Putrescine enhances Fe deficiency-induced accumulation of nitric oxide which ultimately leads to the reutilization of cell wall Fe under Fe-deficient conditions.
Abstract
Plants challenged with abiotic stress show enhanced polyamines levels. Here, we show that the polyamine putrescine (Put) plays an important role to alleviate Fe deficiency. The adc2-1 mutant, which is defective in Put biosynthesis, was hypersensitive to Fe deficiency compared with wild type (Col-1 of Arabidopsis [Arabidopsis thaliana]). Exogenous Put decreased the Fe bound to root cell wall, especially to hemicellulose, and increased root and shoot soluble Fe content, thus alleviating the Fe deficiency-induced chlorosis. Intriguingly, exogenous Put induced the accumulation of nitric oxide (NO) under both Fe-sufficient (+Fe) and Fe-deficient (-Fe) conditions, although the ferric-chelate reductase (FCR) activity and the expression of genes related to Fe uptake were induced only under -Fe treatment. The alleviation of Fe deficiency by Put was diminished in the hemicellulose-level decreased mutant-xth31 and in the noa1 and nia1nia2 mutants, in which the endogenous NO levels are reduced, indicating that both NO and hemicellulose are involved in Put-mediated alleviation of Fe deficiency. However, the FCR activity and the expression of genes related to Fe uptake were still up-regulated under -Fe+Put treatment compared with -Fe treatment in xth31, and Put-induced cell wall Fe remobilization was abolished in noa1 and nia1nia2, indicating that Put-regulated cell wall Fe reutilization is dependent on NO. From our results, we conclude that Put is involved in the remobilization of Fe from root cell wall hemicellulose in a process dependent on NO accumulation under Fe-deficient condition in Arabidopsis.
Iron is an essential element for plant growth and development, and iron deficiency is the most common micronutrient deficiency in the world. To cope with iron deficiency, plants have evolved two distinct mechanisms for Fe acquisition from the rhizosphere. Strategy I, found in all dicots and monocots with the exception of graminaceous species, is characterized by (1) release of protons to acidify the rhizosphere, which is mediated in Arabidopsis (Arabidopsis thaliana) by the proton-translocating ATPase AHA2 (ARABIDOPSIS PLASMA MEMBRANE H+-ATPASE ISOFORM 2; Curie and Briat, 2003; Santi and Schmidt, 2009); (2) inducing ferric chelate reductase activity mediated by FRO2 (FERRIC REDUCTASE OXIDASE2; Robinson et al., 1999); and (3) uptake of Fe2+ by the metal transporter IRT1 (IRON REGULATED TRANSPORTER1; Eide et al., 1996; Vert et al., 2002). Strategy II, utilized by graminaceous monocots (Römheld and Marschner, 1986), is characterized by enhanced release of phytosiderophores that form chelates with Fe(III) (Curie and Briat, 2003). However, in addition to Fe acquisition, the mechanisms underlying the mobilization of Fe(III) also are a major challenge for us to understand.
Recently, accumulating evidence has shown that phenolic compounds are important for iron mobilization. Rodríguez-Celma et al. (2013) showed that secretion of phenolics is critical for Arabidopsis Fe acquisition from low bioavailability sources, and then Fourcroy et al. (2014) and Schmidt et al. (2014) demonstrated that coumarins are the active compounds in this process. Schmid et al. (2014) confirmed that secretion of coumarins is an essential aspect of Arabidopsis Fe acquisition and provided extensive information on metabolomic changes elicited by Fe deficiency. However, under certain conditions Fe is not readily available, and Fe is difficult to mobilize; thus, Fe stored in the plant needs to be reutilized. For example, phenolics are secreted to remobilize the root apoplastic Fe and improve Fe nutrition in red clover (Trifolium pratense) and rice (Oryza sativa) (Jin et al., 2007; Bashir et al., 2011). Moreover, Lei et al. (2014) reported that the cell wall can be an important Fe source during periods of limited Fe supply. As the first barrier to encounter the soil environment, the cell wall is a pivotal site for most cationic ions in plants (Lozano-Rodríguez et al., 1997; Carrier et al., 2003). Hemicellulose contributes to the overall Al/Cd accumulation in the cell wall of Arabidopsis (Zhu et al., 2012, 2013) and also acts as a Fe pool (Lei et al., 2014). Over 75% of Fe in the root is retained in the cell wall (Bienfait et al., 1985), especially in the hemicellulose fraction (Lei et al., 2014). Thus, the cell wall is not only a site to immobilize an element and restrict its entrance into the cell, but also can serve as a pool to provide the nutrient when the supply from the growth medium is limited. However, the upstream mechanism of Fe reutilization through the cell wall, especially hemicellulose, is still far from clear.
The responses to Fe deficiency in plants involve numerous phytohormones and signaling molecules, including auxin (Römheld and Marschner, 1981; Chen et al., 2010), ethylene (García et al., 2010; Wu et al., 2011), and NO (Graziano and Lamattina, 2007; Chen et al., 2010). Polyamines share common substrates with nitric oxide (NO) (Shi and Chan, 2014), and polyamines like spermidine and spermine rapidly induce a burst of NO in various plant species, indicating that NO is a potential intermediate of polyamine-mediated signaling.
Polyamines, including putrescine (Put), spermidine, and spermine, are low Mr natural compounds with nitrogen-containing aliphatic structure and influence basic physiological and developmental events, such as cell division and differentiation, rhizogenesis, leaf senescence, zygotic, somatic embryogenesis, and development of flowers and fruits (Feirer et al., 1984; Galston et al., 1995; Bouchereau et al., 1999; Kakkar et al., 2000; Tun et al., 2001; Shi and Chan, 2014). The metabolism of polyamines in plant tissues is subject to strict regulation, and polyamine levels in plant roots change upon exposure to abiotic stress such as salt, drought, low and high temperature, heavy metals (Cu, Cr, Fe, and Ni), and oxidative stresses (Liu et al., 2005; Cheng et al., 2009; Wimalasekera et al., 2011; Tavladoraki et al., 2012).
Ample evidence demonstrates the involvement of Put in responses to various types of abiotic stress, such as mineral deficiency in barley (Hordeum vulgare) leaves (Smith, 1973), high osmotic pressure in barley, corn, wheat, and wild oat leaves (Flores and Galston, 1982a), low pH in peeled oat (Avena sativa L. var Victory) leaf (Young and Galston, 1983), potassium deficiency in oat shoot and Arabidopsis thaliana (L.) Heynh (Young and Galston, 1984; Watson and Malmberg, 1996), and cadmium toxicity in oat and bean leaves (Weinstein et al., 1986). In animals, Put is produced either from Orn by Orn decarboxylase or from Arg by Arg decarboxylase (ADC) (Hanfrey et al., 2001). As there is no detectable Orn decarboxylase activity in Arabidopsis, the ADC route is critical for Put biosynthesis. Although there are two genes responsible for ADC activity, Urano et al. (2004) reported that the expression of ADC2 correlates well with the increment of free Put, indicating ADC2 plays an important role in Put biosynthesis in Arabidopsis. However, the role of Put under Fe deficiency in plants remains unknown.
In this study, we found that Fe deficiency results in enhanced Put levels. Further, whereas exogenous Put alleviated Fe deficiency, the adc2-1 mutant, in which endogenous Put is decreased, exhibited a Fe deficiency-sensitive phenotype. We demonstrated that Put acts upstream of NO to decrease the Fe binding capacity of the cell wall, especially that of hemicellulose, thus resulting in greater Fe reutilization.
