Abstract
Recently, there has been a lot of interest in the utilization of rhodococci in the bioremediation of petroleum contaminated environments. This study investigates the response of Rhodococcus erythropolis IBBPo1 cells to 1% organic solvents (alkanes, aromatics). A combination of microbiology, biochemical, and molecular approaches were used to examine cell adaptation mechanisms likely to be pursued by this strain after 1% organic solvent exposure. R. erythropolis IBBPo1 was found to utilize 1% alkanes (cyclohexane, n-hexane, n-decane) and aromatics (toluene, styrene, ethylbenzene) as the sole carbon source. Modifications in cell viability, cell morphology, membrane permeability, lipid profile, carotenoid pigments profile and 16S rRNA gene were revealed in R. erythropolis IBBPo1 cells grown 1 and 24 h on minimal medium in the presence of 1% alkanes (cyclohexane, n-hexane, n-decane) and aromatics (toluene, styrene, ethylbenzene). Due to its environmental origin and its metabolic potential, R. erythropolis IBBPo1 is an excellent candidate for the bioremediation of soils contaminated with crude oils and other toxic compounds. Moreover, the carotenoid pigments produced by this nonpathogenic Gram-positive bacterium have a variety of other potential applications.
Key words: Rhodococcus, solvents, morphology, lipids, carotenoids
Introduction
The existence of contaminated areas with highly toxic organic solvents is a clear indication of the lack of biological systems that have the ability to efficiently degrade these compounds. Research in recent years has focused on the search for solvent-tolerant bacteria that have the catabolic potential necessary to remove comprehensively these toxic compounds (Torres et al., 2011). Many of the organic solvents are relatively stable and toxic to bacteria because they are able to bind to the cell membrane (Torres et al., 2011). When organic solvents accumulate in the cell membrane, its integrity is affected, resulting in loss of function as a permeability barrier, as a protein and reaction matrix and as an energy transducer leading concomitantly to damages of the cellular metabolism, growth inhibition, and, finally leading to cell death (Isken and de Bont, 1998; Heipieper et al., 2007). The large genomes of Rhodococcus strains, their redundant and versatile catabolic pathways, their ability to uptake and metabolize hydrophobic compounds, to form biofilms, to persist in adverse conditions, as well as the availability of recently developed tools for genetic engineering in rhodococci make them suitable industrial microorganisms for biotransformations and the biodegradation of many toxic compounds (Larkin et al., 2006; Martínková et al., 2009). Furthermore, these bacteria are capable to produce several carotenoid pigments (e.g., β-carotene, γ-carotene, chlorobactene) with different medical, industrial, and nutritional applications (Tao et al., 2006). Some organic solvent tolerance mechanisms in rhodococci have been proposed (e.g., induction of general stress regulon, production of organic solvent emulsifying or deactivating enzymes, active solvent efflux pumps) (Torres et al., 2011). In response to toxic organic solvents cell morphology alterations and filamentous growth were also observed in some solvent-tolerant bacteria (Torres et al., 2011). The variety of mechanisms that could confer adaptation to organic solvents implies that bacterial solvent tolerance cannot be provided by a single mechanism (Heipieper et al., 2007). Taking into account the interest raised by the utilization of rhodococci in the bioremediation of petroleum contaminated environments, this study investigates the response of Rhodococcus erythropolis IBBPo1 cells to 1% organic solvents (alkanes, aromatics). Cyclohexane, n-hexane, n-decane, toluene, styrene, and ethylbenzene with different log POW values (logarithm of the partition coefficient in octanol-water mixture) have been used as the sole carbon source in minimal medium. The possible cell adaptation mechanisms pursued by this strain after 1% organic solvent (alkanes, aromatics) exposure was studied by following the changes in the cell viability, cell morphology, membrane permeability, lipid profile, carotenoid pigments profile, and 16S rRNA gene.
Materials and Methods
Bacterial strain
The strain used in the present study was R. erythropolis IBBPo1 (KF059972.1), which has been previously isolated in our laboratory from Poeni crude oil-contaminated soil sample (Stancu, 2014). The analysis of the 16S rRNA gene sequence located strain IBBPo1 (KF059972.1) within the genus Rhodococcus, showing 95% similarity to other Rhodococcus strains from the public databases (GenBank/DDBJ/EMBL).
Response of R. erythropolis IBBPo1 cells to organic solvents
Bacterial cells were cultivated on liquid LB medium containing 30 μg mL-1 kanamycin and incubated at 28 °C on a rotary shaker (200 rpm) until they reached the exponential growth phase. Bacterial cells were harvested by centrifugation, washed twice and finally resuspended (106 cells mL-1) in minimal medium (Stancu and Grifoll, 2011). 0.1% (w/v) yeast extract or 1% (v/v) organic solvents (alkanes: cyclohexane, n-hexane, n-decane; aromatics: toluene, styrene, ethylbenzene) were supplied to the cell suspensions as the sole carbon source. Flasks were sealed and incubated for 1 and 24 h respectively at 28 °C on a rotary shaker (200 rpm).
Cell viability
Serial dilutions of bacterial cultures were spotted on LB agar and the number of viable cells (cfu mL-1) was determined, using the method described by Segura et al. (2008). Petri plates were incubated 24 h at 28 °C.
Cell morphology
Cell morphology was studied using scanning electron microscopy (SEM) and transmission electron microscopy (TEM). SEM samples were prepared using the method indicated by Rocha et al. (2011). Samples were fixed on SEM holder and gold-coated with a JEOL JFC-1300 auto fine coater, in a deep vacuum. The samples were examined with a JEOL JSM-6610LV SEM. TEM samples were prepared using the method indicated by Gutiérrez et al. (2009). Thin sections were mounted onto 300-mesh collodion/carbon coated cooper grids and stained with lead citrate and uranyl acetate. Examination of the sectioned material was performed using a JEOL JEM-1400 80 kV TEM.
