Numerous compounds inducing leaf vein pattern defects target auxin biology.
Abstract
The critical role of veins in transporting water, nutrients, and signals suggests that some key regulators of vein formation may be genetically redundant and, thus, undetectable by forward genetic screens. To identify such regulators, we screened more than 5000 structurally diverse small molecules for compounds that alter Arabidopsis (Arabidopsis thaliana) leaf vein patterns. Many compound-induced phenotypes were observed, including vein networks with an open reticulum; decreased or increased vein number and thickness; and misaligned, misshapen, or nonpolar vascular cells. Further characterization of several individual active compounds suggests that their targets include hormone cross talk, hormone-dependent transcription, and PIN-FORMED trafficking.
The directional flow of auxin is the primary agent guiding the formation of vein networks, according to the original auxin canalization model for plant vein patterning (Sachs, 1991; Nelson and Dengler, 1997). Many experimental data now augment this model with mechanistic detail, such as the dynamic localization of auxin transporters during development (Gälweiler et al., 1998; Friml et al., 2003; Benková et al., 2003). Initially, the intercellular flow of auxin is random, but PIN-FORMED (PIN) transporters with polar localization in cell membranes gradually direct auxin flow toward a single “canal” that subsequently differentiates into a connected line of vascular cells. Based on the observed dynamic changes in leaf PIN1 expression and orientation, epidermal auxin convergence points among leaf marginal cells appear to guide the position of major veins. The subsequent inward directional flow of auxin guides vascular strand formation toward preexisting veins (Scarpella et al., 2006).
Numerous regulators of vein patterning in Arabidopsis (Arabidopsis thaliana) have been identified by a combination of genetic screens, inhibitor studies, and vascular cell profiling (Cano-Delgado et al., 2010; Gandotra et al., 2013). Among the venation factors identified are those with roles in auxin signaling and transport (Hardtke and Berleth, 1998; Hobbie et al., 2000; Hamann et al., 2002; Mattsson et al., 1999; Candela et al., 2007; Péret et al., 2012), leaf development (Robles et al., 2010), and cell biological processes (Steinmann et al., 1999; Koizumi et al., 2005; Sieburth et al., 2006; Naramoto et al., 2014), including sterol and lipid biosynthesis (Schrick et al., 2000; Carland et al., 2002; Souter et al., 2002; Carland and Nelson, 2009; Naramoto et al., 2009; Tejos et al., 2014). However, given the critical role of veins for the transport of photosynthate, water, and signaling molecules, it is likely that some components and regulators are present in multiple copies and, thus, undetectable in typical genetic screens. Approximately two-thirds of Arabidopsis genes have at least one close relative, and more than one-third belong to gene families with five or more members (Blackwell and Zhao, 2003).
A chemical biology approach offers an alternative method to identify the components of developmental signaling pathways, including genetically redundant or knockout-lethal ones (Kawasumi and Nghiem, 2007). The ability of bioactive small molecules to act on and to reveal targets specifically, rapidly, and reversibly in a concentration-dependent manner makes them ideal as probes of developmental processes (Blackwell and Zhao, 2003; Yeh and Crews, 2003; Han et al., 2009). The application and removal of active compounds can be limited in time and space, to determine critical periods and to overcome lethality (Tavassoli et al., 2011). For example, this approach has been useful for manipulating the Hedgehog (Hh), Wnt, and bone morphogenic protein signaling pathways (Sakata and Chen, 2011).
A phenotypic screen of chemical libraries is hypothesis-generating, in that candidate compounds are recovered based on their bioactivity, without prior knowledge of their target proteins (Kawasumi and Nghiem, 2007). The bioactivities of libraries of compounds tested under a broad spectrum of conditions are archived in community databases in an ongoing manner (Wagner and Clemons, 2009). Thus far, most compounds have been assayed for the induction of phenotypes on human cell lines, although data are accumulating for the generation of plant phenotypes. In plants, the abscisic acid receptor was identified in a chemical genetic screen after classical genetic strategies were unsuccessful (Park et al., 2009). Chemical agonists and antagonists have similarly been identified for plant endomembrane systems, hormonal signaling pathways, and other features (Yamazoe et al., 2005; Gendron et al., 2008; Tsuchiya et al., 2010; Drakakaki et al., 2011; He et al., 2011; Hicks and Raikhel, 2012; Stokes and McCourt, 2014).
Vein development is regulated by the small molecule hormone auxin, suggesting that the process can be perturbed by exogenous small molecules (Tsuchiya et al., 2010). Indeed, the application of small molecule polar auxin transport inhibitors (PATI) severely disrupts leaf vascular cell polarity and alignment by restricting the flow of auxin to leaf margins (Mattsson et al., 1999; Sieburth, 1999). One such PATI, 1-N-naphthylphthalamic acid (NPA), binds to an actin-interacting peripheral plasma membrane protein that appears to be a regulatory component of the auxin efflux carrier complex (Rubery, 1990; Muday, 2000). A screen for mutants resistant to another compound, the antiauxin p-chlorophenoxyisobutyric acid (PCIB), identified a novel component of auxin signaling (Biswas et al., 2007), reinforcing the value of the chemical interference approach. Bioactive chemicals are likely to expand the range of phenotypes that can inform the theoretical modeling of the development of leaf vein patterns, which are currently limited to the pattern variants produced by genetic mutants (Rolland-Lagan and Prusinkiewicz, 2005).
In this study, we identify compounds able to perturb vein patterns in developing Arabidopsis leaves, using high-throughput phenotypic screening of chemical libraries. We identify a range of specific compounds that can induce novel vein patterns and that point to previously undetected regulators of vascular networks. We analyze nine specific compounds producing particularly strong phenotypes in three classes. Their characterization suggests that their targets include hormone cross talk, hormone-dependent transcription, and PIN trafficking.
RESULTS
High-Throughput Identification of Compounds Affecting Vein Patterning
We identified compounds with activity on vein patterning from a large and diverse collection of small molecules, using a 96-well plate assay that revealed leaf xylem cell abnormalities in 12-d-old seedlings. Tracheary elements, the main conducting cells of xylem, are visible in leaves cleared of chlorophyll and serve as reporters of vein pattern defects (Fig. 1A). We screened three collections of small molecules: (1) the library of compounds active on Arabidopsis (LATCA; approximately 3600 compounds), (2) 800 natural product molecules, and (3) approximately 1120 compounds from a commercially available library of diverse compounds. Numerous compounds affecting vein pattern (CAVPs) were identified in these collections and categorized into phenotypic classes.
Figure 1.
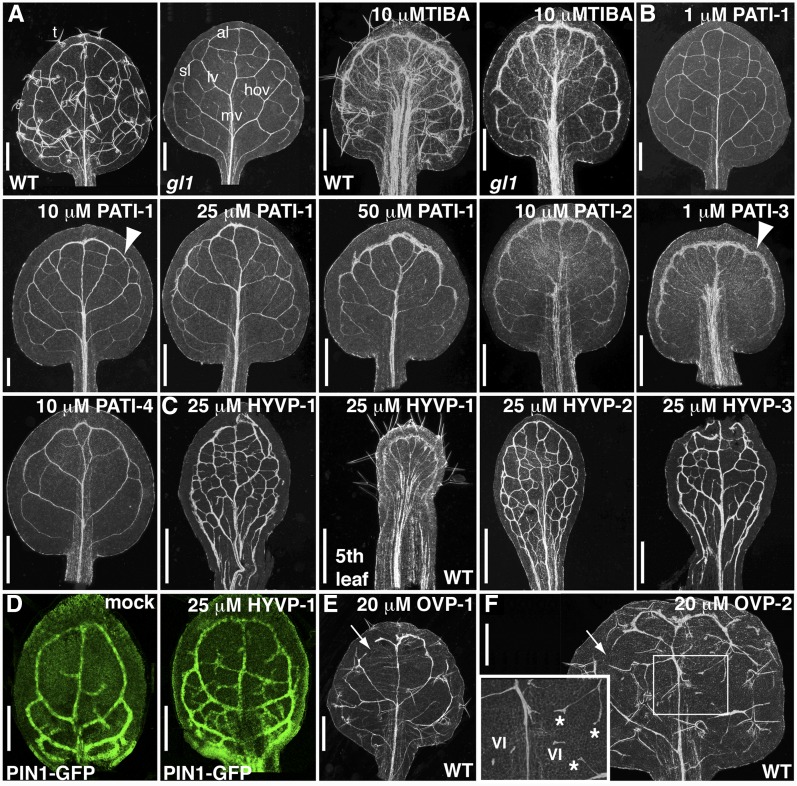
Compound-induced vein pattern defects. A to C, First rosette cleared leaves of 12-d-old gl1 seedlings (unless otherwise noted) from compound-grown plants. A, gl1 has similar compound responsiveness to 10 µm TIBA as wild type (WT). T, Trichomes; mv, midvein; al, apical loop; sl, second loop; lv, lateral vein; hov, higher order veins. B, PATI-induced phenotypes. Arrowhead indicates thick marginal ring. C, HYVP (25 µm)-induced vein patterns to show extensive venation network. Note elongated, wide petiole and reduced lamina of fifth leaf from HYVP-1-treated plants. D, Confocal images of PIN1-GFP-labeled incipient vascular cells in developing leaves shows that the extensive vein branching in 25 µm HYVP-1-treated leaves occurs at an early stage. E and F, First rosette cleared leaves of 12-d-old wild-type seedlings grown on media containing 20 µm OVP-1 (E) or 20 µm OVP-2 (F). Open loops are indicated with arrows. Boxed region of OVP-2 is at higher magnification (inset). VI, Vascular islands (short stretches of unconnected vein). Vascular cells have irregular morphology, bifurcated vein endings, and delayed differentiation (asterisks). Tracheary elements appear as white lines in cleared leaves viewed under dark-field microscopy. Bars, 500 µm (A–C, E, and F), or 100 µm (D).
We selected nine CAVPs representing three phenotypic classes for further study because they demonstrated reproducible effects, acted in a dose-dependent manner, and were commercially available. Compounds were named for their elicited phenotype and included PATI-1 to -4, hypervascular pattern (HYVP)-1 to -3, and open vein pattern (OVP)-1 and -2 (Supplemental Table S1).
PATIs
A large group of previously uncharacterized compounds caused vein patterning changes similar to those of known PATIs and of the pin1 mutant (Mattsson et al., 1999; Sieburth, 1999). In control leaves, lateral or secondary veins branch from the prominent centrally positioned midvein. The later-forming higher order veins, including tertiary and quaternary orders, are positioned between lateral veins to produce a highly reticulated network of connected veins (Fig. 1A). To assist with visualizing leaf vein patterns, we used a glabrous (gl1) background that shows a response to chemicals, such as the PATI 2,3,5-triiodobenzoic acid (TIBA), similar to that of wild type (Fig. 1A). Leaves of plants treated with PATI-1 to -4 were characterized by a thickened marginal vascular ring, decreased higher order vein network, bifurcated apical midvein, and an enlarged, displaced midrib (Fig. 1B; Supplemental Figs. S1, A–C, and S2A). PATI-3 was the most potent compound, with PATI-4, PATI-3, and PATI-1 positioned in the order of decreasing potency. Rosettes were compacted, with shortened petioles and smooth-margined leaves due to loss of leaf serrations. Although cotyledon vein networks are established during embryogenesis, prior to administration of test compounds, they were still susceptible to perturbation. New postgermination vascular growth in the radial axis was affected by compound exposure evidenced by vein number reduction accompanied by misaligned vascular cells (Supplemental Fig. S1, B and C).
HYVP
Plants treated with compounds in the HYVP class exhibited thickened veins and an increase in vein reticularization at screening levels (Fig. 1C; Supplemental Figs. S1, D–F, and S2B). In addition, late-forming leaves (the fifth leaf and above) exhibited PATI-like vein patterning aberrations, including a thickened marginal vein, a reduction in higher vein orders, and a prominent midrib (Fig. 1C). The auxin efflux transporter PIN1 is the earliest known marker for vein formation with expression preceding vascular cell formation (Scarpella et al., 2006). PIN1::GFP localization in HYVP-1-treated seedlings revealed extensive branching in developing leaves, indicating that the highly reticulated pattern is established early in development (Fig. 1D). The HYVP class represents a novel phenotype since no mutants with increased branching have been recovered from traditional screens (Scarpella and Meijer, 2004).
