Abstract
The contribution of human neutrophils to the protection against fungal infections by Aspergillus fumigatus is essential but not fully understood. Whereas healthy people can inhale spores of A. fumigatus without developing disease, neutropenic patients and those receiving immunosuppressive drugs have a higher incidence of invasive fungal infections. To study the role of neutrophils in protection against A. fumigatus infections, we developed an in vitro assay in which the interactions between human neutrophils and A. fumigatus were observed in real time, at single-cell resolution, in precisely controlled conditions. We measured the outcomes of neutrophil-fungus interactions and found that human neutrophils have a limited ability to migrate toward A. fumigatus and block the growth of A. fumigatus conidia (proportion with growth blocked, 69%). The blocking ability of human neutrophils increased to 85.1% when they were stimulated by uniform concentrations of fMLP and was enhanced further, to 99.4%, in the presence of chemoattractant gradients. Neutrophils from patients receiving immunosuppressive treatment after transplantation were less effective against the fungus than those from healthy donors, and broader heterogeneity exists between patients, compared with healthy individuals. Further studies using this microfluidic platform will help understand the relevance of innate immune deficiencies responsible for the higher risk of fungal infections in patients with immunosuppressive disease.
Keywords: neutrophil, microfluidics, A. fumigatus, host-pathogen interaction, chemoattractants
Each year, >500 000 patients in the United States are treated for fungal infections, resulting in substantial patient morbidity and mortality and costing the healthcare system an estimated $8 billion [1]. Patients with primary immunosuppressive diseases or those that are medically acquired through immunosuppressive therapy during transplantation or through cancer therapies are at higher risk [2]. Of the fungal infections, common and particularly challenging are those caused by Aspergillus fumigatus, a saprophytic fungus commonly found in the environment that can cause life-threatening infections in immunocompromised patients.
Several mechanisms contribute to the defenses against A. fumigatus if it is able to reach the alveolar surface, including the physical barriers of the respiratory tract and a well-regulated orchestration of innate and adaptive immune responses [3]. Resting A. fumigatus spores can be phagocytosed by neutrophils, monocytes [4], and dendritic cells [5–9], while lymphocytes also play critical roles in the protection against A. fumigatus in vivo [10, 11]. Neutrophils play a critical role and are rapidly recruited to sites of infection [6]. In the absence of neutrophils, conidia can germinate and grow into an invasive filamentous mass in the lungs [12]. While neutrophil responses can be dampened by T-helper type 2 (Th2) lymphocyte–mediated recruitment of eosinophils [13], the mobilization and priming of human neutrophils by proinflammatory cytokines and chemokines secreted by sentinel cells such as resident macrophages is important in protection from the pathogenesis of A. fumigatus [14]. However, detailed studies of Th1 lymphocyte–mediated priming events involving neutrophils in vivo remain challenging owing to the complex nature of these interactions and the poor ability to control conditions in the local microenvironment of lung tissue. Current in vitro systems to evaluate neutrophil-fungal interactions are qualitative because of their limited ability to monitor the growth of A. fumigatus, which elongates and branches repeatedly during growth.
Here we measured neutrophil-fungi interactions in real time, at single-cell resolution, in the presence of gradients, as well as uniform concentrations of various chemoattractants, in microfluidic devices specifically designed to measure host-pathogen interactions. We focused our studies on LTB4 and fMLP because previous studies identified them as eliciting the fastest, most persistent chemotaxis patterns among the evaluated chemoattractants [15]. We found that human neutrophils can block A. fumigatus growth and that blocking is significantly more effective when the neutrophils are guided toward the fungus by chemoattractant gradients. We tested the new microfluidic tools with human neutrophils from immunosuppressed recipients of kidney transplants and found that these neutrophils were less effective against the fungus than those from healthy donors. Such tools may become useful for estimating the risk for fungal infections in immunosuppressed patients and may have implications for the faster diagnosis and start of antifungal treatment in patients at risk.
METHODS
A. fumigatus Culture
Aspergillus fumigatus strain 293 expressing cytosolic red fluorescent protein or green fluorescent protein was grown on Sabouraud dextrose agar plates supplemented with 100 μg/mL ampicillin at 30°C for 3–4 days. Conidia were harvested by gentle scraping, followed by washing in ice-cold phosphate-buffered saline (PBS) 3 times. Conidia were immediately used or stored at 4°C for up to 1 week before use.
Microfluidic Device Fabrication
Microfluidic devices used to measure leukocyte migration in response to A. fumigatus were manufactured using standard microfabrication techniques. For the devices with chemoattractant gradients, 2 layers of photoresist (SU8; Microchem), the first 10-μm thin (corresponding to the migration channels) and the second 70-μm thick (corresponding to the chemotaxis chambers), were patterned on 1 silicon wafer sequentially, using 2 photolithographic masks and processing cycles according to the instructions from the manufacturer. For the microwell array, a single layer, 70-µm thick, was patterned using photolithographic techniques. The wafer with a patterned photoresist was used as a mold to produce polydimethylsiloxane (Fisher Scientific) devices, which were then bonded to the base of glass-bottomed 12-well plates, using an oxygen plasma machine (Nordson-March).
Human Neutrophil Isolation
Deidentified fresh blood samples (volume, 10 mL) obtained from healthy volunteers aged ≥18 years who were not receiving immunosuppressant agents were purchased from Research Blood Components. Blood samples were collected from kidney transplant recipients at Massachusetts General Hospital (MGH). Venous blood samples from healthy volunteers were collected by phlebotomy, after receipt of informed consent, and the procedures described below were approved by the MGH Institutional Review Board (protocol 2008-P-002123). Peripheral blood samples were collected in 3-mL tubes containing a final concentration of 5 mM ethylenediaminetetraacetic acid (Vacutainer; Becton Dickinson) and processed within 2 hours of collection.
Using a sterile technique, we isolated neutrophils from whole blood by use of HetaSep followed by the EasySep human neutrophil enrichment kit (Stemcell Technologies) in accordance with the manufacturer's protocol. The purity of neutrophils was assessed to be >98%, using the Sysmex KX-21N Hematology Analyzer (Sysmex America). White blood cells (WBCs) were isolated using Hetasep, followed by a 5-minute spin-down cycle and washing with 1 × PBS. WBCs were stained with Hoechst fluorescent dye (32.4 μM; Sigma-Aldrich). The final aliquots of WBCs were resuspended in Roswell Park Memorial Institute (RPMI) medium plus 10% fetal bovine serum (FBS; stock 50 mL of FBS/450 mL of RPMI; Sigma-Aldrich) at a concentration of 4000 cells/2 μL and kept at 37°C. Cells were then immediately introduced into the microfluidic device for the chemotaxis and A. fumigatus assay. All experiments were repeated at least 3 times with neutrophils or WBCs from 3 different healthy donors.
