The functional characterization of homologs of a flowering-time transcription factor provides new insights into the control of floral repression before vernalization in barley.
Abstract
In barley (Hordeum vulgare), PHOTOPERIOD1 (Ppd-H1) acts as a major positive regulator of flowering under long-day conditions, while VERNALIZATION2 (VRN-H2) is a strong repressor of flowering under long days before vernalization. By contrast, CONSTANS (CO) plays a key role in the photoperiodic regulation of flowering in Arabidopsis (Arabidopsis thaliana). Here, we study the role of the closest barley CO homologs, HvCO1 and HvCO2, in the long day-dependent control of flowering and their interactions with Ppd-H1 and VRN-H2. HvCO2 overexpression in spring barley, with a natural deletion of the VRN-H2 locus, caused a Ppd-H1-dependent induction of flowering and FLOWERING LOCUS T1 (HvFT1) expression. In winter barley, which carries the VRN-H2 locus, overexpression of HvCO1/CO2 caused an up-regulation of VRN-H2, resulting in a reduced expression of HvFT1 and delayed flowering under long- and short-day conditions. In addition, natural variation at Ppd-H1 altered the expression of VRN-H2 in wild-type plants under long days. VRN-H2, in turn, was involved in the down-regulation of Ppd-H1 and HvCO2, demonstrating strong reciprocal interactions between HvCO2, Ppd-H1, and VRN-H2. Consequently, this study showed that the induction of the floral repressor VRN-H2 and the floral activator HvFT1 was regulated by the same genes, Ppd-H1 and HvCO1/CO2. Our findings provide a novel insight into the photoperiodic regulation of the vernalization pathway in barley.
Flowering is one of the most critical stages in the life cycle of plants. The coincidence of flowering with favorable conditions ensures that seed production is maximized and enhances the chances of successful reproduction. A key adaptive mechanism to achieve this coincidence is sensing changes in daylength or photoperiod (Greenup et al., 2009). Long photoperiods promote flowering in the model and facultative long-day (LD) plant Arabidopsis (Arabidopsis thaliana) through the activity of CONSTANS (CO), a transcription factor that binds to the promotor of FLOWERING LOCUS T (FT), which, in turn, induces the floral transition (Putterill et al., 1995; Tiwari et al., 2010). CO encodes a protein with two zinc finger B-boxes and a CCT (CONSTANS, CONSTANS-like, and TIMING OF CAB EXPRESSION1 [TOC1]) domain (Robson et al., 2001). CO transcription is regulated by the circadian clock and its components in a way that allows the accumulation of CO mRNA at the end of the light period of long days (LDs) but after dusk in short days (SDs; Imaizumi et al., 2005; Fornara et al., 2009). The CO protein is stabilized by photoreceptors in the light and degraded by the ubiquitin ligase CONSTITUTIVE PHOTOMORPHOGENIC1 during the dark, which allows the accumulation of CO at the end of a long day to induce FT transcription (Jang et al., 2008; Turck et al., 2008).
The function of CO in controlling the photoperiod response is conserved in the short-day (SD) cereal monocot rice (Oryza sativa). Under inductive SDs, Heading date1 (Hd1), the rice ortholog of CO, promotes flowering by inducing the expression of Hd3a, the ortholog of FT (Izawa et al., 2002; Kojima et al., 2002). Under LDs, however, Hd1 represses flowering through the down-regulation of Hd3a (Yano et al., 2000, Izawa et al., 2002; Hayama et al., 2003). Consequently, Hd1 is bifunctional in rice, where it promotes heading under SD conditions and inhibits it under LD conditions (Yano et al., 2000). In barley (Hordeum vulgare), HvCO1 and HvCO2 are the closest homologs of Arabidopsis CO and rice Hd1 (Griffiths et al., 2003). Comparison with wheat (Triticum aestivum), Brachypodium (Brachypodium distachyon) and rice suggests that HvCO1 and HvCO2 are paralogs that have arisen in temperate cereals by segmental duplication. HvCO1 is colinear with Hd1, whereas HvCO2 was lost in rice (Higgins et al., 2010). Overexpression of HvCO1 promoted flowering under LD and SD conditions, which suggested that HvCO1 functions as a floral activator in barley (Campoli et al., 2012). However, the role of HvCO2 in flowering time control in barley has not yet been elucidated.
Comparison of CO function across species demonstrates that CO homologs may act as an LD activator of flowering, as seen in Arabidopsis, or an LD repressor of flowering, as observed in rice. Nemoto et al. (2003) reported that wheat CO complemented hd1 and repressed flowering in rice under LDs, suggesting that functional differences of CO in SD and LD plants are not due to structural variation but rather to trans-acting regulatory mechanisms.
In rice, LD repression of flowering is mediated by two additional CCT domain genes: Hd2/PSEUDO-RESPONSE REGULATOR37 (OsPRR37) and Hd4/GRAIN NUMBER, PLANT HEIGHT, AND HEADING DATE7 (Ghd7; Xue et al., 2008; Koo et al., 2013; Gao et al., 2014). OsPRR37 is orthologous to the Arabidopsis circadian clock gene PRR3/7 and is characterized by a pseudoreceiver and a CCT domain. OsPRR37 is expressed under LD and SD conditions but is only functional to repress Hd3a under LDs (Murakami et al., 2003; Koo et al., 2013; Gao et al., 2014). Interestingly, PHOTOPERIOD1 (Ppd-H1), the barley homolog of the LD repressor OsPRR37, is the major photoperiod response gene in barley and induces flowering under LDs by up-regulating HvFT1, the barley homolog of Hd3a (Turner et al., 2005). Barley carries five FT homologs, of which HvFT1 correlates with flowering time under LD conditions, while a natural deletion at HvFT3 has been associated with floral development under SD conditions (Yan et al., 2006; Faure et al., 2007; Kikuchi et al., 2009). FT1 expression and flowering time are controlled by PPD1 independently of CO1/2 expression in barley and wheat (Wilhelm et al., 2009; Campoli et al., 2012; Shaw et al., 2012). Consequently, CO and PRR37 may act independently and function as floral repressors or activators depending on the species and photoperiod. The genetic basis of this dual role of CO and PRR37 as activators and repressors of flowering is not yet understood.
Ghd7 belongs to the CCT motif family subclass of the CCT gene family with only a single CCT domain (Cockram et al., 2012). Ghd7 is up-regulated under LD conditions and represses Hd3a and flowering time. VERNALIZATION2 (VRN-H2), a barley homolog of the LD repressor Ghd7 in rice, is also up-regulated under LDs and represses HvFT1 and flowering in barley (Trevaskis et al., 2006; Hemming et al., 2008). VRN-H2 shows a diurnal pattern of expression and is not expressed under SD conditions. The repression of VRN-H2 under SDs is controlled by components of the circadian clock. Mutations in the barley clock gene EARLY FLOWERING3 (HvELF3) resulted in the expression of VRN-H2 under SD conditions (Turner et al., 2013). Barley hvelf3 mutants exhibited an early-flowering phenotype independently of the photoperiod due to elevated expression levels of Ppd-H1 and, consequently, HvFT1 (Faure et al., 2012). SD expression of VRN2 was also reported in the day-neutral Ppd-D1a wheat mutant, which carries a deletion in the promoter of Ppd-D1a associated with constitutive expression of the gene (Turner et al., 2013). Similarly, in rice, Ghd7 and OsPRR37, homologous to VRN2 and PPD1, exhibited epistatic interactions in the control of flowering time of rice populations grown in the field under different photoperiods (Fujino and Sekiguchi, 2005; Shibaya et al., 2011). These studies in rice and wheat suggested that PPD1/OsPRR37 and VRN2/Ghd7 might interact; however, the mechanism that controls the activation of VRN2 expression in response to photoperiod remains unclear.
Allelic variation of the two LD response genes Ppd-H1 and VRN-H2, has contributed significantly to the spread of barley cultivation across different environments. A natural mutation in the CCT domain of Ppd-H1 is associated with lower transcript levels of HvFT1 and delayed flowering under LDs compared with the wild-type Ppd-H1 allele, but it is not associated with flowering variation under SDs (Laurie et al., 1995; Decousset et al., 2000; Turner et al., 2005; Hemming et al., 2008). The natural mutation at Ppd-H1 is prevalent in spring barley, which is characterized by deletions of the VRN-H2 locus and does not require vernalization (Dubcovsky et al., 2005). In winter barley, VRN-H2 is down-regulated during vernalization by VRN-H1, an APETALA1/FRUITFUL-like MADS box transcription factor that is induced by vernalization (Trevaskis et al., 2006; Hemming et al., 2008; Alonso-Peral et al., 2011). Variation in the regulatory region of VRN-H1 determines the timing and cold dependency of VRN-H1 activation and thus the repression of VRN-H2 (Hemming et al., 2008, 2009). In the LD cereals wheat and barley, the vernalization and photoperiod response pathways are known to converge on FT1 (Trevaskis et al., 2007; Hemming et al., 2008). However, a recent study has identified potential epistatic interactions between VRN-H2 and HvCO1 in a nested association mapping population (Maurer et al., 2015). Putative interactions of VRN-H2 with Ppd-H1 and HvCO1 suggest that VRN-H2 might also be important for the integration of photoperiod and vernalization signals.