OVP
Two compounds converted the normally closed leaf vein reticulum to an open network. OVP-1 caused irregular vein formation with reduced branching and thickened veins with misaligned and poorly differentiated vascular cells, particularly at higher doses (Fig. 1E; Supplemental Figs. S1, G and H, and S2, C and D). OVP-2 caused an increase in free vein endings composed of irregular vascular cell morphology and differentiation (Fig. 1F; Supplemental Figs. S1I and S2E). In contrast to vein connectivity-reducing mutations such as cvp2 (Carland and Nelson, 2004), forked1(fkd1), and fkd2 (Steynen and Schultz, 2003), the overall vein pattern of OVP-2-treated leaves was dramatically altered and exhibited a reduced number of thickened lateral veins, regions of poorly differentiated vascular cells, and bifurcated vein endings.
The compounds in each of these three classes were subjected to dose-response tests. Subsequent characterization was performed at the minimum dose required to yield representative phenotypes (10 μm for the PATI-1, -2, and -4; 1 μm for PATI-3; 25 μm for HYVPs; and 20 μm for OVPs) to avoid nonspecific effects due to inhibitor promiscuity (Fig. 1; Supplemental Fig. S1). Several of the CAVPs exhibited distinct effects on root and hypocotyl length in addition to their observed effects on leaves (Supplemental Fig. S3, A and B). In HYVP-treated leaves, the petiole was hyperextended with severely reduced lamina formation, sometimes barely exceeding petiole width, particularly at high doses or prolonged exposure (Fig. 1C; Supplemental Figs. S1, D–F, and S3, C–E). An advantage of small molecules is their ability to act temporally so as to manipulate their bioactivity in a conditional manner. The effects of tested CAVPs were completely reversible. When plants were grown on compound-containing media for 17 d and then transferred to compound-free media, new growth resumed at wild-type rates and patterns (Supplemental Fig. S4).
Patterns of Leaf Auxin Accumulation Correlate with CAVP-Induced Vein Pattern Phenotypes
Because auxin influences vein patterning and differentiation, we examined auxin accumulation patterns in the presence of selected active compounds, using the auxin-responsive reporter DR5::GUS (Ulmasov et al., 1997). In light-grown wild-type seedlings, DR5::GUS activity marks incipient veins in developing leaves, progressively diminishing its expression basipetally as veins and leaves mature (Mattsson et al., 2003). In mature leaves and cotyledons, GUS activity is restricted to the apical margin (Fig. 2A). Both PATI and HYVP compounds led to pronounced and expanded domains of GUS reporter expression (Fig. 2B). OVP compounds induced distinct patterns. Young leaves treated with OVP-1 exhibited a normal but reduced auxin accumulation pattern in vascular regions, consistent with the overall compound-induced decrease in vein density (Fig. 2C). Growth on OVP-2-containing media resulted in a persistent and vein-specific auxin accumulation throughout the leaf (excluding the midvein region) and did not diminish progressively from tip to base (Fig. 2D). This sustained DR5::GUS expression pattern parallels the prolonged vascular development associated with increased radial expansion, particularly in the leaf apical regions, and delayed vascular cell differentiation.
Figure 2.
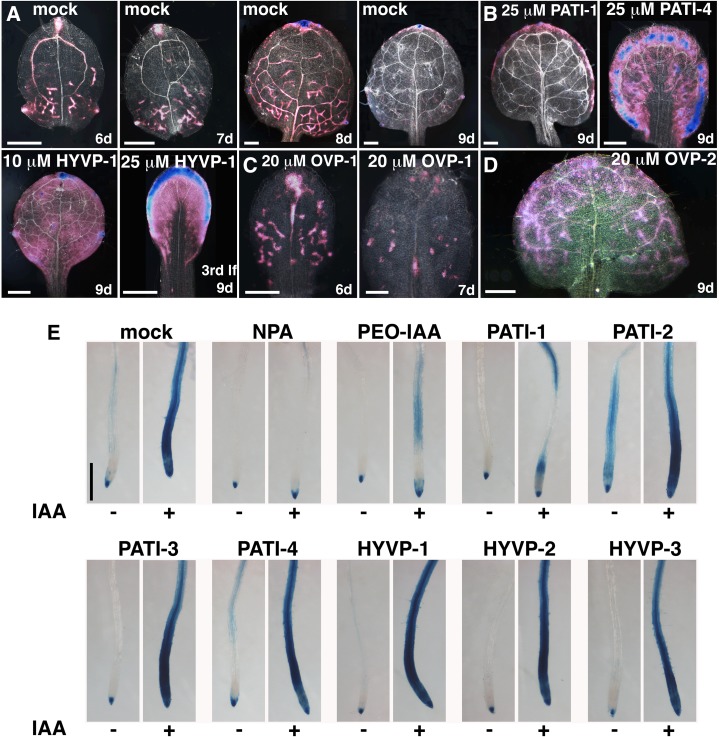
Auxin response to compounds. A to D, Images of GUS-stained DR5::GUS leaves with indicated treatment viewed with dark-field optics. A, Series of a developing wild-type leaf shows progressively diminishing DR5::GUS reporter expression in veins. B, Treatment with PATI-1, PATI-4, and HYVP-1 dramatically broadens the pattern of DR5::GUS expression. lf, Leaf. C, OVP-1 causes reduced DR5::GUS expression consistent with reduced vein number. D, OVP-2-treated leaves exhibit sustained DR5::GUS vascular expression throughout the leaf. Strong and weak GUS is indicated by blue and pink colors, respectively. E, Auxin response in compound-treated roots. Images are of 4-d-old GUS-stained DR5::GUS seedling roots treated with compound in absence (left) or presence (right) of auxin (IAA). PATI-1 inhibits auxin response in contrast to the remaining CAVPs, which have no effect. Concentrations: 1 µm, PATI-3; 10 µm, NPA, PEO-IAA, PATI-1, -2, and -4; 25 µm, HYVPs; 1 µm IAA. Bars, 250 µm (A–D) and 500 µm (E).
To examine whether the compounds acted as auxin mimics or affected auxin response or transport, we used the DR5::GUS reporter to compare auxin activity levels in the root following auxin or CAVP treatment (Fig. 2E). In control seedlings, auxin supplementation induced strong GUS levels throughout the root meristematic and elongation zones. In the presence of NPA, a known PATI, there was no auxin accumulation relative to untreated seedlings. Auxin was restricted to root tips due to failure of auxin redistribution. A similar result was reported for the known PATI TIBA (Surpin et al., 2005). The auxin action inhibitor PCIB (Supplemental Fig. S5) and the specific auxin receptor antagonist PEO-IAA (Hayashi et al., 2012) also strongly prevented DR5::GUS reporter expression, presumably due to inhibition of the auxin response. In tests of CAVP compounds, PATI-1 prevented confluent DR5::GUS expression. PATI-2 showed weak GUS reporter levels in the absence of exogenous auxin and, thus, may possess limited auxin activity, and it did not prevent a robust auxin response. PATI-3 and HYVPs demonstrated auxin responses like that of mock-treated seedlings, even at high concentrations (Supplemental Fig. S5). These results suggest that PATI-2, -3, -4, and HYVPs represent novel compounds able to disrupt leaf vein patterning via mechanisms distinct from the inhibition of auxin transport or auxin responses in the root.
CAVPs Influence Apical Hook Curvature in an Opposing Manner
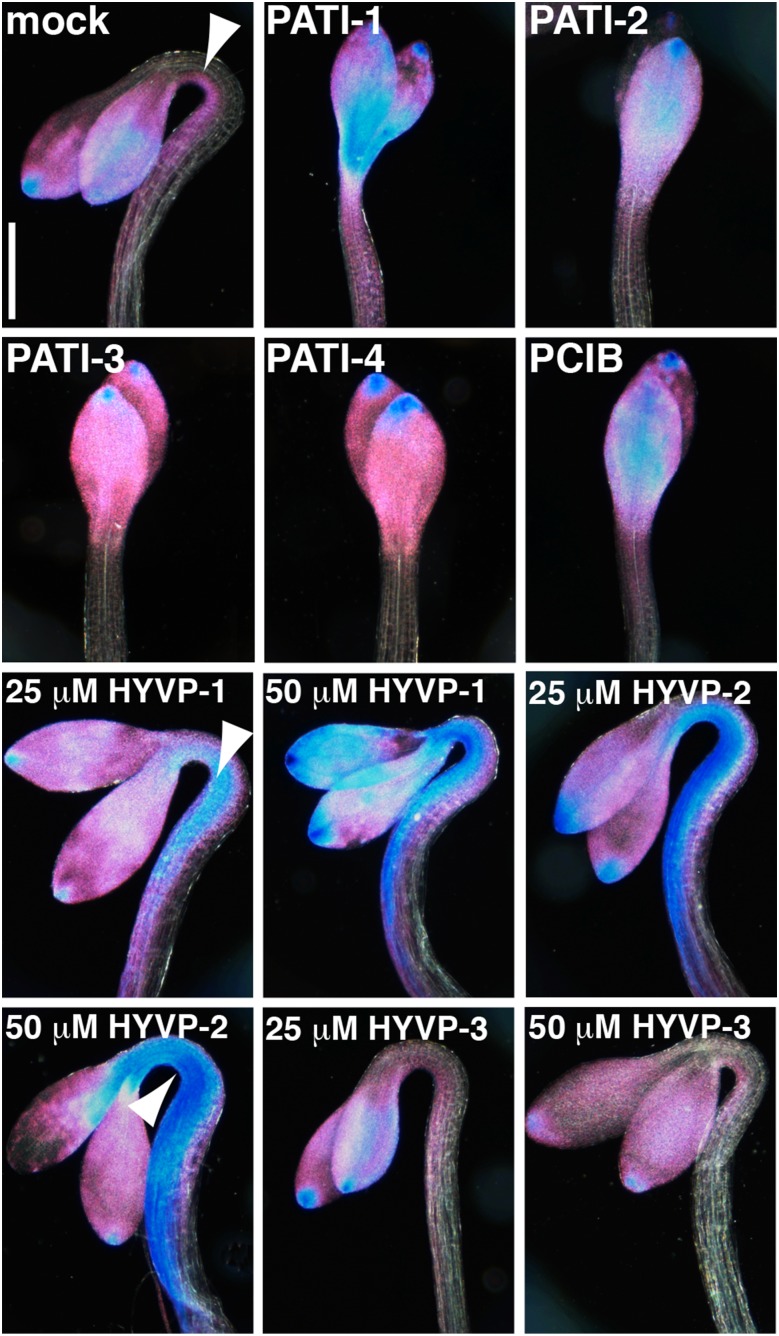
To characterize further the targets of CAVP treatment, we examined whether CAVPs affected the auxin-ethylene network that regulates hypocotyl apical hook formation. In dark-grown seedlings, the apical hook is formed by the interaction between ethylene and auxin. Ethylene is thought to promote auxin transport and synthesis by redistributing auxin efflux carriers and synthesis components to the underside of the hook to allow the nonuniform accumulation of auxin that produces asymmetric cell elongation and, thus, growth curvature (for review, see Muday et al. [2012]). Ethylene-deficient mutants lack apical hooks because they fail to redistribute PINs and auxin. Treatment with PATI-1 through -4 caused loss of apical hook formation, accompanied by symmetric DR5::GUS reporter expression on both hypocotyl sides, a pattern similar to that for the known PATIs TIBA and NPA (Zádníková et al., 2010; Lehman et al., 1996), and for the antiauxin PCIB (Fig. 3). HYVP-3 had no affect on curvature or auxin accumulation. In contrast, HYVP-1 and -2 resulted in hook curvature with an expanded and robust asymmetric DR5::GUS expression domain, particularly at elevated concentrations, compared to mock-treated controls. These results suggest that HYVPs act mechanistically distinct from PATIs and PCIB, possibly by promoting auxin transport, and are consistent with the HYVP-dependent vein pattern defects characterized by an extensively complex thickened vein network.
Figure 3.