Well Array and Chemotaxis-Chamber Microfluidic Device Preparation
Immediately after bonding to the well plate, donut-shaped chemotaxis-chamber devices were filled with A. fumigatus conidia at a concentration of 106 cells/mL and/or chemoattractant solution of fMLP (N-formyl-methionyl-leucyl-phenylalanine; 100 nM; Sigma-Aldrich, St. Louis, Missouri), LTB4 (100 nM; Cayman), or interleukin 8 (IL-8; 100 nM; R & D Systems). The devices were then placed in a vacuum for 15 minutes. The chemoattractant filled all of the focal chemoattractant chambers (FCCs) as the air was displaced. The devices were then vigorously washed 5 times with 1 × PBS to remove any residual A. fumigatus conidia and chemoattractant that was outside of the FCCs. The devices were then submerged in 0.5 mL of cell medium. Neutrophils or WBCs (4000 cells/2 μL) were then pipetted into the cell-loading chamber, using a gel-loading pipette tip. Neutrophil migration into the migration channel toward the FCC started immediately and was recorded using time-lapse imaging on a fully automated Nikon TiE microscope (10 × original magnification) with the biochamber heated to 37°C with 5% carbon dioxide gas (Supplementary Figure 1). Images of cell migration counts and fungi growth were analyzed by hand, using Image J software.
A. fumigatus Shape Factor Analysis
To compare the shape of fungi during growth, we used a shape factor analysis. The shape factor was defined as the ratio between the perimeter length of the fungus cell and the perimeter length of a perfectly circular object with the same area as the fungus cell. Shape factor values are 1 for a perfectly circular object and 0 for an elongated, branched object. We measured the shape factor changes from time-lapse images and defined a threshold shape value of 0.75 for identifying the transition between conidia and hyphae.
Quantification of Neutrophil Recruitment
Image analysis of cell migration counts and fungi growth was analyzed by hand, using Image J software. Neutrophil counts per chamber were counted every 15 minutes for the first 2 hours of the experiment and then every hour for the remaining 16 hours. When using WBCs, neutrophils were counted using Hoechst stain to define cell nucleus.
Quantification of Neutrophil Blocking of A. fumigatus Growth
The percentage of conidia converting to hyphal growth was measured by counting the number of conidia loaded per chamber before neutrophils or WBCs are loaded into the chamber and then counting numbers of these conidia that grow into hyphae by 18 hours. The image-acquisition software used for time-lapse imaging was NIS Elements (Nikon), and analysis of images, such as determination of the hyphal growth velocity, was performed using Image J analysis software. Data from the 16 chambers in >3 devices were analyzed for at least 3 different healthy donors, using GraphPad PRISM software. The normalized capacity to block fungal growth is calculated as follows: ([percentage of fungi killed with chemoattractant]/[percentage of fungi killed without chemoattractant]) × ([number of neutrophils per well or chamber with chemoattractant]/[number of neutrophils per well or chamber without chemoattractant]).
Quantification of Neutrophil Phagocytosis of A. fumigatus
Phagocytosis was verified by measuring pH levels in pHrodo (Life Technologies) in accordance with the manufacturers protocol. Conidia were incubated with the pHrodo dye for 1 hour and are nonfluorescent at neutral pH and fluoresce brightly in acidic environments, such as the phagosome. The fluorescence intensity was quantified using Elements software.
RESULTS
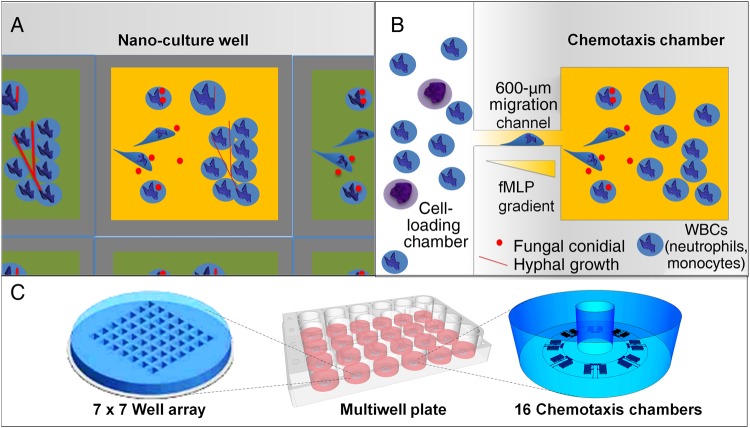
We designed 2 microfluidic platforms with single-cell resolution to investigate the interactions between neutrophils and fungi in controlled microenvironmental conditions (Figure 1). One design consisted of an array of wells filled with a uniform concentration of chemoattractant. The confinement of the well brings the neutrophils and conidia into close proximity at the start of the experiment (well array). This design permitted testing of whether chemoattractants can prime the neutrophils for interactions with A. fumigatus (Figure 1A). The second design requires neutrophils to migrate along a chemoattractant gradient to reach nanoliter-sized chemotaxis chambers (2.6 nL), where they subsequently interact with A. fumigatus (chemotaxis-chamber). This setting replicates the presence of biochemical gradients (eg, LTB4 and fMLP) established in tissues during inflammation and helps probe the role of directed neutrophil migration before interactions with A. fumigatus (Figure 1B). Both microfluidic devices can be run in parallel in glass-bottomed, multiwell plates, using neutrophils from one or multiple donors (Figure 1C).
Figure 1.