Membrane permeability
Membrane permeability was determined using the method indicated by Gaur and Khare (2009). To determine the release of nucleic acid from bacterial cells, the absorbance of cell-free supernatant was measured at 260 nm.
Membrane lipids
Membrane lipids were analyzed by thin layer chromatography (TLC). Lipids were extracted from the culture broths with chloroform-methanol (2:1 v/v). The samples were spotted with a Linomat 5 sample applicator (CAMAG), on a 10 × 20 cm precoated silica gel 60 TLC aluminium sheets (Merck). The separation was performed using chloroform-methanol-acetic acid-water (85:22.5:10:4 v/v/v/v) mixture as mobile phase (Das et al., 2009). For phospholipids visualization, the plates were treated with 10% (w/v) molybdatophosphoric acid hydrate in ethanol, and for glycolipids, the plates were treated with 0.5% (w/v) α-naphthol in methanol-water (1:1 v/v) mixture and sulfuric acid-ethanol (1:1 v/v) mixture. The densitometric scan of dried TLC plates was performed at 500 nm with a TLC Scanner 4 (CAMAG).
Carotenoid pigments
Carotenoid pigments were analyzed by spectrophotometry and thin layer chromatography (TLC). Carotenoids were extracted from the culture broths with acetone (Tao et al., 2006). UV/visible scanning spectra of the samples were recorded between 200 and 800 nm using a SPECORD 200 UV-visible spectrophotometer (Analytik Jena). For TLC analysis, the samples were spotted with a Linomat 5 sample applicator (CAMAG), on a 10 × 20 cm precoated silica gel 60 TLC aluminium sheets (Merck). The separation was performed using chloroform-methanol (90:10 v/v) mixture as mobile phase. The densitometric scan of dried TLC plates was performed at 254 nm with a TLC Scanner 4 (CAMAG).
16S rRNA gene expression
Genomic DNA was extracted using the method of Whyte et al. (1996). For PCR amplification of 16S rRNA gene, 2 μL of DNA extract was added to a final volume of 50 μL reaction mixture, containing: 5 × GoTaq flexi buffer, MgCl2, dNTP mix, bacterial universal primers (27f and 1492r, Marchesi et al., 1998), and GoTaq DNA polymerase (Promega). PCR was performed with a mastercycler proS (Eppendorf). The PCR program consisted in initial denaturation for 10 min at 94 °C, followed by 35 cycles of denaturation at 94 °C for 1 min, annealing at 55 °C for 30 s, extension step at 72 °C for 2 min, and a final extension at 72 °C for 10 min. After separation on 1.5% (w/v) TBE agarose gel (Sambrook et al., 1989) and staining with fast blast DNA stain (Bio-Rad) the PCR products were analyzed. The PCR products were purified using the DNA clean and concentrator-5 kit (Zymo Research). PCR products were digested with EcoRI and XbaI restriction endonucleases (Promega), and the resultant restriction fragments were analyzed by electrophoresis on 2% (w/v) agarose gel. PCR products were also sequenced with the BigDye Terminator v.3.1 cycle sequencing kit (Applied Biosystems). The reactions were performed using the amplification primers. The products were purified using the BigDye XTerminator purification kit (Applied Biosystems). Sequencing reactions were obtained with an Applied Biosystems 3500/3500xL genetic analyzer at the Institute of Biology Bucharest of Romanian Academy. DNA sequencing runs were assembled using the BioEdit software (Hall, 1999). The sequences obtained were compared with respective control using the BLAST program (Altschul et al., 1990). The phylogenetic tree was generated using the neighbor-joining methods in MEGA5.1 program (Tamura et al., 2011).
Reagents used during this study were of reagent grade and purchased from different commercial sources (Merck, Sigma-Aldrich, Promega, Invitrogen, Zymo Research, Applied Biosystems, Bio-Rad Laboratories). The PCR primers were purchased from Integrated DNA Technologies.
Results and Discussion
Response of R. erythropolis IBBPo1 cells to organic solvents
Numerous and complex physiological cellular responses and adaptations involved in organic solvents tolerance have been observed in R. erythropolis IBBPo1 cells grown on LB medium in the presence of 1% organic solvents (Stancu, 2014). This bacterium was adapt to 1% organic solvents by changing surface hydrophobicity, cell morphology, metabolic fingerprinting and the otsA1 (trehalose-6-phosphate synthase) gene sequence. Taking into account the interest raised by the utilization of rhodococci in the bioremediation of petroleum contaminated environments, we continued our previous study with the investigation of the response of R. erythropolis IBBPo1 cells to 1% organic solvents (alkanes, aromatics). In this study, 1% alkanes (cyclohexane, n-hexane, n-decane) and aromatics (toluene, styrene, ethylbenzene) were used as the sole carbon source in minimal medium. The controls were prepared using the same mineral medium, but supplemented with 0.1% (w/v) yeast extract as the carbon source.