DR5::GUS reporter expression in PATI- and HYVP-treated etiolated seedlings. Images are of cleared 3-d-old GUS-stained DR5::GUS seedlings grown in the dark with the indicated compound and viewed with dark-field optics. Arrowhead denotes asymmetric pattern of DR5::GUS expression on the hook underside. PATI and PCIB treatment eliminates hook formation and asymmetric DR5::GUS expression in contrast to HYVP-1 and -2 treatment, which shows strong DR5::GUS asymmetric expression. HYVP-3-treated seedlings are indistinguishable from mock-treated. Strong and weak GUS is indicated by blue and pink colors, respectively. Concentrations: 1 µm, PATI-3; 10 µm, PATI-1, -2, and -4; and 25 μm, PCIB. Bar, 500 µm.
PATI-1 and -2 Disrupt PIN2 Cycling
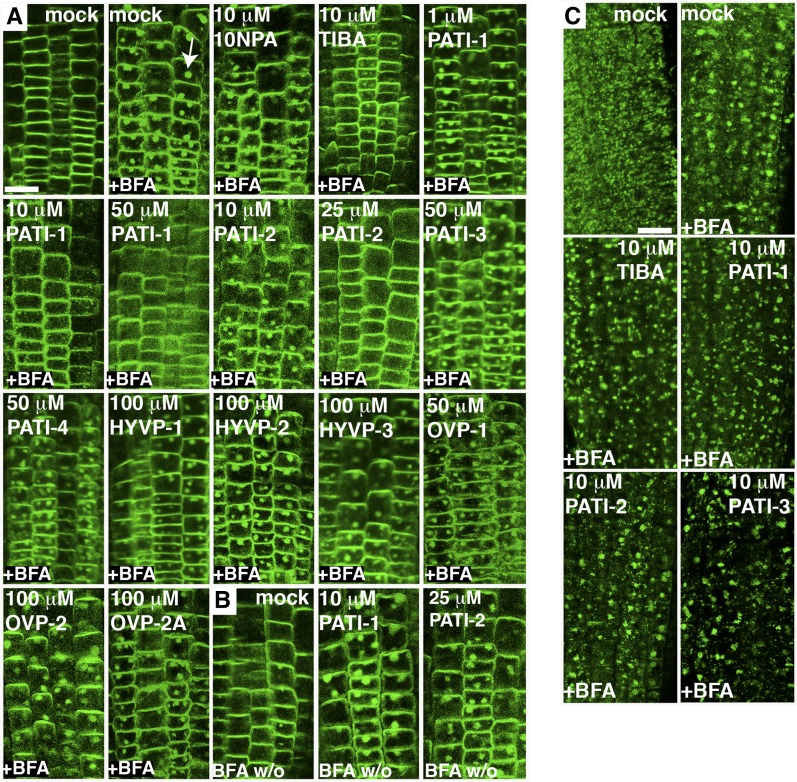
Some previously characterized PATIs appear to inhibit auxin efflux by disrupting intracellular PIN cycling between endosomal and plasma membrane compartments (Geldner et al., 2001). We examined whether CAVPs affected PIN trafficking by monitoring the Brefeldin A (BFA)-mediated aggregation of PIN-GFP-containing endosomes in treated roots. PATI-1 and -2 prevented BFA formation of PIN2-GFP (Xu and Scheres, 2005) in a concentration-dependent manner, similar to the known PATIs TIBA and NPA (Fig. 4A; Supplemental Table S3). They also prevented cycling of PIN2 from the endosomes to the plasma membrane, as revealed by the presence of BFA bodies after BFA wash out (Fig. 4B). In contrast, PATI-3 and -4, HYVP-1 and -2, and OVP-1 and -2 did not affect PIN2-GFP cycling, even at high doses. PIN1-GFP (Benková et al., 2003) responded in a similar manner to the CAVPs (Supplemental Fig. S6). We used the endosomal marker VHA-a1-GFP to investigate whether the PIN trafficking deficient-inducing compounds disrupted movement of additional endosomal proteins (Viotti et al., 2013). TIBA was previously shown to block BFA-dependent endosomal aggregate formation of two additional proteins, suggesting PATIs act by blocking vesicle trafficking (Geldner et al., 2001). Interestingly, as shown in Figure 4C, bioactive levels of TIBA did not inhibit BFA-elicited aggregate formation of VHA-a1-GFP possibly indicating specificity in protein trafficking. Similarly, PATI-1, and PATI-2 had no effect on BFA compartment formation in roots, suggesting that these PATIs may specifically affect PIN cycling rather than affecting membrane trafficking in general. As expected, PATI-3 did not affect BFA-induced VHA-a1-GFP aggregates and served as a control.
Figure 4.
Compound effects on endosomal trafficking. A and B, Confocal images of PIN2-GFP-expressing root epidermal cells with indicated treatments. A, BFA (50 μm) supplementation causes the formation of endosomal aggregates or BFA compartments (arrow). PIN2-GFP-expressing roots were pretreated with compound for 30 min prior to the addition of BFA for an additional hour. B, PATI-1 and -2 retain BFA bodies in washout experiments. Roots were treated with BFA for 2 h, followed by 2 h of washouts in the presence of compound. BFA w/o, BFA washout. C, Compounds do not inhibit BFA-induced VHA-a1-GFP endosomal aggregate formation. Confocal images of VHA-a1-GFP-expressing root epidermal cells with indicated treatments. Bar, 25 µm.
CAVPs Elicit Distinct Effects on PIN Transcription
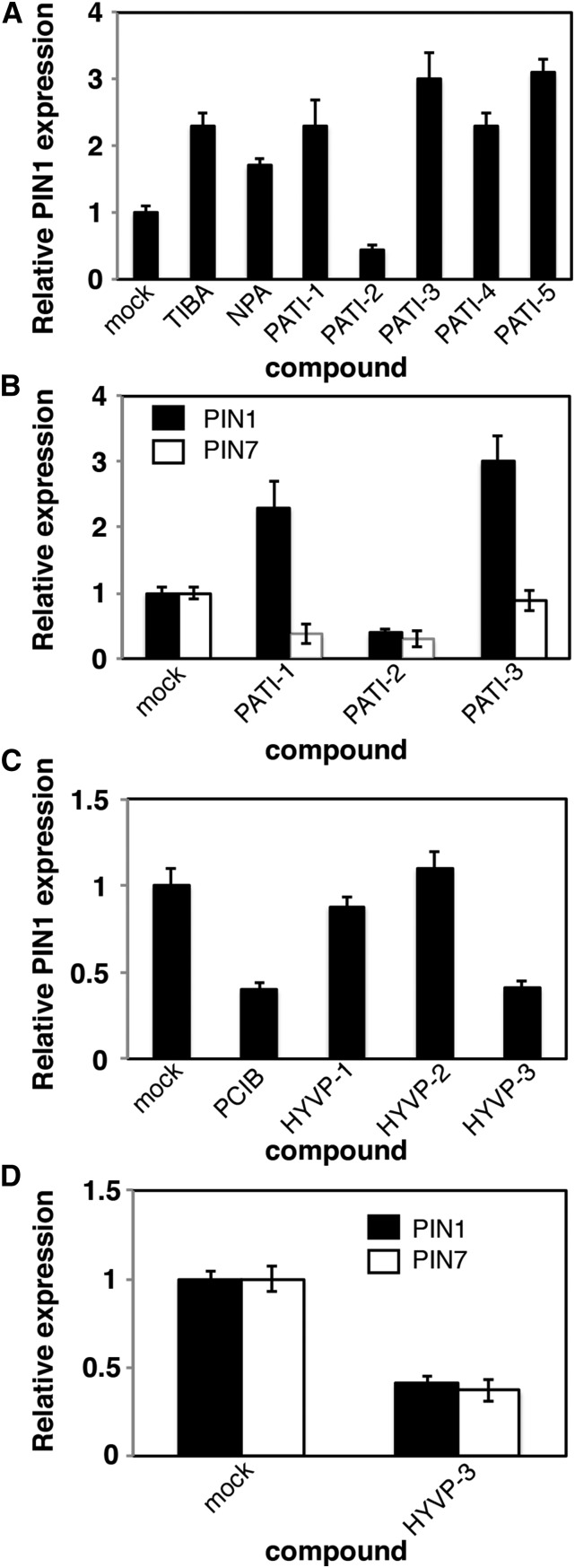
We investigated the PIN RNA levels by quantitative real-time PCR (qRT-PCR) in 7-d-old seedlings following 4-h treatments with CAVPs, TIBA, or NPA. Consistent with previous reports, the known PATIs TIBA and NPA activate PIN1 transcription (Peer et al., 2004; Fig. 5A). Similarly, we found that PATI-1, -3, -4, and -5 (Supplemental Table S2) also increase PIN1 RNA amounts (Fig. 5A). However, PIN1 levels are significantly reduced in the presence of PATI-2. Loss-of-function pin mutants exhibit transcriptional compensation through the activation of additional PIN family members (Vieten et al., 2005). Therefore, we monitored changes in RNA levels of a related member. We found that shoot-expressed PIN7 was reduced by PATI-1 and -2 treatment, relative to the mock-treated control (Fig. 5B). PATI-2 elicits more dramatic vein network defects than PATI-1, suggesting that reduced PIN1 and PIN7 may in part be responsible for the observed PATI-2-mediated severity. Although HYVP-1 and -2 had no effect on PIN1 transcription, HYVP-3 and the antiauxin PCIB reduced PIN1 and in the case of HYVP-3, PIN7 expression levels (Fig. 5, C and D). OVP-1 and -2 had no effect on transcription levels (Supplemental Fig. S7). Etiolated seedlings showed different responses among the HYVPs. The reduction in transcription levels of multiple PIN family members by HYVP-3 maybe contributing to the venation pattern defects. Together, these results suggest that HYVP-3 is mechanistically distinct from HYVP-1 and -2. In summary, PATIs and HYVP-3 affect PIN1 expression generally in an opposing manner.
Figure 5.
Compounds affect PIN transcription. A to D, qRT-PCR analysis of transcriptional levels for indicated genes. RNA was isolated from 7-d-old media-grown seedlings that were treated with compound for 4 h at room temperature. A, PIN1 transcriptional levels. Most PATIs (at 10 µm) increase PIN1 expression level. B, PATI-1 and -2 (at 10 µm) reduce PIN7 expression level. C, HYVPs elicit varying effects on PIN1 and PIN7 transcription, 25 µM compound treatment. D, HYVP-3 (25 µm) reduces PIN1 and PIN7 expression levels. Bars denote ± sd derived from three separate experiments with three technical replicates per experiment.
Candidate Targets for OVPs
OVP-1 Targets a BR-Associated Serine Hydrolase
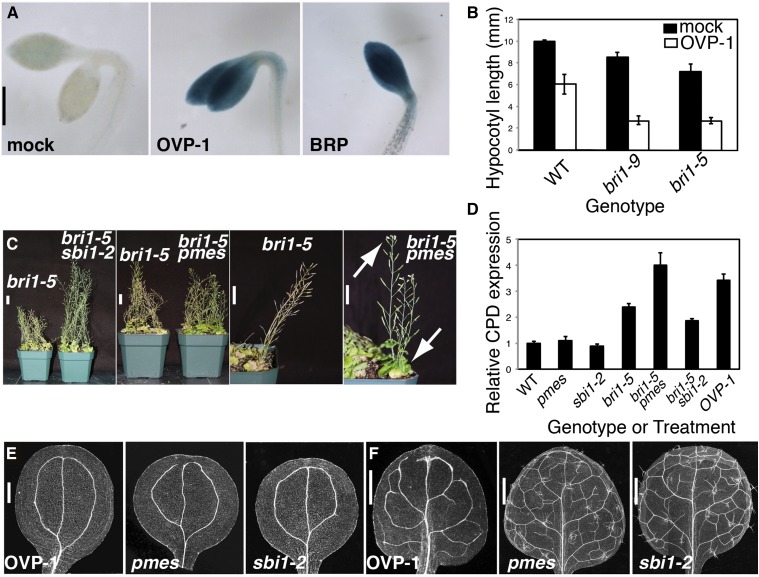
Prolonged exposure to OVP-1 produced phenotypes resembling that of the sterol methyltransferase (smt) mutants, smt1 and smt3, and cotyledon vascular pattern-1 (cvp1; Supplemental Fig. S1H). We tested the response of smt mutants to OVP-1 treatment and observed severely misaligned and poorly differentiated vascular cells (Supplemental Fig. S8). This enhanced phenotype suggests that OVP-1 does not directly act on SMT enzymes, but amplifies the sterol deficiency, possibly by targeting the BR/sterol regulatory circuitry.