RESULTS
Fe Deficiency Induces Put Accumulation in Roots of Arabidopsis
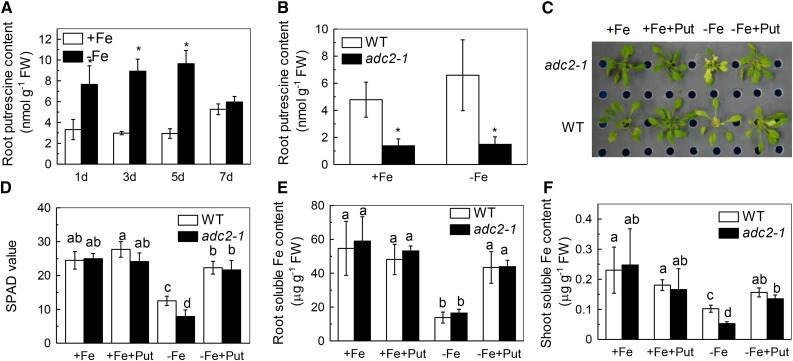
To investigate the effect of Fe deficiency on Put metabolism, we exposed Arabidopsis plants to Fe-deficient (-Fe) nutrient solution for 7 d. Fe deficiency induced Put accumulation in the roots within 1 d (Fig. 1A). Put levels remained high through day 5 and then decreased to the same level as control by day 7 (Fig. 1A). These results demonstrate that Fe deficiency results in the rapid accumulation of Put in the roots of Arabidopsis.
Figure 1.
Effect of endogenous and exogenous Put on Fe-deficiency symptoms in Arabidopsis. Six-week-old wild type and adc2-1 mutant were treated with or without 50 μm Fe for the designed time (A, wild type only) and for 7 d (B), and the root samples were taken to measure Put content. The phenotypes of the plants (C), the SPAD value of the newly expanded leaves (D), and root (E) and shoot (F) soluble Fe content were examined after six-week-old wild type and adc2-1 were placed in the -Fe or +Fe medium with or without 0.1 mm Put for 7 d. Pictures were taken using a digital camera. Error bars represent ± sd (n = 4). Different letters and * represent significant difference at P < 0.05.
Put Alleviates Fe Deficiency
To quantify the effects of exogenous Put application on the response of Arabidopsis to Fe deficiency, plants were treated with or without 0.1 mm Put under Fe deficiency. After 7-d treatments, Fe deprivation resulted in chlorosis in the newly expanded leaves (Fig. 1C). However, this typical Fe deficiency symptom was hardly observed in the -Fe+Put treatment, consistent with the increment in Soil and Plant Analyzer Development (SPAD), an indicator of the total chlorophyll content (Fig. 1D). The soluble Fe in the roots (Fig. 1E) and shoots (Fig. 1F) increased in -Fe+Put relative to -Fe, in good agreement with the phenotype observed (Fig. 1C).
To analyze the role of endogenous Put under Fe deficiency, we used the adc2-1 mutant, in which the free Put content is reduced to about 25% of that in wild-type Columbia ecotype (Fig. 1B; Urano et al., 2004). The SPAD values of wild type and adc2-1 were similar under Fe-sufficient (+Fe) or +Fe+Put treatment. Under -Fe treatment, the leaf chlorosis was more pronounced in adc2-1, but could be alleviated by exogenous Put (Fig. 1C). Furthermore, there was less shoot soluble Fe in adc2-1 under the Fe-deficient condition (Fig. 1F), although there was almost no difference in the root soluble Fe or root and shoot total Fe (Fig. 1E; Supplemental Figure S1, A and B). Put applied exogenously could increase the shoot soluble Fe content in adc2-1 back to the wild-type level under Fe-deficient conditions (Fig. 1F). Moreover, Put application also increased the root and shoot symplastic Fe in the wild-type and adc2-1 mutant, whereas less symplastic Fe was detected in the adc2-1 mutant root and shoot (Supplemental Figure S1, C and D). These results are in accordance with the increased root and shoot soluble Fe content in –Fe+Put versus –Fe treatment in wild type and the decreased shoot soluble Fe content in adc2-1 versus wild type (Fig. 1, C and D), further indicating that the increment of the Put levels under Fe-deficient conditions is beneficial to the Arabidopsis (Fig. 1).
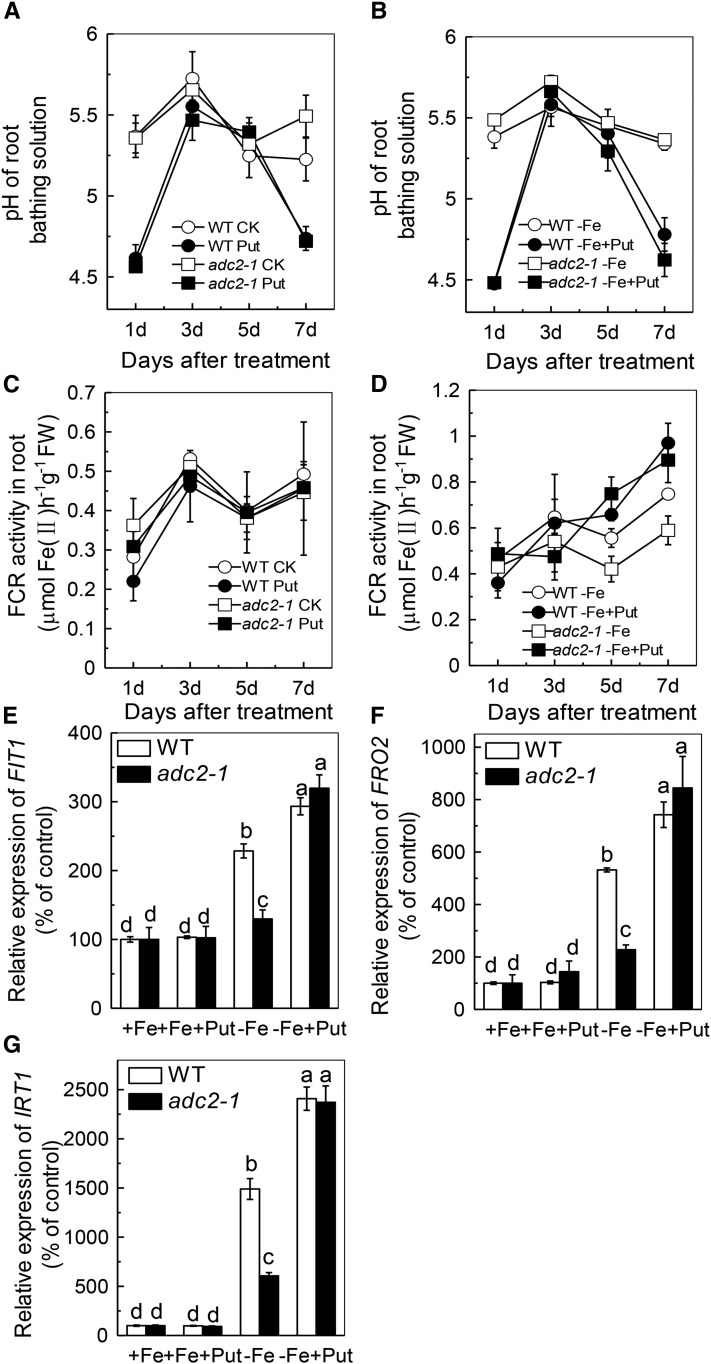
Stimulation of the plasmalemma NADPH-ferric chelate reductase (FCR; Robinson et al., 1999) activity and extrusion of proton to the rhizosphere, which can be measured as changes in pH value of the growth solution (Jin et al., 2007, 2009), are hallmark responses to Fe deficiency in dicots (Robinson et al., 1999; Curie and Briat, 2003). Here, we found that the pH of the -Fe solution was decreased greatly after 1 d of Put treatment (Fig. 2, A and B), while the FCR activity was remarkably enhanced after 5 d of -Fe+Put treatment (Fig. 2, C and D). Moreover, the adc2-1 mutant showed less root FCR activity than wild-type plants and could be rescued by exogenous Put (Fig. 2D). These findings suggest that the increased Put content under Fe-deficient conditions was responsible for the increased FCR activity.