The objectives of this study were to characterize the potential role of HvCO2 in the control of flowering time under different photoperiods and to test if HvCO1/CO2 genetically interact with Ppd-H1 and VRN-H2 to control flowering in barley. We show that HvCO2 overexpression accelerates flowering in spring barley but does not abolish plant sensitivity to inductive LDs. Overexpression of HvCO1 and HvCO2 up-regulated the expression of VRN-H2, which was associated with a delay in flowering under LD and SD conditions as compared with spring transgenic genotypes with a deletion of VRN-H2. In addition, variation at Ppd-H1 controlled VRN-H2 expression. Our data thus suggest that the floral activators HvCO1/CO2 and Ppd-H1 indirectly repress flowering before vernalization by controlling the expression of VRN-H2 under LDs. These findings unravel a degree of functional conservation between HvCO1/CO2 and Ppd-H1 and their rice orthologs Hd1 and OsPRR37, which function as floral repressors under LDs.
RESULTS
Overexpression of HvCO2 Accelerated Flowering Time in a Spring Barley Background
The effect of HvCO2 on time to flowering was investigated by ectopically overexpressing the gene in the spring variety Golden Promise and analyzing flowering time and the expression of major flowering time genes under LDs and SDs.
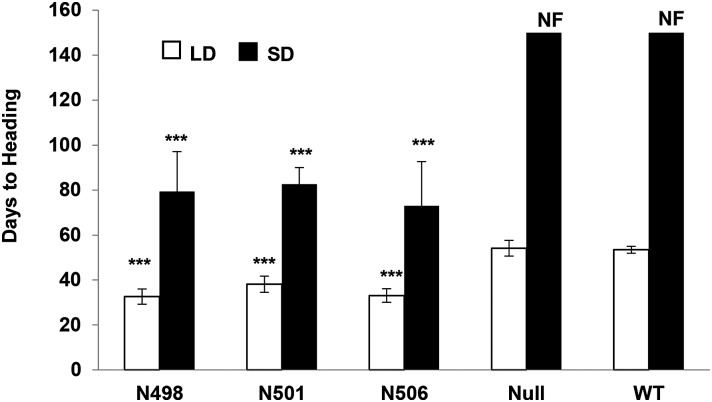
Under LDs, transgenic Ubi::HvCO2 lines flowered on average 36 d after emergence (DAE) and thus significantly earlier than the null segregants and wild-type Golden Promise, which required on average 54 d to flower (Fig. 1). Under SD conditions, overexpression of HvCO2 induced flowering, whereas the null segregants and the wild type had not flowered by 150 DAE, when the experiment was stopped. Ubi::HvCO2 lines flowered on average 78 DAE under SDs and thus significantly later than under LDs.
Figure 1.
Analysis of the flowering time of Ubi::HvCO2 transgenic lines under LD and SD conditions. Flowering time is shown for Ubi::HvCO2 transgenic lines (N498, N501, and N506), the null segregant line (Null), and Golden Promise (WT) grown under LD (white bars; 16 h of light) and SD (black bars; 8 h of light) conditions. Flowering time was measured for five to 20 plants for each of the Ubi::HvCO2 lines, the null segregant line, and the wild type in days from germination until heading. Null and wild-type plants did not flower at the end of the experiment (NF; 150 d) under SDs. Columns represent the average flowering time. Error bars indicate sd. Asterisks refer to significant differences in flowering time of the transgenic lines compared with the null and wild-type plants (***, P < 0.001).
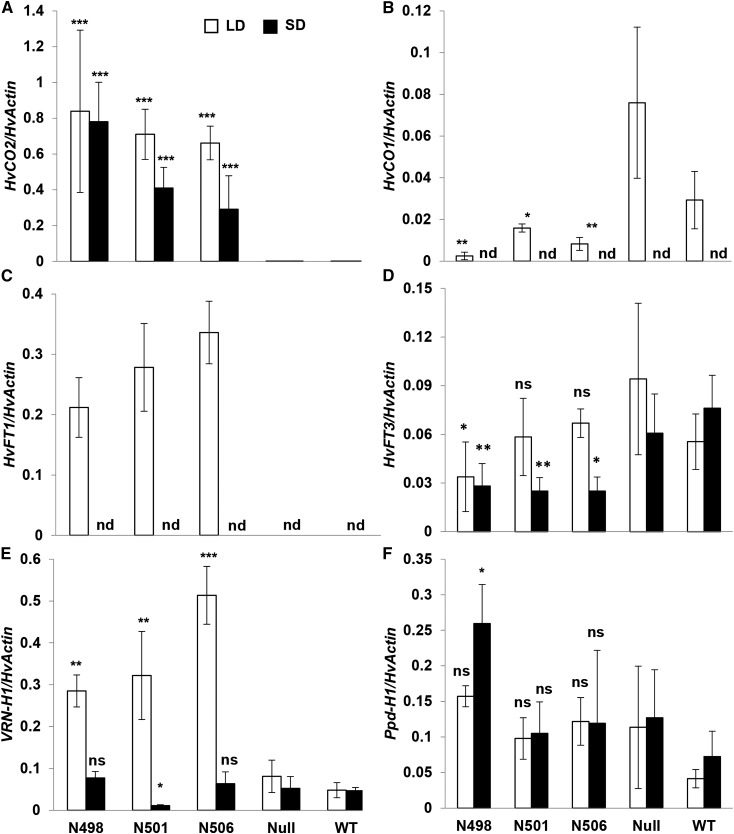
To further characterize the daylength-dependent effects of Ubi::HvCO2 on flowering time, we evaluated the expression of HvCO2, HvCO1, and major flowering time genes such as Ppd-H1, HvFT1, HvFT3, and VRN-H1 in leaf tissue of Ubi::HvCO2 lines and the wild-type controls under LD and SD conditions. Expression of HvCO2 was significantly up-regulated in the transgenic lines compared with the null segregants and the wild type under LD and SD conditions (Fig. 2A). Expression of HvCO1 was significantly reduced in all Ubi::HvCO2 lines compared with the null segregants and the wild type under LDs. Under SDs, the expression of HvCO1 was below the detection limit at the time when the seedlings were sampled (Fig. 2B).
Figure 2.
Expression levels of flowering time genes in Ubi::HvCO2 transgenic lines. Expression levels of flowering time genes are shown for Ubi::HvCO2 transgenic lines (N498, N501, and N506), null segregants (Null), and Golden Promise (WT) under LD (white bars; 16 h of light) and SD (black bars; 8 h of light) conditions. Expression analysis was performed on leaf samples collected 2 h before the end of the light period at day 7 after germination under LDs and SDs. For each transgenic line, the null segregant line, and the wild type, three to seven plants were used as biological replicates. Columns represent the average expression of HvCO2 (A), HvCO1 (B), HvFT1 (C), HvFT3 (D), VRN-H1 (E), and Ppd-H1 (F), all normalized to the expression level of HvACTIN. nd, No expression detected. Error bars indicate sd. Asterisks refer to significant expression differences in the transgenic lines compared with the null and wild-type plants (*, P < 0.05; **, P < 0.01; and ***, P < 0.001). ns, No significant difference in expression at P < 0.05. Statistical comparisons were performed separately for gene expression measured under LDs and SDs.
The expression of HvFT1 was significantly up-regulated in all tested transgenic lines under LDs but was below the detection level in the null segregants and the wild type (Fig. 2C). Under SD conditions, however, the expression of HvFT1 was not detected in any of the tested genotypes. The expression of HvFT3 was not different between transgenic and nontransgenic plants under LDs but was down-regulated in all four Ubi::HvCO2 lines as compared with the wild type and the null segregants under SDs (Fig. 2D). In addition, overexpression of HvCO2 caused a significant up-regulation of VRN-H1 under LDs, whereas differences in VRN-H1 expression between Ubi::HvCO2 lines, the wild type, and the null segregants were not consistent under SDs (Fig. 2E). Expression levels of Ppd-H1 in the Ubi::HvCO2 lines did not differ significantly from those in nontransgenic controls under LDs and SDs (Fig. 2F).
Taken together, overexpression of HvCO2 caused early flowering under LD and SD conditions. However, transgenic lines showed a strong response to the photoperiod, and this was associated with the photoperiod-dependent regulation of the barley flowering time genes HvFT1 and VRN-H1.
Overexpression of HvCO2 Did Not Overcome the Vernalization Requirement
The genetic interactions of HvCO2 with the photoperiod gene Ppd-H1 and the vernalization genes VRN-H1 and VRN-H2 were evaluated by recording flowering time in an F2 population derived from a cross between Ubi::HvCO2 line N506 and the winter variety Igri. The Ubi::HvCO2 line N506 in the background of the spring barley Golden Promise carries a natural mutation at Ppd-H1, a deletion of the VRN-H2 locus, a deletion in the first regulatory intron of VRN-H1, and a functional HvFT3 gene. As a consequence, this genotype does not require vernalization and shows a reduced photoperiod response. In contrast, Igri is characterized by the wild-type allele at Ppd-H1, winter alleles at VRN-H1 and VRN-H2, and a partial deletion of HvFT3. Consequently, Igri requires vernalization to flower and shows a strong photoperiod response. To test whether the overexpression of HvCO2 can overcome the vernalization requirement, F2 plants were grown without vernalization under LDs and scored for flowering time.
Flowering time varied between 23 and 130 d in the F2 population of Ubi::HvCO2 × Igri under LDs (Supplemental Fig. S1). The F2 population showed transgressive segregation, as 37 plants (19%) flowered earlier than the average flowering time (41 d) of the transgenic parent. Only five plants (3%) flowered later than the winter parent Igri, which flowered after 116 d.
We associated genetic variation at the flowering time genes Ubi::HvCO2, Ppd-H1, VRN-H1, VRN-H2, and HvFT3 with time to flowering in the F2 population to estimate the contribution of each of the tested genes to the overall trait variation. To analyze the genetic interaction of Ubi::HvCO2 and Ppd-H1 in the absence of VRN-H2, we also associated the allelic variation of the candidate genes with flowering time in the spring/facultative F2 subpopulation comprising all F2 genotypes with a deletion of the VRN-H2 locus. We designated alleles segregating in the F2 population and derived from the winter parent with W (winter) and alleles derived from the spring barley Golden Promise with S (spring).