Cell viability
R. erythropolis cells contain a large set of important enzymes for bioconversion and biodegradation processes, which enable them to perform oxidations, dehydrogenations, epoxidations, hydrolysis, hydroxylations, dehalogenations, and desulfurizations (de Carvalho et al., 2009). The exposure for 1 and 24 h respectively of R. erythropolis IBBPo1 cells to 1% alkanes (cyclohexane, n-hexane, n-decane) and aromatics (toluene, styrene, ethylbenzene) had different effects on their survival rate (Table 1). One hour after 1% alkanes and aromatics exposure the survival rates were 102-106 cfu mL-1, and after 24 h the survival rates were 104-1010 cfu mL-1. As anticipated, the viability of R. erythropolis IBBPo1 cells 1 and 24 h after 1% alkanes and aromatics exposure was lower, compared with the controls (107, 1011 cfu mL-1). Tolerance of bacteria to organic solvents has been estimated by the solvent parameter log POW, which is an index of biological toxicity (Sikkema et al., 1995). It is generally accepted that solvents with log POW values below 5 are considered extremely toxic because of their high degree of partitioning into the aqueous layer surrounding the cells, and from there into the lipid membrane bilayer (Torres et al., 2011. However the cells that are able to remain viable after the first moments of contact with toxic organic solvents will be able to endure its presence for some time (de Carvalho et al., 2009). In our study the results showed higher survival rates (105-1010 cfu mL-1) when R. erythropolis IBBPo1 cells have been exposed to 1% alkanes (cyclohexane, n-hexane, n-decane) with log POW between 3.35 and 5.98, as compared with the survival rates of cells (102-107 cfu mL-1) exposed to 1% aromatics (toluene, styrene, ethylbenzene) with log POW between 2.64 and 3.17. The survival rates drastically reduced from 107 and 1011 to 102 and 104, respectively, when R. erythropolis IBBPo1 cells were exposed to 1% toluene. This is in agreement with a previous study which found that organic solvents with lower log POW value (e.g., benzene, toluene) bound more abundantly to bacterial cells thus being more toxic for them (Sikkema et al., 1995; Torres et al., 2011).
Table 1. Viability of R. erythropolis IBBPo1 cells after 1% organic solvents exposure.
| Cell viabilitya | Organic solvents (log P OW b) | ||||||
|---|---|---|---|---|---|---|---|
| Control | Cyclohexane (3.35) | n-Hexane (3.86) | n-Decane (5.98) | Toluene (2.64) | Styrene (2.86) | Ethylbenzene (3.17) | |
| 1 h | 4.7 × 107 | 3.0 × 105 | 2.7 × 106 | 2.0 × 106 | 7.3 × 102 | 4.0 × 103 | 3.1 × 105 |
| 24 h | 2.9 × 1011 | 2.7 × 108 | 2.0 × 109 | 3.4 × 1010 | 1.2 × 104 | 3.0 × 106 | 2.2 × 107 |
Legend:
= serial dilutions of cultures were spread on LB agar and the number of viable cells (cfu mL-1) was determined;
= logarithm of the partition coefficient of the solvent in octanol-water mixture.
Cell morphology
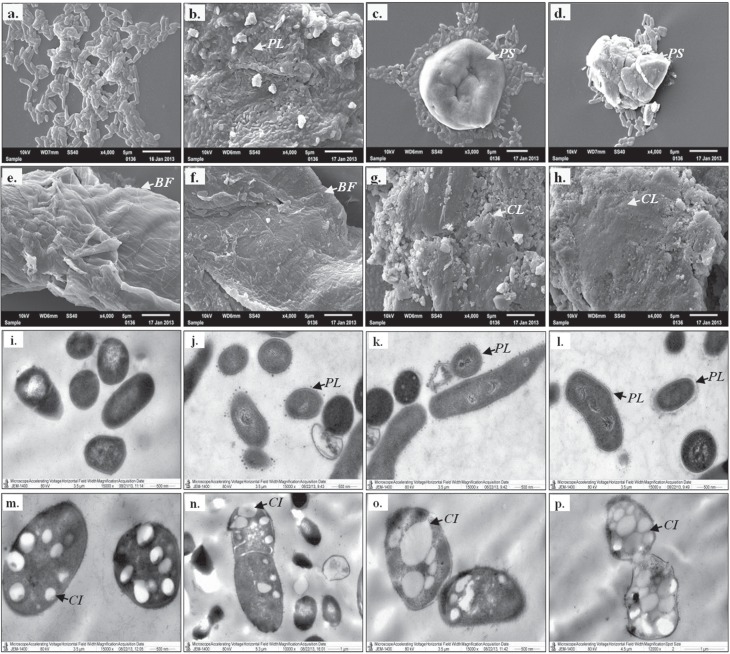
Different adaptation responses of R. erythropolis IBBPo1 cells were observed by SEM (Figure 1a–1h) and TEM (Figure 1i–1p) studies, 1 and 24 h respectively after 1% alkanes (cyclohexane, n-hexane, n-decane) and aromatics (toluene, styrene, ethylbenzene) exposure. R. erythropolis IBBPo1 cells exposed to 1% alkanes and aromatics were free within the water phase as those in the control cells (Figure 1a), embedded in a polymeric layer (Figure 1b), closely grouped around polymeric structures of bacterial origin (Figure 1c, 1d), on the surface of biofilms (Figure 1e, 1f) or linked together as clusters (Figure 1g, 1h). All these structures were not observed in R. erythropolis IBBPo1 control cells. Similar results were previously obtained by Rocha et al. (2011) for a Gram-negative bacterium Pseudomonas aeruginosa ATCC 55925 grown on 0.5% heating oil or pure alkanes (i.e., C7-C18 n-alkanes, C19 branched alkane).
Figure 1. SEM (panels a-h) and TEM (panels i-p) studies of R. erythropolis IBBPo1 cells after 1% organic solvents exposure. Bacterial cells cultivated 24 h in minimal medium (panels a-p); control (panels a, i); alkanes and aromatics (panels b-h, j-p); polymeric layers (PL), polymeric structures (PS), biofilms (BF), cells clusters (CL), cytoplasmatic electron-transparent inclusions (CI).
According to the literature (Urai et al., 2007; Gutiérrez et al., 2009; Rocha et al., 2011; Torres et al., 2011), the structures depicted only when the cells are exposed to hydrocarbons (e.g., biofilms, cells clusters) play a significant role in toxic compounds tolerance and they protect the cells from different environmental stresses. Furthermore, the polymers produced by some bacterial strains play an important role in sequestering molecules of solvent (i.e., benzene) within its immediate environment, thereby reducing solvent contact with its cell membrane and conferring it some degree of tolerance (Gutiérrez et al., 2009).
