized block design with four replicates. Each replicate contained 16 plants. Experimental data were analyzed using INFOSTAT software (professional version 1.1) by means of factorial ANOVA. When interaction terms were significant (P < 0.05), differences between means were analyzed using Tukey comparisons. Significant differences between treatment means are indicated by different letters.
Sequence data from this article can be found in the GenBank/EMBL data libraries under the accession numbers listed in Supplemental Table S3.
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Cold tolerance for plants grown in the dark, R, and blue light conditions.
Supplemental Figure S2. Cold tolerance in CBF1 and CBF1/2/3 gene-silenced tomato plants under L-R/FR (33:67) and H-R/FR (72:28) conditions.
Supplemental Figure S3. The role of phytochromes in L-R/FR-induced (R/FR ratio, 33:67) cold tolerance.
Supplemental Figure S4. The transcript levels of CBF, ABA, and JA pathway-related genes in phyA and phyB1B2 plants at 6 h after cold treatment at 4°C under H-R/FR or L-R/FR conditions.
Supplemental Figure S5. Cold tolerance for plants under white light and R conditions supplemented with different intensities of FR.
Supplemental Figure S6. Changes in the transcript levels for genes involved in CBF, ABA, and JA pathways for plants under white light supplemented with different intensities of FR.
Supplemental Figure S7. Cold tolerance and associated changes in the transcript levels for genes involved in CBF, ABA, and JA pathways for plants under different R/FR ratios before and during the cold stress.
Supplemental Figure S8. Effects of ABA and JA on cold tolerance in tomato plants.
Supplemental Figure S9. Expression of genes involved in the CBF, ABA, and JA pathways in response to H-R/FR or L-R/FR in the not and spr2 mutants under cold stress.
Supplemental Table S1. PCR primer sequences used for vector construction.
Supplemental Table S2. Parameters used for the detection of phytohormones and related compounds by liquid chromatography-tandem mass spectrometry.
Supplemental Table S3. List of primer sequences used for qRT-PCR analysis.
Supplementary Material
Acknowledgments
We thank the Tomato Genetics Resource Center at the University of California and Chuanyou Li (Chinese Academy of Sciences) for tomato seeds, Xiaodan Wu (Analysis Center of Agrobiology and Environmental Sciences, Institute of Agrobiology and Environmental Sciences, Zhejiang University) for assistance with phytohormone analysis, and Jocelyn Rose (Cornell University) for carefully reading and editing the article.
Glossary
- R
red light
- FR
far-red light
- ABA
abscisic acid
- JA
jasmonic acid
- Pn
CO2 assimilation rate
- VIGS
virus-induced gene silencing
- H-R/FR
high red light/far-red light ratio
- L-R/FR
low red light/far-red light ratio
- Fv/Fm
maximum photochemical efficiency of PSII
- MeJA
methyl jasmonate
- qRT
quantitative reverse transcription
- LED
light-emitting diode
Footnotes
This work was supported by the National Natural Science Foundation of China (grant nos. 31372109 and 31430076), the Special Fund for Agro-scientific Research in the Public Interest (grant no. 201203004), the National High Technology R&D Program of China (grant no. 2013AA102406), the Fok Ying-Tong Education Foundation (grant no. 132024), and the BrainBridge Project Plan for Joint Research at Zhejiang University, Eindhoven University of Technology, and Philips Research.
Articles can be viewed without a subscription.