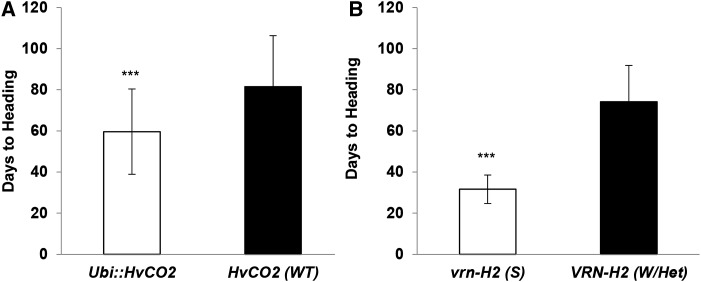
In total, the overexpression of HvCO2 and allelic variation at VRN-H1, VRN-H2, and Ppd-H1 accounted for 89% of the variation identified for flowering time in the F2 population grown under LDs (Supplemental Table S1). Natural variation at HvFT3 did not show any significant effect on flowering time under LDs. The transgene Ubi::HvCO2 accelerated flowering time but explained only 16% of the overall phenotypic variation (Fig. 3A; Supplemental Table S1). In contrast, natural variation at VRN-H2 had the strongest effect on flowering time and accounted for 51% of the flowering time variation (Fig. 3B; Supplemental Table S1). F2 genotypes carrying the winter allele of VRN-H2 flowered on average after 74 d and thus 42 d later than those carrying the deletion (spring allele) of the gene. The vernalization gene VRN-H1 explained 11% of the variation in days to flowering, as the winter allele delayed flowering time by an average of 18 d. Furthermore, the interaction between VRN-H1 and VRN-H2 was significant and explained 3% of the phenotypic variation. The combination of winter alleles at VRN-H2 and VRN-H1 delayed flowering time by an additional 22 d compared with the sum of the effects of the winter alleles at both genes. The vernalization genes VRN-H1 and VRN-H2 and their interaction thus explained in total 65% of flowering time variation in the population. Consequently, the effects of VRN-H2 and VRN-H1 had more pronounced effects on time to flowering than Ubi::HvCO2. Nevertheless, Ubi::HvCO2 reduced days to heading in the winter F2 plants with homozygous and heterozygous winter alleles at VRN-H1 and VRN-H2, respectively, by about 22 d (Supplemental Fig. S2A). Allelic variation at the major photoperiod gene Ppd-H1 explained 5% of the overall variation in days to flowering. The photoperiod-responsive allele reduced time to flowering by 8 d compared with the mutated ppd-H1 allele. In spring or facultative F2 genotypes with a deletion of VRN-H2, Ppd-H1 exerted the strongest effect on flowering time (65%; Supplemental Table S2) even in the presence of the transgene (Supplemental Fig. S2B). The wild-type Ppd-H1 allele accelerated flowering time by 11 d as compared with the mutated ppd-H1 allele in the transgenic F2 genotypes with a deletion of VRN-H2.
Figure 3.
Effects of Ubi::HvCO2 and VRN-H2 on flowering time of the F2 population Ubi::HvCO2 × Igri under LD conditions. Columns represent the average flowering time of F2 genotypes classified according to the presence/absence of the transgene Ubi::HvCO2 (A) and the allelic variation of VRN-H2 (B). S, Spring allele; W/Het, homozygous and heterozygous winter allele; WT, wild type. Error bars indicate sd. Asterisks refer to a significant difference (***, P < 0.001).
Taken together, the repressive effect of VRN-H2 was stronger than the effect of Ubi::HvCO2 on flowering. Nevertheless, the presence of the transgene accelerated flowering time also in the winter genotypes. In transgenic F2 genotypes with a deletion of the VRN-H2 locus, variation at Ppd-H1 had the strongest effect on flowering time, consistent with the observation that transgenic genotypes maintained a strong photoperiod response.
Overexpression of HvCO2 Up-Regulated the Floral Repressor VRN-H2
To further characterize the molecular control of flowering time in the F2 population, we analyzed the effects of Ubi::HvCO2 on the expression levels of selected flowering time regulators.
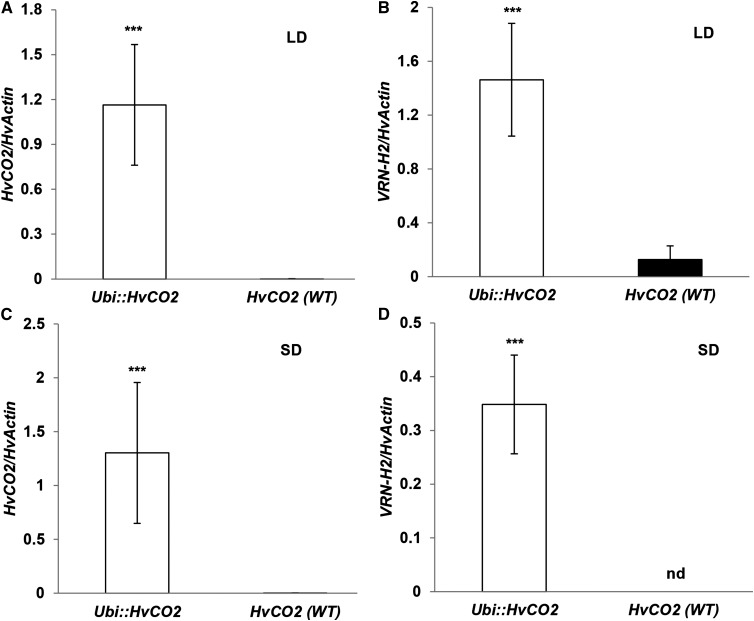
HvCO2 expression in F2 genotypes carrying the transgene was on average 1,000 times higher than in the nontransgenic F2 genotypes (Fig. 4A). Accordingly, the presence/absence of the transgene explained 72% of the variation in HvCO2 expression (Supplemental Table S3). Interestingly, the presence of VRN-H2 was associated with a significant down-regulation of HvCO2 expression in F2 genotypes carrying the wild-type HvCO2 gene (Supplemental Fig. S3). HvCO2 expression levels showed a high negative correlation with days to flowering (−0.58; Supplemental Table S4). In addition, HvCO2 exhibited a high positive correlation with the expression levels of Ppd-H1 (0.60) in the winter F2 population but not in the spring F2 population (Supplemental Table S5).
Figure 4.
Effects of Ubi::HvCO2 on the expression of HvCO2 and VRN-H2 in F2 genotypes of the population Ubi::HvCO2 × Igri grown under LD and SD conditions. Columns represent the average expression of HvCO2 (A and C) and VRN-H2 (B and D), each normalized to HvACTIN in F2 genotypes classified according to the presence/absence of the transgene Ubi::HvCO2 under LD (16 h of light; A and B) and SD (8 h of light; C and D) conditions. F2 genotypes with the deleted VRN-H2 locus were not considered in B and D. Expression analysis was performed on leaf samples collected 2 h before the end of the light period at day 7 after germination. nd, No expression detected; WT, wild type. Error bars indicate sd. Asterisks refer to significant differences (***, P < 0.001).
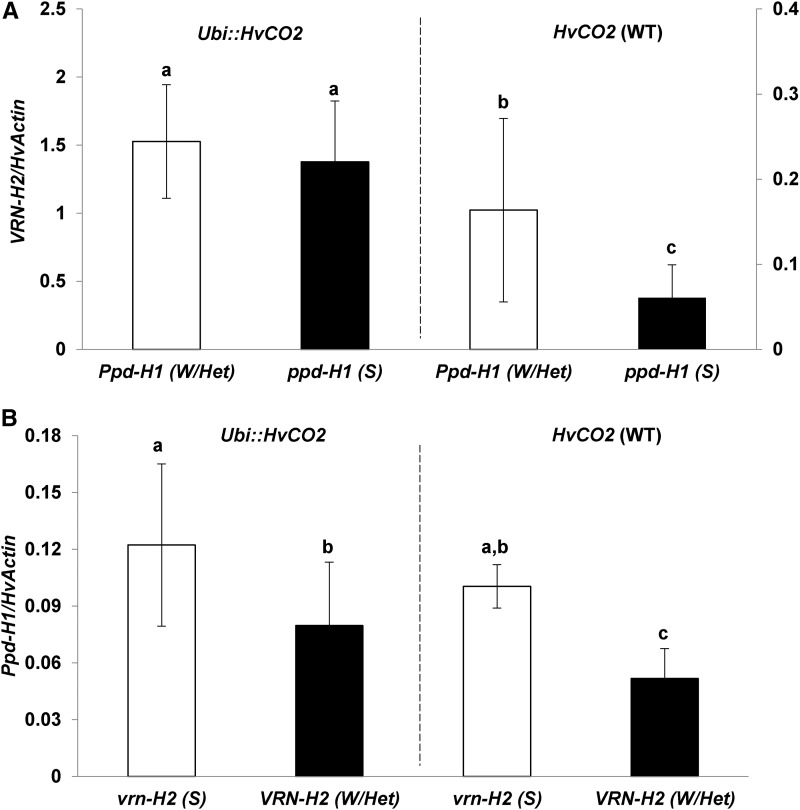
Across the entire population, genetic variation at VRN-H2, HvCO2, and their interactions explained 61%, 6%, and 15% of the variation in VRN-H2 expression, respectively (Supplemental Table S3). Interestingly, winter F2 genotypes carrying the Ubi::HvCO2 transgene showed on average an 11 times higher expression of VRN-H2 than F2 genotypes without the transgene (Fig. 4B). Accordingly, the expression levels of HvCO2 and VRN-H2 were highly correlated (0.79) in winter F2 genotypes (Supplemental Table S5). Despite the strong up-regulation of the flowering repressor VRN-H2 in the presence of Ubi::HvCO2, transgenic winter F2 genotypes flowered earlier than the nontransgenic winter F2 genotypes (Supplemental Fig. S2A). In addition, VRN-H2 was significantly up-regulated by the wild-type allele of Ppd-H1 in the background of nontransgenic F2 genotypes (Fig. 5A). The presence of VRN-H2, in turn, correlated with the down-regulation of Ppd-H1, in particular in the background of the nontransgenic genotypes (Fig. 5B).