Schematics of the well array and chemotaxis-chamber device for quantifying neutrophil interactions with Aspergillus fumigatus. Two polydimethylsiloxane devices for measurement at the nanoliter level were fabricated to compare the antifungal priming effect on human neutrophils of uniform versus gradient concentrations of chemoattractant. A, The well array consists of 49 open wells (300 μm wide × 500 µm long × 50 µm deep), and neutrophils and fungi with or without a uniform concentration of chemoattractant are cultured together. In the well array, the fungi and neutrophils are brought in close proximity by the seeding protocol, which relies on gravity to settle the fungal spores and the neutrophils to the bottom of the wells. The physical constrains of the wells serve to enhance the chances of neutrophils-fungus interaction. B, The chemotaxis assay combines 16 chemotaxis chambers surrounding a central cell-loading chamber, and neutrophils migrate along chemoattractant gradients formed in the migration chamber before interacting with fungi. In the chemotaxis assay, A. fumigatus conidia are loaded into the chemotaxis chambers and cultured. Neutrophils loaded into the cell-loading chamber follow the chemical gradients produced by A. fumigatus to interact with the fungus. Moreover, in some experiments, an additional chemoattractant (fMLP, LTB4, and interleukin 8) is loaded into the chemotaxis chambers with the fungus to further enhance the migration and priming of neutrophils. C, Both devices are mounted inside the glass-bottomed wells of a multiwell plate, and interactions between the host immune cells and A. fumigatus are monitored via time-lapse microscopy. Abbreviation: WBC, white blood cell.
A. fumigatus Growth Rates in Well Arrays and Chemotaxis Chambers
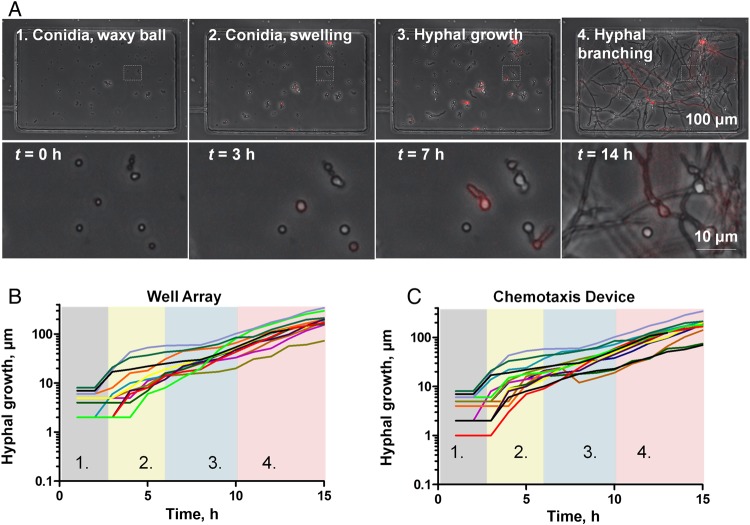
We used time-lapse imaging (1 image was obtained every 15 minutes for 18 hours) to measure the growth of A. fumigatus in well arrays and chemotaxis chambers (Figure 2A and Supplementary Movies 1 and 8). We optimized the microfluidic priming conditions (initial fungi culture preparation, 105 conidia/mL) to observe a mean (±standard deviation [SD]) of 15.9 ± 3.7 A. fumigatus conidia per well in the well arrays and per chamber in the fungi chemotaxis-chamber devices (Supplementary Figure 2). To quantitatively follow the changes in morphology from conidia to hyphae, we calculated the shape factor for 10 fungus cells over time. Immediately after conidia were loaded in the well array (t = 0), they were round (mean shape factor [±SD], 0.95 ± 0.02). By approximately 3 hours conidia had begun to swell, and by 7 hours they had grown into hyphae (mean shape factor [±SD], 0.4 ± 0.09). Approximately 14 hours after loading in the devices, we observed hyphal branching. These measurements of growth showed comparable dynamics in both devices (Figure 2B and 2C) and were in agreement with the literature [16]. They confirm that morphologic transition in A. fumigatus from conidia to hyphae is not restricted by nutrient limitation or other factors in either in vitro microfluidic platform [17]. Growth speed in the hyphae stage was comparable in the well array and chemotaxis-chamber device (mean value [±SD], 10 ± 7 μm/hour) and increased (to 25.9 ± 12.8 μm/hour) in the branching phase.
Figure 2.
Quantification of Aspergillus fumigatus growth on microfluidic platforms in the absence of neutrophils. A, Time-lapse images of A. fumigatus strain 293 (expressing cytosolic red fluorescent protein) growing inside chemotaxis chambers. Conidia are loaded into the chemotaxis chambers during the priming step. At approximately 3 h, conidia begin to swell. At approximately7 h, hyphal growth begins, and at approximately14 h, hyphal branching initiates (scale bar, 100 µm). B, Fungal growth measurements of 10 different conidia over time in the well array. Colored vertical bands indicate the different stages of fungal growth, showing (1) small conidia, (2) swollen conidia, (3) hyphae, and (4) branched hyphae. C, Fungal growth measurements of 10 different conidia over time inside the chemotaxis device show comparable timing of morphological changes.