Figure 2.
Effect of endogenous and exogenous Put on Fe deficiency response in Arabidopsis. Six-week-old wild type and adc2-1 were treated with or without 0.1 mm Put in the presence or absence of 50 μm Fe for the designated time for pH measurement (A, B), and root samples were taken to measure the FCR activity (C, D). The expression of genes related to Fe uptake (E, F, G) were examined after six-week-old wild type and adc2-1 were placed in the -Fe or +Fe medium with or without 0.1 mm Put for 7 d. Expression levels in the +Fe-treated sample for each gene were set to 100%, and levels in the tested sample were calculated relative to the corresponding control value. Error bars represent ± sd (n = 4). Different letters and * represent significant difference at P < 0.05.
Interestingly, Put applied alone also leads to a decrease in the pH value (Fig. 2A). The solubility of Fe decreases up to 1000-fold for each unit increase in pH (Santi and Schmidt; 2009), but as there was no Fe in the nutrient solution, this reduction of the pH cannot explain the alleviation of Fe deficiency symptom (Fig. 1C). Furthermore, when the solution was buffered at pH 5.6 with 10 mm MES, the root and shoot soluble Fe content showed no significant difference from those in the nonbuffered solution (Supplemental Figure S2, A and B). Santi and Schmidt (2009) suggested that the rhizosphere acidification in response to Fe deficiency is chiefly mediated by AHA2. Thus, if lower pH increases the availability of Fe, there should be more soluble Fe in wild-type plants compared with the aha2 (Salk_022010) mutant under Fe-deficient conditions. However, we observed almost no difference in the root and shoot soluble Fe content between wild type and aha2, neither under -Fe nor under -Fe+Put treatment (Supplemental Figure S2, C and D). Therefore, the effect of Put observed here is not attributable to the effects of a lower pH in the media, but rather is caused by the synergetic action of Put and -Fe.
The FCR activity reached a maximum level at 7 d (Fig. 2D), as did the expression of FIT1, FRO2, and IRT1 (Fig. 2, E-G). We wondered whether exogenous Put would affect FCR activity under Fe-sufficient conditions. Neither the relative expression of FIT1, FRO2, and IRT1 nor the root FCR activity was affected by +Fe+Put treatment compared with +Fe treatment (Fig. 2), indicating that Put can trigger root FCR activity only under Fe deficient conditions.
Put Acts Upstream of NO Accumulation
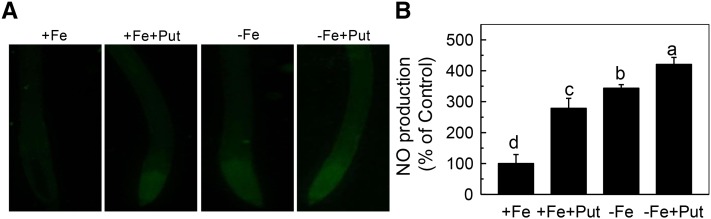
It has been previously demonstrated that NO was involved in the induction of FCR activity (Graziano and Lamattina, 2007). To examine whether Put acts upstream or downstream of NO under Fe-deficient conditions, we first analyzed the effects of exogenous application of Put on NO accumulation in wild type. As shown in Figure 3, Put treatment significantly increased the NO accumulation under both Fe-sufficient and Fe-deficient conditions. However, the increment of NO accumulation under Fe-sufficient conditions may not be efficient to induce the FCR activity compared with the increment of NO accumulation under Fe-deficient conditions (Fig. 3 and Fig. 2).
Figure 3.
Effect of Put on NO accumulation in wild type. A, NO production reported as green fluorescence by using DAF-FM DA probes in representative roots. B, NO production expressed as relative fluorescence intensity. Fluorescence intensity in the +Fe-treated root was set to 100%, and intensity in the tested root was calculated relative to the corresponding control value. Error bars represent ± sd (n = 4). Different letters represent significant difference at P < 0.05.
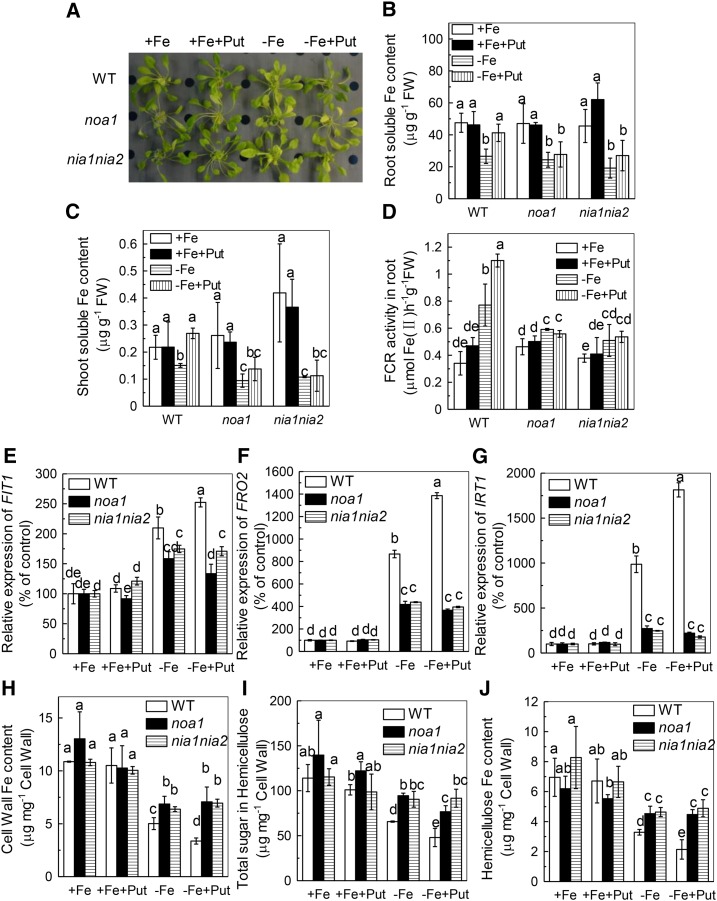
We further analyzed the effect of Put in noa1 and nia1nia2 mutants, both of which are defective in NO biosynthesis (Guo, 2006; Wilson et al., 2008; Chen et al., 2010). Put failed to alleviate the Fe deficiency symptoms in both mutants (Fig. 4A). Consistent with this, there was no increment of FCR activity (Fig. 4D), no change in the root and shoot soluble Fe content in either mutant (Fig. 4, B and C), and no induction of the relative expression of FIT1, FRO2, and IRT1 (Fig. 4, E-G). All of these results indicate that both NOA-dependent and NR-dependent production of NO acts downstream of Put under Fe-deficient conditions.
Figure 4.