The bioactivity of OVP-1 in other systems supports the hypothesis that it acts on a BR/sterol-related network. We found that OVP-1 and some other CAVPs have bioactivities previously measured in other organisms, compiled in PubChem BioAssay (http://pubchem.ncbi.nlm.nih.gov), SciFinder, and other comprehensive bioactivity databases. (Supplemental Table S1). OVP-1 had been identified as an inhibitor of human protein phosphatase methylesterase1 (hsPME1), a serine hydrolase (SH), which demethylates the catalytic subunit of heterotrimeric PP2A (PP2A-C; Bachovchin et al., 2009). PP2A signaling is highly conserved among eukaryotes (Moorhead et al., 2009). Indeed, there is extensive homology between Arabidopsis protein phosphatase methylesterase (PMES; At4g10050) and human PME1 (Xing et al., 2008; Supplemental Fig. S9). Although Arabidopsis PP2A-C has not yet been shown to interact physically with the PP2A signaling complex, the five-member family of AtPP2A-C (C1–C5) has a role in hormonal pathways (DeLong, 2006). In Arabidopsis, redundant PP2A-C3 and PP2A-C4 regulate auxin transport during embryogenesis, and PP2A-C5 influences BR signaling (Tang et al., 2010; Ballesteros et al., 2013). Furthermore, a C subunit methylation-dependent mechanism is implicated in BR signaling with the identification of leucine carboxyl methyltransferase (LCMT) as a suppressor of bri-5 (sbi1; Wu et al., 2011). LCMT methylates PP2A-C and, thus, has an antagonistic function to PME1.
Initially, we tested whether BR signaling was deficient in compound-grown etiolated seedlings using the BR-repressed reporter CPD::GUS (Mathur et al., 1998). Surprisingly, most CAVPs did not disrupt BR signaling, indicated by the absence of CPD:GUS activation, although there is significant overlap between auxin and BR hormonal signaling (Nemhauser et al., 2004; Supplemental Fig. S10). In contrast, OVP-1 showed bioactivity at levels comparable to the positive control, the BR biosynthetic inhibitor brassinopride (BRP; Gendron et al., 2008; Fig. 6A).
Figure 6.
OVP-1 targets PMES to affect BR signaling and vein patterning. A, Images of 3-d-old GUS-stained CPD::GUS seedlings grown on 20 μm compound in the dark. B, OVP-1 enhances the hypocotyl defect in dark-grown bri1 seedlings. Values represent three biological replicates with approximately 35 seedlings per experiment. Bars are ± SD. C, Adult mutant plants exhibit suppression of the dwarf bri1 defect by sbi1-2, and delayed growth of bri1-5 pmes double mutants are shown with close-ups. Arrows denote flowers and green rosette of bri1-5 pmes mutant plants in contrast to fully senesced bri1-5 plants. D, OVP-1 and its target PMES affect CPD expression level. Transcript levels were measured with qRT-PCR from 7-d-old seedlings without treatment (mutants) or with 4-h incubation with 20 µm OVP-1 (OVP-1). Bars denote ± SD derived from three separate experiments with three technical replicates per experiment. E and F, Images of cleared cotyledons (E) or leaves (F). OVP-1 induces similar cotyledon vein pattern defects as those observed in pmes and sbi1-2; however, the mutants display a wild-type (WT) leaf vein pattern. Bars: A, 500 µm; C, 2 cm; E, 250 µm; and F, 500 µm.
We further identified an insertion mutant in single-copy PMES, predicting that a PME inhibitor would mimic a pmes loss-of-function mutant. Both PMES and OVP-1 enhanced bri1-5 growth defects producing opposite phenotypes than sbi1, which was confirmed with CPD transcriptional analysis (Figs 6, B–D; Supplemental Fig. S11). These results support a BRI1-dependent role for PP2A-C subunit methylation in growth and development and illustrate the close relationship between BR and auxin signaling. Although OVP-1-treated cotyledons mimicked the hemivenata (Candela et al., 2007) phenotype of pmes and sbi1 mutants, the mutants did not show leaf vein pattern alterations (Fig. 6, E and F). These data suggest that OVP-1 may inhibit pmes, as well as affecting additional SHs that collectively affect leaf vein patterns. In addition, our results suggest that OVP-1 acts early to influence BR gene expression, which may subsequently affect vein patterning.
OVP-2 and Plant MAPKKK Signaling
OVP-2 had been previously identified as an inhibitor of the human MAPKKK, TAK1 kinase (Lockman et al., 2011). Because OVP-2-induced properties included an increase in free vein endings and, thus, phenocopied the lipid signaling cvp2 mutant (Carland and Nelson, 2004) and because MAPK signaling is integrated in the lipid network, we tested OVP-2 response in cvp2 mutants (Supplemental Fig. S12). cvp2 mutant cotyledons showed characteristics of both compound-induced and cvp2 mutants. Assuming OVP-2 is an antagonist, this result suggests that the small molecule and CVP2 act in independent pathways. To examine structure function relationships, we also tested commercially available OVP-2 analogs with minimal activity toward human TAK1 Kinase (Supplemental Table S2). As predicted, OVP-2B had no bioactivity in the hypocotyl bioassay or in vein patterning. Surprisingly, OVP-2A induced multiple responses distinct from OVP-2 but resembling those of PATIs. These included an open apical hook under dark-grown conditions and a PATI-like vein pattern (Supplemental Fig. S13). However, OVP-2A had no effect on PIN cycling and therefore may interfere with other cellular events (Fig. 4A). Together, these results suggest that OVP-2 is not targeting CVP2 but may be affecting a single or multiple MAPKKK family members. In the latter case, chemical modifications of OVP2A resulted in novel activities, possibly due to the substituent group that compromised its ability to inhibit multiple MAPKKKs. Additional functions associated with CAVP chemicals derived from databases are listed in Supplemental Table S1.
Auxin and Ethylene Signaling Mutants Exhibit Insensitivity to PATI and HYVP Compounds; gnom Mutants Are Highly Sensitive to PATIs
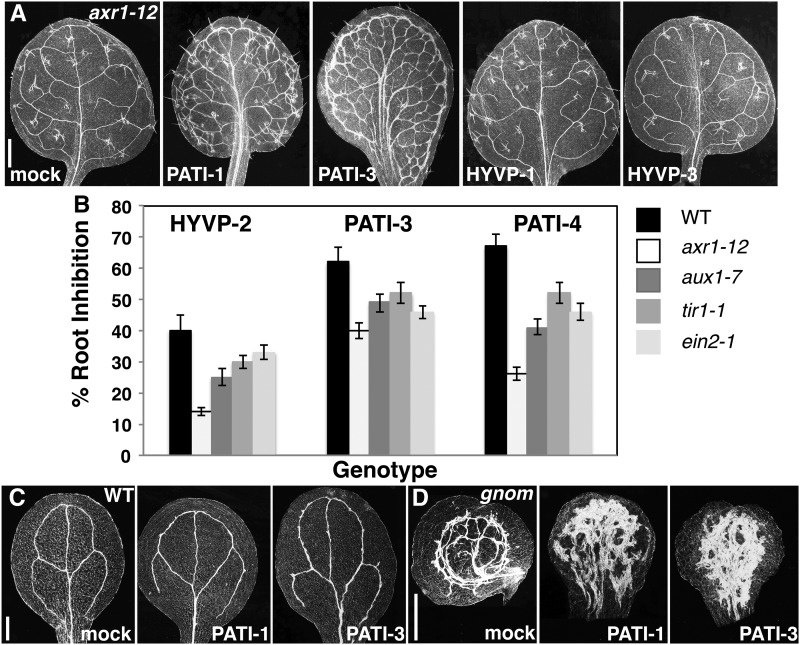
Auxin signaling mutants are reported to exhibit insensitivity to the PATI NPA (Ruegger et al., 1997); therefore, we tested auxin resistant1-12 (axr1-12; Lincoln et al., 1990) response to PATI-1 and -3, as well as HYVP-1 and -2, as there was overlap in their bioactivity. In the presence of the tested PATIs, axr1-12 displayed increased resistance characterized by a less severe compound-induced response (Fig. 7A). axr1-12 was dramatically insensitive to HYVP-1 and -2, closely resembling reduced vascular branching pattern of axr1-12. The insensitivity of auxin and ethylene signaling mutants to PATIs and HYVPs was also observed in root inhibition assays (Fig. 7B). This suggests that auxin signaling is required for compound-induced vascular alterations and that the compounds may be targeting components of that signaling. The ADP ribosylation factor guanine exchange factor (ARF-GEF) GNOM is a BFA-sensitive mediator of PIN cycling (Steinmann et al., 1999; Geldner et al., 2003). Because some PATIs disrupted PIN trafficking, we investigated the responsiveness of the gnom mutant to these compounds. PATI-1 and -3 treatment elicited a dramatic response in which cotyledon vascular cells over-proliferated to form an extensive vascularized mass (Fig. 7C). This synergistic interaction implies a role for PATI-1 and -3 in an overlapping pathway with GNOM, possibly as inhibitors of additional redundant ARF-GEFs or more generally in vesicle trafficking (Richter et al., 2007). We were unable to test the responsiveness of seedling-lethal gnom to HYVPs because these compounds do not elicit any cotyledon phenotypes.
Figure 7.
Auxin and ethylene signaling mutants and gnom mutants display opposing effects to compounds. A, Images of axr1-12 cleared leaves from plants grown in the presence of indicated compounds viewed with dark-field optics. axr1 suppresses PATI- and HYVP-induced venation pattern defects. Compare to Figure 1, A to C. B, Compound-induced root growth inhibition of auxin and ethylene signaling mutants to HYVP-2, PATI-3, and PATI-4 indicate insensitivity of mutants to compounds. C and D, Wild-type (WT; C) or gnom cotyledons (D) on indicated compounds show PATI-1 and -3 synergistic effects on vein pattern. Bars: A, 500 μm; and C and D, 250 μm.
PATI-1 Functions as an Actin Depolymerizing Agent
We hypothesized that endosome transport-associated actin could be a target of PATI-1 because it affected vesicle transport of GFP tagged-PIN1 and -PIN2 and elicited such a dramatic response in gnom mutants. We investigated the effect of PATI-1 on actin dynamics using GFP-labeled F-actin (Voigt et al., 2005). As previously reported (Geldner et al., 2001), lantrunculin B prevented BFA body formation by depolymerizing actin (Supplemental Fig. S14, A and B). TIBA had no effect on actin structure in hypocotyl epidermal cells. This result conflicts with studies on root epidermal cells, suggesting there is tissue specificity of action (Dhonukshe et al., 2008). In contrast, PATI-1 induced the same effects as lantrunculin B and, thus, appears to act as an actin depolymerizer (Supplemental Fig. S14, C–G). The actin-destabilizing effect of PATI-1 is consistent with its observed phenotypes, particularly its disruption of PIN endosomal trafficking.