Figure 5.
Reciprocal interaction between Ppd-H1 and VRN-H2 in the F2 population Ubi::HvCO2 × Igri under LD conditions. A, Columns represent the average expression of VRN-H2 normalized to HvACTIN in F2 genotypes classified according to the presence/absence of Ubi::HvCO2 and allelic variation at Ppd-H1. F2 genotypes with the deleted VRN-H2 locus were not considered. B, Columns represent the average expression of Ppd-H1 normalized to HvACTIN in F2 genotypes classified according to the presence/absence of Ubi::HvCO2 and VRN-H2. S, Spring allele; W/Het, homozygous and heterozygous winter allele; WT, wild type. Expression analysis was performed on leaf samples collected 2 h before the end of the light period in LDs (16 h of light) at day 7 after germination. Error bars indicate sd. Letters on top of each column indicate significant differences in expression levels at P < 0.05.
Allelic variation at VRN-H2 exerted a strong effect on HvFT1 expression levels. In the presence of VRN-H2, HvFT1 expression was completely repressed in all F2 genotypes independent of the transgene (Supplemental Fig. S4). On the other hand, F2 genotypes with the Ubi::HvCO2 transgene showed higher expression levels of HvFT1 in the F2 genotypes with a deletion of the VRN-H2 locus (Fig. 6B). Accordingly, across the entire population, variation in HvFT1 expression was mainly controlled by VRN-H2 (35%) and Ubi::HvCO2 (22%; Supplemental Table S3). Interestingly, the photoperiod-responsive allele of Ppd-H1 significantly up-regulated the expression of HvFT1 in the spring/facultative F2 genotypes with a deletion of VRN-H2 in the presence of the transgene Ubi::HvCO2 (Supplemental Fig. S5). HvFT1 expression levels strongly correlated with days to flowering (−0.70) and with the expression levels of VRN-H1 (0.57), Ppd-H1 (0.33), and VRN-H2 (−0.47; Supplemental Table S4).
Figure 6.
Effects of Ubi::HvCO2 and allelic variation of VRN-H2 on flowering time and HvFT1 expression in Ubi::HvCO2 × Igri F2 genotypes under SD conditions. The F2 genotypes are classified according to the presence/absence of the overexpressed HvCO2 and allelic variation of VRN-H2. S, Spring allele; W/Het, homozygous and heterozygous winter allele; WT, wild type. A, Columns represent the average flowering time of the different genotypic classes. Nontransgenic F2 genotypes did not flower at the end of the experiment (NF; 200 d). B, Columns represent the average HvFT1 expression of the different genotypic classes normalized to the expression of HvACTIN. Expression was analyzed in leaf samples harvested 2 h before the end of the light period from a subset of F2 genotypes that did not flower by the date of sampling (75 DAE). nd, No expression detected. Error bars indicate sd. Asterisks refer to significant differences (***, P < 0.001).
In summary, Ubi::HvCO2 caused a strong up-regulation of VRN-H2. In addition, variation at Ppd-H1 affected VRN-H2 expression in the nontransgenic F2 subpopulation. VRN-H2, in turn, was involved in the down-regulation of Ppd-H1 and HvCO2. Our findings thus suggest strong reciprocal interactions between HvCO2, Ppd-H1, and VRN-H2. Ubi::HvCO2 and Ppd-H1 exhibited additive effects on HvFT1 expression in the absence of VRN-H2.
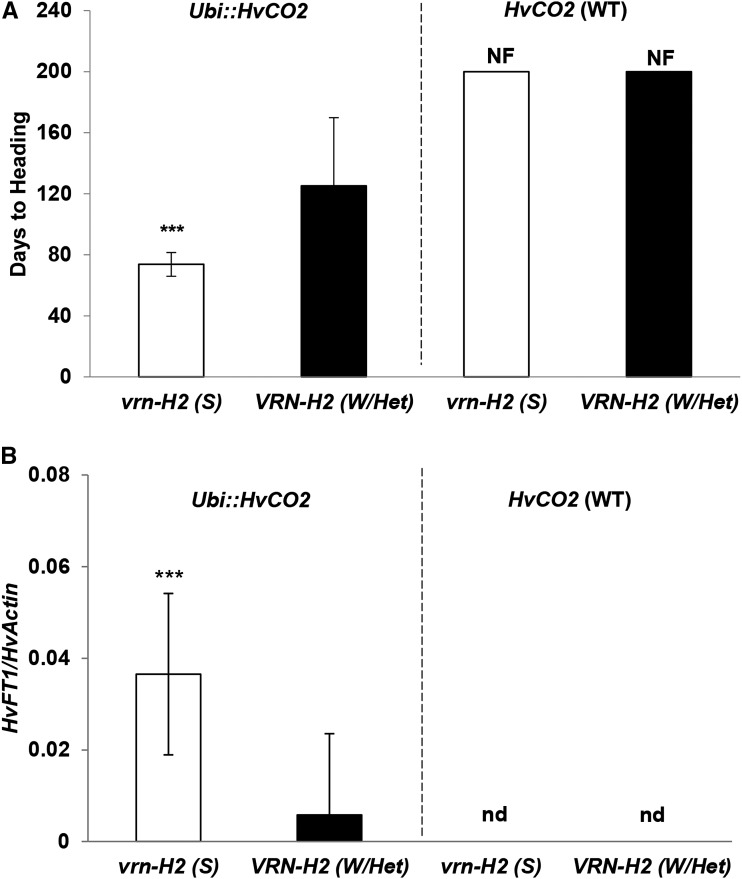
Overexpression of HvCO2 and HvCO1 Induced the Expression of VRN-H2 and HvFT1 under SD Conditions
As the overexpression of HvCO2 up-regulated the expression of VRN-H2 under LDs, we further tested if Ubi::HvCO2 also up-regulated VRN-H2 expression under SDs, when the gene is usually not expressed. For this purpose, 168 F2 genotypes derived from the cross Ubi::HvCO2 × Igri were grown in the greenhouse under SD conditions (8–10 h of light) and scored for flowering time and gene expression.
Overexpression of HvCO2 caused an up-regulation of VRN-H2 in the transgenic F2 genotypes also under SDs at 7 DAE, while no VRN-H2 expression was detected in the nontransgenic F2 genotypes (Fig. 4, C and D). Transgenic F2 genotypes with the VRN-H2 locus flowered on average after 125 DAE, while transgenic F2 genotypes with a deletion of VRN-H2 required on average 74 d to flower under SDs (Fig. 6A). All nontransgenic F2 genotypes failed to flower up to 200 DAE (when the experiment was stopped). The expression of VRN-H2 as mediated by Ubi::HvCO2 was thus associated with a significant delay in flowering also under SDs. Accordingly, Ubi::HvCO2 and VRN-H2 explained 48% and 11% of the observed variation in flowering time (Supplemental Table S6). Expression levels of HvFT1 were under the detection limit at 7 DAE but were later (75 DAE) detected in transgenic F2 genotypes under SDs (Fig. 6B). Transgenic F2 genotypes with a deletion of VRN-H2 had 6-fold increased expression levels of HvFT1 as compared with their siblings with the winter allele of VRN-H2. Expression of HvFT1 was not detected in the nontransgenic F2 genotypes. Variation in HvFT1 expression was thus mainly controlled by VRN-H2 (29%) and Ubi::HvCO2 (16%; Supplemental Table S6). Finally, variation at Ppd-H1 affected flowering time and HvFT1 expression in the transgenic F2 genotypes under SDs, when Ppd-H1 does usually not have any effect on time to flowering (Supplemental Table S6).
We further tested if overexpression of HvCO1, as the closest homolog of HvCO2, could also influence VRN-H2 expression under LD and noninductive SD conditions. The up-regulation of HvCO1 expression in Ubi::HvCO1 × Igri F2 genotypes carrying the Ubi::HvCO1 transgene was associated with an up-regulation of VRN-H2 under LDs and SDs (Supplemental Fig. S6).
Taken together, Ubi::HvCO1 and Ubi::HvCO2 up-regulated the expression of VRN-H2 under LDs and SDs. Up-regulation of VRN-H2 in Ubi::HvCO2 genotypes under SDs was associated with a delay in flowering time and a reduction in HvFT1 expression as compared with Ubi::HvCO2 genotypes with a deletion of VRN-H2. Finally, variation at Ppd-H1 affected time to flowering and HvFT1 expression in transgenic, but not wild-type, F2 genotypes under SDs.