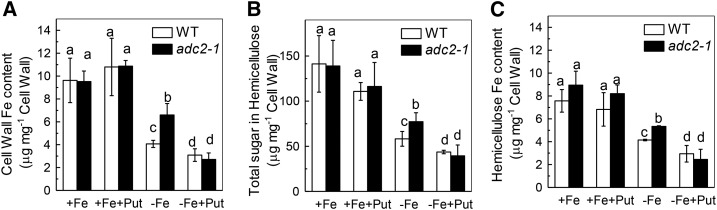
Effect of exogenous Put on Fe deficiency symptoms in Arabidopsis wild type and noa1 and nia1nia2 mutants. Six-week-old seedlings were treated with or without 0.1 mm Put in the presence or absence of 50 μm Fe for 7 d, the Fe deficiency symptoms (A) were taken using a digital camera, and root and shoot samples were taken to measure the root soluble Fe content (B), shoot soluble Fe content (C), root FCR activity (D), the expression of genes related to Fe uptake (E, F, G), cell wall Fe content (H), cell wall hemicellulose content (I), and Fe content in the hemicellulose (J). Expression levels in the +Fe-treated sample for each gene were set to 100%, and levels in the tested sample were calculated relative to the corresponding control value. Error bars represent ± sd (n = 4). Different letters represent significant difference at P < 0.05.
Put Induces Fe Remobilization from the Root Cell Wall Hemicellulose
As there was no available Fe in the nutrient solution, the increased FCR activity could not be responsible for the increased root soluble Fe content under Fe-deficient conditions. This raised the question of the source of the soluble Fe. Since the cell wall plays pivotal roles in Fe reutilization in Arabidopsis during Fe deficiency (Lei et al., 2014), we wondered whether Put treatment led to a release of the cell wall-retained Fe. Indeed, we found that the cell wall fractions of wild-type and adc2-1 roots contained about 4.1 and 6.6 µg bound Fe/mg under Fe-deficient conditions (Fig. 5A), but only about 3 µg/mg in the -Fe+Put treatment (Fig. 5A). These results indicate that Put induces Fe remobilization from the root cell walls.
Figure 5.
Effect of endogenous and exogenous Put on the cell wall Fe content (A), cell wall hemicellulose content (B), and cell wall hemicellulose Fe content (C). Six-week-old wild type and adc2-1 were placed in +Fe or -Fe medium with or without 0.1 mm Put for 7 d. Error bars represent ± sd (n = 4). Different letters represent significant difference at P < 0.05.
We previously demonstrated that hemicellulose contributes greatly to the metal binding in cell walls of rice and Arabidopsis (Yang et al., 2008, 2011; Zhu et al., 2012, 2013). Here, we found that -Fe+Put treatment significantly decreased hemicellulose content compared with -Fe treatment alone (Fig. 5B). In accordance with the changes in the cell wall components, the Fe content in the hemicellulose fraction was also much lower with -Fe+Put treatment than with -Fe treatment alone (Fig. 5C), suggesting that Put alleviated Fe deficiency through reutilization of Fe from root cell wall hemicellulose.
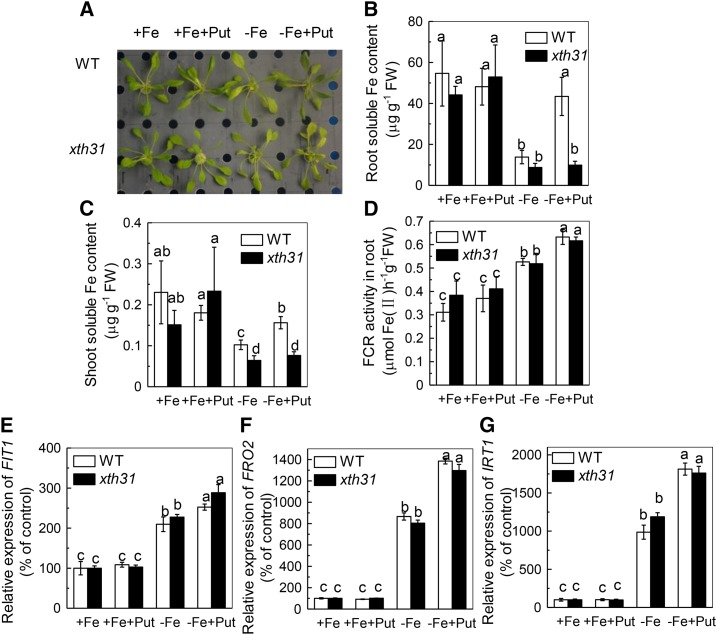
To further clarify the role of hemicellulose in the Put-mediated alleviation of Fe deficiency, we used the xth31 mutant, in which the endogenous hemicellulose content is decreased (Zhu et al., 2012). As shown in Figure 6, Put was unable to alleviate the Fe deficiency phenotype in the xth31 mutant (Fig. 6A), with no change in the root and shoot soluble Fe content (Fig. 6, B and C), although there was still an induction of the FCR activity and the expression of Fe uptake related genes: FIT1, FRO2, and IRT1 (Fig. 6, D-G). These results indicate that the ability of Put to alleviate Fe deficiency is dependent on hemicellulose.
Figure 6.
Effect of exogenous Put treatment on the xth31 mutant with decreased hemicellulose content. Phenotypes of the plants (A), root soluble Fe content (B), shoot soluble Fe content (C), FCR activity (D), and the expression of genes related to Fe uptake (E, F, G). Six-week-old wild type and xth31 were placed in +Fe or -Fe medium with or without 0.1 mm Put for 7 d. Expression levels in the +Fe-treated sample for each gene were set to 100%, and levels in the tested sample were calculated relative to the corresponding control value. Error bars represent ± sd (n = 4). Different letters represent significant difference at P < 0.05.
The Hemicellulose-Mediated Function of Put in Fe Deficiency Is Dependent on NO Accumulation
In this study, we found that Put could not alleviate Fe deficiency in NO-related mutants (noa1 and nia1nia2) or in a hemicellulose content-reduced mutant (xth31) (Fig. 4 and 6). This prompted us to explore the relationship between NO and hemicellulose in Put alleviation of Fe deficiency. There was no difference in cell wall hemicellulose content or cell wall hemicellulose Fe content between noa1 and nia1nia2 mutants under -Fe+Put or -Fe treatment (Fig. 4, H-J). The fact that there was still an induction of FCR activity and the expression of Fe-related genes in the xth31 mutant (Fig. 6) indicates that the effect of Put is mediated through NO, as shown in the model proposed in Figure 7.
Figure 7.
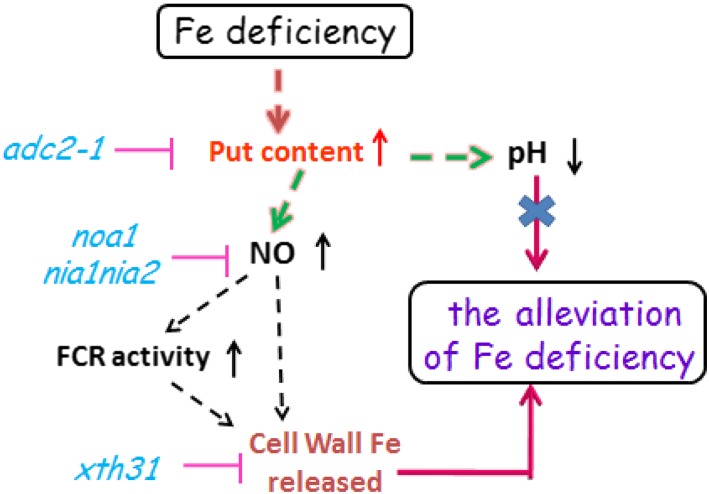
Proposed model illustrating the link between Put and alleviation of Fe deficiency. Fe deficiency induces the increment of root Put level, which results in the pH reduction and NO accumulation. Moreover, cell wall hemicellulose acts downstream of NO in the Put-mediated Fe deficiency response. However, the reduced pH is not involved in the Fe remobilization induced by Put during Fe deficiency.
