Abstract
Background. Passively acquired respiratory syncytial virus (RSV) neutralizing antibody protects against RSV-associated lower respiratory infections, but placental malaria (PM) and maternal hypergammaglobulinemia might interfere with transplacental immunoglobulin transport.
Methods. We measured RSV plaque-reduction neutralization (PRN) antibody in 300 full-term maternal/cord serum pairs in 2 cohorts in malaria-endemic Papua New Guinea: Alexishafen (2005–2008) and the Fetal Immunity Study (FIS) (2011–2013). We defined impaired transport as a cord-to-maternal titer ratio <1.0 and a protective RSV PRN titer (PRNT) ≥1:200.
Results. PM and hypergammaglobulinemia occurred in 60% and 54% of Alexishafen mothers versus 8% and 9% of FIS mothers, respectively. 34% of Alexishafen and 32% of FIS pairs demonstrated impaired transport. Multivariate modeling revealed significant associations between increasing maternal IgG (log2) and impaired transport (adjusted OR, Alexishafen: 2.68 [1.17–6.14], FIS: 6.94 [1.94–24.8]) but no association with PM. 34% of Alexishafen and 31% of FIS cord PRNTs were <1:200.
Conclusions. Impaired RSV antibody transport was observed in approximately one-third of maternal/cord pairs. Hypergammaglobulinemia, but not PM, was associated with impaired transport, particularly among women with low RSV PRNT. Detection of RSV PRNT <1:200 in one-third of cord sera confirms the need to increase levels of RSV neutralizing antibody in pregnant women through maternal immunization.
Keywords: hypergammaglobulinemia, maternal immunization, placental malaria, RSV, transplacental transfer of antibody
Pneumonia is the leading global cause of morbidity and mortality in children under 5 years of age [1]. As access to vaccines for bacterial pneumonia improves, viruses such as influenza and respiratory syncytial virus (RSV) are becoming increasingly important contributors to acute lower respiratory illness (ALRI) in pediatric populations. RSV is the most frequent cause of ALRI and bronchiolitis in infants [2, 3]. A recent global estimate of the annual burden attributed 33.8 million episodes of illness in children under 5 years of age and 253 000 deaths in children under 1 year to RSV; 99% of deaths are estimated to occur in developing countries [1, 3].
Protection against RSV-associated disease is primarily antibody (Ab)–mediated [4, 5], and passively acquired RSV neutralizing Ab can protect infants against RSV ALRI [6–9]. To address the global burden of RSV, promising vaccines are in development with a focus on maternal immunization (to prevent RSV ALRI in the first months of life) and infant immunization (to prevent RSV ALRI in later infancy and early childhood) [10–12].
Protection of infants via maternal immunization requires efficient transplacental transport of maternal immunoglobulin G (IgG) [13]. Transport begins during the second trimester and increases until delivery, with peak transfer occurring after 32 weeks gestation [13]. In term infants, cord IgG typically equals or exceeds maternal IgG [13]. Preterm birth, maternal malaria (specifically, placental malaria [PM]), human immunodeficiency virus (HIV), and hypergammaglobulinemia have been associated with impaired transplacental transfer of IgG for a variety of pathogens [13–26]; however, data are limited on transplacental transport of RSV-specific Ab [9, 15, 16, 27–29], as well as conditions that may impede its transport [15, 16]. Therefore, we sought to determine the impact of PM and maternal hypergammaglobulinemia on transplacental transport of RSV neutralizing Ab. Furthermore, we restricted our study to term infants in order to evaluate the effects of PM independent of its association with preterm birth. We conducted our study using specimens from mother-infant pairs residing on the north coast of Papua New Guinea (PNG), where both Plasmodium falciparum and P. vivax malaria are prevalent and pneumonia is the leading cause of child mortality, accounting for 17% of deaths in children under 5 [30].
METHODS
Populations and Specimens
Our study was nested within 2 studies of malaria in pregnancy conducted by the Papua New Guinea Institute of Medical Research (PNGIMR), the University of Melbourne, and Case Western Reserve University. These studies were conducted in rural and peri-urban areas on the north coast of Madang Province, PNG (Supplementary Figure 1). We analyzed 300 pairs of maternal and cord serum samples collected at delivery from term pregnancies (gestational age [GA] ≥37 weeks).
The first cohort, “Alexishafen,” provided 157 maternal/cord pairs obtained between 2005 and 2008 near Madang town (Supplementary Figure 1), prior to intensified malaria control interventions and when the rate of malaria transmission was very high [31, 32]. The second cohort, from the Fetal Immunity Study (“FIS”), provided 143 pairs from a clinical trial of intermittent preventive treatment of malaria in pregnancy (NCT01136850) [33]. Pregnant women from regions in or near Madang town were enrolled between 2011 and 2013. As this was subsequent to the initiation of malaria control measures in 2009–2010 [34], the rate of malaria in pregnancy was expected to be substantially lower than in the Alexishafen cohort.
GA at delivery was assessed using Ballard scores in the Alexishafen cohort [35], and composite measures of GA based on last menstrual period, fundal height, Ballard scores, and ultrasound data, when available, in the FIS cohort [33].
Placental Histology
Histologic characterization of placentae was performed using methods developed by Rogerson et al [33]. Placentae were graded 1–5 to indicate acute (stages 1 and 2), chronic (3), past (4), or no (5) infection. We defined PM as the presence of parasites, malaria pigment, or both (stages 1–4). Polymerase chain reaction (PCR) and blood smears for malaria were also performed on maternal peripheral blood and placentae using methods described elsewhere for Alexishafen [31] and FIS [33].