Structural Relationships among CAVPs
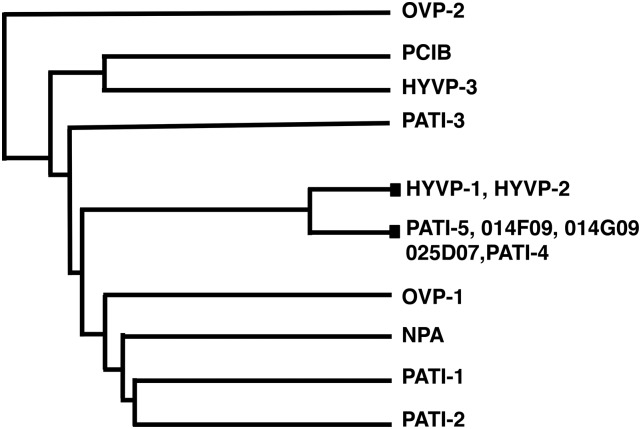
To examine structure activity relationships among the compounds, we grouped compounds into a dendrogram using PubChem cluster analysis (Fig. 8; Supplemental Fig. S16). Seven compounds cluster based on chemical properties, including HYVP-1, -2, PATI-4, -5 (Supplemental Table S2; Fig. 5A), and the three inactive compounds LATCA 014F09, LATCA 014G09, and LATCA 025D07 (Supplemental Table S2). OVPs and PATI-1 to -3 are distantly related, consistent with their diverse structures and distinct profiles. Interestingly, PATI-4 and the closely related PATI-5 tightly group with HYVP-1 and -2, suggesting an overlap in their mechanism of action. The lack of bioactivity of individual small molecules may be due to limitations in their uptake as well as to functional differences. Inactive LATCA compounds 025D07, 014F09, and 014G09 (Supplemental Table S2) are structurally similar to those in the cluster but lack bioactivity at screening concentrations. We tested higher concentrations of 014G09 and 014F09 to overcome uptake issues and found that 014G09 elicited a PATI-like phenotype, characterized by bifurcated midveins and reduced higher order veins (Supplemental Fig. S15, A and B). In addition, 014G09 reduced hypocotyl length in a dose-dependent manner, in contrast to the inactivity of 014F09 (Supplemental Fig. S15C). Thus, 014G09 exhibited some activity at elevated concentrations, although this may be due to inhibitor promiscuity typically resulting from aggregate formation (Seidler et al., 2003). 014F09 bioactivity was consistent with nonspecific compromised growth, presumably due to off target effects. Because compound grouping indicates a lack of small molecule specificity, it is plausible that HYVP-1 and -2 and, likewise, PATI-4 and -5 act on the same target. However, the remaining CAVPs are distantly related, suggesting that most CAPV compounds have distinct targets.
Figure 8.
HYVP-1 and -2 cluster with specific PATI compounds. Dendrogram of chemical structures using PubChem clustal analysis shows relationships among compounds. Closed box designates position of two or more compounds.
DISCUSSION
This study demonstrates the utility of a phenotype-based small molecule screen to identify novel chemical probes for a plant developmental process and to identify candidate components of the process as the specific targets of those probes. This approach can overcome the potential redundancy in components that might confound a direct genetic analysis. The bioactivity and targets of specific chemicals, screened systematically from compound libraries, are being compiled across many species and biological assays, driven in part by drug-discovery projects and accelerated by emerging chemogenomics methods (Wang and Xie, 2014). These bioactivity databases make it possible to generate hypotheses about the biological targets of specific compounds found to be active in a plant, based on the already-characterized targets of that compound in yeast, mammalian cells, or in other plant species.
The regulation of vein patterning and vascular cell differentiation is responsive at many points to inputs from auxin, steroids, and other endogenous small molecules. Therefore, chemical screens are likely to yield exogenous compounds that can act as agonists or antagonists on the same biological targets. Such bioactive small compounds can be valuable experimental tools, since they can be administered and removed at precise dosages, times, and locations, independently of the programing of endogenous developmental signals. The following sections expand on the value of specific compounds in the PATI, HYVP, and OVP classes recovered in our screens for further understanding of vein patterning.
PATI Class Compounds Affect Distinct Targets
We identified PATIs based on their ability to elicit the defective venation patterns previously induced by mutants or inhibitors of auxin transport. Each of these small molecules induced effects rapidly, within the 10-min to 4-h time scales of our cell biology and transcriptional assays. The novel PATI compounds appeared to induce rapid changes in gene expression of PIN gene family members. Compounds PATI-1 and -3 decreased PIN7 expression, accompanied by an increase in PIN1. A similar apparent compensation among PIN family members was previously observed in mutant studies (Vieten et al., 2005). The compound PATI-2 decreased expression of both PIN1 and PIN7 and produced a more severely aberrant vein pattern.
Each compound in the PATI phenotypic class exhibited a distinct behavior profile in the range of bioassays we performed, implying a distinct target. PATI-1 through -4 all eliminated hook curvature in etiolated seedlings. However, only PATI-1 and PATI-2 disrupted PIN::GFP localization. Only compound PATI-1 depolymerized actin and reduced the auxin response in auxin-treated roots. Only compound PATI-2 reduced both PIN1 and PIN7 transcription levels. These distinct bioassay profiles, each associated with a distinct chemical structure, imply that the PATI family compounds target diverse auxin transport components and, thus, expand the tools and targets for investigation of auxin transport.
The venation defects induced by PATI compounds have not yet in every case been associated with discrete cell biological targets. PIN trafficking occurs through GNOM-mediated PIN cycling to and from the endosomal compartment and the plasma membrane. Accordingly, gnom mutants exhibit irregular vein patterns with erratic areoles consisting of poorly aligned vascular cells. However, PATI-3 causes a severe vein pattern defect resembling a PIN disruption, but it does not apparently affect PIN::GFP trafficking, suggesting that it may target PIN activity rather than localization. In contrast, PATI-1 induces mild vein network abnormalities, apparently due to its actin-depolymerizing activity. PATIs have been reported to be modified in planta, for example, by glycosylation and hydrolysis (Katekar and Geissler, 1980; Murphy and Taiz, 1999). Therefore, it is plausible that PATI-1 is modified to a less active form during plant growth (undetected in the short time frames in our bioassays) and, thus, causes fewer abnormalities. Assuming PATI-1 and -3 are antagonists, they act synergistically with GNOM. PATI-1 and-3-grown gnom cotyledons exhibit uncontrolled vascular cell initiation and fail to establish continuous veins. Collectively, these results suggest a role for PATI-1 and -3 in an overlapping pathway or similar pathway with GNOM, possibly targeting additional ARF-GEFs. Furthermore, GNOM may be necessary for the proposed in planta detoxification of PATI-1.
HYVP Class Molecules Appear to Be Novel Agonists of Auxin Transport Able to Affect Vein Patterns
The HYVP class of compounds increased leaf vein thickness and the number of open and closed areoles, accompanied by leaf and petiole morphological abnormalities. Auxin levels, monitored by the DR5-GUS reporter, were increased in treated leaves and etiolated hypocotyls. It was previously reported (Scarpella et al., 2006) that elevated auxin levels initially expand PIN1 expression domains (PEDs), which are rapidly narrowed down to one or more conduits. In that study, chemical inhibitors delayed the narrowing of PEDs, and differentiated veins were broad. In addition, bipolar PED cells, in which PIN is localized to both ends of a cell, allowed the formation of closed loops. We propose that HYVP-1 and its structural variant HYVP-2 cause increased leaf vein PEDs and bipolar cells, based on the observed auxin accumulation in leaves and exaggerated curvature accompanied by hyper-accumulated auxin in etiolated hypocotyls. In contrast to PATIs, which slow the restriction of PEDs, HYVPs appear to act as agonists to increase auxin transport capabilities. As has been shown with computer simulations (Feugier et al., 2005), enhanced auxin transport would increase the formation of bifurcated veins, presumably by reallocation of auxin carriers to form bipolar cells, in order to guide auxin away from a linear path. With increased auxin flow, directional auxin transport would be maintained until the vein tip merged with an existing vein to form closed areoles. Consistent with this, low levels of NPA application generated thicker veins and increased tertiary vein number (Mattsson et al., 1999). Thus, although there is some overlap in phenotype induced by HYVP-1 and -2 and NPA, it is likely that the HYVPs act on a target distinct from that of NPA because they do not disrupt PIN cycling or cause any other NPA-induced characteristics, even at high concentrations. Similarly, they exhibit distinct profiles from the antiauxin PCIB. It is plausible that HYVP-1 and -2 may act on the auxin influx carriers AUX1 and LAX3, which have also been associated with hook formation and ethylene signals (Vandenbussche et al., 2010).
HYVP-3-induced pattern phenotypes are less severe than those HYVP-1 and-2, and it uniquely causes a reduction in PIN1and PIN7 transcriptional levels and hypocotyl auxin levels. This reduction of PIN1and PIN7 transcriptional levels in HYVP-3-treated seedlings may be the primary factor contributing to the phenotype. Although the reduced PIN expression appears inconsistent with the phenotype of increased PED, it has been reported that PIN polarity does not depend on its transcription but is dependent on the AUX/IAA signaling pathway (Sauer et al., 2006). This is consistent with the resistance of auxin signaling mutants to HYVPs. The compound HYVP-3 is reported to target an amine transporter in mammalian cells, which is consistent with its having a transport target in plants. If additional carriers, such as the auxin transporting multidrug-resistance-like/P-glycoproteins type ATP-binding cassette proteins, are activated, a consequence may be to lower PIN levels either directly or indirectly. Mutants in additional members of the ATP-binding cassette transporter family exhibit venation pattern defects (Le Hir et al., 2013).
Although HYVPs exhibit profiles distinctly different from PATIs, there is some overlap in that continuous exposure to HYVPs caused a PATI vein pattern in late-forming leaves. We propose that this represents more global changes in auxin flow beyond leaf marginal transporting cells. Decreased lamina size and reduced lateral organ expansion are characteristics of pin mutants, which exhibit limited organ fusion in early leaves but dramatically altered inflorescences (Okada et al., 1991). Reduced inflorescence auxin quantities correlate with preclusion of organ outgrowth. With prolonged HYVP exposure during vegetative development, elevated source-to-sink forces gradually drive auxin away from the leaf, thus mirroring transport inhibitors. It is plausible that the continuous restriction of auxin flow results in the observed thick marginal veins and limited lateral vein differentiation, as well as the restricted lamina lateral outgrowth.
OVP-1 Functions as a Modifier of SHs
The compound OVP-1 is documented in bioactivity databases as an inhibitor of hsPME1, which antagonistically functions with LCMT to confer the methylation status of the catalytic subunit of trimeric PP2A-C. In animal systems, the methylation status may positively or negatively influence PP2A activity B (Bachovchin et al., 2009). In Arabidopsis, there are five members of the C subunit family. However, none has been shown to physically interact with PP2A-A (DeLong, 2006). Although PMES has not been studied in plants, a role for C subunit methylation in guiding BRI receptor localization and, thus, activity in the BR signaling pathway has been reported. Methylated PP2A-C subunit, specified by the PME antagonist, SBI1/LCMT, promoted the dephosphorylation of BRI1 to reduce its signaling capability; thus, PP2A acted as a negative regulator (Wu et al., 2011). Further downstream, PP2A has an additional function as a positive regulator of the BR signal transduction pathway in activation of BR-regulated gene expression by dephosphorylating the BZR1 transcription factor (Tang et al., 2011). Loss-of-function mutations in the PP2A-C5 subunit had elevated CPD transcription levels and enhanced response to BRZ. Similarly, OVP-1 activated CPD expression and enhanced the etiolated response of weak bri1 alleles. Likewise, pmes mutants enhanced a weak bri1 allele, which was validated with elevated CPD expression. Furthermore, OVP1-grown plants and pmes had simpler venation patterns. From our results, it appears that PMES has a positive role in regulation of BR signaling, suggesting that demethylated PP2A is the active form acting on downstream components. Together, these results suggest a role for OVP1 as an inhibitor of PMES and that it affects cotyledon vein pattern by disrupting BR signaling. BRs influence tracheary element differentiation (Yamamoto et al., 1997) and polar auxin transport (Li et al., 2005; Ibañes et al., 2009). The vascular-specific BR receptors, BRASSINOSTEROID RECEPTOR LIKE1 (BRL1) and BRASSINOSTEROID RECEPTOR LIKE3 (BRL3), affect venation patterning (Caño-Delgado et al., 2004). The significant overlap between auxin and BR signaling networks may explain the novel venation defects elicited by OVP-1. Alternatively, because PP2A functions antagonistically with PINOID kinase to regulate PIN phosphorylation (Michniewicz et al., 2007), mismethylated PP2A-C may disrupt normal PIN activity and contribute to the foliar vein defects.
The super family of SHs, of which PME1 is a member, consists of a diverse group of proteins, including subtilases, lipases, and esterases that constitute approximately 1% of the total proteome in all living organisms (Simon and Cravatt, 2010). Although SH antagonists may be highly selective, particularly those identified with the activity-based protein profiling platform, a single SH inhibitor may disrupt more than one SH. Indeed, many hsPME1 antagonists affect multiple SH family members (Simon and Cravatt, 2010; Kaschani et al., 2012). However, there is also value in high-quality, broad-spectrum probes because such compounds can be exploited to inhibit multiple members within a given enzyme class and thereby reveal novel phenotypes. Because the PMES loss-of-function mutant shared several, but not all, properties with OVP-1-mediated phenotypes, it is likely that OVP-1 interferes with PMES and additional SH(s). The involvement of this gene in vein patterning is unlikely to have been revealed by methods other than this chemical biology approach.