DISCUSSION
Put Alleviated Fe Deficiency
The experiments described here demonstrate the beneficial effect of Put on plants exposed to Fe-deficient conditions. The chlorosis induced by Fe deficiency was obviously alleviated upon treatment with exogenous Put (Fig. 1). Further, the Put level-reduced mutant adc2-1 showed more pronounced chlorosis than did wild type (Fig. 1). These results suggest that high levels of Put under Fe deficiency are causally related to the tolerance of Fe-deficient conditions by Arabidopsis.
Put Alleviates Fe Deficiency by Inducing Reutilization of Cell Wall Fe, Particularly from Hemicellulose
Accumulating evidence shows that the cell wall plays a significant role in regulating plant adaptive responses to Fe deficiency stress. Jin et al. (2007) provided the first evidence, to our knowledge, that Fe stored in the apoplast can be remobilized via Fe deficiency-induced secretion of phenolics in red clover and that the remobilized Fe can be found in the shoots; thus, the Fe nutrition of the shoots was improved. Lei et al. (2014) reported that the content of Fe bound to the cell wall decreases under Fe-deficient conditions in Arabidopsis. Exogenous application of ABA to Fe-deficient Arabidopsis significantly decreases cell wall Fe content, and as a result, more Fe is released and the plant becomes more Fe deficiency resistant. Here, we found that the amount of root cell wall Fe was significantly increased in the Put level-reduced adc2-1 mutant and decreased in -Fe+Put treatment (Fig. 5A), indicating that Put promotes the remobilization of root cell wall Fe.
The cell wall is composed mainly of the matrix polysaccharides, including cellulose, hemicellulose, and pectin (Cosgrove, 2005). Pectin has been regarded as the main source of the negative charges of the cell wall and is generally attributed to the binding of cations, such as Al in tobacco (Chang et al., 1999), wheat (Zheng et al., 2004), maize (Eticha et al., 2005), rice (Yang et al., 2008), and Arabidopsis (Yang et al., 2011). However, recent evidence from Zhu et al. (2012) showed that hemicellulose make the greatest contribution to Al retention in the cell wall. Hemicellulose, which is tightly bound to the surface of cellulose to form a strong and resilient network, is synthesized in the Golgi and consists of a group of neutral or slightly acidic polysaccharides, such as xyloglucan, mannan, and arabinoxylan (Carpita and Gibeaut, 1993; Cosgrove, 2005; Zhu et al., 2012). In our previous study, we measured the Fe retained in different cell wall components and found that the largest pool of Fe was bound to hemicellulose (Lei et al., 2014). Upon treatment with exogenous Put, significantly less Fe was found that retained in hemicellulose (Fig. 5C). In fact, the hemicellulose content was significantly decreased by Put treatment (Fig. 5B), indicating that cell wall hemicellulose contributed greatly to the Put-mediated alleviation of Fe deficiency. Furthermore, we found that Put failed to alleviate Fe deficiency in the hemicellulose-reduced xth31 mutant (Fig. 6), implying that a certain hemicellulose level is necessary for Put to relieve Fe deficiency.
Put Acts Upstream of NO under Fe Deficiency
How does Put affect the hemicellulose content under Fe-deficient conditions? Put and NO share the common substrates, and NO has been demonstrated to function as a signal molecule in plant responses to low Fe conditions (Chen et al., 2010). For example, NO can induce many important genes regulating Fe acquisition, such as FRO1 expression in tomato (Graziano and Lamattina, 2007) and FRO2 and GENERAL REGULATORY FACTOR11 (GRF11) expression in Arabidopsis (Chen et al., 2010; Yang et al., 2013). Both Put and NO content were increased in Fe-deficient roots (Fig. 1A and Fig. 3), does one event precede the other? In this study, we found that either Put applied exogenously or Fe deficiency could increase the root NO levels (Fig. 3) and that NO accumulation was further increased under -Fe+Put treatment (Fig. 3). Consistent with this, FCR activity was further induced under -Fe+Put compared with -Fe (Fig. 2D), in concert with the up-regulation of the expression of Fe uptake-related genes, FIT1, FRO2, and IRT1 (Fig. 2, E-G), which is in accordance with Chen et al. (2010). These findings indicate that Put may act upstream of NO to induce the expression of FRO2 and the activity of FCR. This conclusion was further demonstrated by the lack of FCR induction in both noa1 and nia1nia2 mutants upon Put treatment under Fe-deficient conditions (Fig. 4D). It is interesting that the increment of NO accumulation under +Fe+Put treatment failed to induce FCR activity compared with +Fe treatment (Fig. 2C and 3). The possibility that there is a tight threshold of NO accumulation that leads to the induction of the FCR activity awaits further study.
Put-Mediated Changes in Cell Wall Hemicellulose Content under Fe Deficiency Is Dependent on NO
As Put could influence both NO accumulation and hemicellulose content, we explored the relationship between NO and hemicellulose content. We monitored the hemicellulose content and Fe retention in the cell wall of noa1 and nia1nia2 mutants under -Fe and -Fe+Put treatment. Put influenced neither the hemicellulose content nor the cell wall Fe content in noa1 and nia1nia2 mutants; thus, root and shoot soluble Fe content remained unchanged (Fig. 4). This result further indicated that Put-mediated changes in cell wall hemicellulose contents under Fe deficiency depend on the NO level.
In conclusion, we propose a model to link the Fe deficiency-induced accumulation of Put level to Fe deficiency responses (Fig. 7). Previous reports showed that both NO accumulation and hemicellulose reduction are involved in response to Fe deficiency (Chen et al., 2010; Lei et al., 2014). Here, we further demonstrated that Fe deficiency induces Put accumulation in roots and that Put is involved in the remobilization of Fe from root cell wall hemicellulose through the accumulation of NO. As a result, there is more soluble Fe in the roots and leaves, thus alleviating the Fe deficiency-induced chlorosis. In other words, plants with less endogenous Put have less Fe reutilization from the root cell wall and thus are more sensitive to Fe deficiency.
MATERIALS AND METHODS
Plant Material and Growth Conditions
The Columbia ecotype (wild-type) of Arabidopsis (Arabidopsis thaliana) and the mutants generated in this background, including adc2-1 (Salk_026916C), aha2 (Salk_022010), xth31 mutant (decreased hemicellulose levels), NOA-deficient mutant noa1, and the NR-null-deficient double mutant nia1nia2 were used in this study. Seedlings were germinated on a sponge in Murashige and Skoog nutrient solution for 3 weeks in an environmental controlled growth chamber. Subsequently, seedlings of similar rosette diameters were transferred to nutrient solution containing Murashige and Skoog salts for another 3 weeks. These 6-week-old plants were subjected to the following treatments: +Fe (the complete nutrient solution as control), +Fe+Put (complete nutrient solution plus 0.1 mm Put), -Fe (control solution without Fe), and -Fe+Put (-Fe solution plus 0.1 mm Put). Put standard (Putrescine dihydrochloride, ref: P7505) was purchased from Sigma Chemical, according to Urano et al. (2004). For the pH buffered and nonbuffered solution, 10 mm MES was included or not in each of the above nutrient solutions (+Fe, +Fe+Put, -Fe, and -Fe+Put). All treatment solutions were renewed every 3 d. After 1 week, the plants were harvested and separated into roots and shoots and washed with deionized water. The nutrient medium consisted of the following macronutrients in mM: KNO3, 6.0; Ca(NO3)2, 4.0; MgSO4, 1; NH4H2PO4, 0.1, and the following micronutrients in μM: Fe(III)-EDTA, 50; H3BO3, 12.5; MnSO4, 1; CuSO4, 0.5; ZnSO4, 1; H2MoO4, 0.1; NiSO4, 0.1 according to Murashige and Skoog (1962). The final pH was adjusted to 5.6 with 1 m KOH. The growth conditions for the seedlings were at a temperature of 24°C, a light intensity of 140 µmol m−2 S−1, and a 16-h/8-h day/night rhythm. The growth conditions were the same for all experiments unless otherwise specified.