Human Neutrophils Phagocytose Conidia in Well Arrays
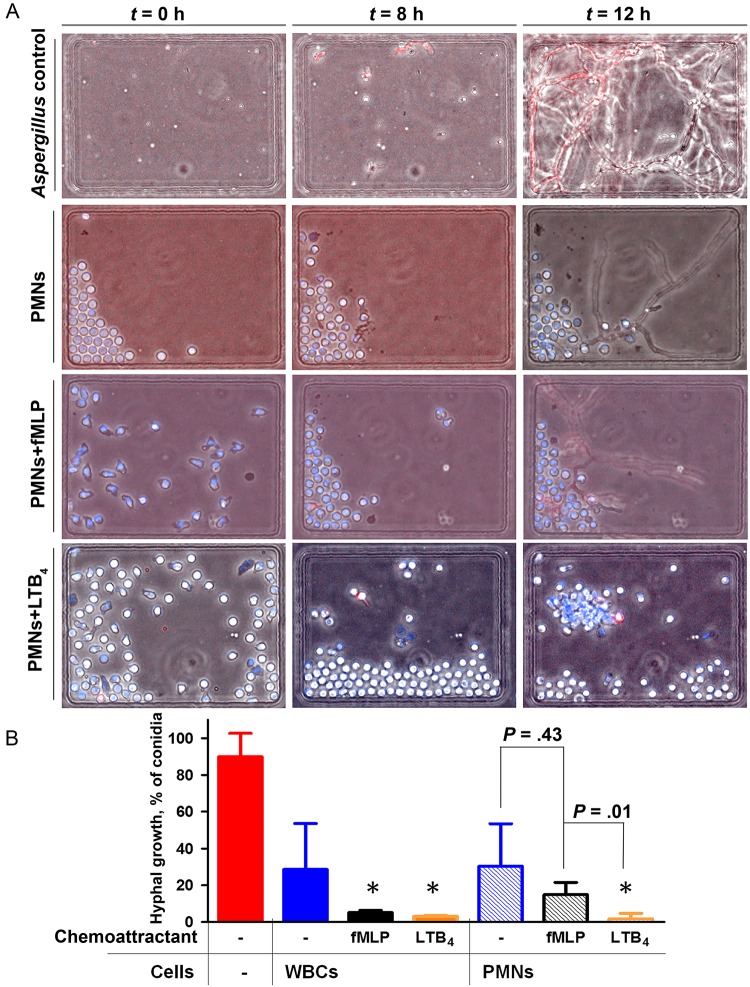
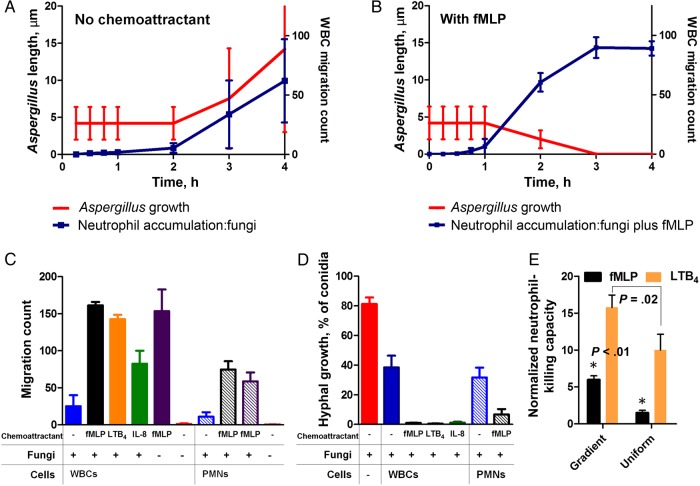
To measure the interactions between human neutrophils and fungi, we confined these interactions inside wells (300 µm wide × 500 µm long × 50 µm deep). We loaded fungi in the presence and absence of chemoattractants into the wells and then added either human WBCs or isolated human neutrophils to the wells, with an average concentration of 100 neutrophils/well. We monitored the interactions between neutrophils and fungi for 18 hours (Figure 3A). To further verify the ability of neutrophils to kill conidia after phagocytosis, we monitored the acidification of the phagosome, using pH-sensitive fluorescent dyes (Supplementary Figure 3), and monitored the wells for up to 48 hours to determine whether any hyphae grew from the phagocytized conidia. When WBCs were added to the well array, they were able to reduce hyphae growth, and only 28.7% of conidia formed hyphae (n = 3; Figure 3B). The growth of conidia into hyphae was further blocked in the presence of fMLP or LTB4, which primed the WBCs to significantly block hyphae growth (3.2% vs 5.2% of conidia formed hyphae, respectively; n = 3). When isolated human neutrophils were added to the well arrays, in the absence of chemoattractants they were able to reduce hyphae growth, and 30.3% of conidia formed hyphae (n = 3; Figure 3B and Supplementary Movie 2). Their efficiency was comparable to that of the entire WBC population in buffy coat, highlighting the major role of neutrophils in innate immune protection against fungal infections. The ability of neutrophils to block the growth of conidia into hyphae was enhanced in the presence of a uniform concentration of chemoattractants. In the presence of fMLP, the efficiency by which neutrophils phagocytosed conidia and blocked hyphal growth was not statistically different from that of neutrophils alone (14.9% [n = 3]; P = .43; Supplementary Movie 3). With isolated neutrophils and in the presence of uniform concentrations of LTB4, hyphal growth is reduced to a mean proportion (±SD) of 2% ± 1% of the initial number of conidia (n = 3; Supplementary Movie 4). Without chemoattractant activation, neutrophils will change morphology to a rounded shape and distribute heterogeneously inside the well. Often, neutrophils would crowd one edge of the device, reflecting the lack of adhesion to the cell culture plate that is moving continuously on the microscope stage during imaging at multiple locations (Figure 3A).
Figure 3.
Neutrophil activity against Aspergillus fumigatus in well arrays in the presence of uniform chemoattractant concentrations. A, Panel of phase contrast and merged fluorescent images showing fungal growth even in the presence of a uniform concentration of fMLP (100 nM) and neutrophils. In contrast, fungal growth is not observed in the presence of a uniform gradient of LTB4 (see the corresponding time-lapse videos in Supplementary Movies 1–4). B, Quantification of white blood cell (WBC) or neutrophil ability to kill fungi is measured as the fraction of conidia that grow into hyphae. Measurements of hyphae growth are made without chemoattractant or with uniform concentrations of fMLP (100 nM) or LTB4 (100 nM). A uniform concentration of fMLP (no gradient) does not significantly lower the ability of neutrophils to kill A. fumigatus, compared with the control condition. *P < .05, by 1-way analysis of variance, for the difference between the control in the absence of chemoattractant and conditions with uniform concentrations of chemoattractant. Abbreviation: PMN, polymorphonuclear leukocyte.