References
- Adie BA, Pérez-Pérez J, Pérez-Pérez MM, Godoy M, Sánchez-Serrano JJ, Schmelz EA, Solano R (2007) ABA is an essential signal for plant resistance to pathogens affecting JA biosynthesis and the activation of defenses in Arabidopsis. Plant Cell 19: 1665–1681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alba JM, Schimmel BC, Glas JJ, Ataide LM, Pappas ML, Villarroel CA, Schuurink RC, Sabelis MW, Kant MR (2015) Spider mites suppress tomato defenses downstream of jasmonate and salicylate independently of hormonal crosstalk. New Phytol 205: 828–840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JP, Badruzsaufari E, Schenk PM, Manners JM, Desmond OJ, Ehlert C, Maclean DJ, Ebert PR, Kazan K (2004) Antagonistic interaction between abscisic acid and jasmonate-ethylene signaling pathways modulates defense gene expression and disease resistance in Arabidopsis. Plant Cell 16: 3460–3479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballaré CL. (2014) Light regulation of plant defense. Annu Rev Plant Biol 65: 335–363 [DOI] [PubMed] [Google Scholar]
- Ballaré CL, Scopel AL, Sánchez RA (1990) Far-red radiation reflected from adjacent leaves: an early signal of competition in plant canopies. Science 247: 329–332 [DOI] [PubMed] [Google Scholar]
- Burbidge A, Grieve TM, Jackson A, Thompson A, McCarty DR, Taylor IB (1999) Characterization of the ABA-deficient tomato mutant notabilis and its relationship with maize Vp14. Plant J 17: 427–431 [DOI] [PubMed] [Google Scholar]
- Cagnola JI, Ploschuk E, Benech-Arnold T, Finlayson SA, Casal JJ (2012) Stem transcriptome reveals mechanisms to reduce the energetic cost of shade-avoidance responses in tomato. Plant Physiol 160: 1110–1119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao WH, Liu J, He XJ, Mu RL, Zhou HL, Chen SY, Zhang JS (2007) Modulation of ethylene responses affects plant salt-stress responses. Plant Physiol 143: 707–719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerrudo I, Keller MM, Cargnel MD, Demkura PV, de Wit M, Patitucci MS, Pierik R, Pieterse CM, Ballaré CL (2012) Low red/far-red ratios reduce Arabidopsis resistance to Botrytis cinerea and jasmonate responses via a COI1-JAZ10-dependent, salicylic acid-independent mechanism. Plant Physiol 158: 2042–2052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers PA, Spence DH (1984) Diurnal changes in the ratio of underwater red to far red light in relation to aquatic plant photoperiodism. J Ecol 72: 495–503 [Google Scholar]
- Chen F, Li B, Li G, Charron JB, Dai M, Shi X, Deng XW (2014) Arabidopsis phytochrome A directly targets numerous promoters for individualized modulation of genes in a wide range of pathways. Plant Cell 26: 1949–1966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chico JM, Fernández-Barbero G, Chini A, Fernández-Calvo P, Díez-Díaz M, Solano R (2014) Repression of jasmonate-dependent defenses by shade involves differential regulation of protein stability of MYC transcription factors and their JAZ repressors in Arabidopsis. Plant Cell 26: 1967–1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit M, Spoel SH, Sanchez-Perez GF, Gommers CMM, Pieterse CMJ, Voesenek LACJ, Pierik R (2013) Perception of low red:far-red ratio compromises both salicylic acid- and jasmonic acid-dependent pathogen defences in Arabidopsis. Plant J 75: 90–103 [DOI] [PubMed] [Google Scholar]
- Dubois PG, Olsefski GT, Flint-Garcia S, Setter TL, Hoekenga OA, Brutnell TP (2010) Physiological and genetic characterization of end-of-day far-red light response in maize seedlings. Plant Physiol 154: 173–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durgbanshi A, Arbona V, Pozo O, Miersch O, Sancho JV, Gómez-Cadenas A (2005) Simultaneous determination of multiple phytohormones in plant extracts by liquid chromatography-electrospray tandem mass spectrometry. J Agric Food Chem 53: 8437–8442 [DOI] [PubMed] [Google Scholar]