Measurement of Chlorophyll Fluorescence
SPAD values (total chlorophyll content) were determined on the youngest fully expanded leaves of seedlings with a portable chlorophyll meter (SPAD-502; Minolta Sensing).
Put Measurement
Free polyamines were analyzed by HPLC according to Flores and Galston (1982b) and Morgan (1998) with some modifications. After treatment, roots were homogenized in 5% HClO4 in chilled mortars and pestles. After the homogenates were incubated in ice-cold water for 1 h, 10 µL benzoyl chloride and 2 mL saturated sodium chloride were added, and the samples were mixed vigorously to disperse the benzoyl chloride. Then the reaction was allowed to proceed at room temperature for 30 min. The polyamines were subsequently extracted with 2 mL 2 m NaOH and 2 mL diethyl ether and centrifuged briefly to separate the layers after vigorous vortexing for 30 s. The mixtures were separated into two phases, aqueous and organic. The organic phase, containing the polyamines, was completely dried using a rotary evaporator at 40°C, redissolved in 100 µL methanol, and assayed immediately. Aliquots of samples were diluted 5- to 20-fold before injection into the HPLC, with excitation at 350 nm and emission at 495 nm. Standards were treated in a similar way.
Solution pH Measurement
The pH in nutrient solution was measured every other day with a pH electrode (METROHM).
Gene Expression Analysis
Plants were grown in the same conditions used for inductively coupled plasma-atomic emission spectrometry (ICP-AES) analysis. Root tissues were collected 7 d after treatments began and were immediately frozen in liquid nitrogen before total RNA extraction. Total RNA was isolated from the root using TRIzol (Invitrogen). cDNA was prepared from 1 µg of total RNA using the PrimeScript RT reagent kit (Takara). For quantitative real-time PCR analysis, 1 µL 10-fold-diluted cDNA was used for quantitative analysis of gene expression performed with SYBR Premix ExTaq (Takara) with the following pairs of gene-specific primers. FIT (forward: 5′-GGAGAAGGTGTTTGTCCATCTC-3′; reverse: 5′-GGTTAGGCAAGTTTAAGCTCTG-3′); FRO2 (forward: 5′-CGTTGCACGAGCGATTCTTG-3′; reverse: 5′-GCGACTTGTAGTGCGGCTATG-3′); IRT1 (forward: 5′-CGGTTGGACTTCTAAATGC-3′; reverse: 5′-CGATAATCGACATTCCACCG-3′). Each cDNA sample was run in triplicate. Expression data were normalized to the expression of TUBULIN (forward: 5′-AAGTTCTGGGAAGTGGTT-3′; reverse: 5′-CTCCCAATGAGTGACAAA-3′) (Chen et al., 2010).
Cell Wall Extraction and Fractionation
Extraction of crude cell wall materials and subsequent fractionation of cell wall components were carried out according to Zhong and Lauchli (1993) with minor modifications according to Yang et al. (2011). After treatment, roots were ground in liquid nitrogen with a mortar and pestle, homogenized by incubation in 75% ethanol for 20 min in an ice-cold water bath, and then centrifuged at 8,000g for 10 min and the supernatant was removed. Then, the pellet was homogenized and washed with acetone, methanol:chloroform (1:1), and methanol for 20 min each, with the supernatant removed after centrifugation.
Cell wall samples (about 2 mg) were treated three times with 1 mL water at 100°C for 1 h, and the residue was further extracted twice with 1 mL 24% (w/v) KOH and 0.02% (w/v) KBH4 at room temperature for 12 h. The two supernatants were combined in a 2-mL tube and centrifuged at 12,000g for 10 min to recover the hemicellulose fraction.
Total Polysaccharide Measurement
The total polysaccharide contents in the hemicellulose fractions were determined by the phenol sulfuric acid method (Dubois et al., 1956) and expressed as Glc equivalents. Briefly, 200-μL hemicellulose extracts were incubated with 1 mL 98% H2SO4 and 10 μL 80% phenol at room temperature for 15 min, and then the mixed solutions were incubated at 100°C for 15 min. After chilling, the absorbance was measured spectrophotometrically at 490 nm.
Measurements of Fe Content in Plants
Extraction of water-soluble Fe was performed according to Cassin et al. (2009). Briefly, after plants were treated as indicated, 0.5- to 1-g root and shoot samples were ground in liquid nitrogen and extracted with 5 volumes of deionized water at room temperature. After centrifugation, the supernatant was collected. The Fe concentration in the supernatant was measured as the root and shoot soluble Fe content. For total root and shoot Fe content assays, materials were digested with HNO3/HClO4 (4:1, v/v). For symplastic Fe content analysis, the root and shoot tissues were excised after washing three times with 0.5 mm CaCl2 and then Put in a Ultra free-MC Centrifugal filter units (Millipore) and centrifuged at 3,000g for 10 min at 4°C to remove apoplastic solution. The roots were then frozen at −80°C overnight. The root-cell sap solution was obtained by thawing the samples at room temperature, and then the liquid was collected after centrifuging at 20,600g for 10 min according to Zhu et al. (2013). The soluble Fe, total Fe, and symplastic Fe content were determined by ICP-AES (IRIS/AP optical emission spectrometer).
Measurements of Cell Wall and Cell Wall Hemicellulose Fe Content in Plants
Fe in cell wall was extracted by 2 n HCl for 24 h with occasional shaking. Fe concentrations in these cell wall extracts and Fe content in the above extracted hemicellulose were determined by ICP-AES (IRIS/AP optical emission spectrometer).
Root FCR Activity Determination
FCR activity was determined according to Grusak (1995) and Lei et al. (2014). Briefly, whole excised roots were placed into a test tube filled with 5 mL assay solution consisting of 0.5 mm CaSO4, 0.1 mm 4-morpholineethanesulfonic acid, 0.1 mm bathophenanthroline-disulfonic acid disodium salt hydrate, and 100 mm Fe-EDTA at pH 5.5 adjusted by 1 m NaOH. The tubes were placed in a dark room at 25°C for 1 h, with periodic hand swirling at 10-min intervals. The absorbance of the assay solutions was recorded by a spectrophotometer at 535 nm, and the concentration of Fe(II)[bathophenanthroline-disulfonic acid disodium salt hydrate]3 was quantified using an extinction coefficient of 22.14 mM−1 cm−1.
Determination of NO Content in Roots
The endogenous levels of NO in roots were determined using 4-amino-5-methylamino-2′, 7′-difluorofluorescein diacetate (DAF-FM DA) probes and epifluorescence microscopy. The root tips were incubated with 5 μm DAF-FM DA in dark for 30 min, washed three times in PBS (pH 7.4), and analyzed microscopically. The fluorescent intensity was recorded using PhotoShop 7.0 (Adobe Systems) according to Besson-Bard et al. (2009).
Statistical Analysis
Each experiment was repeated at least three times, and one set of data are shown in the Results. Data were analyzed by one-way ANOVA and the means were compared by Duncan’s multiple range test. Different letters and asterisks on the histograms indicate that the means were statistically different at the P < 0.05 level.
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Effect of endogenous and exogenous Put on Fe-deficiency symptoms in Arabidopsis.