OVP-2 as an Interfering Agent of MAPK Signaling
OVP-2 was recovered in a chemical screen for antagonists of the human mitogen-activated protein 3 kinase (MAP3K), TGF-β-activating kinase1(TAK1/MAP3K7). MAPKs function in signaling cascade modules shared among all eukaryotes. In plants, these protein phosphorylation events connect external cues to a multitude of cellular responses, including abiotic and biotic stresses, and hormonal and developmental processes (Colcombet and Hirt, 2008). In Arabidopsis, the MAP3K family is highly redundant, consisting of 60 members divided into two main groups with 15 subdivisions. This suggests that OVP-2 may inhibit multiple MAP3Ks to produce its novel phenotype (Ichimura et al., 2002). Phylogenetic analysis indicated that two subgroups, containing five MAP3Ks, are most closely related to human TAK1 and may be OVP2 targets in Arabidopsis. Studies of loss-of-function and gain-of-function mutants revealed roles for MAPK cascade signaling in auxin biology and vein patterning. Overexpression of MKK7 modifies polar auxin transport resulting in a less reticulated venation pattern (Dai et al., 2006). ibr5, a MAPK phosphatase (MKP) mutant recovered by its auxin-resistant phenotype, also simplifies vein patterning (Monroe-Augustus et al., 2003). Surprisingly, the chemically modified OVP-2A, which had low activity against human TAK1, retained some bioactivity but differed from that of OVP-2, suggesting that it targets fewer MAP3K family members. Alternatively, the modification may produce novel activities that act on an additional class of enzymes.
OVP-2 dramatically perturbed the overall vein network, producing enlarged vertically-oriented apical lateral veins, which appear “midvein-like,” and increased freely ending veinlets and thus, reduced closed loops. Based on the observed pattern of vascular specific DR5-GUS reporter expression, vascular strands flanking the midvein appear to differentiate throughout leaf development, significantly deviating from the typical vein maturation process. The positioning of the lower region of the apical loop is initiated by an internalization of auxin from a convergence point of bidirectional auxin flow in epidermal cells (Scarpella et al., 2006). Vein continuity and loop formation is established by auxin transport directed toward the midvein. Bidirectional auxin flow, evidenced by bipolar PIN localization, is reiterated within cells destined to form vein connections. According to this model, the positioning of leaf marginal auxin convergence points appears to be randomized or possibly restricted to the leaf apex in OVP-2-treated leaves. We also predict a decrease in bipolar PIN localization that would allow vasculature loop formation. Detailed analyses of PIN1 subcellular studies will aid in determining the primary defect induced by OVP-2 and provide additional support for convergence points in defining vein positional cues and continuity.
MATERIALS AND METHODS
Plant Materials, Growth Conditions, and Chemical Screen
Seed stocks Columbia, Landsberg erecta, Wassilewskija, aux1-7, axr1-12, ein2-1, gl1-1, tir1-1, pmes (At4g10050; SALK_079539), sbi1-2 (At1g02100; SALK_079466), and PIN1-GFP were obtained from the Arabidopsis Biological Resource Center. Primers used to genotype SALK lines are listed in Supplemental Table S2. Additional seed stocks were provided by the following individuals: Franz Tax (bri1-5 and bri1-9), Boris Voigt (GFP-FABD2), Miklos Szekeres (CPD::GUS), Gloria Muday with permission from Ben Scheres (PIN2-GFP), and Hiroo Fukuda (gnom/emb30-7). All mutant seeds were in Arabidopsis (Arabidopsis thaliana) ecotype Columbia background with the exception of the Landsberg erecta allele of gnom and the Wassilewskija alleles of bri1-5 and bri1-9. The VHA-a1-GFP construct was provided by Karin Schumacher, introduced into Agrobacterium tumefaciens strain GV3101 by electroporation, and transformed into Arabidopsis ecotype Columbia using the floral dipping method (Clough and Bent, 1998).
For all vein patterning and root petiole length studies, seed was surface sterilized, plated on germination media without compound or with compound for untreated (mock) and treated samples, respectively, and incubated at 4°C for approximately 3 d prior to placement in a growth chamber as previously described (Carland et al., 2010). In hypocotyl studies, plates were prepared as above but were wrapped in foil and subsequently incubated for 3 d at 22°C in the dark. For qRT-PCR, auxin response, and cell biology studies, seedlings were grown on germination media and transferred to compound-containing 0.5× liquid germination media and incubated for the indicated times. In all assays, the following concentrations were used unless otherwise noted: 1 μm PATI-3, 10 μm PATI-1, -2, -4, NPA, TIBA, and PEO-IAA, 20 μm OVP-1, -2 (and OVP-2 analogs), and BRP, and 25 μm HYVP-1, -2, -3, and PCIB. The mock control media was supplemented with 0.1% DMSO/0.1% ethanol in a 1:1 ratio. Root inhibition assays were previously described (Carland and Nelson, 2009).
For the chemical screen, 96-well plates were loaded with 100 μL of germination media. 25 μm (1 μL of 2.5 mm stock) of each compound was added to individual wells. Following the pipetting of three to four sterile seeds into each well, plates were cold treated and incubated under lights. After approximately 12 d, plants were screened for phenotypic alterations and fixed in 3 ethanol:1 acetic acid. After overnight fixation, plants were transferred to 70% ethanol and screened for vein pattern defects using a Zeiss Stemi-2000C dissecting microscope equipped with dark-field capabilities. Selected plants were transferred to 100% ethanol, cleared with 10% NaOH at 37°C for 1 h, dissected and mounted on glass slides, and imaged with a Zeiss Axiophot using dark-field optics. Small molecules of interest were purchased from either Ryan Scientific or Hit2Lead for Maybridge (PATI-1, OVP-1) or Chembridge (PATI-2 –PATI-4, HYVP-1, HYVP-2, OVP-2, OVP-2A, OVP-2B, and BRP) compounds. Purchased small molecules were retested for validation and used for further studies. HYVP-3, PCIB, NPA, TIBA, and BFA were purchased from Sigma. PEO-IAA was kindly provided by Ken-ichiri Hayashi. All chemicals were resuspended in DMSO as 50 to 100 mg/mL stock solutions and further diluted in ethanol.
GUS Histochemical Staining
For leaf and hypocotyl GUS studies, specimens were incubated in GUS staining solution overnight or 5 h for DR5:GUS or CPD:GUS, respectively, at 37°C, then mounted and imaged as previously described (Carland and Nelson, 2009). For root auxin response studies, 4-d-old roots were incubated with compound and with or without 1 μm IAA in liquid germination medium for 5 h at 22°C and GUS stained.
qRT-PCR
Seven-day-old seedlings were incubated with compounds for 4 h at 22°C. Total RNA was extracted from 1.3 g of tissue by the TRIzol method (Ambion) and subjected to DNAse-treatment (Turbo; Ambion), followed by purification with the RNeasy MinElute Cleanup Kit (Qiagen). First strand cDNA was synthesized with the RETROscript kit (Ambion) from 2 μg of RNA using oligo(dT) according to the manufacturer’s instructions. cDNA was tested with PCR using tubulin primers (26 cycles; Supplemental Table S2) to monitor RNA quality and quantity. For qRT-PCR transcript analysis of PMES, PIN1, PIN7, and CPD, equal amounts of cDNA (1 μL in 20 μL reaction volume) were amplified using Actin 8 and Tubulin2 as reference primers or TaqMan gene expression assay probes following the manufacturer’s guidelines (Applied Biosystems). Amplifications were conducted on a BioRad C1000 Thermal Cycler using preset conditions for TaqMan assays. Primers, or assay identification numbers for TaqMan amplicons, are provided in Supplemental Table S2. Data were analyzed using CFX Manager v1.6 software (Bio-Rad) and fulfilled all quality control requirements. All samples were run in triplicate in at least three independent experiments.
Confocal Microscopy
Imaging was performed on a Zeiss laser scanning microscope 510 META using 20× or 40× water immersion objectives as previously described (Carland et al., 2010). Roots of 3-d-old PIN2-GFP and 7-d-old PIN1-GFP and VHA-a1-GFP were treated with compound with or without BFA for 1 h, fixed in 4% paraformaldehyde, pH 7.0, in 1× phosphate buffered saline (1.4 m NaCl, 0.1 m phosphate, pH 7.4, 0.03 m KCl) and mounted in 10% glycerol for imaging. Hypocotyls of 3-d-old GFP-FABD2 seedlings were treated for 20 min with 5 μm jasplakinolide or 10 min with 1 μm lantrunculin B, or small molecules at indicated concentrations. Jasplakinolide and lantruculin B were purchased from Sigma.
Compound Analysis
SMILES (Simplified Molecular-Input Line-Entry System) and molecular formulas for LATCA small molecules were obtained from the ChemMine database (chemminedb.ucr.edu). LATCA SMILES were entered in PubChem (pubchem.ncbi.nlm.nih.gov) for bioassay searches and chemical names.
Structures were drawn in SciFinder to obtain CAS numbers, additional published literature, and patent information. Both PubChem and SciFinder were mined for structurally similar compounds and for vendor availability of compounds.
For the compound dendrogram, compound identifiers (CID) obtained from the PubChem and ChemMine databases were used as input for structure clustering tool with 3D tanimoto similarity of chemical shape and feature. Structures were drawn with ChemBioDraw Version 13.0 (Perkin Elmer).
Supplemental Data
The following supplemental materials are available.
Supplemental Table S1. Structure and bioactivity of compounds characterized.
Supplemental Table S2. Additional compounds used.
Supplemental Table S3. Quantification of BFA body number in root epidermal cells after compound treatment.
Supplemental Table S4. Primers used for genotyping and expression analysis.
Supplemental Figure S1. Dose-dependence of compound action.
Supplemental Figure S2. Quantification of compound-induced vein pattern phenotypes.
Supplemental Figure S3. Compounds induce additional phenotypes.
Supplemental Figure S4. Compound effects are reversible.
Supplemental Figure S5. Increased doses of HYVP compounds do not affect DR5::GUS root response to auxin.
Supplemental Figure S6. PATI-1 but not HYVP-1 and -2 affect PIN1-GFP cycling in the root stele.
Supplemental Figure S7. OVPs do not alter PIN1 gene expression level.
Supplemental Figure S8. Sterol mutants are hypersensitive to OVP-1.
Supplemental Figure S9. Arabidopsis PMES is highly conserved with human PME1.
Supplemental Figure S10. BR signaling/biosynthesis is unaffected in PATI- and HYVP-treated etiolated seedlings.
Supplemental Figure S11. Seedling phenotypes of bri1-5 sbi1-2 and bri1-5 pmes double mutants.
Supplemental Figure S12. OVP-2 does not target CVP2.
Supplemental Figure S13. The OVP-2 analog OVP-2A induces distinct vein phenotypes.
Supplemental Figure S14. PATI-1 depolymerizes GFP-Fimbrin.
Supplemental Figure S15. Reduced bioactivity with HYVP/PATI-related compounds.
Supplemental Figure S16. Heat map of compound dendrogram with tanimoto similarity.
Acknowledgments
We thank the Arabidopsis Biological Resource Center, Ken-ichiri Hayashi, Karin Schumacher, Frans Tax, Miklos Szekeres, and Boris Voigt for providing materials. We are grateful for the assistance of Mariya Kolesnikova, Denton Hoyer, and Jane Merkel at the Yale Center for Molecular Discovery.
Glossary
- PATI
polar auxin transport inhibitor
- NPA
1-N-naphthylphthalamic acid
- PCIB
p-chlorophenoxyisobutyric acid
- CAVP
compound affecting vein pattern
- HYVP
hypervascular pattern
- OVP
open vein pattern
- LCMT
Leu carboxylmethyltransferase
- BRP
brassinopride
- ARF-GEF
ADP ribosylation factor guanine exchange factor
- qRT-PCR
quantitative real-time PCR
- PED
PIN1 expression domain
- SH
Ser hydrolase
- TIBA
Triiodobenzoic acid
- BFA
Brefeldin A
Footnotes
This work was supported by the National Science Foundation (award nos. IOS–1021188 to T.N. and IOS–1258175 to S.C.).
This article is available without a subscription.