- Ekengren SK, Liu Y, Schiff M, Dinesh-Kumar SP, Martin GB (2003) Two MAPK cascades, NPR1, and TGA transcription factors play a role in Pto-mediated disease resistance in tomato. Plant J 36: 905–917 [DOI] [PubMed] [Google Scholar]
- Finlayson SA, Krishnareddy SR, Kebrom TH, Casal JJ (2010) Phytochrome regulation of branching in Arabidopsis. Plant Physiol 152: 1914–1927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler SG, Cook D, Thomashow MF (2005) Low temperature induction of Arabidopsis CBF1, 2, and 3 is gated by the circadian clock. Plant Physiol 137: 961–968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin KA. (2009) Light and temperature signal crosstalk in plant development. Curr Opin Plant Biol 12: 63–68 [DOI] [PubMed] [Google Scholar]
- Franklin KA, Quail PH (2010) Phytochrome functions in Arabidopsis development. J Exp Bot 61: 11–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin KA, Toledo-Ortiz G, Pyott DE, Halliday KJ (2014) Interaction of light and temperature signalling. J Exp Bot 65: 2859–2871 [DOI] [PubMed] [Google Scholar]
- Franklin KA, Whitelam GC (2004) Light signals, phytochromes and cross-talk with other environmental cues. J Exp Bot 55: 271–276 [DOI] [PubMed] [Google Scholar]
- Franklin KA, Whitelam GC (2007) Light-quality regulation of freezing tolerance in Arabidopsis thaliana. Nat Genet 39: 1410–1413 [DOI] [PubMed] [Google Scholar]
- Gilmour SJ, Thomashow MF (1991) Cold acclimation and cold-regulated gene expression in ABA mutants of Arabidopsis thaliana. Plant Mol Biol 17: 1233–1240 [DOI] [PubMed] [Google Scholar]
- Griebel T, Zeier J (2008) Light regulation and daytime dependency of inducible plant defenses in Arabidopsis: phytochrome signaling controls systemic acquired resistance rather than local defense. Plant Physiol 147: 790–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gururani MA, Venkatesh J, Ganesan M, Strasser RJ, Han Y, Kim JI, Lee HY, Song PS (2015) In vivo assessment of cold tolerance through chlorophyll-a fluorescence in transgenic zoysiagrass expressing mutant phytochrome A. PLoS ONE 10: e0127200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusta LV, Trischuk R, Weiser CJ (2005) Plant cold acclimation: the role of abscisic acid. J Plant Growth Regul 24: 308–318 [Google Scholar]
- Hsieh HL, Okamoto H (2014) Molecular interaction of jasmonate and phytochrome A signalling. J Exp Bot 65: 2847–2857 [DOI] [PubMed] [Google Scholar]
- Hsieh TH, Lee JT, Yang PT, Chiu LH, Charng YY, Wang YC, Chan MT (2002) Heterology expression of the Arabidopsis C-repeat/dehydration response element binding factor 1 gene confers elevated tolerance to chilling and oxidative stresses in transgenic tomato. Plant Physiol 129: 1086–1094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Jiang L, Wang F, Yu D (2013) Jasmonate regulates the inducer of cbf expression-C-repeat binding factor/DRE binding factor1 cascade and freezing tolerance in Arabidopsis. Plant Cell 25: 2907–2924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao Y, Lau OS, Deng XW (2007) Light-regulated transcriptional networks in higher plants. Nat Rev Genet 8: 217–230 [DOI] [PubMed] [Google Scholar]
- Kasuga M, Miura S, Shinozaki K, Yamaguchi-Shinozaki K (2004) A combination of the Arabidopsis DREB1A gene and stress-inducible rd29A promoter improved drought- and low-temperature stress tolerance in tobacco by gene transfer. Plant Cell Physiol 45: 346–350 [DOI] [PubMed] [Google Scholar]
- Kebrom TH, Brutnell TP, Finlayson SA (2010) Suppression of sorghum axillary bud outgrowth by shade, phyB and defoliation signalling pathways. Plant Cell Environ 33: 48–58 [DOI] [PubMed] [Google Scholar]