Supplemental Figure S2. Effect of pH on the Put-mediated alleviation of Fe-deficiency in Arabidopsis.
Supplementary Material
Acknowledgments
We thank N.M. Crawford (University of California, San Diego, La Jolla, CA) for noa1 seeds, W.J. Zhang (Beijing Institute for Biological Science, China) for nia1nia2 seeds, and Z.L. Shang (Hebei Normal University, China) for aha2 seeds. Thanks are also given to two anonymous reviewers for their valuable comments to improve the quality of our work.
Glossary
- ADC
Arg by Arg decarboxylase
- FCR
ferric-chelate reductase
- +Fe
Fe-sufficient
- -Fe
Fe-deficient
- ICP-AES
inductively coupled plasma-atomic emission spectrometry
- NO
nitric oxide
- Put
putrescine
Footnotes
This work was supported by the National Key Basic Research Program of China (No. 2014CB441000), ‘Strategic Priority Research Program’ of the Chinese Academy of Sciences (Nos. XDB15030302 and XDB15030202).
Articles can be viewed without a subscription.
References
- Bashir K, Ishimaru Y, Shimo H, Kakei Y, Senoura T, Takahashi R, Satob Y, Uozumib N, Nakanishi H, Nishizawa NK (2011) Rice phenolics efflux transporter 2 (PEZ2) plays an important role in solubilizing apoplasmic iron. Soil Sci Plant Nutr 57: 803–812 [Google Scholar]
- Besson-Bard A, Gravot A, Richaud P, Auroy P, Duc C, Gaymard F, Taconnat L, Renou JP, Pugin A, Wendehenne D (2009) Nitric oxide contributes to cadmium toxicity in Arabidopsis by promoting cadmium accumulation in roots and by up-regulating genes related to iron uptake. Plant Physiol 149: 1302–1315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienfait HF, van den Briel W, Mesland-Mul NT (1985) Free space iron pools in roots: generation and mobilization. Plant Physiol 78: 596–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchereau A, Aziz A, Larger F, Martin-Tanguy J (1999) Polyamines and environmental challenges: recent developments. Plant Sci 140: 103–125 [Google Scholar]
- Carpita NC, Gibeaut DM (1993) Structural models of primary cell walls in flowering plants: consistency of molecular structure with the physical properties of the walls during growth. Plant J 3: 1–30 [DOI] [PubMed] [Google Scholar]
- Carrier P, Baryla A, Havaux M (2003) Cadmium distribution and microlocalization in oilseed rape (Brassica napus) after long-term growth on cadmium-contaminated soil. Planta 216: 939–950 [DOI] [PubMed] [Google Scholar]
- Cassin G, Mari S, Curie C, Briat JF, Czernic P (2009) Increased sensitivity to iron deficiency in Arabidopsis thaliana overaccumulating nicotianamine. J Exp Bot 60: 1249–1259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang YC, Yamamoto Y, Matsumoto H (1999) Accumulation of aluminium in the cell wall pectin in cultured tobacco (Nicotiana tabacum L.) cells treated with a combination of aluminium and iron. Plant Cell Environ 22: 1009–1017 [Google Scholar]
- Chen WW, Yang JL, Qin C, Jin CW, Mo JH, Ye T, Zheng SJ (2010) Nitric oxide acts downstream of auxin to trigger root ferric-chelate reductase activity in response to iron deficiency in Arabidopsis. Plant Physiol 154: 810–819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng L, Zou Y, Ding S, Zhang J, Yu X, Cao J, Lu G (2009) Polyamine accumulation in transgenic tomato enhances the tolerance to high temperature stress. J Integr Plant Biol 51: 489–499 [DOI] [PubMed] [Google Scholar]
- Cosgrove DJ. (2005) Growth of the plant cell wall. Nat Rev Mol Cell Biol 6: 850–861 [DOI] [PubMed] [Google Scholar]
- Curie C, Briat JF (2003) Iron transport and signaling in plants. Annu Rev Plant Biol 54: 183–206 [DOI] [PubMed] [Google Scholar]
- Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28: 350–356 [Google Scholar]
- Eide D, Broderius M, Fett J, Guerinot ML (1996) A novel iron-regulated metal transporter from plants identified by functional expression in yeast. Proc Natl Acad Sci USA 93: 5624–5628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eticha D, Stass A, Horst WJ (2005) Cell-wall pectin and its degree of methylation in the maize root-apex: significance for genotypic differences in aluminium resistance. Plant Cell Environ 28: 1410–1420 [Google Scholar]
- Feirer RP, Mignon G, Litvay JD (1984) Arginine decarboxylase and polyamines required for embryogenesis in the wild carrot. Science 223: 1433–1435 [DOI] [PubMed] [Google Scholar]
- Flores HE, Galston AW (1982a) Polyamines and plant stress: activation of putrescine biosynthesis by osmotic shock. Science 217: 1259–1261 [DOI] [PubMed] [Google Scholar]
- Flores HE, Galston AW (1982b) Analysis of polyamines in higher plants by high performance liquid chromatography. Plant Physiol 69: 701–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fourcroy P, Sisó-Terraza P, Sudre D, Savirón M, Reyt G, Gaymard F, Abadía A, Abadia J, Alvarez-Fernández A, Briat JF (2014) Involvement of the ABCG37 transporter in secretion of scopoletin and derivatives by Arabidopsis roots in response to iron deficiency. New Phytol 201: 155–167 [DOI] [PubMed] [Google Scholar]
- Galston AW, Kaur-Sawhney R, Altabella T, Tiburcio AF (1995) Plant polyamines in reproductive activity and response to abiotic stress. Bot Acta 110: 197–207 [Google Scholar]
- García MJ, Lucena C, Romera FJ, Alcántara E, Pérez-Vicente R (2010) Ethylene and nitric oxide involvement in the up-regulation of key genes related to iron acquisition and homeostasis in Arabidopsis. J Exp Bot 61: 3885–3899 [DOI] [PubMed] [Google Scholar]
- Graziano M, Lamattina L (2007) Nitric oxide accumulation is required for molecular and physiological responses to iron deficiency in tomato roots. Plant J 52: 949–960 [DOI] [PubMed] [Google Scholar]
- Grusak MA. (1995) Whole-root iron(III)-reductase activity throughout the life cycle of iron-grown Pisum sativum L. (Fabaceae): relevance to the iron nutrition of developing seeds. Planta 197: 111–117 [Google Scholar]
- Guo FQ. (2006) Response to Zemojtel et al: plant nitric oxide synthase:AtNOS1 is just the beginning. Trends Plant Sci 11: 527–528 [DOI] [PubMed] [Google Scholar]
- Hanfrey C, Sommer S, Mayer MJ, Burtin D, Michael AJ (2001) Arabidopsis polyamine biosynthesis: absence of ornithine decarboxylase and the mechanism of arginine decarboxylase activity. Plant J 27: 551–560 [DOI] [PubMed] [Google Scholar]
- Jin CW, Du ST, Chen WW, Li GX, Zheng SJ (2009) Elevated carbon dioxide improves plant Fe nutrition through enhancing the iron-deficiency-induced responses under iron-limited conditions in tomato (Lycopersicon esculentum M.). Plant Physiol 150: 272–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin CW, You GY, He YF, Tang C, Wu P, Zheng SJ (2007) Iron deficiency-induced secretion of phenolics facilitates the reutilization of root apoplastic iron in red clover. Plant Physiol 144: 278–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakkar RK, Nagar PK, Ahuja PS, Rai VK (2000) Polyamines and plant morphogenesis. Biol Plant 43: 1–11 [Google Scholar]