Maternal IgG Measurement
Maternal IgG levels were measured by radial immunodiffusion (Binding Site, Birmingham, UK). Maternal hypergammaglobulinemia was defined as ≥1700 mg/dL, based on comparisons to levels in healthy adults [36].
RSV-specific Antibody Measurement
RSV Ab was measured using a complement-enhanced 60% plaque-reduction neutralization (PRN) assay [37]. Plaque-reduction neutralization titers (PRNTs) are expressed arithmetically and as reciprocal log2.
Cord-to-maternal titer ratios (CMTRs) (cord PRNT/maternal PRNT) were calculated for each mother-infant pair. CMTRs in healthy, full-term pregnancies range from 1.0 to 1.2 [13]. Therefore, for this study, we defined normal transfer as CMTR ≥1.0, impaired transfer as CMTR <1.0, and severely impaired transfer as CMTR <0.8.
Cord PRNTs were also assessed as an outcome of interest. A cord PRNT that correlates with protection of infants against RSV ALRI has yet to be precisely defined. To determine an appropriate threshold for our analysis, we reviewed data from studies in cotton rats and in infants of protection afforded by intravenous administration of immunoglobulin containing high titers of RSV neutralizing Ab (RSV-IGIV) measured using a similar PRN assay [4, 6]. In cotton rats, PRNTs of 1:200–1:400 were associated with protection against pulmonary infection [4]. In high-risk infants receiving monthly doses of RSV-IGIV, trough levels of serum RSV Ab as measured by PRNT were generally >1:200 in infants in whom protection against RSV ALRI was observed [6]. Therefore, we categorized infants with cord blood PRNT ≥1:200 as having the putative minimal Ab level required for protection against RSV ALRI at birth.
Statistical Analysis
Statistical analyses were performed using Stata version 11 (StataCorp LP, College Station, Texas). Fisher's exact, χ2, Mann–Whitney, and Student's t-test were used for pairwise comparisons as appropriate. Linear regression was used for continuous outcomes, while logistic regression was used for dichotomous outcomes. P < .05 was considered significant. Log2-transformed maternal RSV PRNT, categorical maternal age, and gravidity (dichotomized as primigravid or multigravid) were included in final models a priori. Total IgG was analyzed as both a continuous and dichotomized predictor.
Ethical Review
The Institutional Review Boards of University Hospitals Case Medical Center (No. 05-11-02), the Papua New Guinea Medical Research Advisory Committee (No. 11.33), and the Johns Hopkins School of Public Health approved this work.
RESULTS
Mothers and Infants
In Alexishafen and FIS, the mean maternal age was 25 years; range, 16–49 years (Alexishafen) and 16–42 years (FIS) (Table 1). Fewer women in Alexishafen were primigravid compared to FIS (34.4% vs 48.9%; P = .012). At delivery, maternal anemia (hemoglobin ≤9 g/dL) was substantially higher in Alexishafen than in FIS (40% vs 17%; P < .001, Table 1). Approximately 7% of infants in each cohort were low birth weight (<2500 g) (Table 1).
Table 1.
Baseline Clinical Characteristics of Mother-Infant Pairs
| Characteristic | Alexishafen Cohort | FIS Cohort | P Value |
|---|---|---|---|
| Number of pairs | 157 | 143 | … |
| Maternal characteristics | |||
| Maternal age in years, mean (range) | 25.4 (16–49) | 25 (16–42) | .543 |
| Gravidity, mean (range) | 2.77 (1–10) | 1.8 (1–8) | <.001 |
| Primigravid, n (%) | 54 (34.4%) | 70 (48.9%) | .012 |
| Maternal hemoglobin at delivery (g/dL), mean (range) | 9.3 (4.2–14.2) | 9.67 (5.5–13.9) | .097 |
| Anemic (≤9 g/dL), n (%) | 63 (40%) | 25 (17%) | <.001 |
| Infant characteristics | |||
| Gestational age at birth in days, mean (range) | 273 (245–294) | 277 (255–300) | <.001 |
| Birth weight in grams, mean (range) | 3007 (2050–5700) | 3057 (2100–4400) | .324 |
| Low birth weight (<2500 g), n (%) | 11 (7%) | 10 (6.9%) | .996 |
| Female, n (%) | 70 (46.4%) | 69 (48.3%) | .7451 |
| Malaria exposure | |||
| Placental malaria by histology | |||
| Number of pairs with histology available | 153 | 137 | … |
| Placental malaria positive (stages 1–4), n (%) | 93 (60.2%) | 12 (8.8%) | <.001 |
| Placental malaria negative (stage 5), n (%) | 60 (39.2%) | 125 (91.2%) | <.001 |
| Stage 1—acute: parasites only, no pigment in monocytes or fibrin, n (%) | 58 (37.9%) | 7 (5.1%) | <.001 |
| Stage 2—acute: parasites, pigment in monocytes +/− fibrin, n (%) | 20 (13%) | 0 | <.001 |
| Stage 3—chronic: parasites, pigment in fibrin, n (%) | 3 (2.0%) | 1 (0.7%) | .361 |
| Stage 4—past infection, n (%) | 12 (7.8%) | 4 (2.9%) | .062 |
| Peripheral blood PCR | |||
| No positive PCR test in pregnancy, n (%) | 13 (8.2%) | 130 (90.9%) | <.001 |
| Any positive PCR test in pregnancy (P. falciparum, P. vivax, or P. ovale), n (%) | 144 (91.7%) | 13 (9.1%) | <.001 |
| Negative by PCR at delivery (maternal peripheral blood), n (%) | 90 (57.3%) | 136 (97.1%) | <.001 |
| Positive by PCR at delivery (maternal peripheral blood), n (%) | 67 (42.7%) | 4 (2.86%) | <.001 |
| Maternal IgG | |||
| Mean (range) | 2004 (727.8–7514.3) | 1110 (501–2726.2) | <.001 |
| Hypergammaglobulinemia (>1700 mh/dL), n (%) | 85 (54%) | 12 (8.4%) | <.001 |
Abbreviations: FIS, Fetal Immunity Study; IgG, immunoglobulin G; PCR, polymerase chain reaction.