DISCUSSION
Overexpression of HvCO2 Causes Photoperiod-Dependent Early Flowering in Barley
In Arabidopsis, CO is an important promoter of flowering in response to LDs (Koornneef et al., 1991; Putterill et al., 1995). Arabidopsis plants constitutively overexpressing CO were early flowering and almost completely insensitive to daylength (Onouchi et al., 2000). In our study, overexpression of HvCO2, which represents with HvCO1 the closest barley orthologs of AtCO (Griffiths et al., 2003), also caused early flowering in spring barley under LDs and SDs. However, transgenic plants overexpressing HvCO2 retained a strong response to daylength and flowered significantly earlier under LDs than under SDs. Accordingly, HvFT1 up-regulation in the transgenic lines occurred significantly later under SDs when compared with LD conditions. Analysis of flowering time and gene expression in the cross Ubi::HvCO2 × Igri suggested that the photoperiod response of transgenic lines was influenced by Ppd-H1. Variation at Ppd-H1 affected flowering time and the expression of HvFT1 in transgenic spring F2 genotypes under LDs. Consequently, Ppd-H1 controlled flowering time downstream of HvCO2 expression under LDs (Fig. 7). Similarly, transgenic lines overexpressing HvCO1 retained a photoperiod response, and Ppd-H1 exerted a significant effect on flowering time in Ubi::HvCO1 transgenic lines grown under LDs (Campoli et al., 2012). Consequently, both HvCO1 and HvCO2 accelerate flowering, but their effects are modified by daylength and natural variation at Ppd-H1. In contrast, up-regulation of Ppd-H1 expression in a barley mutant with a nonfunctional HvELF3 gene was associated with photoperiod-independent early flowering (Faure et al., 2012). Moreover, natural mutations in the promoters of the homologous PPD1 genes in wheat and consequent up-regulation of PPD1 expression caused photoperiod insensitivity and early flowering (Beales et al., 2007). These reports, together with our results, indicate that the expression variation of Ppd1 has a stronger impact on photoperiodic flowering than expression changes of CO1/CO2. Similarly, a rice line with a nonfunctional allele at Hd1 (rice CO) retained sensitivity to daylength, and complete daylength insensitivity was only observed when alleles at both Hd1 (rice CO) and Hd2 (OsPRR37) were nonfunctional (Lin et al., 2000). On the other hand, variation at PRR37 orthologs affected flowering time only in the background of a functional CO ortholog in sorghum (Sorghum bicolor) and rice (Lin et al., 2000; Yang et al., 2014). This suggests that the ability of PRR37-like genes to control flowering is dependent on CO. In barley, variation at Ppd-H1 only affected flowering time under LDs; however, in the background of Ubi::HvCO2 plants, variation at Ppd-H1 regulated HvFT1 expression and influenced flowering time also under SDs. The effect of Ppd-H1 on flowering time was thus modified by the overexpression of HvCO2, which suggested that, also in barley, Ppd-H1 interacted with HvCO2. In Arabidopsis, factors controlling CO protein stability have a strong impact on flowering time. The photoreceptors CRY1/2 and PHYA stabilize CO, whereas PHYB destabilizes the protein; accordingly, cry2 and phyA mutants are late flowering while phyB mutants are early flowering (Turck et al., 2008). Interestingly, a Ppd-H1 homolog in Arabidopsis, PRR3, was shown to stabilize the TOC1 protein, which shares the CCT domain with CO (Para et al., 2007). The induction of HvFT1 by Ppd-H1 may thus be dependent on the posttranscriptional modification of HvCO1/CO2. The availability of barley lines with nonfunctional alleles at HvCO1 and HvCO2 would further help to dissect the genetic interactions of Ppd-H1 and HvCO1/CO2 in barley.
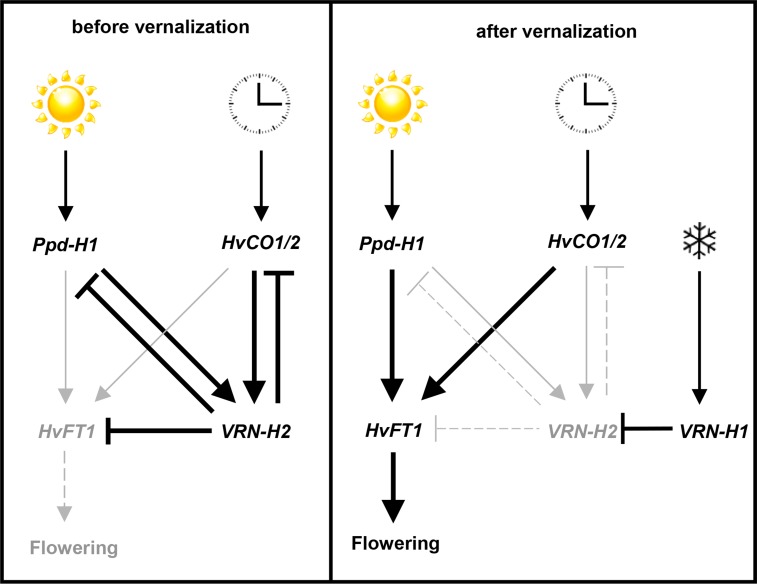
Figure 7.
Model for the coregulation of VRN-H2 and HvFT1 by HvCO1/CO2 and Ppd-H1 in winter barley under LD conditions before and after vernalization. HvCO1/CO2 and Ppd-H1 induce the expression of VRN-H2, which acts as a strong repressor of HvFT1 and flowering time in winter barley before vernalization. Up-regulation of VRN-H1 during vernalization represses VRN-H2. In the absence of VRN-H2 after vernalization (or in spring barley), Ppd-H1 and HvCO1/CO2 up-regulate HvFT1 and induce flowering under LD conditions.
HvCO1/CO2 and Ppd-H1 Coregulate VRN-H2 Expression
In Arabidopsis, overexpression of the photoperiod response gene CO could largely overcome the delay in flowering caused by the overexpression of the major vernalization gene FLC (Hepworth et al., 2002). However, we show that, in barley, flowering time was delayed by the winter alleles at VRN-H2 and VRN-H1 even in the presence of Ubi::HvCO2. Interestingly, overexpression of HvCO1/CO2 caused an up-regulation of the flowering repressor VRN-H2 under inductive LDs but also under SD conditions, when the gene is normally not expressed. VRN-H2 was functional, repressed HvFT1 expression, and delayed flowering time under LDs and SDs. Consequently, HvCO1/CO2 are involved in mediating the photoperiodic regulation of VRN-H2. As such, HvCO1/CO2 acted as a promoter of flowering in a spring barley background but as an indirect repressor of flowering in a winter barley line with a functional VRN-H2 gene. Hd1, the rice ortholog of CO, was also proposed to have these two opposite functions of repressing and promoting flowering by inhibiting and inducing Hd3a expression (FT ortholog) under LDs and SDs, respectively (Yano et al., 2000; Hayama et al., 2003). Consequently, the involvement of CO in the LD repression of flowering seems to be partially conserved between rice and barley, despite the opposite flowering behavior of the two cereal crops under LD conditions. In rice, floral repression under LDs is mediated by Ghd7, a CCT domain gene that, like Vrn-H2, is up-regulated under LDs and represses the expression of Hd3a (Xue et al., 2008). It was suggested that Ghd7 and Hd1 independently control the photoperiod response in rice (Xue et al., 2008; Tsuji et al., 2011). However, Saito et al. (2012) showed that OsElf3 controlled the expression of both Hd1 and Ghd7 and suggested that both genes may interact to control Hd3a. In addition, Shibaya et al. (2011) demonstrated that Ghd7 interacted with Hd2, which was identified as OsPRR37, the rice homolog of Ppd-H1. Interestingly, our expression analysis revealed that the functional allele of Ppd-H1 was associated with higher expression levels of VRN-H2 under LDs in the nontransgenic F2 genotypes with a winter allele at VRN-H2. Although allelic variation at Ppd-H1 has not yet been associated with VRN-H2 expression levels, barley hvelf3 and wheat Ppd-D1a mutants, in which Ppd-H1 and Ppd-D1 are constitutively expressed, exhibited up-regulated expression levels of VRN-H2 under noninductive SDs (Turner et al., 2013). Moreover, expression studies in wheat phyC mutants revealed a correlated down-regulation of PPD1 and VRN-H2 (Chen et al., 2014). These findings indicate that Ppd-H1 is involved in the regulation of VRN-H2. Thus, Ppd-H1 may also act as an indirect repressor of flowering by up-regulating VRN-H2 under LDs before vernalization. We propose that, before vernalization, Ppd-H1 functions as a floral repressor under LDs, as has been shown for the rice and sorghum orthologs of Ppd-H1, OsPRR37 and SbPRR37 (Murphy et al., 2011; Koo et al., 2013).
Our results showed that functional allelic diversity at Ppd-H1 and overexpression of HvCO1/CO2 could influence VRN-H2 expression (Fig. 7). This supports our previous suggestion that Ppd-H1 and HvCO1/CO2 might interact posttranscriptionally to control downstream targets. However, we also observed that overexpression of HvCO2 was associated with an up-regulation of Ppd-H1 under LDs, indicating that both genes may also have interacted transcriptionally. Furthermore, the expression levels of Ppd-H1 and HvCO2 were repressed by VRN-H2, indicating the presence of negative feedback loops from VRN-H2 to Ppd-H1 and HvCO2. Consequently, the expression levels of the three genes were strongly interdependent. Each of the three genes encodes a protein with a CCT domain that is known to be important for the function of the protein and for protein-protein interactions (Robson et al., 2001; Yan et al., 2004; Turner et al., 2005; Distelfeld et al., 2009; Li et al., 2011). Li et al. (2011) demonstrated that the CCT domains of VRN2 and CO2 proteins in wheat interacted with the same set of Heme Activator Protein (HAP)/Nuclear Factor Y (NF-Y) proteins. The authors suggested that the competitive interactions of VRN2 and CO2 with NF-Y proteins played an important role in the integration of seasonal signals for the transcriptional regulation of VRN3 (TaFT) in wheat. HAP/NF-Y proteins are known to regulate flowering in Arabidopsis (Wenkel et al., 2006; Kumimoto et al., 2008, 2010) and rice (Wei et al., 2010; Yan et al., 2011; Dai et al., 2012). In addition, NF-Y proteins are involved in plant responses to various environmental stresses, such as drought and osmotic stress (Nelson et al., 2007; Stephenson et al., 2007; Li et al., 2008). Reciprocal transcriptional activation and repression of CO, PPD1, and VRN2 may help to prioritize environmental signals, whereas competitive interactions of these genes with HAP/NF-Y factors could provide a complex system to integrate the seasonal cues with multiple stress signals to fine-tune the regulation of flowering time.