In R. erythropolis IBBPo1 cells exposed to 1% alkanes (cyclohexane, n-hexane, n-decane), the cell membrane was intact (Figure 1j–1l) just like in the case of the control cells (Figure 1i), and no accumulation of solvents was seen in the cytoplasm. In cells exposed to 1% aromatics (toluene, styrene, ethylbenzene), the accumulation of solvent in the cytoplasm of cells with a disturbed cell membrane and an increase in the cell size were observed (Figure 1m–1p). The observed cytoplasmatic electron-transparent inclusions were similar to those reported previously for other bacterial strains, and their formation is a general cell adaptation response to hydrocarbon growth (Rocha et al., 2011). When the large solvent inclusions occupied all the cytoplasm and the cell wall integrity was altered, the death of R. erythropolis IBBPo1 cells was also observed (Figure 1p).
Membrane permeability
In general, solvents exert their toxic effect by altering the cell membrane permeability (Gaur and Khare, 2009), which leads to the inactivation and denaturation of membrane-embedded proteins, and the promotion of leakages of ions and intracellular macromolecules, such as nucleic acids (Isken and de Bont, 1998). The exposure for 1 and 24 h of R. erythropolis IBBPo1 cells to 1% alkanes (cyclohexane, n-hexane, n-decane) and aromatics (toluene, styrene, ethylbenzene) had different effects on membrane permeability (Table 2).
Table 2. Nucleic acid release by R. erythropolis IBBPo1 cells after 1% organic solvents exposure.
| Cell permeabilitya | Organic solvents (log P OW b) | ||||||
|---|---|---|---|---|---|---|---|
| Control | Cyclohexane (3.35) | n-Hexane (3.86) | n-Decane (5.98) | Toluene (2.64) | Styrene (2.86) | Ethylbenzene (3.17) | |
| 1 h | 0.156 | 0.277 | 0.244 | 0.257 | 0.509 | 0.567 | 0.597 |
| 24 h | 0.234 | 0.362 | 0.370 | 0.333 | 0.834 | 0.778 | 0.695 |
the absorbance of cell-free supernatant was measured at 260 nm;
logarithm of the partition coefficient of the solvent in octanol-water mixture.
The release of nucleic acids was higher after aromatics (0.509-0.834) and alkanes (0.277-0.370) exposure, compared with the controls (0.156, 0.234). Similar results were earlier obtained by Gaur and Khare (2009) for a Gram-negative bacterium Pseudomonas aeruginosa PseA grown in the presence of cyclohexane and tetradecane. These two organic solvents affect membrane integrity and structure dramatically, thereby altering the permeability and incurring toxicity. The higher release of nucleic acid into the growth medium when R. erythropolis IBBPo1 cells were grown in minimal medium in the presence of 1% aromatics (toluene, styrene, ethylbenzene) supports the results of TEM studies, which showed changes in the cytoplasmic membrane integrity of cells exposed to these toxic solvents.
Membrane lipids
Changes in the composition of phospholipids, glycolipids and mycolic acids of R. erythropolis have been suggested to depend on the availability and structure of the carbon source (de Carvalho et al., 2009). The TLC analysis revealed the existence of some differences between phospholipids and glycolipids extracted from R. erythropolis IBBPo1 control cells and those extracted from cells exposed 1 and 24 h to 1% alkanes (cyclohexane, n-hexane, n-decane) and aromatics (toluene, styrene, ethylbenzene) (Figure 2a–2d).
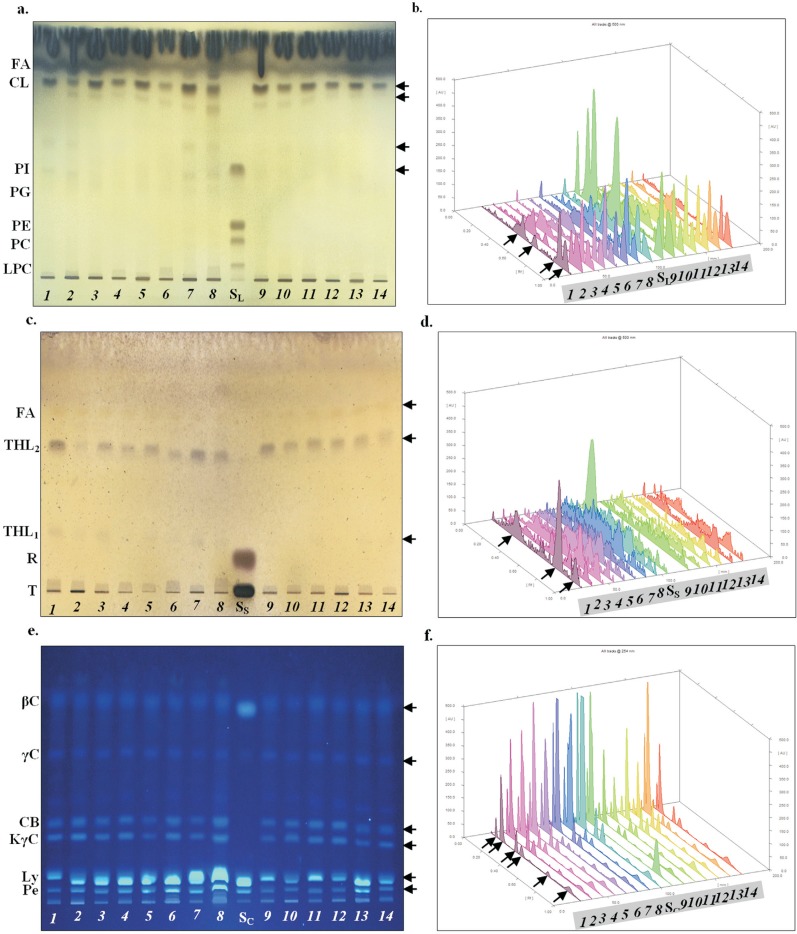
Figure 2. Phospholipids (panels a, b), glycolipids (panels c, d) and carotenoids (panels e, f) of R. erythropolis IBBPo1 cells after 1% organic solvents exposure. The TLC plates were visualized (panels a, c, e) and scanned (panels b, d, f) under a 500 nm visible white light (panels a-d) or under a 254 nm ultraviolet light (panels e, f); bacterial cells cultivated 1 h (lanes 1, 3, 5, 7, 9, 11, 13) and 24 h (lanes 2, 4, 6, 8, 10, 12, 14) in minimal medium; control (lanes 1, 2), cyclohexane (lanes 3, 4), n-hexane (lanes 5, 6), n-decane (lanes 7, 8), toluene (lanes 9, 10), styrene (lanes 11, 12), ethylbenzene (lanes 13, 