Malaria in Pregnancy
The burden of malaria differed substantially between cohorts. In Alexishafen, 92% of mothers had ≥1 PCR+ assay for malaria in peripheral blood, 43% were malaria-positive at delivery by blood smear, and 60% of placentae showed evidence of chronic or acute PM. In contrast, the frequency of malaria in FIS was substantially lower: 9% of pregnant women had ≥1 PCR+ assay for malaria in peripheral blood, 2.9% of mothers were malaria-positive at delivery by blood smear, and 8.8% of placentae for which histology was available showed evidence of PM (Table 1).
CMTRs, Placental Malaria, and Hypergammaglobulinemia
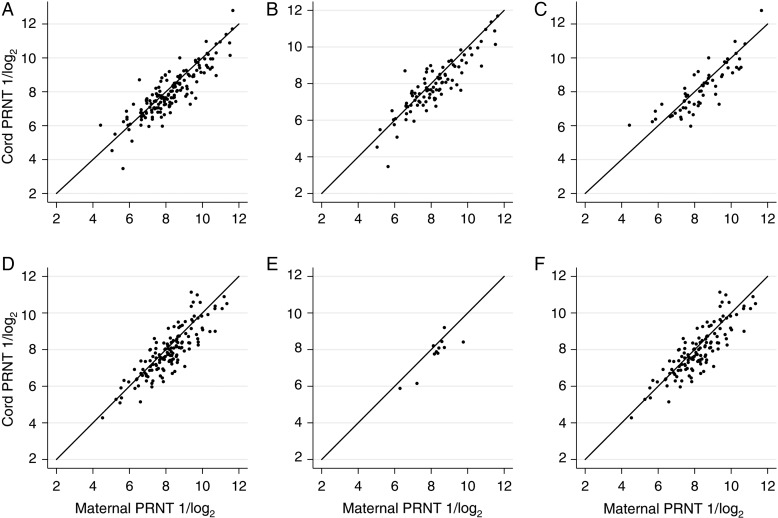
The distribution of CMTRs was very similar across the 2 cohorts: geometric mean (GM) 1.19 for Alexishafen (range, 0.22–4.54) and GM 1.22 (range, 0.29–3.88) for FIS. Despite the vast difference in rates of PM between the cohorts, proportions of maternal-cord pairs with impaired transfer were nearly identical. Approximately one-third (34% in Alexishafen, 32% in FIS) of maternal-cord pairs in each cohort had CMTR <1.0 [Table 2; Figure 1A and 1D]. Furthermore, the proportions with impaired transfer were not statistically significantly different after stratification by PM exposure: 37% of PM-exposed pairs in Alexishafen and 25% in FIS had CMTRs <1.0 (Table 2, Figure 1B and 1E), compared to 32% and 34%, respectively, in PM-unexposed pairs (Table 2, Figure 1C and 1F). Severely impaired transfer (CMTR <0.8) was observed in 17% of Alexishafen and 18% of FIS pairs and likewise was not significantly different after stratification by PM exposure (Table 2). The relationship between PM and CMTR was further explored through univariate and multivariate logistic regression, adjusting for maternal PRNT, maternal IgG, maternal age, and gravidity (Table 3). These analyses confirmed that PM was not significantly associated with impaired transfer in either cohort. Regression analyses using PCR-based definitions of malaria exposure (any positive PCR test in antenatal care [ANC] or at delivery) were also conducted; no significant associations were observed (data not shown).
Table 2.
RSV PRN Titers and CMTRs by Placental Malaria Status and Hypergammaglobulinemia
| Maternal PRNT GM (range) | Cord PRNT GM (range) | CMTR GM (range) | Proportion of CMTRs <0.8 | Proportion of CMTRs <1.0 | |
|---|---|---|---|---|---|
| Alexishafen cohort | |||||
| All | 254.3 (11–7150) | 301.7 (21.4–3257) | 1.19 (0.22–4.54) | 0.17 | 0.34 |
| Placental malaria + | 220.4 (23–7150) | 256.0 (33–3257) | 1.16 (0.22–4.24) | 0.17 | 0.37 |
| Placental malaria − | 303.0 (11–3329) | 371.7 (21.4–3191) | 1.23 (0.32–4.54) | 0.17 | 0.32 |
| P = .02 | P = .007 | P = .8 | OR 1.04 [0.40–2.77] P = .9 | OR 1.24 [0.59–2.64] P = .53 | |
| Hypergammaglobulinemia + | 305.1 (11–7150) | 332.4 (21.4–3257) | 1.09 (0.22–4.55) | 0.22 | 0.42 |
| Hypergammaglobulinemia − | 205.1 (23–1973) | 269.1 (33–2870) | 1.31 (0.40–3.99) | 0.11 | 0.25 |
| P = .004 | P = .159 | P = .014 | OR 2.3 [0.88–6.5] P = .06 | OR 2.2 [1.05–4.66] P = .02 | |
| FIS cohort | |||||
| All | 239.1 (19.5–2259) | 291.0 (23.1–2592) | 1.22 (0.29–3.88) | 0.18 | 0.32 |
| Placental malaria + | 229.9 (58.7–587.9) | 300.9 (78.3–869.3) | 1.30 (0.71–2.55) | 0.08 | 0.25 |
| Placental malaria − | 246.0 (19.5–2259) | 295.2 (23.1–2592.8) | 1.2 (0.29–3.88) | 0.2 | 0.34 |
| P = .81 | P = .78 | P = .52 | OR 0.36 [0.008–2.74] P = .46 | OR 0.64 [0.11–2.73] P = .75 | |
| Hypergammaglobulinemia + | 368.5 (87.1–2032) | 353.2 (192.4–836) | 0.96 (0.41–2.58) | 0.50 | 0.67 |
| Hypergammaglobulinemia − | 229.8 (19.5–2259) | 285.9 (23.1–2598) | 1.24 (0.29–3.88) | 0.15 | 0.29 |
| P = .055 | P = .278 | P = .065 | OR 5.55 [1.31–22.7] P = .009 | OR 4.89 [1.2–23.3] P = .019 | |
Abbreviations: CMTRs, cord-to-maternal titer ratios; FIS, Fetal Immunity Study; GM, geometric mean; OR, odds ratio; PRNT, plaque-reduction neutralization titer; RSV, respiratory syncytial virus.