CONCLUSION
HvCO2 overexpression enhanced HvFT1 expression and accelerated flowering time, but the expression levels of HvFT1 and daylength sensitivity were controlled by Ppd-H1 downstream of HvCO2 overexpression (Fig. 7). HvCO1/CO2 and Ppd-H1 coregulated HvFT1 but also VRN-H2, which revealed a dual function of CO orthologs and Ppd-H1 as activator/repressor of flowering depending on the presence of VRN-H2. LDs repress flowering before vernalization through the function of Ppd-H1, HvCO, and VRN-H2 but activate flowering after vernalization when VRN-H2 is down-regulated. Consequently, floral repression through VRN-H2 and floral activation through HvFT1 are regulated by the same set of genes, Ppd-H1 and HvCO. Our work suggests that the LD repression of flowering by PRR and CO genes is conserved between rice and barley and possibly among other grasses. Finally, the genetic interactions between HvCO and Ppd-H1 with VRN-H2 are important to consider for cereal breeding programs, as manipulation of the photoperiod response pathway also affects the vernalization response.
MATERIALS AND METHODS
Generation of Transgenic Ubi::HvCO2 Lines and Their Growth Conditions
Barley (Hordeum vulgare) plants of the spring variety Golden Promise were transformed with an overexpression construct generated with the complementary DNA (cDNA) clones of HvCO2 (AF490470) driven by the maize (Zea mays) ubiquitin promoter (Christensen et al., 1992). The overexpression cassette was inserted into the pWBVEC8 binary vector (Wang et al., 1998) and introduced into Agrobacterium tumefaciens. A. tumefaciens-mediated transformation was then performed on excised barley embryos (Tingay et al., 1997; Matthews et al., 2001).
Independent barley transformants were regenerated, and T1 and T2 plants were screened for the presence of the transgene using two pairs of primers that bind to the hygromycin selectable marker gene and the HvCO2 cDNA sequence (Supplemental Table S7). The generation of transgenic Ubi::HvCO1 lines is described by Campoli et al. (2012).
Three independent transgenic T2 families, designated Ubi::HvCO2 lines N498, N501, and N506, a null segregant control line that lost the transgene, and the wild-type Golden Promise were sown in soil and grown under LD (16 of light/8 h of dark) and SD (8 h of light/16 h of dark) conditions in the greenhouse (temperature, 20°C/16°C days/nights). Five to 20 plants of the transgenic line, the null segregants, and the wild type were used to score flowering time, which was measured in days from emergence until heading (DAE). Heading was scored as the spike awns emerged from the sheath of the main shoot flag leaf (Zadoks stage 49; Zadoks et al., 1974). Leaf material from three to seven plants (biological replicates) for each tested line was collected for RNA extraction and gene expression analysis. The samples were harvested 7 DAE 2 h before the end of the light period under LDs and SDs (ZT14 [for Zeitgeber time] under LDs and ZT6 under SDs).
Generation of Ubi::HvCO2 × Igri and Ubi::HvCO1 × Igri F2 Populations and Their Growth Conditions
For generation of the F2 populations, each of the transgenic lines Ubi::HvCO2 N506 and Ubi::HvCO1 N2330 was crossed with the winter barley Igri. Wild-type Golden Promise, the genetic background of the transgenic lines, carries the spring allele of Ppd-H1 with a mutation in the CCT domain. This mutation causes reduced photoperiod sensitivity and delays flowering under LDs. In addition, the wild type is characterized by a spring allele at VRN-H1 and a deletion of the VRN-H2 locus and, consequently, does not require vernalization for the induction of flowering. Finally, Golden Promise carries a functional HvFT3 gene that accelerates development under SDs (Laurie et al., 1995; Faure et al., 2007). In contrast, Igri carries the dominant Ppd-H1 allele with a strong photoperiod response and winter alleles at VRN-H1 and VRN-H2 and, thus, needs vernalization to flower. Furthermore, Igri is characterized by a partial deletion of HvFT3. In the resulting F2 populations, alleles derived from the winter parent Igri are designated with W and alleles derived from the spring parent Golden Promise are designated with S.
One hundred ninety-one F2 plants and 168 F2 plants derived from the cross Ubi::HvCO2 × Igri were sown in soil and grown in the greenhouse (temperature, 20°C/16°C days/nights) under LD (16 h of light/8 h of dark) and SD (8 h of light/16 h of dark) conditions, respectively. After 50 d in 8-h SD conditions, the light period was extended to 10 h to accelerate plant development. Seedlings were not subjected to vernalization, and flowering time was scored as number of days from emergence until heading (Zadoks stage 49). Leaf material was harvested from parental lines and 71 F2 genotypes 7 DAE at ZT14 under LDs and from all 168 F2 genotypes 7 DAE at ZT6 under SDs and subsequently used for RNA extraction and gene expression analysis. The selection of F2 genotypes for gene expression analysis under LDs was based on the genotypic information to balance the number of plants within each genotypic class at the analyzed flowering time genes (the transgene, Ppd-H1, VRN-H1, and VRN-H2). Additional leaf samples for gene expression analysis were harvest from 55 F2 genotypes grown under SDs 75 DAE (25 d after extending the photoperiod to 10 h). Selection of the genotypes was also based on genotypic information and excluded genotypes that had already flowered by the time of sampling.
Similarly, 80 F2 genotypes derived from the cross Ubi::HvCO1 × Igri were grown under the same LD and SD conditions. The F2 genotypes were genotyped for the transgene Ubi::HvCO1 and VRN-H2. Expression analysis was performed on a subset of 20 F2 genotypes under LDs and 12 F2 genotypes under SDs, which had been selected for the dominant winter allele VRN-H2 but segregated for the presence of the transgene. The expression of HvCO2 and VRN-H2 was quantified in leaf samples harvested 22 and 11 DAE under LDs (ZT14) and SDs (ZT6), respectively.
DNA Extraction and Genotyping of the Segregating Populations
Genomic DNA of individual F2 genotypes was extracted from leaf samples following the Biosprint DNA extraction protocol (Qiagen). F2 genotypes of all analyzed populations were genotyped for the presence of the transgene and the allelic diversity of the major flowering genes Ppd-H1 (Turner et al., 2005), VRN-H1 (Hemming et al., 2009), VRN-H2 (Dubcovsky et al., 2005), and HvFT3 (Faure et al., 2007; Kikuchi et al., 2009). PCR was performed as described in the original references (primers are listed in Supplemental Table S7).
RNA Extraction, cDNA Synthesis, and Quantitative Real-Time PCR
Total RNA extraction, first-strand cDNA synthesis, and quantitative real-time PCR for individual F2 plants were performed as described by Campoli et al. (2012). Quantitative real-time PCR was performed using gene-specific primers (Supplemental Table S7). Two technical replicates were used for each cDNA sample, and starting amounts for each data point were calculated based on the titration curve for each target gene and the reference gene (HvACTIN) using the LightCycler 480 Software (version 1.5; Roche).
Statistical Analysis
The statistical significance of differences in flowering time and gene expression levels between each of the Ubi::HvCO2 genotypes and the wild type and the null controls (wild type + null combined) grown under LDs and SDs was determined using Student’s t test. A fixed-model ANOVA for unbalanced designs was used to calculate significant effects and two-way interaction effects of the transgene and allelic variation at Ppd-H1, VRN-H1, VRN-H2, and HvFT3 on flowering time and gene expression in all tested F2 populations. Pearson correlation coefficients were calculated between flowering time and gene expression values in the tested populations.
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Flowering time of the F2 population Ubi::HvCO2 × Igri under LDs.
Supplemental Figure S2. Effects of Ubi::HvCO2 and Ppd-H1 on flowering time of different F2 subpopulations of Ubi::HvCO2 × Igri under LDs.
Supplemental Figure S3. Effects of VRN-H2 on expression of HvCO2 in the F2 population Ubi::HvCO2 × Igri under LDs.
Supplemental Figure S4. Effects of Ubi::HvCO2 and VRN-H2 on expression of HvFT1 in the F2 population Ubi::HvCO2 × Igri under LDs.
Supplemental Figure S5. Effect of Ppd-H1 on expression levels of HvFT1 in the transgenic spring/facultative F2 subpopulation under LDs.
Supplemental Figure S6. Effects of Ubi::HvCO1 on expression of HvCO1 andVRN-H2 in the F2 population Ubi::HvCO1 × Igri under LDs and SDs.
Supplemental Table S1. Analysis of variance (ANOVA) of flowering time of the F2 population Ubi::HvCO2 × Igri under LDs.
Supplemental Table S2. ANOVA of flowering time of the spring/facultative subpopulation Ubi::HvCO2 × Igri F2 population under LDs.
Supplemental Table S3. ANOVA for expression of flowering time genes in the F2 population Ubi::HvCO2 × Igri LDs.
Supplemental Table S4. Pearson correlation coefficients of flowering time and expression of flowering genes in the F2 population Ubi::HvCO2 × Igri under LDs.