- Lei GJ, Zhu XF, Wang ZW, Dong F, Dong NY, Zheng SJ (2014) Abscisic acid alleviates iron deficiency by promoting root iron reutilization and transport from root to shoot in Arabidopsis. Plant Cell Environ 37: 852–863 [DOI] [PubMed] [Google Scholar]
- Liu J, Jiang MY, Zhou YF, Liu YL (2005) Production of polyamines is enhanced by endogenous abscisic acid in maize seedlings subjected to salt stress. J Integr Plant Biol 47: 1326–1334 [Google Scholar]
- Lozano-Rodríguez EN, Hernández LA, Bonne P, Carpena RO (1997) Distribution of Cd in shoot and root tissues of maize and pea plants: physiological disturbances. J Exp Bot 48: 123–128 [Google Scholar]
- Morgan DM. (1998) Determination of polyamines as their benzoylated derivatives by HPLC. Methods Mol Biol 79: 111–118 [DOI] [PubMed] [Google Scholar]
- Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol Plant 15: 473–496 [Google Scholar]
- Robinson NJ, Procter CM, Connolly EL, Guerinot ML (1999) A ferric-chelate reductase for iron uptake from soils. Nature 397: 694–697 [DOI] [PubMed] [Google Scholar]
- Rodríguez-Celma J, Lin WD, Fu GM, Abadía J, López-Millán AF, Schmidt W (2013) Mutually exclusive alterations in secondary metabolism are critical for the uptake of insoluble iron compounds by Arabidopsis and Medicago truncatula. Plant Physiol 162: 1473–1485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Römheld V, Marschner H (1981) Iron deficiency stress induced morphological and physiological changes in root tips of sunflower. Plant Physiol 53: 354–360 [Google Scholar]
- Römheld V, Marschner H (1986) Mobilization of iron in the rhizosphere of different plant species. Adv Plant Nutr 2: 155–204 [Google Scholar]
- Santi S, Schmidt W (2009) Dissecting iron deficiency-induced proton extrusion in Arabidopsis roots. New Phytol 183: 1072–1084 [DOI] [PubMed] [Google Scholar]
- Schmid NB, Giehl RFH, Döll S, Mock HP, Strehmel N, Scheel D, Kong X, Hider RC, von Wirén N (2014) Feruloyl-CoA 6′-Hydroxylase1-dependent coumarins mediate iron acquisition from alkaline substrates in Arabidopsis. Plant Physiol 164: 160–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt H, Günther C, Weber M, Spörlein C, Loscher S, Böttcher C, Schobert R, Clemens S (2014) Metabolome analysis of Arabidopsis thaliana roots identifies a key metabolic pathway for iron acquisition. PLoS One 9: e102444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H, Chan Z (2014) Improvement of plant abiotic stress tolerance through modulation of the polyamine pathway. J Integr Plant Biol 56: 114–121 [DOI] [PubMed] [Google Scholar]
- Smith TA. (1973) Amine levels in mineral-deficient Hordeum vulgare leaves. Phytochemistry 12: 2093–2100 [Google Scholar]
- Tavladoraki P, Cona A, Federico R, Tempera G, Viceconte N, Saccoccio S, Battaglia V, Toninello A, Agostinelli E (2012) Polyamine catabolism: target for antiproliferative therapies in animals and stress tolerance strategies in plants. Amino Acids 42: 411–426 [DOI] [PubMed] [Google Scholar]
- Tun NN, Holk A, Scherer GFE (2001) Rapid increase of NO release in plant cell cultures induced by cytokinin. FEBS Lett 509: 174–176 [DOI] [PubMed] [Google Scholar]
- Urano K, Yoshiba Y, Nanjo T, Ito T, Yamaguchi-Shinozaki K, Shinozaki K (2004) Arabidopsis stress-inducible gene for arginine decarboxylase AtADC2 is required for accumulation of putrescine in salt tolerance. Biochem Biophys Res Commun 313: 369–375 [DOI] [PubMed] [Google Scholar]
- Vert G, Grotz N, Dédaldéchamp F, Gaymard F, Guerinot ML, Briat JF, Curie C (2002) IRT1, an Arabidopsis transporter essential for iron uptake from the soil and for plant growth. Plant Cell 14: 1223–1233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson MB, Malmberg RL (1996) Regulation of Arabidopsis thaliana (L.) Heynh Arginine decarboxylase by potassium deficiency stress. Plant Physiol 111: 1077–1083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein LH, Kaur-Sawhney R, Rajam MV, Wettlaufer SH, Galston AW (1986) Cadmium-induced accumulation of putrescine in oat and bean leaves. Plant Physiol 82: 641–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson ID, Neill SJ, Hancock JT (2008) Nitric oxide synthesis and signalling in plants. Plant Cell Environ 31: 622–631 [DOI] [PubMed] [Google Scholar]
- Wimalasekera R, Tebartz F, Scherer GFE (2011) Polyamines, polyamine oxidases and nitric oxide in development, abiotic and biotic stresses. Plant Sci 181: 593–603 [DOI] [PubMed] [Google Scholar]
- Wu J, Wang C, Zheng L, Wang L, Chen Y, Whelan J, Shou H (2011) Ethylene is involved in the regulation of iron homeostasis by regulating the expression of iron-acquisition-related genes in Oryza sativa. J Exp Bot 62: 667–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JL, Li YY, Zhang YJ, Zhang SS, Wu YR, Wu P, Zheng SJ (2008) Cell wall polysaccharides are specifically involved in the exclusion of aluminum from the rice root apex. Plant Physiol 146: 602–611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JL, Zhu XF, Peng YX, Zheng C, Li GX, Liu Y, Shi YZ, Zheng SJ (2011) Cell wall hemicellulose contributes significantly to aluminum adsorption and root growth in Arabidopsis. Plant Physiol 155: 1885–1892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JL, Chen WW, Chen LQ, Qin C, Jin CW, Shi YZ, Zheng SJ (2013) The 14-3-3 protein GENERAL REGULATORY FACTOR11 (GRF11) acts downstream of nitric oxide to regulate iron acquisition in Arabidopsis thaliana. New Phytol 197: 815–824 [DOI] [PubMed] [Google Scholar]
- Young ND, Galston AW (1983) Putrescine and acid stress: induction of arginine decarboxylase activity and putrescine accumulation by low pH. Plant Physiol 71: 767–771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young ND, Galston AW (1984) Physiological control of arginine decarboxylase activity in k-deficient oat shoots. Plant Physiol 76: 331–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng SJ, Lin XY, Yang JL, Liu Q, Tang C (2004) The kinetics of aluminum adsorption and desorption by root cell walls of an aluminum resistance wheat (Triticum aestivum L.) cultivar. Plant Soil 261: 85–90 [Google Scholar]
- Zhong H, Lauchli A (1993) Changes of cell wall component and polymer size in primary roots of cotton seedlings under high salinity. J Exp Bot 44: 773–778 [Google Scholar]
- Zhu XF, Shi YZ, Lei GJ, Fry SC, Zhang BC, Zhou YH, Braam J, Jiang T, Xu XY, Mao CZ, Pan YJ, Yang JL, et al. (2012) XTH31, encoding an in vitro XEH/XET-active enzyme, regulates aluminum sensitivity by modulating in vivo XET action, cell wall xyloglucan content, and aluminum binding capacity in Arabidopsis. Plant Cell 24: 4731–4747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu XF, Lei GJ, Wang ZW, Shi YZ, Braam J, Li GX, Zheng SJ (2013) Coordination between apoplastic and symplastic detoxification confers plant aluminum resistance. Plant Physiol 162: 1947–1955 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.