Human Neutrophils Phagocytose Conidia in Chemotaxis-Chamber Devices
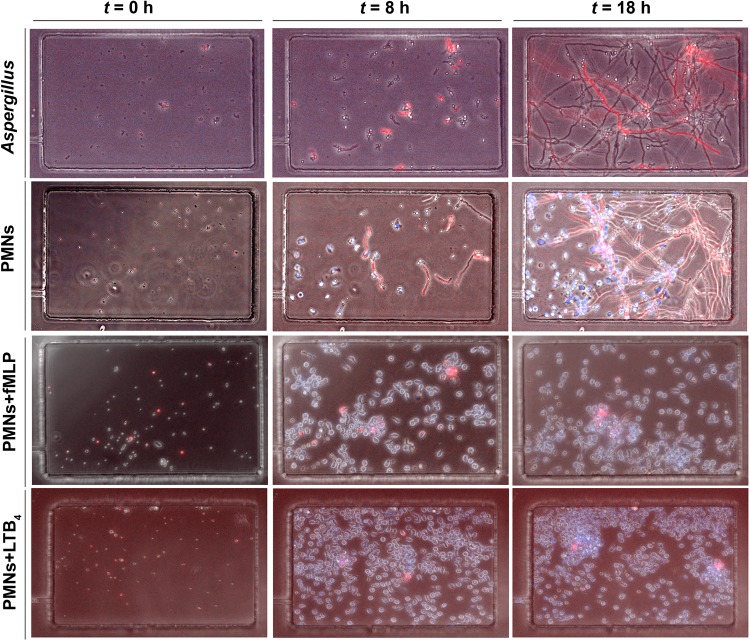
In the chemotaxis-chamber devices, A. fumigatus spores (conidia) were loaded in 16 chambers in each device, in the presence or absence of chemoattractant. In the absence of neutrophils, the conidia germinated and grew into hyphae at rates that were similar to those in open-well devices. Neutrophils, loaded at 4000 cells/µL in the central cell-loading chamber, can reach the fungus only after migrating along a 600-µm–long channel between the cell-loading chamber and the chemotaxis chambers containing the fungus. In the absence of chemoattractant, we observed that many neutrophils migrated spontaneously toward A. fumigatus conidia in the chemotaxis chambers. A mean (±SD) of 15 ± 5 neutrophils were attracted and arriving to the chemotaxis chambers in about 2–3 hours and reached the conidia after it began to swell (Figure 4, Figure 5C, and Supplementary Movie 6). The migration of neutrophils from the cell-loading well to the fungal cells in the chemotaxis chambers appeared to be guided by a chemoattractant gradient produced by the fungi, between the chemotaxis chambers and the cell-loading chamber. We observed no neutrophil migration toward the chemotaxis chambers in the absence of fungi in the chemotaxis chambers (Figure 5C). We observed that, at 4 hours, all conidia in the chemoattractant chambers had been phagocytosed. However, 18 hours later, many conidia inside neutrophils had swollen and eventually formed hyphae. Overall, neutrophils alone were able to reduce hyphal formation by half (from a mean value [±SD] of 80% ±5% to 35% ± 4%; n = 3; Figure 4 and Figure 5D). We observed large variations in the number of neutrophils migrating, possibly reflecting differences between individuals in their responses to A. fumigatus. When WBCs were loaded in the devices, in the absence of external chemoattractants, A. fumigatus alone attracted a mean (±SD) of 25 ± 20 neutrophils (Figure 5A). We confirmed that all attracted cells were neutrophils by staining the nucleus of the WBCs with Hoechst DNA-intercalating dye and observing the characteristic polymorphic shape of the neutrophil nucleus for all cells reaching the chemotaxis chambers. The evaluation of nuclear shape continued over time, enabling us to observe the shape from multiple orientations in the moving cells. The neutrophils arriving to the chemotaxis chamber reduced the number of growing hyphae to about half (from a mean value [±SD] of 80% ± 5% in control conditions to 38% ± 7% in the presence of WBCs; n = 3). The reduction in hyphal growth in the presence of neutrophils attracted from WBCs samples was comparable to that in the presence of isolated neutrophils.
Figure 4.
fMLP and LTB4 gradients attract and prime neutrophils for blocking Aspergillus fumigatus growth. Panels of phase contrast and merged fluorescent images show the control experiments, in which the fungus grows in the absence (top row) and presence (middle and bottom rows) of neutrophils, in the absence (second row) and presence (third row) of an fMLP chemoattractant gradient, and the presence of a LTB4 chemoattractant gradient (bottom row). In the absence of chemoattractant gradients, neutrophils migrate alone from the reservoir to the chamber and phagocytose conidia in <4 h. However, by 18 h several hyphae have grown from inside the neutrophils. Additional neutrophils recruited around the growing hyphae cannot stop their growth. In the presence of fMLP (100 nM) or LTB4 (100 nM), neutrophils (blue; Hoechst stain) accumulate in the chemotaxis chamber by 4 h, and fungal growth is blocked at 18 h (bottom 2 rows). The corresponding time-lapse videos are available in Supplementary Movies 5–8. Abbreviation: PMN, polymorphonuclear leukocyte.
Figure 5.
Chemoattractant gradients prime human neutrophils to effectively block Aspergillus fumigatus growth. A, In the absence of chemoattractant, low numbers of neutrophils begin accumulating in the chemotaxis chamber at 3 h, soon after conidia start swelling. On average, around 50 neutrophils accumulate per chamber at 6 h (blue line). However, the presence of neutrophils does not hinder hyphal growth (red line). B, In the presence of fungus and fMLP (100 nM), neutrophils begin to accumulate at 1 h. Two-fold more neutrophils migrate to the chemotaxis chamber, compared with fungus alone (blue line; P < .001). Neutrophils phagocytose first the swollen conidia (reflected by the decrease in average size of free conidia in the chemotaxis chamber; red line), and by 3 h all conidia are cleared from the chambers. The growth from conidia to hyphae is blocked for the remaining 14 h of the experiment. C, The average white blood cell (WBC) count (solid bars) and isolated neutrophils (striped bars) per chemotaxis chamber (2 devices with 16 chambers each) is presented at 14 h in the presence of A. fumigatus alone (blue bar) or with fMLP (black bar), LTB4 (yellow bar), or interleukin 8 (IL-8; green bar). Positive (purple) and negative (red) control bars represent the number of neutrophils migrating toward chemotaxis chambers with and those without fMLP, respectively. D, WBCs (buffy coat) significantly decrease fungal growth alone (P < .001), reducing growth from 80% (red bar) to 40% (blue bar) of conidia into hyphae. In the presence of fMLP (black bar), LTB4 (yellow bar), or IL-8 (green bar), <1% of conidia convert to hyphal growth. Isolated neutrophils reduce hyphae growth to 40% (blue stripe) and to <5% with fMLP (black stripe). All data are from 18 h. E, Normalized neutrophil fungus growth–blocking capacities in the presence of fMLP (P < .01) and LTB4 (P = .02, by the nonparametric t test) gradients are significantly larger than in the presence of uniform concentrations. Neutrophil-blocking capacity is calculated as follows: ([percentage of fungi killed with chemoattractant]/[percentage of fungi killed without chemoattractant]) × ([number of neutrophils per well or chamber with chemoattractant]/[number of neutrophils per well or chamber without chemoattractant]).