Supplemental Table S5. Pearson correlation coefficients of flowering time and expression of flowering genes in the spring/facultative and winter subpopulations of the F2 population Ubi::HvCO2 × Igri under LDs.
Supplemental Table S6. ANOVA of HvFT1 expression and flowering time of the F2 population Ubi::HvCO2 × Igri under SDs.
Supplemental Table S7. List of primers used in this study.
Supplementary Material
Acknowledgments
We thank Kerstin Luxa, Caren Dawidson, and Andrea Lossow for excellent technical assistance.
Glossary
- LD
long-day
- SD
short-day
- SDs
short days
- LDs
long days
- DAE
days after emergence
- cDNA
complementary DNA
Footnotes
This work was supported by the Max Planck Society, by the Deutsche Forschungsgemeinschaft (grant no. SPP1530), by the Excellence Cluster EXC1028, and by the German Academic Exchange Service (DAAD fellowship to M.A.M.).
References
- Alonso-Peral MM, Oliver SN, Casao MC, Greenup AA, Trevaskis B (2011) The promoter of the cereal VERNALIZATION1 gene is sufficient for transcriptional induction by prolonged cold. PLoS One 6: e29456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beales J, Turner A, Griffiths S, Snape JW, Laurie DA (2007) A pseudo-response regulator is misexpressed in the photoperiod insensitive Ppd-D1a mutant of wheat (Triticum aestivum L.). Theor Appl Genet 115: 721–733 [DOI] [PubMed] [Google Scholar]
- Campoli C, Drosse B, Searle I, Coupland G, von Korff M (2012) Functional characterisation of HvCO1, the barley (Hordeum vulgare) flowering time ortholog of CONSTANS. Plant J 69: 868–880 [DOI] [PubMed] [Google Scholar]
- Chen A, Li C, Hu W, Lau MY, Lin H, Rockwell NC, Martin SS, Jernstedt JA, Lagarias JC, Dubcovsky J (2014) Phytochrome C plays a major role in the acceleration of wheat flowering under long-day photoperiod. Proc Natl Acad Sci USA 111: 10037–10044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen AH, Sharrock RA, Quail PH (1992) Maize polyubiquitin genes: structure, thermal perturbation of expression and transcript splicing, and promoter activity following transfer to protoplasts by electroporation. Plant Mol Biol 18: 675–689 [DOI] [PubMed] [Google Scholar]
- Cockram J, Thiel T, Steuernagel B, Stein N, Taudien S, Bailey PC, O’Sullivan DM (2012) Genome dynamics explain the evolution of flowering time CCT domain gene families in the Poaceae. PLoS One 7: e45307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai X, Ding Y, Tan L, Fu Y, Liu F, Zhu Z, Sun X, Sun X, Gu P, Cai H, Sun C (2012) LHD1, an allele of DTH8/Ghd8, controls late heading date in common wild rice (Oryza rufipogon). J Integr Plant Biol 54: 790–799 [DOI] [PubMed] [Google Scholar]
- Decousset L, Griffiths S, Dunford RP, Pratchett N, Laurie DA (2000) Development of STS markers closely linked to the Ppd-H1 photoperiod response gene of barley (Hordeum vulgare L.). Theor Appl Genet 101: 1202–1206 [Google Scholar]
- Distelfeld A, Tranquilli G, Li C, Yan L, Dubcovsky J (2009) Genetic and molecular characterization of the VRN2 loci in tetraploid wheat. Plant Physiol 149: 245–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubcovsky J, Chen C, Yan L (2005) Molecular characterization of the allelic variation at the VRN-H2 vernalization locus in barley. Mol Breed 15: 395–407 [Google Scholar]
- Faure S, Higgins J, Turner A, Laurie DA (2007) The FLOWERING LOCUS T-like gene family in barley (Hordeum vulgare). Genetics 176: 599–609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faure S, Turner AS, Gruszka D, Christodoulou V, Davis SJ, von Korff M, Laurie DA (2012) Mutation at the circadian clock gene EARLY MATURITY 8 adapts domesticated barley (Hordeum vulgare) to short growing seasons. Proc Natl Acad Sci USA 109: 8328–8333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornara F, Panigrahi KC, Gissot L, Sauerbrunn N, Rühl M, Jarillo JA, Coupland G (2009) Arabidopsis DOF transcription factors act redundantly to reduce CONSTANS expression and are essential for a photoperiodic flowering response. Dev Cell 17: 75–86 [DOI] [PubMed] [Google Scholar]
- Fujino K, Sekiguchi H (2005) Mapping of QTLs conferring extremely early heading in rice (Oryza sativa L.). Theor Appl Genet 111: 393–398 [DOI] [PubMed] [Google Scholar]
- Gao H, Jin M, Zheng XM, Chen J, Yuan D, Xin Y, Wang M, Huang D, Zhang Z, Zhou K, Sheng P, Ma J, et al. (2014) Days to heading 7, a major quantitative locus determining photoperiod sensitivity and regional adaptation in rice. Proc Natl Acad Sci USA 111: 16337–16342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenup A, Peacock WJ, Dennis ES, Trevaskis B (2009) The molecular biology of seasonal flowering-responses in Arabidopsis and the cereals. Ann Bot (Lond) 103: 1165–1172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths S, Dunford RP, Coupland G, Laurie DA (2003) The evolution of CONSTANS-like gene families in barley, rice, and Arabidopsis. Plant Physiol 131: 1855–1867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayama R, Yokoi S, Tamaki S, Yano M, Shimamoto K (2003) Adaptation of photoperiodic control pathways produces short-day flowering in rice. Nature 422: 719–722 [DOI] [PubMed] [Google Scholar]
- Hemming MN, Fieg S, Peacock WJ, Dennis ES, Trevaskis B (2009) Regions associated with repression of the barley (Hordeum vulgare) VERNALIZATION1 gene are not required for cold induction. Mol Genet Genomics 282: 107–117 [DOI] [PubMed] [Google Scholar]
- Hemming MN, Peacock WJ, Dennis ES, Trevaskis B (2008) Low-temperature and daylength cues are integrated to regulate FLOWERING LOCUS T in barley. Plant Physiol 147: 355–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepworth SR, Valverde F, Ravenscroft D, Mouradov A, Coupland G (2002) Antagonistic regulation of flowering-time gene SOC1 by CONSTANS and FLC via separate promoter motifs. EMBO J 21: 4327–4337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JA, Bailey PC, Laurie DA (2010) Comparative genomics of flowering time pathways using Brachypodium distachyon as a model for the temperate grasses. PLoS One 5: e10065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imaizumi T, Schultz TF, Harmon FG, Ho LA, Kay SA (2005) FKF1 F-box protein mediates cyclic degradation of a repressor of CONSTANS in Arabidopsis. Science 309: 293–297 [DOI] [PubMed] [Google Scholar]
- Izawa T, Oikawa T, Sugiyama N, Tanisaka T, Yano M, Shimamoto K (2002) Phytochrome mediates the external light signal to repress FT orthologs in photoperiodic flowering of rice. Genes Dev 16: 2006–2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang S, Marchal V, Panigrahi KC, Wenkel S, Soppe W, Deng XW, Valverde F, Coupland G (2008) Arabidopsis COP1 shapes the temporal pattern of CO accumulation conferring a photoperiodic flowering response. EMBO J 27: 1277–1288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi R, Kawahigashi H, Ando T, Tonooka T, Handa H (2009) Molecular and functional characterization of PEBP genes in barley reveal the diversification of their roles in flowering. Plant Physiol 149: 1341–1353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima S, Takahashi Y, Kobayashi Y, Monna L, Sasaki T, Araki T, Yano M (2002) Hd3a, a rice ortholog of the Arabidopsis FT gene, promotes transition to flowering downstream of Hd1 under short-day conditions. Plant Cell Physiol 43: 1096–1105 [DOI] [PubMed] [Google Scholar]
- Koo BH, Yoo SC, Park JW, Kwon CT, Lee BD, An G, Zhang Z, Li J, Li Z, Paek NC (2013) Natural variation in OsPRR37 regulates heading date and contributes to rice cultivation at a wide range of latitudes. Mol Plant 6: 1877–1888 [DOI] [PubMed] [Google Scholar]
- Koornneef M, Hanhart CJ, van der Veen JH (1991) A genetic and physiological analysis of late flowering mutants in Arabidopsis thaliana. Mol Gen Genet 229: 57–66 [DOI] [PubMed] [Google Scholar]
- Kumimoto RW, Adam L, Hymus GJ, Repetti PP, Reuber TL, Marion CM, Hempel FD, Ratcliffe OJ (2008) The Nuclear Factor Y subunits NF-YB2 and NF-YB3 play additive roles in the promotion of flowering by inductive long-day photoperiods in Arabidopsis. Planta 228: 709–723 [DOI] [PubMed] [Google Scholar]
- Kumimoto RW, Zhang Y, Siefers N, Holt BF III (2010) NF-YC3, NF-YC4 and NF-YC9 are required for CONSTANS-mediated, photoperiod-dependent flowering in Arabidopsis thaliana. Plant J 63: 379–391 [DOI] [PubMed] [Google Scholar]
- Laurie DA, Pratchett N, Bezant JH, Snape JW (1995) RFLP mapping of five major genes and eight QTL controlling flowering time in a winter × spring barley cross. Genome 38: 575–585 [DOI] [PubMed] [Google Scholar]