References
- Bachovchin DA, Brown SJ, Rosen H, Cravatt BF (2009) Identification of selective inhibitors of uncharacterized enzymes by high-throughput screening with fluorescent activity-based probes. Nat Biotechnol 27: 387–394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballesteros I, Domínguez T, Sauer M, Paredes P, Duprat A, Rojo E, Sanmartín M, Sánchez-Serrano JJ (2013) Specialized functions of the PP2A subfamily II catalytic subunits PP2A-C3 and PP2A-C4 in the distribution of auxin fluxes and development in Arabidopsis. Plant J 73: 862–872 [DOI] [PubMed] [Google Scholar]
- Benková E, Michniewicz M, Sauer M, Teichmann T, Seifertová D, Jürgens G, Friml J (2003) Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell 115: 591–602 [DOI] [PubMed] [Google Scholar]
- Biswas KK, Ooura C, Higuchi K, Miyazaki Y, Van Nguyen V, Rahman A, Uchimiya H, Kiyosue T, Koshiba T, Tanaka A, Narumi I, Oono Y (2007) Genetic characterization of mutants resistant to the antiauxin p-chlorophenoxyisobutyric acid reveals that AAR3, a gene encoding a DCN1-like protein, regulates responses to the synthetic auxin 2,4-dichlorophenoxyacetic acid in Arabidopsis roots. Plant Physiol 145: 773–785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwell HE, Zhao Y (2003) Chemical genetic approaches to plant biology. Plant Physiol 133: 448–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candela H, Alonso-Peral MM, Ponce MR, Micol JL (2007) Role of HEMIVENATA and the ubiquitin pathway in venation pattern formation. Plant Signal Behav 2: 258–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cano-Delgado A, Lee J-Y, Demura T (2010) Regulatory mechanisms for specificity and patterning of plant vascular tissues. Annu Rev Cell Dev Biol 26: 605–637 [DOI] [PubMed] [Google Scholar]
- Caño-Delgado A, Yin Y, Yu C, Vafeados D, Mora-García S, Cheng J-C, Nam KH, Li J, Chory J (2004) BRL1 and BRL3 are novel brassinosteroid receptors that function in vascular differentiation in Arabidopsis. Development 131: 5341–5351 [DOI] [PubMed] [Google Scholar]
- Carland F, Nelson T (2009) CVP2- and CVL1-mediated phosphoinositide signaling as a regulator of the ARF GAP SFC/VAN3 in establishment of foliar vein patterns. Plant J 59: 895–907 [DOI] [PubMed] [Google Scholar]
- Carland F, Fujioka S, Nelson T (2010) The sterol methyltransferases SMT1, SMT2, and SMT3 influence Arabidopsis development through nonbrassinosteroid products. Plant Physiol 153: 741–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carland FM, Nelson T (2004) Cotyledon vascular pattern2-mediated inositol (1,4,5) triphosphate signal transduction is essential for closed venation patterns of Arabidopsis foliar organs. Plant Cell 16: 1263–1275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carland FM, Fujioka S, Takatsuto S, Yoshida S, Nelson T (2002) The identification of CVP1 reveals a role for sterols in vascular patterning. Plant Cell 14: 2045–2058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Colcombet J, Hirt H (2008) Arabidopsis MAPKs: a complex signalling network involved in multiple biological processes. Biochem J 413: 217–226 [DOI] [PubMed] [Google Scholar]
- Dai Y, Wang H, Li B, Huang J, Liu X, Zhou Y, Mou Z, Li J (2006) Increased expression of MAP KINASE KINASE7 causes deficiency in polar auxin transport and leads to plant architectural abnormality in Arabidopsis. Plant Cell 18: 308–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLong A. (2006) Switching the flip: protein phosphatase roles in signaling pathways. Curr Opin Plant Biol 9: 470–477 [DOI] [PubMed] [Google Scholar]
- Dhonukshe P, Grigoriev I, Fischer R, Tominaga M, Robinson DG, Hasek J, Paciorek T, Petrásek J, Seifertová D, Tejos R, Meisel LA, Zazímalová E, et al. (2008) Auxin transport inhibitors impair vesicle motility and actin cytoskeleton dynamics in diverse eukaryotes. Proc Natl Acad Sci USA 105: 4489–4494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drakakaki G, Robert S, Szatmari A-M, Brown MQ, Nagawa S, Van Damme D, Leonard M, Yang Z, Girke T, Schmid SL, Russinova E, Friml J, et al. (2011) Clusters of bioactive compounds target dynamic endomembrane networks in vivo. Proc Natl Acad Sci USA 108: 17850–17855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feugier FG, Mochizuki A, Iwasa Y (2005) Self-organization of the vascular system in plant leaves: inter-dependent dynamics of auxin flux and carrier proteins. J Theor Biol 236: 366–375 [DOI] [PubMed] [Google Scholar]
- Friml J, Vieten A, Sauer M, Weijers D, Schwarz H, Hamann T, Offringa R, Jürgens G (2003) Efflux-dependent auxin gradients establish the apical-basal axis of Arabidopsis. Nature 426: 147–153 [DOI] [PubMed] [Google Scholar]
- Gandotra N, Coughlan SJ, Nelson T (2013) The Arabidopsis leaf provascular cell transcriptome is enriched in genes with roles in vein patterning. Plant J 74: 48–58 [DOI] [PubMed] [Google Scholar]
- Gälweiler L, Guan C, Müller A, Wisman E, Mendgen K, Yephremov A, Palme K (1998) Regulation of polar auxin transport by AtPIN1 in Arabidopsis vascular tissue. Science 282: 2226–2230 [DOI] [PubMed] [Google Scholar]
- Geldner N, Anders N, Wolters H, Keicher J, Kornberger W, Muller P, Delbarre A, Ueda T, Nakano A, Jürgens G (2003) The Arabidopsis GNOM ARF-GEF mediates endosomal recycling, auxin transport, and auxin-dependent plant growth. Cell 112: 219–230 [DOI] [PubMed] [Google Scholar]
- Geldner N, Friml J, Stierhof YD, Jürgens G, Palme K (2001) Auxin transport inhibitors block PIN1 cycling and vesicle trafficking. Nature 413: 425–428 [DOI] [PubMed] [Google Scholar]
- Gendron JM, Haque A, Gendron N, Chang T, Asami T, Wang Z-Y (2008) Chemical genetic dissection of brassinosteroid-ethylene interaction. Mol Plant 1: 368–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamann T, Benková E, Bäurle I, Kientz M, Jürgens G (2002) The Arabidopsis BODENLOS gene encodes an auxin response protein inhibiting MONOPTEROS-mediated embryo patterning. Genes Dev 16: 1610–1615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han L, Wang Y, Bryant SH (2009) A survey of across-target bioactivity results of small molecules in PubChem. Bioinformatics 25: 2251–2255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardtke CS, Berleth T (1998) The Arabidopsis gene MONOPTEROS encodes a transcription factor mediating embryo axis formation and vascular development. EMBO J 17: 1405–1411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi K, Neve J, Hirose M, Kuboki A, Shimada Y, Kepinski S, Nozaki H (2012) Rational design of an auxin antagonist of the SCF(TIR1) auxin receptor complex. ACS Chem Biol 7: 590–598 [DOI] [PubMed] [Google Scholar]
- He W, Brumos J, Li H, Ji Y, Ke M, Gong X, Zeng Q, Li W, Zhang X, An F, Wen X, Li P, et al. (2011) A small-molecule screen identifies L-kynurenine as a competitive inhibitor of TAA1/TAR activity in ethylene-directed auxin biosynthesis and root growth in Arabidopsis. Plant Cell 23: 3944–3960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks GR, Raikhel NV (2012) Small molecules present large opportunities in plant biology. Annu Rev Plant Biol 63: 261–282 [DOI] [PubMed] [Google Scholar]
- Hobbie L, McGovern M, Hurwitz LR, Pierro A, Liu NY, Bandyopadhyay A, Estelle M (2000) The axr6 mutants of Arabidopsis thaliana define a gene involved in auxin response and early development. Development 127: 23–32 [DOI] [PubMed] [Google Scholar]
- Ibañes M, Fàbregas N, Chory J, Caño-Delgado AI (2009) Brassinosteroid signaling and auxin transport are required to establish the periodic pattern of Arabidopsis shoot vascular bundles. Proc Natl Acad Sci USA 106: 13630–13635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaschani F, Nickel S, Pandey B, Cravatt BF, Kaiser M, van der Hoorn RAL (2012) Selective inhibition of plant serine hydrolases by agrochemicals revealed by competitive ABPP. Bioorg Med Chem 20: 597–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katekar GF, Geissler AE (1980) Auxin Transport Inhibitors: IV. Evidence of a common mode of action for a proposed class of auxin transport inhibitors: the phytotropins. Plant Physiol 66: 1190–1195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasumi M, Nghiem P (2007) Chemical genetics: elucidating biological systems with small-molecule compounds. J Invest Dermatol 127: 1577–1584 [DOI] [PubMed] [Google Scholar]
- Ichimura K, Shinozaki K, Tena G, Sheen J, Henry Y, Champion A, Kreis M, Zhang S, Hirt H, Wilson C, Herberle-Bors E, Ellis BE, et al. (2002) Mitogen-activated protein kinase cascades in plants: a new nomenclature. Trends Plant Sci 7: 301–308 [DOI] [PubMed] [Google Scholar]
- Koizumi K, Naramoto S, Sawa S, Yahara N, Ueda T, Nakano A, Sugiyama M, Fukuda H (2005) VAN3 ARF-GAP-mediated vesicle transport is involved in leaf vascular network formation. Development 132: 1699–1711 [DOI] [PubMed] [Google Scholar]
- Le Hir R, Sorin C, Chakraborti D, Moritz T, Schaller H, Tellier F, Robert S, Morin H, Bako L, Bellini C (2013) ABCG9, ABCG11 and ABCG14 ABC transporters are required for vascular development in Arabidopsis. Plant J 76: 811–824 [DOI] [PubMed] [Google Scholar]
- Lehman A, Black R, Ecker JR (1996) HOOKLESS1, an ethylene response gene, is required for differential cell elongation in the Arabidopsis hypocotyl. Cell 85: 183–194 [DOI] [PubMed] [Google Scholar]
- Li L, Xu J, Xu Z-H, Xue H-W (2005) Brassinosteroids stimulate plant tropisms through modulation of polar auxin transport in Brassica and Arabidopsis. Plant Cell 17: 2738–2753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lincoln C, Britton JH, Estelle M (1990) Growth and development of the axr1 mutants of Arabidopsis. Plant Cell 2: 1071–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockman JW, Reeder MD, Robinson R, Ormonde PA, Cimbora DM, Williams BL, Willardsen JA (2011) Oxindole derivatives as inhibitors of TAK1 kinase. Bioorg Med Chem Lett 21: 1724–1727 [DOI] [PubMed] [Google Scholar]
- Mathur J, Molnár G, Fujioka S, Takatsuto S, Sakurai A, Yokota T, Adam G, Voigt B, Nagy F, Maas C, Schell J, Koncz C, et al. (1998) Transcription of the Arabidopsis CPD gene, encoding a steroidogenic cytochrome P450, is negatively controlled by brassinosteroids. Plant J 14: 593–602 [DOI] [PubMed] [Google Scholar]
- Mattsson J, Sung ZR, Berleth T (1999) Responses of plant vascular systems to auxin transport inhibition. Development 126: 2979–2991 [DOI] [PubMed] [Google Scholar]
- Mattsson J, Ckurshumova W, Berleth T (2003) Auxin signaling in Arabidopsis leaf vascular development. Plant Physiol 131: 1327–1339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michniewicz M, Zago MK, Abas L, Weijers D, Schweighofer A, Meskiene I, Heisler MG, Ohno C, Zhang J, Huang F, Schwab R, Weigel D, et al. (2007) Antagonistic regulation of PIN phosphorylation by PP2A and PINOID directs auxin flux. Cell 130: 1044–1056 [DOI] [PubMed] [Google Scholar]
- Monroe-Augustus M, Zolman BK, Bartel B (2003) IBR5, a dual-specificity phosphatase-like protein modulating auxin and abscisic acid responsiveness in Arabidopsis. Plant Cell 15: 2979–2991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorhead GB, De Wever V, Templeton G, Kerk D (2009) Evolution of protein phosphatases in plants and animals. Biochem J 417: 401–409 [DOI] [PubMed] [Google Scholar]
- Muday GK. (2000) Maintenance of asymmetric cellular localization of an auxin transport protein through interaction with the actin cytoskeleton. J Plant Growth Regul 19: 385–396 [DOI] [PubMed] [Google Scholar]