Figure 1.
Relationship of maternal respiratory syncytial virus (RSV) plaque-reduction neutralizing titer (PRNT) to cord RSV PRNT in the presence and absence of placental malaria (PM). PRNTs are expressed as reciprocal log2. In each graph, the solid line represents a cord-to-maternal titer ratio of 1.0. A, Alexishafen cohort, all pairs. B, Alexishafen cohort, PM-positive pairs. C, Alexishafen cohort, PM-negative pairs. D, FIS cohort, all pairs. E, FIS cohort, PM-positive pairs. F, FIS cohort, PM-negative pairs. Abbreviation: FIS, Fetal Immunity Study.
Table 3.
Multiple Logistic Regression for Association Between Placental Malaria and Maternal IgG With CMTRs
| Alexishafen |
FIS |
|||
|---|---|---|---|---|
| Adjusted Odds Ratio (CI) | P Value | Adjusted Odds Ratio (CI) | P Value | |
| CMTR <0.8 | ||||
| Placental malaria | 1.11 (.43–2.87) | .822 | 0.237 (.024–2.27) | .212 |
| Maternal IgG | 2.93 (1.10–7.74) | .03 | 5.95 (1.32–26.77) | .02 |
| CMTR <1.0 | ||||
| Placental malaria | 1.36 (.647–2.87) | .415 | 0.44 (.099–1.94) | .280 |
| Maternal IgG | 2.68 (1.17–6.14) | .020 | 6.94 (1.94–24.8) | .003 |
Placental malaria is included in these models as a dichotomous variable, while maternal IgG (measured in mg/dL) is a log2-transformed continuous measure.
Abbreviations: CI, confidence interval; CMTRs, cord-to-maternal titer ratios; FIS, Fetal Immunity Study; IgG, immunoglobulin G.
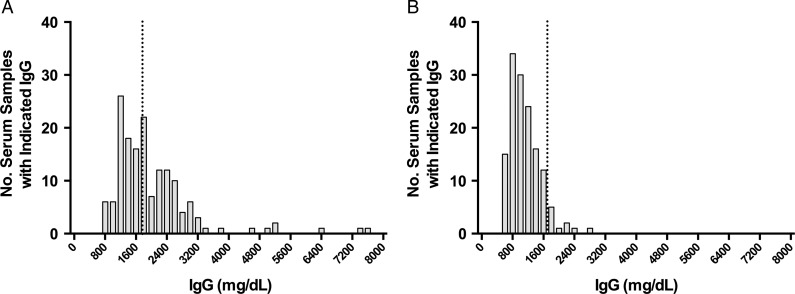
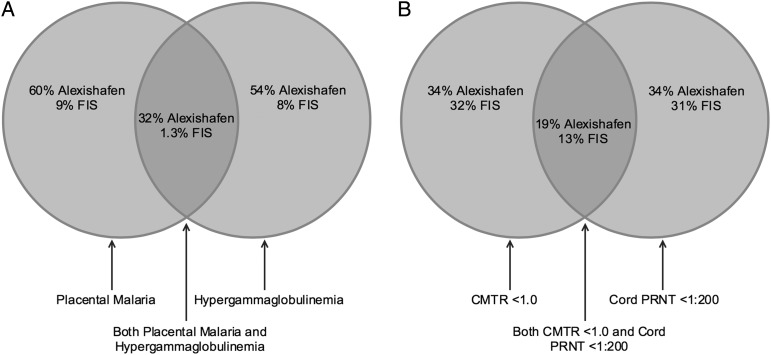
Maternal IgG levels were much greater in Alexishafen (GM: 2004 [range, 727–7514 mg/dL]) than FIS (GM: 1110 [range, 501–2762 mg/dL]; P < .001]) as was the proportion of women with hypergammaglobulinemia (54% vs 8%; P < .001; Figure 2). Interestingly, PM and hypergammaglobulinemia were not invariably associated: of 300 total women studied, 105 had PM and 97 had hypergammaglobulinemia, but only 53 had both (Figure 3A).
Figure 2.
Distributions of maternal IgG in each study cohort. Alexishafen cohort (A); FIS cohort (B). IgG levels are expressed as mg/dL. The dashed line represents the threshold for hypergammaglobulinemia (1700 mg/dL). Abbreviations: FIS, Fetal Immunity Study; IgG, immunoglobulin G.
Figure 3.