Chemoattractant Gradients Prime Neutrophils for Blocking A. fumigatus Growth
When fMLP, LTB4, or IL-8 was used in the chemotaxis-chamber devices, the WBC recruitment at 14 hours was increased 7-fold, 6-fold, and 4-fold, respectively, compared with no-gradient conditions (Figure 5C). Only neutrophils were attracted to the chemotaxis chambers. The enhanced effect of chemotaxis on fungus neutralization was systematic, and the fMLP, LTB4, or IL-8 chemoattractant gradients each significantly enhanced the ability of neutrophils to block the growth of A. fumigatus. Compared with the presence of no chemoattractant, when >40% of conidia transitioned to hyphae, in the presence of chemoattractant gradients, <1% of conidia transitioned to hyphae (n = 3; Figure 5D). In these microfluidic devices, the effects of fMLP are dominant over the products of A. fumigatus alone and it is possible that the fMLP and Aspergillus are not synergistic [18–20]. Control experiments in the absence of fungus showed consistent WBC recruitment in fMLP control (purple bar) and no recruitment in no-chemoattractant, negative controls (red).
We also compared the responses of isolated neutrophils to A. fumigatus in the presence and absence of fMLP and LTB4 gradients (Figure 5C and 5D). In the absence of chemoattractant, at approximately7 hours, conidia ingested by the neutrophils continued to grow into hyphae inside the neutrophils, eventually lysing these neutrophils (Supplementary Movie 6). In the presence of fMLP (Supplementary Movie 7) or LTB4 (Supplementary Movie 8) chemoattractant gradients, approximately 1 hour after loading the neutrophils in the cell-loading chamber, neutrophils reached the chemotaxis chambers and began interacting with the conidia. Within 2–3 hours, the majority of conidia had been phagocytosed by neutrophils (Figure 4 and Supplementary Figure 4). On average, hyphal growth was reduced, with only a mean (±SD) of 4% ± 2% of conidia forming hyphae (Figure 5D). This value was higher than in the presence of WBCs, indicating that neutrophils play a key role in blocking fungal growth but that other WBC types are also important. While we verified that all WBCs reaching the chamber were neutrophils, it is possible that the other WBCs primed the neutrophils before and during their migration toward the chemotaxis chamber. Also, the neutrophils phagocytosed swollen conidia first, as evidenced by the decreasing average size of A. fumigatus cells inside the chemotaxis chambers (Figure 5B).
We verified that the timing of neutrophil interaction with swollen conidia in the chemotaxis assay was not the source of the difference in neutrophil-blocking activity. For this, we compared the activity of human neutrophils in uniform concentrations of fMLP when added to the well arrays 0 and 4 hours after conidia loading. We found no significant differences in the ability of neutrophils to block the growth of A. fumigatus conidia (t = 0) or swollen conidia (t = 4 hours; Supplementary Figure 5). When normalized to the number of neutrophils in the well array, the fMLP gradient primed human neutrophils more effectively. Neutrophils after chemotaxis toward fMLP were 5-fold more efficient (P < .01) at blocking A. fumigatus growth than when fMLP was present at a uniform concentration in the wells (Figure 5E). Surprisingly, LTB4 gradients were even more efficient at priming the neutrophils than fMLP. The normalized blocking efficiency of human neutrophils after chemotaxis in LTB4 gradients was only 1.6-fold higher (P = .02) than in the presence of uniform concentrations of LTB4 (Figure 5E).
One interesting observation in our study was the large variation between individuals for WBCs or isolated neutrophil chemotaxis toward fungi in the absence of chemoattractants. We found that some human donors had neutrophils that migrated at high rates toward A. fumigatus and were able to phagocytes all conidia in the chamber. In several healthy donor samples, only 1–2 neutrophils migrated toward fungi alone (significantly lower than the average migration count of 25.2 neutrophils). Occasionally, in human donors, hours after the initial chemotaxis in the fMLP gradient and phagocytosis of conidia, 1 hypha might occasionally begin to grow, and swarms of 10–20 neutrophils would gather around the hypha and neutralize it (data not shown).
Neutrophils from patients receiving immunosuppressive therapy were less effective at blocking the growth of A. fumigatus in the presence of chemoattractant gradients, compared with neutrophils from healthy volunteers.
We compared the activity of neutrophils in buffy coat against A. fumigatus in the presence and absence of fMLP gradients, using neutrophils from 5 healthy donors and those from 6 immunosuppressed kidney transplant recipients. Figure 6A and 6B present results from 1 healthy volunteer and 1 immunosuppressed patient to outline the difference in the number of neutrophils recruited and the fraction of conidia germinating in the presence of these neutrophils. In some patients, fungal growth was reduced by >50% in the absence of external chemoattractant gradients and by >99% in the presence of a gradient of fMLP (100 nM). In others, neutrophils had high intrinsic ability to kill A. fumigatus in the absence of chemoattractant gradients but were less responsive to stimulation by gradients of fMLP (Table 1). With WBCs from healthy donors, we observed a mean reduction (±SD) in the number of conidia growing into hyphae by 73.9% ± 11.5% and almost complete elimination (99.4% ± 0.5%) of growth in the presence of a gradient of fMLP (n = 5; Figure 6C). With WBCs from immunosuppressed patients, the number of conidia growing into hyphae was also reduced by a mean (±SD) of 55% ± 21% and 94% ± 10.3% in the absence and presence of fMLP, respectively (n = 6). Overall, 2-fold fewer neutrophils were recruited, and 10-fold more conidia grew into hyphae in samples from patients, compared with those from healthy donors. We also noted greater heterogeneity in the ability of neutrophils from transplant recipients to block the growth of A. fumigatus, compared with those from healthy volunteers, which may require further investigation. The cells recruited to the chemoattractant chambers were exclusively neutrophils, as determined by examining the shape of their nucleus after staining with Hoechst dye.
Figure 6.