14). Panels a, b. Phospholipids standards (SL), lysophosphatidylcholine (LPC), phosphatidylcholine (PC), phosphatidylethanolamine (PE), phosphatidylglycerol (PG), phosphatidylinositol (PI), cardiolipin (CL), fatty acids (FA). Panels c, d. Sugars standards (SS), trehalose (T), L-rhamnose (R), trehalolipids (THL1, THL2), fatty acids (FA). Panels e, f. Carotenoids standards (SC), phytoene (Pe), lycopene (Ly or ψ, ψ-carotene), 4-keto-γ-carotene (KγC), chlorobactene (CB or Φ, ψ-carotene), γ-carotene (γC), β-carotene (βC). The phospholipids, glycolipids and carotenoids spots and their corresponding peak have been marked by arrows.
The phospholipids (Figure 2a, 2b) found in R. erythropolis IBBPo1 control cells were identified based on their R f (retardation factor) values as phosphatidylinositol (PI with R f 0.45), cardiolipin (CL with R f 0.75) and fatty acids (FA with R f 0.79). The phosphatidylethanolamine (PE) was not detected in these extracts. Although the major lipid components of coryneform and nocardioform bacteria are cardiolipin and phosphatidylethanolamine (Kolomytseva et al., 2005), only cardiolipin was detected in the R. erythropolis IBBPo1 cells extracts. The phospholipids found in R. erythropolis IBBPo1 cells 1 and 24 h after 1% alkanes and aromatics exposure were cardiolipin (with R f 0.70-0.75) and fatty acids (with R f 0.76-0.80). Phosphatidylinositol was detected only in barely detectable quantities in all extracts, except n-decane. An elevated level of cardiolipin was detected in extracts of R. erythropolis IBBPo1 cells 1 and 24 h after 1% alkanes and aromatics exposure (except styrene, ethylbenzene), as compared with those of the respective controls; no such changes were observed in cells exposed to styrene and ethylbenzene. It is known that phospholipids, as the major component of bacterial cells membranes, are responsible for their structural organization and selective permeability. Therefore, the increase in the content of cardiolipin and fatty acids in the membranes of cells cultured in the presence of the toxic compounds is the result of adaptation of the bacterial cells and has a defensive nature (Kolomytseva et al., 2005; Martínková et al., 2009).
The glycolipids (Figure 2c, 2d) found, based on their R f values, in R. erythropolis IBBPo1 control cells were trehalolipids (THL1 with R f 0.30, THL2 with R f 0.61) and fatty acids (FA with R f 0.72). The glycolipids found in R. erythropolis IBBPo1 cells 1 and 24 h after 1% alkanes and aromatics exposure were trehalolipids (THL1 with R f 0.26-0.29, THL2 with R f 0.57-0.63) and fatty acids (FA with R f 0.70-0.74). THL1 was not detected in extracts of cells exposed 1 h to toluene and for 24 h to cyclohexane, toluene and styrene. R f values of THL1 and THL2 were similar to data given in the literature, and these value correspond to trehalose monomycolate and trehalose dimycolate, which are regular extractable components of the rhodococcal cell envelope (Niescher et al., 2006).
An elevated level of THL2 was detected in extracts of R. erythropolis IBBPo1 control cells, as compared with those of the cells exposed for 1 and 24 h to 1% alkanes and aromatics. This is not surprising because the trehalolipids could be released in high quantities into the growth medium when the cells are exposed to 1% organic solvents, as compared with the control cells. We observed previously that R. erythropolis IBBPo1 is a good biosurfactant producer (emulsification index E24 = 100%), compared with other Rhodococcus strains (Stancu, 2014). The biosurfactants released into the growth medium modify the cell surface hydrophobicity and/or promote emulsification and/or solubilization of organic solvents thus accelerating their biodegradation (Philp et al., 2002; Gesheva et al., 2010). Such solvent-tolerant bacteria provide the key for the use of otherwise toxic solvents in whole-cell two-phase biotransformations, by overcoming the toxic effects of substrates and products (Heipieper et al., 2007).