A, The relationship between placental malaria (PM) and hypergammaglobulinemia. Left circle, proportion of mothers with PM by histology; right circle, proportion of mothers with hypergammaglobulinemia; shaded middle area, proportion of mothers with both PM and hypergammaglobulinemia. B, The relationship between cord-to-maternal titer ratios (CMTRs) and cord plaque-reduction neutralization titers (PRNTs). Left circle, proportion of maternal/cord pairs exhibiting CMTR <1.0; right circle, proportion of cord specimens with PRNT <1:200, shaded middle area, proportion of cord specimens where CMTR was <1.0 and PRNT was <1:200. Abbreviation: FIS, Fetal Immunity Study.
In contrast to PM, high levels of maternal IgG were associated with impaired transfer of RSV Ab in these populations. We found that mean CMTRs were slightly lower when maternal hypergammaglobulinemia was present than when it was absent (GM CMTR 1.07; 95% confidence interval [CI], .96–1.19 vs 1.26; 95% CI, 1.19–1.34; P = .05) (Supplementary Figure 2). Furthermore, multivariate analyses that included IgG and CMTR as continuous variables and adjusted for maternal PRNT, maternal age, gravidity, and PM demonstrated that increasing maternal IgG was significantly associated with a reduction in CMTR in both cohorts. Likewise, increasing maternal IgG was associated with increased odds of impaired CMTR when we dichotomized at CMTRs of 1.0 and 0.8. For every doubling of maternal IgG, the odds of a CMTR <1.0 increased substantially in both cohorts (adjusted odd ratio for CMTR <1.0 for Alexishafen: 2.68; 95% CI, 1.17–6.14, P = .02; FIS: 6.94; 95% CI, 1.94–24.8, P = .003; Table 3). Hypergammaglobulinemia was not invariably associated with high maternal levels of RSV PRNT: 36% of women with IgG levels >1700 had RSV PRNT <1:200 (Supplementary Figure 3). Furthermore, the effect of hypergammaglobulinemia on transplacental transport appeared to be greatest in those with the lowest titers: among women with both hypergammaglobulinemia and maternal RSV PRNT <1:200, 39% had CMTR <1.0, whereas only 21% of women with RSV PRNT <1:200 and normal IgG levels had CMTR <1.0 (P = .04).
Titers of RSV neutralizing Antibodies in Maternal and Cord Serum Samples
Maternal RSV PRNT GMT were nearly identical in both cohorts (Alexishafen: GMT 254, range 11–7150; FIS: GMT 239, range 19–2259), as were cord RSV PRNT GMTs (Alexishafen: GMT 302, range 21–3257; FIS: GMT 291, range 23–2592). The point estimates for mean maternal PRNT were lower in women with PM than in those without PM in Alexishafen and FIS, but these findings were not statistically significant (Table 2). As expected, maternal and cord PRNTs were highly correlated (Pearson's r: 0.82 [P < .001] in Alexishafen, 0.73 [P < .001] in FIS, Figure 1).
Thirty-four percent of cord serum samples from Alexishafen and 31% from FIS had RSV PRNT <1:200 (Figure 4). While not evaluated in these cohorts, studies conducted elsewhere estimate the half-life of maternally derived RSV Ab to be approximately 28 days [28, 29]. Using this decay rate, the proportion of 1-month-old infants with a cord PRNT <1:200 would increase to 66% and 68% in Alexishafen and FIS, respectively. Interestingly, the infant specimens with cord PRNT <1:200 and the maternal/infant paired specimens with a CMTR <1.0 were somewhat distinct: only 55% of Alexishafen infants and 41% of FIS infants met both criteria (Figure 3B).
Figure 4.
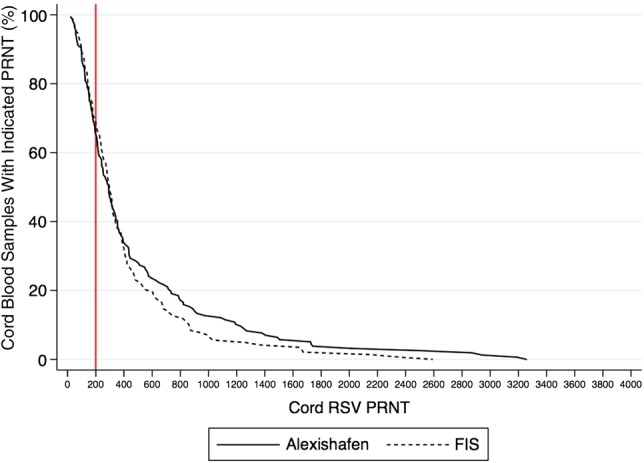
Reverse cumulative distribution of cord respiratory syncytial virus (RSV) plaque-reduction neutralization titers (PRNTs) by cohort. The solid line represents the Alexishafen cohort and the dotted line represents the FIS cohort. The vertical solid line indicates a cord PRNT of 1:200. Abbreviation: FIS, Fetal Immunity Study.
DISCUSSION
As maternal immunization becomes an increasingly important strategy for protection of very young infants, identification of factors that could impact vaccine effectiveness by modifying the efficiency of transplacental Ab transport will be critical. This study shows that increasing maternal IgG, but not PM, was associated with impaired transport of RSV neutralizing Ab in mothers and full-term infants in PNG, and that impaired transport occurred in approximately one-third of maternal-cord pairs.
These cohorts provided an unusual opportunity to examine associations between maternal conditions and impaired Ab transport. Participants were from catchment areas in close proximity to one another but were enrolled in 2005–2008 (Alexishafen) or in 2011–2013 (FIS). During this time interval, regional intensification of malaria control led to substantial reduction in rates of malaria [31], yet the pattern of transplacental transport of RSV neutralizing Ab remained nearly identical, with no statistically significant associations between PM and impaired transport demonstrated in either univariate or multivariate models.