- Kebrom TH, Burson BL, Finlayson SA (2006) Phytochrome B represses Teosinte Branched1 expression and induces sorghum axillary bud outgrowth in response to light signals. Plant Physiol 140: 1109–1117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kegge W, Weldegergis BT, Soler R, Vergeer-Van Eijk M, Dicke M, Voesenek LACJ, Pierik R (2013) Canopy light cues affect emission of constitutive and methyl jasmonate-induced volatile organic compounds in Arabidopsis thaliana. New Phytol 200: 861–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, Kim YK, Park JY, Kim J (2002) Light signalling mediated by phytochrome plays an important role in cold-induced gene expression through the C-repeat/dehydration responsive element (C/DRE) in Arabidopsis thaliana. Plant J 29: 693–704 [DOI] [PubMed] [Google Scholar]
- Knight H, Zarka DG, Okamoto H, Thomashow MF, Knight MR (2004) Abscisic acid induces CBF gene transcription and subsequent induction of cold-regulated genes via the CRT promoter element. Plant Physiol 135: 1710–1717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau OS, Deng XW (2010) Plant hormone signaling lightens up: integrators of light and hormones. Curr Opin Plant Biol 13: 571–577 [DOI] [PubMed] [Google Scholar]
- Lee CM, Thomashow MF (2012) Photoperiodic regulation of the C-repeat binding factor (CBF) cold acclimation pathway and freezing tolerance in Arabidopsis thaliana. Proc Natl Acad Sci USA 109: 15054–15059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SC, Luan S (2012) ABA signal transduction at the crossroad of biotic and abiotic stress responses. Plant Cell Environ 35: 53–60 [DOI] [PubMed] [Google Scholar]
- Li C, Liu G, Xu C, Lee GI, Bauer P, Ling HQ, Ganal MW, Howe GA (2003) The tomato suppressor of prosystemin-mediated responses2 gene encodes a fatty acid desaturase required for the biosynthesis of jasmonic acid and the production of a systemic wound signal for defense gene expression. Plant Cell 15: 1646–1661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Schiff M, Dinesh-Kumar SP (2002) Virus-induced gene silencing in tomato. Plant J 31: 777–786 [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔΔCT method. Methods 25: 402–408 [DOI] [PubMed] [Google Scholar]
- Mantyla E, Lang V, Palva ET (1995) Role of abscisic acid in drought-induced freezing tolerance, cold acclimation, and accumulation of LT178 and RAB18 proteins in Arabidopsis thaliana. Plant Physiol 107: 141–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews S. (2006) Phytochrome-mediated development in land plants: red light sensing evolves to meet the challenges of changing light environments. Mol Ecol 15: 3483–3503 [DOI] [PubMed] [Google Scholar]
- Melan MA, Dong X, Endara ME, Davis KR, Ausubel FM, Peterman TK (1993) An Arabidopsis thaliana lipoxygenase gene can be induced by pathogens, abscisic acid, and methyl jasmonate. Plant Physiol 101: 441–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura K, Jin JB, Lee J, Yoo CY, Stirm V, Miura T, Ashworth EN, Bressan RA, Yun DJ, Hasegawa PM (2007) SIZ1-mediated sumoylation of ICE1 controls CBF3/DREB1A expression and freezing tolerance in Arabidopsis. Plant Cell 19: 1403–1414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Möglich A, Yang X, Ayers RA, Moffat K (2010) Structure and function of plant photoreceptors. Annu Rev Plant Biol 61: 21–47 [DOI] [PubMed] [Google Scholar]
- Moons A, Prinsen E, Bauw G, Van Montagu M (1997) Antagonistic effects of abscisic acid and jasmonates on salt stress-inducible transcripts in rice roots. Plant Cell 9: 2243–2259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno JE, Tao Y, Chory J, Ballaré CL (2009) Ecological modulation of plant defense via phytochrome control of jasmonate sensitivity. Proc Natl Acad Sci USA 106: 4935–4940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neff MM, Street IH, Turk EM, Ward JM (2006) Interaction of light and hormone signalling to mediate photomorphogenesis. In Schafer E, Nagy F, eds, Photomorphogenesis in Plants and Bacteria. Springer, Dordrecht, The Netherlands, pp 439–473 [Google Scholar]