Carotenoid pigments
Carotenoids are hydrophobic molecules typically associated with cytoplasmic membrane and/or noncovalently bound to specific proteins. These pigments form an integral part of the complex membrane structure of some bacteria and influence membrane fluidity, by increasing its rigidity and mechanical strength (Godinho and Bhosle, 2008). It has been suggested that the presence of carotenoids may change the effectiveness of the membrane as a barrier to water, oxygen, and other molecules. Some bacteria may be accumulating carotenoids as part of their responses to various environmental stresses, and thus aiding their survival in this habitat (Godinho and Bhosle, 2008). Therefore, we further investigated the effect of the 1% alkanes (cyclohexane, n-hexane, n-decane) and aromatics (toluene, styrene, ethylbenzene) to the carotenoids synthesis in R. erythropolis IBBPo1 cells. The UV/visible absorption scanning spectra of the pigment extract of R. erythropolis IBBPo1 cells showed absorption maxima at 340 nm. Carotenoid pigments extracted from R. erythropolis IBBPo1 cells 1 and 24 h after 1% alkanes and aromatics exposure showed the same absorption maxima. The TLC plate developed with chloroform-methanol mixture showed the separation of the carotenoid pigments into 6 fluorescent spots (Figure 2e). The carotenoids synthesized by different R. erythropolis strains were previously characterized to be 4-keto-γ-carotene as the major carotenoid and sometimes γ-carotene as the minor carotenoids (Tao et al., 2006). However, other carotenoids (e.g., phytoene, lycopene, chlorobactene, β-carotene) were also described in R. erythropolis (Tao et al., 2006). The carotenoids (Figure 2e, 2f) found, based on their R f values, in R. erythropolis IBBPo1 control cells were phytoene (Pe with R f 0.04), lycopene (Ly or ψ, ψ-carotene with R f 0.10), 4-keto-γ-carotene (KγC with R f 0.23), chlorobactene (CB or Φ, ψ-carotene with R f 0.29), γ-carotene (γC with R f 0.53), β-carotene (βC with R f 0.73). The carotenoids found in R. erythropolis IBBPo1 cells 1 and 24 h after 1% alkanes and aromatics exposure were phytoene (with R f 0.04-0.06), lycopene (with R f 0.07-0.10), 4-keto-γ-carotene (with R f 0.20-0.24), chlorobactene (with R f 0.26-0.30), γ-carotene (with R f 0.51-0.53), β-carotene (with R f 0.71-0.73). An elevated level of lycopene (which is an important intermediate in the biosynthesis of γ-carotene and β-carotene) was detected in extracts of R. erythropolis IBBPo1 cells 1 and 24 h after 1% alkanes exposure (especially in the case of n-decane), as compared with those of the control cells; no such changes were observed in cells exposed to 1% aromatics (except ethylbenzene).
16S rRNA gene expression
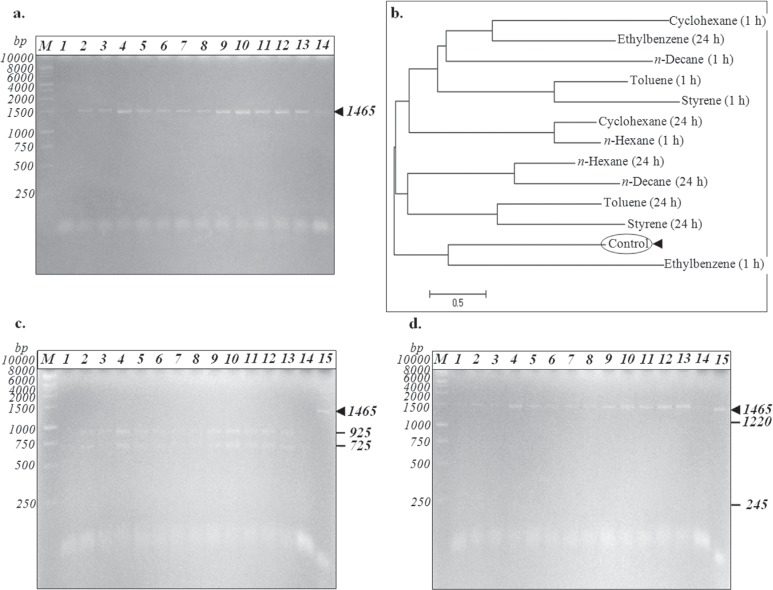
Because rhodococci commonly exhibit considerable genomic instabilities that can be specifically selected (Larkin et al., 2006), we further investigated the expression of 16S rRNA gene after R. erythropolis IBBPo1 cells exposure to organic solvents. Genomic DNA extracted from R. erythropolis IBBPo1 control cells and those extracted from cells 1 and 24 h after 1% alkanes and aromatics exposure was used as template for PCR amplification of 16S rRNA gene. The expected PCR product size was 1465 bp for this gene (Figure 3a).
Figure 3. Detection of 16S rRNA gene (panels a, c, d) and phylogenetic tree based on 16S rRNA gene sequences (panel b) of R. erythropolis IBBPo1 cells after 1% organic solvents exposure. Panel a. 16S rRNA gene; Panel c. 16S rRNA gene digested with restriction endonuclease EcoRI; Panel d. 16S rRNA gene digested with restriction endonuclease XbaI; bacterial cells cultivated 1 h (lanes 1, 3, 5, 7, 9, 11, 13) and 24 h (lanes 2, 4, 6, 8, 10, 12, 14) in minimal medium; control (lanes 1, 2), cyclohexane (lanes 3, 4), n-hexane (lanes 5, 6), n-decane (lanes 7, 8), toluene (lanes 9, 10), styrene (lanes 11, 12), ethylbenzene (lanes 13, 14); undigested 16S rRNA gene (lane 15); 1 kb DNA ladder (lane M). Panel b. The phylogenetic tree was obtained using the neighbor-joining method in MEGA5.1 program. The scale bar indicates substitutions per nucleotide position.
PCR product of 16S rRNA gene (1465 bp fragment) from R. erythropolis IBBPo1 cells was digested by EcoRI and XbaI restriction endonucleases (Figure 3c, 3d). The EcoRI recognition site exists in the sequence of 16S rRNA gene from R. erythropolis IBBPo1, while XbaI recognition site did not exist in this bacterium. When 16S rRNA gene from R. erythropolis IBBPo1 cells (control) was digested by EcoRI, two distinct bands (925+725 bp) were observed (Figure 3c). The same RFLP pattern was obtained for R. erythropolis IBBPo1 cells exposed 1 and 24 h to 1% alkanes (cyclohexane, n-hexane, n-decane) and aromatics (toluene, styrene, ethylbenzene).