Our findings differ from previous reports of the effect of PM on transplacental Ab transfer. Studies of Ab transfer for measles, varicella, Haemophilus influenzae, Streptococcus pneumoniae, and tetanus in the Gambia [14, 15], Malawi [18], and Kenya [20] have found associations between impaired transport and PM, as did a study of tetanus Ab transfer among women with malaria in PNG [21]. Data on PM and transplacental transport of RSV Ab are more limited; however, 1 study of 213 mother-infant pairs in the Gambia [15] found that both PM (defined by histology and blood smear of placental tissue) and hypergammaglobulinemia (defined as total IgG ≥1500 g/dL) were associated with a significant reduction in RSV IgG transfer as measured by enzyme-linked immunosorbent assay.
There are several possible explanations for the differences between these earlier findings and ours. First, we restricted our study to term infants to assess the effect of PM independent of preterm birth. In contrast, the study in The Gambia [15] only excluded infants born at <24 weeks gestation, and therefore 27% of infants studied were preterm. PM is a known cause of preterm birth, and preterm birth is associated with impaired Ab transfer [13]. We wanted to evaluate associations between PM and impaired transfer independent of preterm birth. Second, we measured RSV neutralizing Ab (rather than total RSV IgG) because it correlates with protection against RSV-ALRI [6–9]. It is possible that the IgG subclass distribution found when measuring RSV neutralizing Ab is different from the distribution found when measuring total RSV IgG (which presumably would contain both neutralizing and nonneutralizing Ab), and that PM might have a differential effect on subclass transport. Finally, the study in The Gambia evaluated the effects of both PM and hypergammaglobulinemia, but 94% of the hypergammaglobulinemic women also had PM, complicating the assessment of those exposures independently. In contrast, only 56% of the hypergammaglobulinemic women in our study had PM, allowing us to more readily examine the separate effects of these 2 conditions (Figure 3A). Our evaluation of maternal IgG as a continuous predictor may have also helped us retain important detail in these associations, especially in the FIS cohort, where few women had IgG values >1700 mg/dL. Of interest, we found that mean RSV PRNT was lower in mothers with PM compared to those without PM, though these differences were not significant (Table 2). This finding should be explored further in future studies.
We showed that increasing maternal IgG was significantly associated with impaired transport of RSV neutralizing Ab independent of maternal age, gravidity, PM, and maternal RSV PRNT, and that its impact may be more pronounced among women with lower RSV PRNT. Hypergammaglobulinemia is believed to interfere with active receptor-mediated transfer of maternal Ab via saturation of placental FcRN receptors [13], and impaired Ab transfer has been well described in a number of maternal conditions that are associated with hypergammaglobulinemia, such as HIV infection [19, 23]. Hypergammaglobulinemia has been associated with impaired transport of other virus-specific Abs, including measles [14, 17, 18, 38]. Interestingly, women in Alexishafen and FIS had strikingly different rates of hypergammaglobulinemia (54% vs 8%) despite being separated by only a few years in time. While some general health indicators have improved in this interval [30], the substantial decrease in malaria prevalence in this area of PNG is likely the greatest change and may partially explain this difference. Thus, while malaria does not appear to impair Ab transfer directly (ie, through infection of the placenta), it may impair transfer indirectly through the induction of hypergammaglobulinemia. Further studies into the causes of hypergammaglobulinemia in this region are needed.
Despite the association between hypergammaglobulinemia and impaired transport, cord PRN GMTs were strikingly similar in the Alexishafen and FIS cohorts. This is likely because maternal RSV PRNT remains the most important predictor of cord RSV PRNT on a population basis. In both cohorts, we found that approximately one-half of mothers and one-third of infants had RSV PRNTs below the putative protective threshold of 1:200. Taking into account the decay of maternal antibodies after birth, the proportion of susceptible infants would increase to 66%–68% by 1 month of age. These data suggest that a large proportion of young infants in PNG may be susceptible to severe RSV infection. Additional studies to determine the epidemiology of RSV in this population are underway.
This work was subject to several limitations. Specimens were obtained from studies designed for other purposes, so our ability to evaluate additional exposures related to hypergammaglobulinemia is limited. HIV status was not available for either cohort, but a study among women seeking ANC in Madang during 2011–2013 found HIV prevalence to be 1.1% [33]. Syphilis serostatus was not available for women in Alexishafen, but only 2 women in FIS had serologic evidence of active syphilis infection at enrollment. Preterm birth was an exclusion criterion for this study. For a large proportion of participants, methods other than ultrasound were used to estimate GA. Imprecise GA assessment could result in failure to exclude preterm infants; however, recent evidence suggests that these clinical methods have a high specificity for preterm birth [39]. Finally, while PRN is considered the gold standard for measurement of RSV Ab, it is a bioassay and subject to variability. We attempted to minimize this by using standardized protocols and reagents and by testing maternal and cord serum samples in duplicate and on the same plate to ensure consistency.
In summary, we have evaluated transplacental transfer of RSV neutralizing Ab in northern coastal PNG. We showed that increasing maternal IgG, but not PM, was associated with impaired transport among full-term infants. Moreover, we showed that approximately one-third of full-term infants were born without protective levels of RSV neutralizing Ab. These data suggest that approaches to enhance passive protection against RSV in very young infants in populations such as this might help prevent ALRI in early life. As maternal immunization strategies against RSV and other pathogens are developed and implemented, it will be important to continue to assess factors that affect transplacental transfer of Ab and to determine whether sufficient levels of Ab can be induced to overcome any potential deficits in transport.