Capacity of neutrophils healthy donors and immunosuppressed patients to block the growth of Aspergillus fumigatus. A, Large numbers of white blood cells (WBCs) isolated from 1 healthy donor reach the chemotaxis chamber in the presence of fungus and fMLP, and fMLP alone. Significantly fewer WBCs migrate toward the fungus alone or control conditions with no chemoattractant. The WBCs arriving to the chemotaxis chamber reduce the number of growing hyphae by 2-fold, from 80% (solid red bar) to 40% (checkered red bar). In the presence of a gradient of fMLP (100 nM), fungal growth was almost entirely eliminated (0.2% conidia grow into hyphae). All WBCs entering the chemotaxis chamber were neutrophils. Data represent average from 2 devices, each with 16 chemotaxis chambers. B, More WBCs from patients migrate toward fungus alone, compared with WBCs from healthy donors. Fewer WBCs from patient accumulate toward fungus and fMLP or fMLP alone, compared to WBCs from healthy donors. The ability of WBCs to reduce fungal growth is also decreased, from 80% (solid red bar) to 56% (checkered red bar). The presence of a gradient of fMLP (100 nM) enhanced the ability of WBCs from the patient to reduce fungal growth, but the fraction of conidia growing into hyphae (2%) was 10 times that in the healthy donor. All WBCs entering the chemotaxis chamber were neutrophils. Data represent the average from 2 devices, each with 16 chemotaxis chambers. C, Summary of the ability of neutrophils to suppress the growth of A. fumigatus conidia data are for 5 samples from healthy controls and 6 samples from immunosuppressed patients. The trajectory arrow represents the decreased amount fungi growing when more neutrophils are recruited in the presence of fMLP chemoattractant gradients, compared with fungi alone (ie, with no chemoattractant gradient). In the presence and absence of fMLP gradients, larger numbers of neutrophils from healthy volunteers are recruited (empty symbols), compared with neutrophils from immunosuppressed patients (filled symbols). Ten times more fungal cells grow into hyphae in the presence of neutrophils from patients than from healthy volunteers. Moreover, we noted higher heterogeneity in the response of neutrophils from patients, and in some patients the response of neutrophils against fungus after stimulation in chemoattractant gradients was clearly deficient. Please note the logarithmic scale for the fraction of fungus growing after interaction with the neutrophils.
Table 1.
Response of Neutrophils From Kidney Transplant Recipients to Aspergillus fumigatus
| Patient | Time Since Transplantation | ANC, ×1000 cells/µL blood | Treatment (Daily Dose) | Neutrophil Response to Aspergillus |
|||
|---|---|---|---|---|---|---|---|
| Without fMLP |
With fMLP |
||||||
| Neutrophils Recruited, No. | Fraction of Fungus Growing, % | Neutrophils Recruited, No. | Fraction of Fungus Growing, % | ||||
| 1 | 6 mo | 12.24 | Prograf (4 mg), prednisone (20 mg), MMF (1 g) | 222 | 56.6 | 1648 | 1.9 |
| 2 | 6 y | 1.93 | Prograf (4.5 mg), prednisone (5 mg), MMF (1 g) | 447 | 3.6 | 806 | 0.4 |
| 3 | 14 y | 4.58 | Prograf (0.5 mg), prednisone (2.5 mg), Cellcept (500 mg) | 18 | 61.7 | 1166 | 2 |
| 4 | 1 mo | 0.95 | Prograf (6 mg), prednisone (15 mg), Cellcept (750 mg), cefepime, Valcyte, Bactrim |
17 | 55.7 | 52 | 29.3 |
| 5 | 7 y | 2.47 | Prograf (3 mg), Cellcept (500 mg), prednisone (5 mg) | 71 | 43.2 | 1496 | 5.1 |
| 6 | 3 y | 9.34 | Cyclosporine (125 mg), Cellcept (500 mg), prednisone (5 mg) | 26 | 53.3 | 1152 | 0 |
| Overall, mean ± SD | |||||||
| Transplant recipients | … | … | … | 133 ± 172 | 44.4 ± 21.5 | 1053 ± 572 | 6.5 ± 11.3 |
| Healthy controls | … | … | … | 194 ± 223 | 26.1 ± 11 | 1965 ± 354 | 0.6 ± 0.5 |
Responses were measured in vitro, using chemoattractant-chamber devices, with and without fMLP gradients.
Abbreviations: ANC, absolute neutrophil count; MMF, mycophenolate mofetil.
DISCUSSION
Innate immunity is known to be the predominant host defense against A. fumigatus. Neutrophils are professional phagocytes of the innate immune system that are essential to control fungal growth. A. fumigatus is a potent activator of the complement system and human monocyte-derived dendritic cells, which contribute to the protective Th1-dominated immune response, including neutrophil chemotaxis and activation [21, 22]. A. fumigatus antigens also enhance the phagocytosis and production of interleukin IL-8 in granulocytes [22]. Studies have revealed a prominent role of neutrophils in engulfing and killing A. fumigatus through a variety of effector mechanisms, including oxidative burst [23] and elastase secretion [24]. These findings are consistent with clinical studies suggesting that neutrophils are essential to the control of A. fumigatus growth. Patients with chronic granulomatous disease have a dramatically high risk of invasive aspergillosis [25, 26]. These findings are not without controversy, however, considering that some studies have suggested that neutrophils are unable to kill resting conidia of A. fumigatus [27]. A few studies have demonstrated the significant increase in the numbers of conidia ingested by active neutrophils in the presence of chemoattractants, including IL-8 and fMLP [14, 28]. Most of these studies, using traditional transwell assay, required neutrophils to fall from the membrane to the bottom of the dish, where the fungus was located. This represents an unusual behavior for neutrophils, which increase integrin expression and become more adhesive when stimulated by chemoattractants [29]. In vitro studies show that healthy neutrophils were found to damage hyphae, whereas neutrophils from patients with chronic granulomatous disease and myeloperoxidase deficiency did not [30].
We observed, at single-cell resolution, in real time, and in precisely controlled conditions, the interactions between human neutrophils and A. fumigatus. We quantified the outcome of these interactions with high precision in the presence of uniform concentrations and gradients of chemoattractants (fMLP, IL-8, and LTB4). We observed that a low number of neutrophils migrated toward and ingested A. fumigatus conidia even in the absence of chemoattractants. Previous studies have shown that soluble β-d-glucan shed from the cell wall of fungi induces neutrophil chemotaxis and subsequent enhanced expression of proinflammatory proteins [29, 31]. In the absence of a chemoattractant gradient, a considerable number of conidia phagocytosed by neutrophils continue to grow and eventually become hyphae. This indicates that phagocytosis is not enough to kill the conidia and that additional priming of the neutrophil is required (ie, upregulation of reactive oxygen species). However, it is important to note that even when neutrophils do not kill fungi, they effectively delay fungi germination from 3 to 8 hours.