In the 16S rRNA phylogenetic tree obtained using the neighbor-joining method (Figure 3b), control (KF059972.1) formed a tight cluster with only 16S rRNA gene sequence of the cells exposed 1 h to ethylbenzene. Moreover, considerable modifications in 16S rRNA gene sequence of R. erythropolis IBBPo1 cells were observed 24 h after ethylbenzene exposure, 1 and 24 h after 1% alkanes (cyclohexane, n-hexane, n-decane) and aromatics (toluene, styrene) exposure, as compared with the control (Figure 3b). It is not surprising to observe DNA sequence modification after organic solvent exposure, because these toxic compounds are metabolically activated in cells to yield highly reactive bay region dihydrodiol epoxide derivatives which can effectively attack DNA, leading to disruption of normal cellular functions (Wei et al., 1984).
Conclusions
Solvent-tolerant bacteria belonging to the genus Rhodococcus are increasingly recognized as very good candidates for the biodegradation of toxic compounds, because of their ability to degrade a wide range of organic compounds, hydrophobic cell surfaces, biosurfactant production, and ubiquity and robustness in the environment (Pini et al., 2007; Martinkova et al., 2009; Torres et al., 2011).
Organic solvents with log POW values below 5 are considered extremely toxic to bacteria. However we have previously shown that R. erythropolis IBBPo1 cells had a good tolerance (40-100%) to both alkanes (cyclohexane, n-hexane, n-decane) and aromatics (toluene, styrene, ethylbenzene) with log P OW values between 2.64 and 5.98. Additionally, R. erythropolis IBBPo1 was found to utilize these toxic organic solvents as the sole carbon source. Modifications in the cell viability, cell morphology, membrane permeability, lipid profile, carotenoid pigments profile and 16S rRNA gene were revealed in R. erythropolis IBBPo1 cells grown 1 and 24 h on minimal medium in the presence of 1% alkanes (cyclohexane, n-hexane, n-decane) and aromatics (toluene, styrene, ethylbenzene). The acquired results showed higher survival rates when R. erythropolis IBBPo1 cells were exposed to 1% alkanes (cyclohexane, n-hexane, n-decane) with log POW between 3.35 and 5.98, compared with those of the cells exposed to 1% aromatics (toluene, styrene, ethylbenzene) with log POW between 2.64 and 3.17. Due to its environmental origin and its metabolic potential, R. erythropolis IBBPo1 is an excellent candidate for bioremediation of soils contaminated with crude oils and other toxic compounds. Bioremediation of soil contaminated with 5% (w/v) Poeni crude oil was studied for a period of 30 days, under laboratory condition (data not shown). The amount of crude oil degraded by R. erythropolis IBBPo1 after 15 and 30 days incubation were 34% and 85%, respectively. Moreover, the carotenoids produced by this nonpathogenic Gram-positive bacterium can have a variety of other potential applications (medicine, cosmetics, food industry).
Acknowledgments
This study was funded by project no. RO1567-IBB05/2014 from the Institute of Biology Bucharest of Romanian Academy. The author is grateful to Ana Dinu and Alexandru Brînzan for technical support.
References
- Altschul SF, Gish W, Miller W, et al. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Das P, Mukherjee S, Sen R. Substrate dependent production of extracellular biosurfactant by a marine bacterium. Biores Technol. 2009;100:1015–1019. doi: 10.1016/j.biortech.2008.07.015. [DOI] [PubMed] [Google Scholar]
- Carvalho CCCR de, Wick LY, Heipieper HJ. Cell wall adaptations of planktonic and biofilm Rhodococcus erythropolis cells to growth on C5 to C16 n-alkane hydrocarbons. Appl Microbiol Biotechnol. 2009;82:311–320. doi: 10.1007/s00253-008-1809-3. [DOI] [PubMed] [Google Scholar]
- Gaur R, Khare SK. Cellular response mechanisms in Pseudomonas aeruginosa PseA during growth in organic solvents. Lett Appl Microbiol. 2009;49:372–377. doi: 10.1111/j.1472-765X.2009.02671.x. [DOI] [PubMed] [Google Scholar]
- Gesheva V, Stackebrandt E, Vasileva-Tonkova E. Biosurfactant production by halotolerant Rhodococcus fascians from Casey Station, Wilkes Land, Antarctica. Curr Microbiol. 2010;61:112–117. doi: 10.1007/s00284-010-9584-7. [DOI] [PubMed] [Google Scholar]
- Godinho A, Bhosale S. Carotenes produced by alkaliphilic orange-pigmented strain of Microbacterium arborescens - AGSB isolated from coastal sand dunes. Indian J Mar Sci. 2008;37:307–312. [Google Scholar]
- Gutiérrez T, Learmonth R, Couperwhite I. Analysis of benzene-induced effects on Rhodococcus sp. 33 reveals that constitutive processes play a major role in conferring tolerance. Sci World J. 2009;9:209–223. doi: 10.1100/tsw.2009.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall TA. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucl Acids Symp Ser. 1999;41:95–98. [Google Scholar]
- Heipieper HJ, Neumann G, Cornelissen S, et al. Solvent-tolerant bacteria for biotransformations in two-phase fermentation systems. Appl Microbiol Biotechnol. 2007;74:961–973. doi: 10.1007/s00253-006-0833-4. [DOI] [PubMed] [Google Scholar]