Supplementary Data
Supplementary materials are available at http://jid.oxfordjournals.org. Consisting of data provided by the author to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the author, so questions or comments should be addressed to the author.
Notes
Acknowledgments. The authors wish to acknowledge the Alexishafen and Fetal Immunity Study (FIS) study participants as well as the Papua New Guinea Institute of Medical Research field and laboratory staff for their contribution to this work, including Henson Dima, and Dr Regina Wangnapi. We thank Dr James Beeson for providing specimens from the Alexishafen study; and Dr Ivo Mueller, Dr Danielle Stanisic, and Dr Alex Umbers for their contributions to the design and implementation of the Alexishafen and FIS studies. We also thank Dr Indu Malhotra for her guidance and facilitation of this project, and the staff of the King and Karron laboratories at Case Western Reserve University and Johns Hopkins University, respectively, for their technical assistance. We would also like to acknowledge Deborah Higgins at PATH (formerly Program for Appropriate Technology in Health) for her ongoing support and engagement in this work.
Financial support. This work was supported by PATH as well as the National Institute of Allergy and Infectious Diseases at the National Institutes of Health (NIH) (AI AI064687 and DMID #11-0037).
Potential conflicts of interest. J. E. A. reports grants from PATH Vaccines, during the conduct of the study; personal fees from PATH and personal fees from Gavi outside the submitted work. S. J. R. reports grants from Malaria in Pregnancy Consortium, grants and nonfinancial support from Pfizer Inc, during the conduct of the study; grants from National Health and Medical Research Council of Australia outside the submitted work. R. A. K. reports grants from PATH Vaccines, during the conduct of the study; grants from NIH outside the submitted work. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Liu L, Oza S, Hogan D et al. Global, regional, and national causes of child mortality in 2000–13, with projections to inform post-2015 priorities: an updated systematic analysis. Lancet 2014; 6736:2013–4. [DOI] [PubMed] [Google Scholar]
- 2.Hall CB, Weinberg GA, Poehling KA, Erdman D, Grijalva CG, Zhu Y. The burden of respiratory syncytial virus infection in young children. N Engl J Med 2009; 360:588–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nair H, Nokes JD, Gessner BD et al. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet 2010; 375:1545–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hemming VG, Prince GA, Groothuis JR, Siber GR. Hyperimmune globulins in prevention and treatment of respiratory syncytial virus infections. Clin Microbiol Rev 1995; 8:22–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Siber GR, Leombruno D, Leszczynski J et al. Comparison of antibody concentrations and protective activity of respiratory syncytial virus immune globulin and conventional immune globulin. J Infect Dis 1994; 169:1368–73. [DOI] [PubMed] [Google Scholar]
- 6.Groothuis JR, Simoes EA, Levin MJ et al. Prophylactic Administration of RSV immune globulin to high-risk infants and young children. N Engl J Med 1993; 329:1524–30. [DOI] [PubMed] [Google Scholar]
- 7.Glezen WP, Paredes A, Allison JE, Taber LH, Frank AL. Risk of respiratory syncytial virus infection for infants from low-income families in relationship to age, sex, ethnic group, and maternal antibody level. J Pediatr 1981; 98:708–15. [DOI] [PubMed] [Google Scholar]
- 8. IMpact-RSV Study Group, IMpact Study Group. Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in high-risk infants. Pediatrics 1998; 102:531–7. [PubMed] [Google Scholar]
- 9.Eick A, Karron R, Shaw J et al. The role of neutralizing antibodies in protection of American Indian infants against respiratory syncytial virus disease. Pediatr Infect Dis J 2008; 27:207–12. [DOI] [PubMed] [Google Scholar]
- 10.Anderson LJ, Dormitzer PR, Nokes DJ, Rappuoli R, Roca A, Graham BS. Strategic priorities for respiratory syncytial virus (RSV) vaccine development. Vaccine 2013; 31:B209–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Graham BS, Anderson LJ. Challenges and opportunities for respiratory syncytial virus vaccines. Cur Top Microbiol Immunol 2013; 372:391–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karron RA. Respiratory syncytial virus and parainfluenza virus. In: Plotkin S, Orenstein W, Offit P, eds. Vaccines. 6th ed Philadelphia: Elsevier Saunders Inc., 2012: 1146–53. [Google Scholar]
- 13.Palmeira P, Quinello C, Silveira-Lessa AL, Zago CA, Carneiro-Sampaio M. IgG placental transfer in healthy and pathological pregnancies. Clin Dev Immunol 2012; doi:10.1155/2012/985646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Okoko BJ, Wesuperuma LH, Ota MO et al. Influence of placental malaria infection and maternal hypergammaglobulinaemia on materno-foetal transfer of measles and tetanus antibodies in a rural West African population. J Heal Popul Nutr 2001; 19:59–65. [PubMed] [Google Scholar]
- 15.Okoko BJ, Wesumperuma LH, Ota MO et al. The influence of placental malaria infection and maternal hypergammaglobulinemia on transplacental transfer of antibodies and IgG subclasses in a rural West African population. J Infect Dis 2001; 184:627–32. [DOI] [PubMed] [Google Scholar]
- 16.Okoko JB, Wesumperuma HL, Hart CA. The influence of prematurity and low birthweight on transplacental antibody transfer in a rural West African population. Trop Med Int Health 2001; 6:529–34. [DOI] [PubMed] [Google Scholar]