- Muday GK, Rahman A, Binder BM (2012) Auxin and ethylene: collaborators or competitors? Trends Plant Sci 17: 181–195 [DOI] [PubMed] [Google Scholar]
- Murphy A, Taiz L (1999) Naphthylphthalamic acid is enzymatically hydrolyzed at the hypocotyl-root transition zone and other tissues of Arabidopsis thaliana seedlings. Plant Physiol Biochem 37: 413–430 [Google Scholar]
- Naramoto S, Nodzyłski T, Dainobu T, Takatsuka H, Okada T, Friml J, Fukuda H (2014) VAN4 encodes a putative TRS120 that is required for normal cell growth and vein development in Arabidopsis. Plant Cell Physiol 55: 750–763 [DOI] [PubMed] [Google Scholar]
- Naramoto S, Sawa S, Koizumi K, Uemura T, Ueda T, Friml J, Nakano A, Fukuda H (2009) Phosphoinositide-dependent regulation of VAN3 ARF-GAP localization and activity essential for vascular tissue continuity in plants. Development 136: 1529–1538 [DOI] [PubMed] [Google Scholar]
- Nelson T, Dengler N (1997) Leaf vascular pattern formation. Plant Cell 9: 1121–1135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemhauser JL, Mockler TC, Chory J (2004) Interdependency of brassinosteroid and auxin signaling in Arabidopsis. PLoS Biol 2: E258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada K, Ueda J, Komaki MK, Bell CJ, Shimura Y (1991) Requirement of the auxin polar transport system in early stages of Arabidopsis floral bud formation. Plant Cell 3: 677–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S-Y, Fung P, Nishimura N, Jensen DR, Fujii H, Zhao Y, Lumba S, Santiago J, Rodrigues A, Chow TF, Alfred SE, Bonetta D, et al. (2009) Abscisic acid inhibits type 2C protein phosphatases via the PYR/PYL family of START proteins. Science 324: 1068–1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peer WA, Bandyopadhyay A, Blakeslee JJ, Makam SN, Chen RJ, Masson PH, Murphy AS (2004) Variation in expression and protein localization of the PIN family of auxin efflux facilitator proteins in flavonoid mutants with altered auxin transport in Arabidopsis thaliana. Plant Cell 16: 1898–1911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Péret B, Swarup K, Ferguson A, Seth M, Yang Y, Dhondt S, James N, Casimiro I, Perry P, Syed A, Yang H, Reemmer J, et al. (2012) AUX/LAX genes encode a family of auxin influx transporters that perform distinct functions during Arabidopsis development. Plant Cell 24: 2874–2885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter S, Geldner N, Schrader J, Wolters H, Stierhof Y-D, Rios G, Koncz C, Robinson DG, Jürgens G (2007) Functional diversification of closely related ARF-GEFs in protein secretion and recycling. Nature 448: 488–492 [DOI] [PubMed] [Google Scholar]
- Robles P, Fleury D, Candela H, Cnops G, Alonso-Peral MM, Anami S, Falcone A, Caldana C, Willmitzer L, Ponce MR, Van Lijsebettens M, Micol JL (2010) The RON1/FRY1/SAL1 gene is required for leaf morphogenesis and venation patterning in Arabidopsis. Plant Physiol 152: 1357–1372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolland-Lagan A-G, Prusinkiewicz P (2005) Reviewing models of auxin canalization in the context of leaf vein pattern formation in Arabidopsis. Plant J 44: 854–865 [DOI] [PubMed] [Google Scholar]
- Rubery PH. (1990) Phytotropins: receptors and endogenous ligands. Symp Soc Exp Biol 44: 119–146 [PubMed] [Google Scholar]
- Ruegger M, Dewey E, Hobbie L, Brown D, Bernasconi P, Turner J, Muday G, Estelle M (1997) Reduced naphthylphthalamic acid binding in the tir3 mutant of Arabidopsis is associated with a reduction in polar auxin transport and diverse morphological defects. Plant Cell 9: 745–757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs T. (1991) Cell polarity and tissue patterning in plants. Development 113: 83–93 [Google Scholar]
- Sakata T, Chen JK (2011) Chemical ‘Jekyll and Hyde’s: small-molecule inhibitors of developmental signaling pathways. Chem Soc Rev 40: 4318–4331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer M, Balla J, Luschnig C, Wiśniewska J, Reinöhl V, Friml J, Benková E (2006) Canalization of auxin flow by Aux/IAA-ARF-dependent feedback regulation of PIN polarity. Genes Dev 20: 2902–2911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarpella E, Meijer AH (2004) Pattern formation in the vascular system of monocot and dicot plant species. New Phytol 164: 209–242 [DOI] [PubMed] [Google Scholar]
- Scarpella E, Marcos D, Friml J, Berleth T (2006) Control of leaf vascular patterning by polar auxin transport. Genes Dev 20: 1015–1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrick K, Mayer U, Horrichs A, Kuhnt C, Bellini C, Dangl J, Schmidt J, Jürgens G (2000) FACKEL is a sterol C-14 reductase required for organized cell division and expansion in Arabidopsis embryogenesis. Genes Dev 14: 1471–1484 [PMC free article] [PubMed] [Google Scholar]
- Seidler J, McGovern SL, Doman TN, Shoichet BK (2003) Identification and prediction of promiscuous aggregating inhibitors among known drugs. J Med Chem 46: 4477–4486 [DOI] [PubMed] [Google Scholar]
- Sieburth LE. (1999) Auxin is required for leaf vein pattern in Arabidopsis. Plant Physiol 121: 1179–1190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieburth LE, Muday GK, King EJ, Benton G, Kim S, Metcalf KE, Meyers L, Seamen E, Van Norman JM (2006) SCARFACE encodes an ARF-GAP that is required for normal auxin efflux and vein patterning in Arabidopsis. Plant Cell 18: 1396–1411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon GM, Cravatt BF (2010) Activity-based proteomics of enzyme superfamilies: serine hydrolases as a case study. J Biol Chem 285: 11051–11055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souter M, Topping J, Pullen M, Friml J, Palme K, Hackett R, Grierson D, Lindsey K (2002) hydra Mutants of Arabidopsis are defective in sterol profiles and auxin and ethylene signaling. Plant Cell 14: 1017–1031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmann T, Geldner N, Grebe M, Mangold S, Jackson CL, Paris S, Gälweiler L, Palme K, Jürgens G (1999) Coordinated polar localization of auxin efflux carrier PIN1 by GNOM ARF GEF. Science 286: 316–318 [DOI] [PubMed] [Google Scholar]
- Steynen QJ, Schultz EA (2003) The FORKED genes are essential for distal vein meeting in Arabidopsis. Development 130: 4695–4708 [DOI] [PubMed] [Google Scholar]
- Stokes ME, McCourt P (2014) Towards personalized agriculture: what chemical genomics can bring to plant biotechnology. Front Plant Sci 5: 344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surpin M, Rojas-Pierce M, Carter C, Hicks GR, Vasquez J, Raikhel NV (2005) The power of chemical genomics to study the link between endomembrane system components and the gravitropic response. Proc Natl Acad Sci USA 102: 4902–4907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang W, Yuan M, Wang R, Yang Y, Wang C, Oses-Prieto JA, Kim T-W, Zhou H-W, Deng Z, Gampala SS, Gendron JM, Jonassen EM, et al. (2011) PP2A activates brassinosteroid-responsive gene expression and plant growth by dephosphorylating BZR1. Nat Cell Biol 13: 124–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang W, Deng Z, Wang ZY (2010) Proteomics shed light on the brassinosteroid signaling mechanisms. Curr Opin Plant Biol 13: 27–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavassoli A, Hamilton AD, Spring DR (2011) Small molecules in biology. Chem Soc Rev 40: 4269–4270 [DOI] [PubMed] [Google Scholar]
- Tejos R, Sauer M, Vanneste S, Palacios-Gomez M, Li H, Heilmann M, van Wijk R, Vermeer JEM, Heilmann I, Munnik T, Friml J (2014) Bipolar plasma membrane distribution of phosphoinositides and their requirement for auxin-mediated cell polarity and patterning in Arabidopsis. Plant Cell 26: 2114–2128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchiya Y, Vidaurre D, Toh S, Hanada A, Nambara E, Kamiya Y, Yamaguchi S, McCourt P (2010) A small-molecule screen identifies new functions for the plant hormone strigolactone. Nat Chem Biol 6: 741–749 [DOI] [PubMed] [Google Scholar]
- Ulmasov T, Murfett J, Hagen G, Guilfoyle TJ (1997) Aux/IAA proteins repress expression of reporter genes containing natural and highly active synthetic auxin response elements. Plant Cell 9: 1963–1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenbussche F, Petrásek J, Zádníková P, Hoyerová K, Pešek B, Raz V, Swarup R, Bennett M, Zazímalová E, Benková E, Van Der Straeten D (2010) The auxin influx carriers AUX1 and LAX3 are involved in auxin-ethylene interactions during apical hook development in Arabidopsis thaliana seedlings. Development 137: 597–606 [DOI] [PubMed] [Google Scholar]
- Vieten A, Vanneste S, Wiśniewska J, Benková E, Benjamins R, Beeckman T, Luschnig C, Friml J (2005) Functional redundancy of PIN proteins is accompanied by auxin-dependent cross-regulation of PIN expression. Development 132: 4521–4531 [DOI] [PubMed] [Google Scholar]
- Viotti C, Krüger F, Krebs M, Neubert C, Fink F, Lupanga U, Scheuring D, Boutté Y, Frescatada-Rosa M, Wolfenstetter S, Sauer N, Hillmer S, et al. (2013) The endoplasmic reticulum is the main membrane source for biogenesis of the lytic vacuole in Arabidopsis. Plant Cell 25: 3434–3449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voigt B, Timmers AC, Samaj J, Müller J, Baluska F, Menzel D (2005) GFP-FABD2 fusion construct allows in vivo visualization of the dynamic actin cytoskeleton in all cells of Arabidopsis seedlings. Eur J Cell Biol 84: 595–608 [DOI] [PubMed] [Google Scholar]
- Wagner BK, Clemons PA (2009) Connecting synthetic chemistry decisions to cell and genome biology using small-molecule phenotypic profiling. Curr Opin Chem Biol 13: 539–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Xie X-Q (2014) Computational target fishing: what should chemogenomics researchers expect for the future of in silico drug design and discovery? Future Med Chem 6: 247–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G, Wang X, Li X, Kamiya Y, Otegui MS, Chory J (2011) Methylation of a phosphatase specifies dephosphorylation and degradation of activated brassinosteroid receptors. Sci Signal 4: ra29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing Y, Li Z, Chen Y, Stock JB, Jeffrey PD, Shi Y (2008) Structural mechanism of demethylation and inactivation of protein phosphatase 2A. Cell 133: 154–163 [DOI] [PubMed] [Google Scholar]
- Xu J, Scheres B (2005) Dissection of Arabidopsis ADP-RIBOSYLATION FACTOR 1 function in epidermal cell polarity. Plant Cell 17: 525–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto R, Demura T, Fukuda H (1997) Brassinosteroids induce entry into the final stage of tracheary element differentiation in cultured Zinnia cells. Plant Cell Physiol 38: 980–983 [DOI] [PubMed] [Google Scholar]
- Yamazoe A, Hayashi K, Kepinski S, Leyser O, Nozaki H (2005) Characterization of terfestatin A, a new specific inhibitor for auxin signaling. Plant Physiol 139: 779–789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh JR, Crews CM (2003) Chemical genetics: adding to the developmental biology toolbox. Dev Cell 5: 11–19 [DOI] [PubMed] [Google Scholar]
- Zádníková P, Petrásek J, Marhavý P, Raz V, Vandenbussche F, Ding Z, Schwarzerová K, Morita MT, Tasaka M, Hejátko J, Van Der Straeten D, Friml J, et al. (2010) Role of PIN-mediated auxin efflux in apical hook development of Arabidopsis thaliana. Development 137: 607–617 [DOI] [PubMed] [Google Scholar]