- Oh SJ, Song SI, Kim YS, Jang HJ, Kim SY, Kim M, Kim YK, Nahm BH, Kim JK (2005) Arabidopsis CBF3/DREB1A and ABF3 in transgenic rice increased tolerance to abiotic stress without stunting growth. Plant Physiol 138: 341–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen JE, Junttila O, Nilsen J, Eriksson ME, Martinussen I, Olsson O, Sandberg G, Moritz T (1997) Ectopic expression of oat phytochrome A in hybrid aspen changes critical daylength for growth and prevents cold acclimatization. Plant J 12: 1339–1350 [Google Scholar]
- Peña-Cortés H, Prat S, Atzorn R, Wasternack C, Willmitzer L (1996) Abscisic acid-deficient plants do not accumulate proteinase inhibitor II following systemin treatment. Planta 198: 447–451 [Google Scholar]
- Quail PH. (2002a) Phytochrome photosensory signalling networks. Nat Rev Mol Cell Biol 3: 85–93 [DOI] [PubMed] [Google Scholar]
- Quail PH. (2002b) Photosensory perception and signalling in plant cells: new paradigms? Curr Opin Cell Biol 14: 180–188 [DOI] [PubMed] [Google Scholar]
- Reddy SK, Holalu SV, Casal JJ, Finlayson SA (2013) Abscisic acid regulates axillary bud outgrowth responses to the ratio of red to far-red light. Plant Physiol 163: 1047–1058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts MR, Paul ND (2006) Seduced by the dark side: integrating molecular and ecological perspectives on the influence of light on plant defence against pests and pathogens. New Phytol 170: 677–699 [DOI] [PubMed] [Google Scholar]
- Robson F, Okamoto H, Patrick E, Harris SR, Wasternack C, Brearley C, Turner JG (2010) Jasmonate and phytochrome A signaling in Arabidopsis wound and shade responses are integrated through JAZ1 stability. Plant Cell 22: 1143–1160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santino A, Taurino M, De Domenico S, Bonsegna S, Poltronieri P, Pastor V, Flors V (2013) Jasmonate signaling in plant development and defense response to multiple (a)biotic stresses. Plant Cell Rep 32: 1085–1098 [DOI] [PubMed] [Google Scholar]
- Sawada Y, Aoki M, Nakaminami K, Mitsuhashi W, Tatematsu K, Kushiro T, Koshiba T, Kamiya Y, Inoue Y, Nambara E, et al. (2008) Phytochrome- and gibberellin-mediated regulation of abscisic acid metabolism during germination of photoblastic lettuce seeds. Plant Physiol 146: 1386–1396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schepens I, Duek P, Fankhauser C (2004) Phytochrome-mediated light signalling in Arabidopsis. Curr Opin Plant Biol 7: 564–569 [DOI] [PubMed] [Google Scholar]
- Smith H. (1982) Light quality, photoperception, and plant strategy. Annu Rev Plant Physiol 33: 481–518 [Google Scholar]
- Su H, Abernathy SD, White RH, Finlayson SA (2011) Photosynthetic photon flux density and phytochrome B interact to regulate branching in Arabidopsis. Plant Cell Environ 34: 1986–1998 [DOI] [PubMed] [Google Scholar]
- Thomashow MF. (2010) Molecular basis of plant cold acclimation: insights gained from studying the CBF cold response pathway. Plant Physiol 154: 571–577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Hettenhausen C, Meldau S, Baldwin IT (2007) Herbivory rapidly activates MAPK signaling in attacked and unattacked leaf regions but not between leaves of Nicotiana attenuata. Plant Cell 19: 1096–1122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia XJ, Wang YJ, Zhou YH, Tao Y, Mao WH, Shi K, Asami T, Chen Z, Yu JQ (2009) Reactive oxygen species are involved in brassinosteroid-induced stress tolerance in cucumber. Plant Physiol 150: 801–814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Wang J, Shi K, Xia XJ, Zhou YH, Yu JQ (2012) Hydrogen peroxide is involved in the cold acclimation-induced chilling tolerance of tomato plants. Plant Physiol Biochem 60: 141–149 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.