- 17.de Moraes-Pinto MI, Almeida AC, Kenj G et al. Placental transfer and maternally acquired neonatal IgG immunity in human immunodeficiency virus infection. J Infect Dis 1996; 173:1077–84. [DOI] [PubMed] [Google Scholar]
- 18.de Moraes-pinto MI, Verhoeff F, Chimsuku L et al. Placental antibody transfer: influence of maternal HIV infection and placental malaria. Arch Dis Child Fetal Neonatal Ed 1998; 79:F202–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scott S, Cumberland P, Shulman CE et al. Neonatal measles immunity in rural Kenya: the influence of HIV and placental malaria infections on placental transfer of antibodies and levels of antibody in maternal and cord serum samples. J Infect Dis 2005; 191:1854–60. [DOI] [PubMed] [Google Scholar]
- 20.Cumberland P, Shulman CE, Maple PAC et al. Maternal HIV infection and placental malaria reduce transplacental antibody transfer andtetanus antibody levels in newborns in Kenya. J Infect Dis 2007; 196:550–7. [DOI] [PubMed] [Google Scholar]
- 21.Brair M-E, Brabin BJ, Milligan P, Maxwell S, Hart CA. Reduced transfer of tetanus antibodies with placental malaria. Lancet 1994; 343:208–9. [DOI] [PubMed] [Google Scholar]
- 22.Wesumperuma H, Perera A, Pharoah P, Hart C. The influence of prematurity and low birthweight on transplacental antibody transfer in Sri Lanka. Ann Trop Med Parasitol 1999; 93:169–77. [DOI] [PubMed] [Google Scholar]
- 23.Jones CE, Naidoo S, De Beer C, Esser M, Kampmann B, Hesseling AC. Maternal HIV infection and antibody responses against vaccine-preventable diseases in uninfected infants. JAMA 2011; 305:576–84. [DOI] [PubMed] [Google Scholar]
- 24.Gupta A, Mathad JS, Yang W-T et al. Maternal pneumococcal capsular IgG antibodies and transplacental transfer are low in South Asian HIV-infected mother-infant pairs. Vaccine 2014; 32:1466–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Van Den Berg JP, Westerbeek EA, Berbers GA, Van Gageldonk PG, Van Der Klis FR, Van Elburg RM. Transplacental transport of IgG antibodies specific for pertussis, diphtheria, tetanus, Haemophilus influenzae type b, and Neisseria meningitidis serogroup C is lower in preterm compared with term infants. Pediatr Infect Dis J 2010; 29:801–5. [DOI] [PubMed] [Google Scholar]
- 26.Van Den Berg JP, Westerbeek EA, Smits GP, Van Der Klis FR, Berbers GA, Van Elburg RM. Lower transplacental antibody transport for measles, mumps, rubella and varicella zoster in very preterm infants. PLOS One 2014; 9:e94714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suara R, Piedra PA, Glezen WP et al. Prevalence of neutralizing antibody to respiratory syncytial virus in sera from mothers and newborns residing in the Gambia and in The United States. Clin Vaccine Immunol 1996; 3:477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ochola R, Sande C, Fegan G et al. The level and duration of RSV-specific maternal IgG in infants in Kilifi Kenya. PLOS One 2009; 4:e8088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chu HY, Steinhoff MC, Magaret A et al. Respiratory syncytial virus transplacental antibody transfer and kinetics in mother-infant pairs in Bangladesh. J Infect Dis 2014; 210:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. World Health Organization. Papua New Guinea: Health Profile [Internet], 2012. http://www.who.int/gho/countries/png.pdf Accessed 8 March 2015.
- 31.Teo A, Hasang W, Randall LM et al. Decreasing malaria prevalence and its potential consequences for immunity in pregnant women. J Infect Dis 2014; 210:1444–55. [DOI] [PubMed] [Google Scholar]
- 32.Stanisic DI, Moore KA, Baiwog F et al. Risk factors for malaria and adverse birth outcomes in a prospective cohort of pregnant women resident in a high malaria transmission area of Papua New Guinea. Trans R Soc Trop Med Hyg 2015; 109:313–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Unger HW, Ome-Kaius M, Wangnapi RA et al. Sulphadoxine-pyrimethamine plus azithromycin for the prevention of low birthweight in Papua New Guinea: a randomised controlled trial. BMC Med 2015; doi:10.1186/s12916-014-0258-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Senn N, Rarau P, Stanisic DI et al. Intermittent preventive treatment for malaria in Papua New Guinean infants exposed to Plasmodium falciparum and P. vivax: a randomized controlled trial. PLOS Med 2012; doi:10.137/journal.pmed.1001195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Umbers AJ, Boeuf P, Clapham C et al. Placental malaria-associated inflammation disturbs the insulin-like growth factor axis of fetal growth regulation. J Infect Dis 2011; 203:561–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jollift CR, Cost KM, Stivrins PC et al. Reference intervals for serum lgG, IgA, IgM, C3, and C4 as determined by rate nephelometry. Clin Chem 1982; 28:126–8. [PubMed] [Google Scholar]
- 37.Coates H, Alling D, Chanock R. An antigenic analysis of respiratory syncytial virus isolates by a plaque reduction neutralization test. Am J Epidemiol 1966; 83:299–313. [DOI] [PubMed] [Google Scholar]
- 38.Hartter HK, Oyedele OI, Dietz K, Kreis S, Hoffman JP, Muller CP. Placental transfer and decay of maternally acquired antimeasles antibodies in Nigerian children. Pediatr Infect Dis J 2000; 19:635–41. [DOI] [PubMed] [Google Scholar]
- 39.Karl S, Li Wai Suen CS, Unger HW et al. Preterm or not - an evaluation of estimates of gestational age in a cohort of women from rural Papua New Guiena. PLOS One 2015; 10:e0124286. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.