- Li C, Distelfeld A, Comis A, Dubcovsky J (2011) Wheat flowering repressor VRN2 and promoter CO2 compete for interactions with NUCLEAR FACTOR-Y complexes. Plant J 67: 763–773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li WX, Oono Y, Zhu J, He XJ, Wu JM, Iida K, Lu XY, Cui X, Jin H, Zhu JK (2008) The Arabidopsis NFYA5 transcription factor is regulated transcriptionally and posttranscriptionally to promote drought resistance. Plant Cell 20: 2238–2251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin HX, Yamamoto T, Sasaki T, Yano M (2000) Characterization and detection of epistatic interactions of 3 QTLs, Hd1, Hd2, and Hd3, controlling heading date in rice using nearly isogenic lines. Theor Appl Genet 101: 1021–1028 [Google Scholar]
- Matthews PR, Wang M, Waterhouse PM, Thornton S, Fieg SJ, Gubler F, Jacobsen JV (2001) Marker gene elimination from transgenic barley, using co-transformation with adjacent twin ‘T-DNAs’ on a standard Agrobacterium transformation vector. Mol Breed 7: 195–202 [Google Scholar]
- Maurer A, Draba V, Jiang Y, Schnaithmann F, Sharma R, Schumann E, Kilian B, Reif JC, Pillen K (2015) Modelling the genetic architecture of flowering time control in barley through nested association mapping. BMC Genomics 16: 290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami M, Ashikari M, Miura K, Yamashino T, Mizuno T (2003) The evolutionarily conserved OsPRR quintet: rice pseudo-response regulators implicated in circadian rhythm. Plant Cell Physiol 44: 1229–1236 [DOI] [PubMed] [Google Scholar]
- Murphy RL, Klein RR, Morishige DT, Brady JA, Rooney WL, Miller FR, Dugas DV, Klein PE, Mullet JE (2011) Coincident light and clock regulation of pseudoresponse regulator protein 37 (PRR37) controls photoperiodic flowering in sorghum. Proc Natl Acad Sci USA 108: 16469–16474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson DE, Repetti PP, Adams TR, Creelman RA, Wu J, Warner DC, Anstrom DC, Bensen RJ, Castiglioni PP, Donnarummo MG, Hinchey BS, Kumimoto RW, et al. (2007) Plant nuclear factor Y (NF-Y) B subunits confer drought tolerance and lead to improved corn yields on water-limited acres. Proc Natl Acad Sci USA 104: 16450–16455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemoto Y, Kisaka M, Fuse T, Yano M, Ogihara Y (2003) Characterization and functional analysis of three wheat genes with homology to the CONSTANS flowering time gene in transgenic rice. Plant J 36: 82–93 [DOI] [PubMed] [Google Scholar]
- Onouchi H, Igeño MI, Périlleux C, Graves K, Coupland G (2000) Mutagenesis of plants overexpressing CONSTANS demonstrates novel interactions among Arabidopsis flowering-time genes. Plant Cell 12: 885–900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Para A, Farré EM, Imaizumi T, Pruneda-Paz JL, Harmon FG, Kay SA (2007) PRR3 Is a vascular regulator of TOC1 stability in the Arabidopsis circadian clock. Plant Cell 19: 3462–3473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putterill J, Robson F, Lee K, Simon R, Coupland G (1995) The CONSTANS gene of Arabidopsis promotes flowering and encodes a protein showing similarities to zinc finger transcription factors. Cell 80: 847–857 [DOI] [PubMed] [Google Scholar]
- Robson F, Costa MMR, Hepworth SR, Vizir I, Piñeiro M, Reeves PH, Putterill J, Coupland G (2001) Functional importance of conserved domains in the flowering-time gene CONSTANS demonstrated by analysis of mutant alleles and transgenic plants. Plant J 28: 619–631 [DOI] [PubMed] [Google Scholar]
- Saito H, Ogiso-Tanaka E, Okumoto Y, Yoshitake Y, Izumi H, Yokoo T, Matsubara K, Hori K, Yano M, Inoue H, Tanisaka T (2012) Ef7 encodes an ELF3-like protein and promotes rice flowering by negatively regulating the floral repressor gene Ghd7 under both short- and long-day conditions. Plant Cell Physiol 53: 717–728 [DOI] [PubMed] [Google Scholar]
- Shaw LM, Turner AS, Laurie DA (2012) The impact of photoperiod insensitive Ppd-1a mutations on the photoperiod pathway across the three genomes of hexaploid wheat (Triticum aestivum). Plant J 71: 71–84 [DOI] [PubMed] [Google Scholar]
- Shibaya T, Nonoue Y, Ono N, Yamanouchi U, Hori K, Yano M (2011) Genetic interactions involved in the inhibition of heading by heading date QTL, Hd2 in rice under long-day conditions. Theor Appl Genet 123: 1133–1143 [DOI] [PubMed] [Google Scholar]
- Stephenson TJ, McIntyre CL, Collet C, Xue GP (2007) Genome-wide identification and expression analysis of the NF-Y family of transcription factors in Triticum aestivum. Plant Mol Biol 65: 77–92 [DOI] [PubMed] [Google Scholar]
- Tingay S, McElroy E, Kalla R, Fieg S, Wang M, Thornton S, Brettell R (1997) Agrobacterium tumefaciens-mediated barley transformation. Plant J 11: 1369–1376 [Google Scholar]
- Tiwari SB, Shen Y, Chang HC, Hou Y, Harris A, Ma SF, McPartland M, Hymus GJ, Adam L, Marion C, Belachew A, Repetti PP, et al. (2010) The flowering time regulator CONSTANS is recruited to the FLOWERING LOCUS T promoter via a unique cis-element. New Phytol 187: 57–66 [DOI] [PubMed] [Google Scholar]
- Trevaskis B, Hemming MN, Dennis ES, Peacock WJ (2007) The molecular basis of vernalization-induced flowering in cereals. Trends Plant Sci 12: 352–357 [DOI] [PubMed] [Google Scholar]
- Trevaskis B, Hemming MN, Peacock WJ, Dennis ES (2006) HvVRN2 responds to daylength, whereas HvVRN1 is regulated by vernalization and developmental status. Plant Physiol 140: 1397–1405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji H, Taoka K, Shimamoto K (2011) Regulation of flowering in rice: two florigen genes, a complex gene network, and natural variation. Curr Opin Plant Biol 14: 45–52 [DOI] [PubMed] [Google Scholar]
- Turck F, Fornara F, Coupland G (2008) Regulation and identity of florigen: FLOWERING LOCUS T moves center stage. Annu Rev Plant Biol 59: 573–594 [DOI] [PubMed] [Google Scholar]
- Turner A, Beales J, Faure S, Dunford RP, Laurie DA (2005) The pseudo-response regulator Ppd-H1 provides adaptation to photoperiod in barley. Science 310: 1031–1034 [DOI] [PubMed] [Google Scholar]
- Turner AS, Faure S, Zhang Y, Laurie DA (2013) The effect of day-neutral mutations in barley and wheat on the interaction between photoperiod and vernalization. Theor Appl Genet 126: 2267–2277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Li Z, Matthews PR, Upadhyaya NM, Waterhouse PM (1998) Improved vectors for Agrobacterium tumefaciens-mediated transformation of monocot plants. Acta Hortic 461: 401–407 [Google Scholar]
- Wei X, Xu J, Guo H, Jiang L, Chen S, Yu C, Zhou Z, Hu P, Zhai H, Wan J (2010) DTH8 suppresses flowering in rice, influencing plant height and yield potential simultaneously. Plant Physiol 153: 1747–1758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenkel S, Turck F, Singer K, Gissot L, Le Gourrierec J, Samach A, Coupland G (2006) CONSTANS and the CCAAT box binding complex share a functionally important domain and interact to regulate flowering of Arabidopsis. Plant Cell 18: 2971–2984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm EP, Turner AS, Laurie DA (2009) Photoperiod insensitive Ppd-A1a mutations in tetraploid wheat (Triticum durum Desf.). Theor Appl Genet 118: 285–294 [DOI] [PubMed] [Google Scholar]
- Xue W, Xing Y, Weng X, Zhao Y, Tang W, Wang L, Zhou H, Yu S, Xu C, Li X, Zhang Q (2008) Natural variation in Ghd7 is an important regulator of heading date and yield potential in rice. Nat Genet 40: 761–767 [DOI] [PubMed] [Google Scholar]
- Yan L, Fu D, Li C, Blechl A, Tranquilli G, Bonafede M, Sanchez A, Valarik M, Yasuda S, Dubcovsky J (2006) The wheat and barley vernalization gene VRN3 is an orthologue of FT. Proc Natl Acad Sci USA 103: 19581–19586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan L, Loukoianov A, Blechl A, Tranquilli G, Ramakrishna W, SanMiguel P, Bennetzen JL, Echenique V, Dubcovsky J (2004) The wheat VRN2 gene is a flowering repressor down-regulated by vernalization. Science 303: 1640–1644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan WH, Wang P, Chen HX, Zhou HJ, Li QP, Wang CR, Ding ZH, Zhang YS, Yu SB, Xing YZ, Zhang QF (2011) A major QTL, Ghd8, plays pleiotropic roles in regulating grain productivity, plant height, and heading date in rice. Mol Plant 4: 319–330 [DOI] [PubMed] [Google Scholar]
- Yang S, Weers BD, Morishige DT, Mullet JE (2014) CONSTANS is a photoperiod regulated activator of flowering in sorghum. BMC Plant Biol 14: 148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano M, Katayose Y, Ashikari M, Yamanouchi U, Monna L, Fuse T, Baba T, Yamamoto K, Umehara Y, Nagamura Y, Sasaki T (2000) Hd1, a major photoperiod sensitivity quantitative trait locus in rice, is closely related to the Arabidopsis flowering time gene CONSTANS. Plant Cell 12: 2473–2484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zadoks JC, Chang TT, Konzak CF (1974) A decimal code for the growth stages of cereals. Weed Res 14: 415–421 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.