- Isken S, Bont JAM de. Bacteria tolerant to organic solvents. Extremophiles. 1998;2:229–238. doi: 10.1007/s007920050065. [DOI] [PubMed] [Google Scholar]
- Kolomytseva MP, Solyanikova IP, Golovlev EL, et al. Heterogeneity of Rhodococcus opacus 1CP as a response to stress induced by chlorophenols. Appl Biochem Microbiol. 2005;41:474–479. [PubMed] [Google Scholar]
- Larkin MJ, Kulakov LA, Allen CCR. Biodegradation by members of the genus Rhodococcus: biochemistry, physiology, and genetic adaptation. Adv Appl Microbiol. 2006;59:1–29. doi: 10.1016/S0065-2164(06)59001-X. [DOI] [PubMed] [Google Scholar]
- Marchesi JR, Sato T, Weightman AJ, et al. Design and evaluation of useful bacterium-specific PCR primers that amplify genes coding for bacterial 16S rRNA. Appl Environ Microbiol. 1998;64:795–799. doi: 10.1128/aem.64.2.795-799.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínková L, Uhnáková B, Pátek M, et al. Biodegradation potential of the genus Rhodococcus . Environ Int. 2009;35:162–177. doi: 10.1016/j.envint.2008.07.018. [DOI] [PubMed] [Google Scholar]
- Niescher S, Wray V, Lang S, et al. Identification and structural characterisation of novel trehalose dinocardiomycolates from n-alkane-grown Rhodococcus opacus 1CP. Appl Microbiol Biotechnol. 2006;70:605–611. doi: 10.1007/s00253-005-0113-8. [DOI] [PubMed] [Google Scholar]
- Philp JC, Kuyukina MS, Ivshina IB, et al. Alkanotrophic Rhodococcus ruber as a biosurfactant producer. Appl Microbiol Biotechnol. 2002;59:318–324. doi: 10.1007/s00253-002-1018-4. [DOI] [PubMed] [Google Scholar]
- Pini F, Grossi C, Nereo S, et al. Molecular and physiological characterisation of psychrotrophic hydrocarbon-degrading bacteria isolated from Terra Nova Bay (Antarctica) Eur J Soil Biol. 2007;43:368–379. [Google Scholar]
- Rocha CA, Pedregosa AM, Laborda F. Biosurfactant-mediated biodegradation of straight and methyl-branched alkanes by Pseudomonas aeruginosa ATCC 55925. AMB Express. 2011;1:1–10. doi: 10.1186/2191-0855-1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning, A Laboratory Manual. 2nd ed. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, New York: 1989. [Google Scholar]
- Segura A, Hurtado A, Rivera B, et al. Isolation of new toluene-tolerant marine strains of bacteria and characterization of their solvent-tolerance properties. J Appl Microbiol. 2008;104:1408–1416. doi: 10.1111/j.1365-2672.2007.03666.x. [DOI] [PubMed] [Google Scholar]
- Sikkema J, Bont JAM de, Poolman B. Mechanisms of membrane toxicity of hydrocarbons. Microbiol Rev. 1995;59:201–222. doi: 10.1128/mr.59.2.201-222.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stancu MM, Grifoll M. Multidrug resistance in hydrocarbon-tolerant Gram-positive and Gram-negative bacteria. J Gen Appl Microbiol. 2011;57:1–18. doi: 10.2323/jgam.57.1. [DOI] [PubMed] [Google Scholar]
- Stancu MM. Physiological cellular responses and adaptations of Rhodococcus erythropolis IBBPo1 to toxic organic solvents. J Environ Sci. 2014;26 doi: 10.1016/j.jes.2014.08.006. in press. [DOI] [PubMed] [Google Scholar]
- Tamura K, Peterson D, Peterson N, et al. MEGA5: Molecular Evolutionary Genetics Analysis using Maximum Likelihood, Evolutionary Distance, and Maximum Parsimony Methods. Molec Biol Evol. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao L, Wagner LW, Rouvière PE, et al. Metabolic engineering for synthesis of aryl carotenoids in Rhodococcus . Appl Microbiol Biotechnol. 2006;70:222–228. doi: 10.1007/s00253-005-0064-0. [DOI] [PubMed] [Google Scholar]
- Torres S, Pandey A, Castro GR. Organic solvent adaptation of Gram positive bacteria: applications and biotechnological potentials. Biotechnol Adv. 2011;29:442–452. doi: 10.1016/j.biotechadv.2011.04.002. [DOI] [PubMed] [Google Scholar]
- Urai M, Yoshizaki H, Anzai H, et al. Structural analysis of an acidic, fatty acid ester-bonded extracellular polysaccharide produced by a pristine assimilating marine bacterium, Rhodococcus erythropolis PR4. Carbohydr Res. 2007;342:933–942. doi: 10.1016/j.carres.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Wei SJC, Desai SM, Harvey RG, et al. Use of short DNA oligonucleotides for determination of DNA sequence modifications induced by benzo[a]pyrene diol epoxide. Proc Nati Acad Sci USA. 1984;81:5936–5940. doi: 10.1073/pnas.81.19.5936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whyte LG, Greer CW, Inniss WE. Assessment of the biodegradation potential of psychrotrophic microorganisms. Can J Microbiol. 1996;42:99–106. doi: 10.1139/m96-016. [DOI] [PubMed] [Google Scholar]
- Yoo M, Kim D, Choi KY, et al. Draft genome sequence and comparative analysis of the superb aromatic-hydrocarbon degrader Rhodococcus sp. strain DK17. J Bacteriol. 2012;194:4440–4440. doi: 10.1128/JB.00844-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Qin F, Qiao J, et al. Draft genome sequence of Rhodococcus sp. strain P14, a biodegrader of high-molecular-weight polycyclic aromatic hydrocarbons. J Bacteriol. 2012;194:3546–3546. doi: 10.1128/JB.00555-12. [DOI] [PMC free article] [PubMed] [Google Scholar]