Importantly, we found that neutrophils that migrate up chemoattractant gradients have an enhanced ability to neutralize A. fumigatus, compared with those in the absence of chemoattractant or which experience only uniform concentrations of the chemoattractant. This is a somewhat surprising finding, since after migration, once in the fungal chambers, the neutrophils are exposed to uniform levels of chemoattractant. This finding suggests that the process of neutrophil migration affects their physiological state not just during migration but for a period thereafter, as well. This scenario resembles the in vivo innate immune response in which neutrophils extravagate from blood vessels and migrate along chemoattractant gradients to the site of tissue injury or infection, where the chemoattractant concentrations are more uniform. This scenario is also supported by observations in vitro, in which human neutrophils continue their persistent migration after chemotaxis and can undergo retrotaxis [20], and by corresponding studies of neutrophil reversed migration in vivo, in zebrafish [32] and mice [33]. Overall, these reports suggest that human neutrophils may have a so-called memory of migration that is reflected in a more active phenotype, including migration and phagocytosis.
Fungal infections in solid organ transplant recipients are of concern because of the related high mortality and morbidity. A. fumigatus is the predominant fungal pathogen of immunocompromised patients. Even with the use of antifungal agents, the mortality rates associated with invasive aspergillosis remain around 50%, making the development of novel treatment strategies imperative. Previous studies found that more than half of the neutrophil samples from glucocorticoid-treated patients had a reduced ability to kill P. aeruginosa [34] and that in vitro neutrophil adherence was downregulated after in vivo prednisone [35]. Although we have so far studied only a small number of patients, our measurement of antifungal activity of neutrophils isolated from kidney transplant recipients undergoing immunosuppressive therapy revealed reduced levels of neutrophil activity against fungi. Even though the spontaneous neutrophil responses were only slightly different between patients and healthy volunteers, after stimulation with chemoattractant gradients these differences increased and revealed higher heterogeneity in the patient population. Whereas 2-fold fewer neutrophils were recruited toward fungus in the presence of fMLP, 10-fold higher numbers of hyphae were growing after interaction with the neutrophils. This suggests that other differences, in addition to recruitment by chemoattractants, exist between the neutrophils from patients and healthy volunteers.
In conclusion, we found that neutrophils alone can block A. fumigatus growth, that this inhibition is significantly enhanced in the presence of uniform concentrations of chemoattractants, and that the inhibition is further enhanced by gradients of chemoattractants. The microfluidic platform developed in this study may eventually become a useful tool for measuring the ability of neutrophils from patients to mount effective immune responses against fungi in vitro. This tool may help better estimate the risk for fungal infections for each patient and have implications for the faster diagnosis and earlier start of antifungal treatment during infections.
Supplementary Data
Supplementary materials are available at http://jid.oxfordjournals.org. Consisting of data provided by the author to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the author, so questions or comments should be addressed to the author.
Notes
Acknowledgments. We thank Drs H. Shaw Warren and Martin Springer, for useful discussions; Dr Felix Ellett, for assistance with the phagosome pH levels evaluation; Kerry Crisalli, RN, for assistance collecting patient blood samples; and Nida Khan, for assistance in the culture of A. fumigatus.
Financial support. This work was supported by Cidara Therapeutics under a research agreement managed by the Massachusetts General Hospital (to D. I. and J. M. V.) and by the National Institutes of Health (grant GM092804 to D. I. and grant EB002503 to the BioMEMS Resource Center [for all microfabrication work]).
Potential conflicts of interest. D. I., M. C. P., and J. M. V. have received consulting fees from Cidara Therapeutics. K. F. and K. J. were employees of Cidara Therapeutics during the conduct of the study. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Menzin J, Meyers JL, Friedman M et al. . The economic costs to United States hospitals of invasive fungal infections in transplant patients. Am J Infect Control 2011; 39:e15–20. [DOI] [PubMed] [Google Scholar]
- 2.Segal BH. Aspergillosis. N Engl J Med 2009; 360:1870–84. [DOI] [PubMed] [Google Scholar]
- 3.Hasenberg M, Stegemann-Koniszewski S, Gunzer M. Cellular immune reactions in the lung. Immunol Rev 2013; 251:189–214. [DOI] [PubMed] [Google Scholar]
- 4.Swamydas M, Break TJ, Lionakis MS. Mononuclear phagocyte-mediated antifungal immunity: the role of chemotactic receptors and ligands. Cell Mol Life Sci 2015; 72:2157–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lapp K, Vodisch M, Kroll K et al. . Characterization of the Aspergillus fumigatus detoxification systems for reactive nitrogen intermediates and their impact on virulence. Front Microbiol 2014; 5:469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bruns S, Kniemeyer O, Hasenberg M et al. . Production of extracellular traps against Aspergillus fumigatus in vitro and in infected lung tissue is dependent on invading neutrophils and influenced by hydrophobin RodA. PLoS Pathog 2010; 6:e1000873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Behnsen J, Narang P, Hasenberg M et al. . Environmental dimensionality controls the interaction of phagocytes with the pathogenic fungi Aspergillus fumigatus and Candida albicans. PLoS Pathog 2007; 3:e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lother J, Breitschopf T, Krappmann S et al. . Human dendritic cell subsets display distinct interactions with the pathogenic mould Aspergillus fumigatus. Int J Med Microbiol 2014; 304:1160–8. [DOI] [PubMed] [Google Scholar]
- 9.Brown GD. Innate antifungal immunity: the key role of phagocytes. Annu Rev Immunol 2011; 29:1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Romani L. Immunity to fungal infections. Nat Rev Immunol 2004; 4:1–23. [DOI] [PubMed] [Google Scholar]
- 11.Chamilos G, Ganguly D, Lande R et al. . Generation of IL-23 producing dendritic cells (DCs) by airborne fungi regulates fungal pathogenicity via the induction of T(H)-17 responses. PLoS One 2010; 5:e12955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cunha C, Kurzai O, Loffler J, Aversa F, Romani L, Carvalho A. Neutrophil responses to Aspergillosis: new roles for old players. Mycopathologia 2014; 178:387–93. [DOI] [PubMed] [Google Scholar]
- 13.O'Dea EM, Amarsaikhan N, Li H et al. . Eosinophils are recruited in response to chitin exposure and enhance Th2-mediated immune pathology in Aspergillus fumigatus infection. Infect Immun 2014; 82:3199–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Richardson MD, Patel M. Stimulation of neutrophil phagocytosis of Aspergillus fumigatus conidia by interleukin-8 and N-formylmethionyl-leucylphenylalanine. J Med Vet Mycol 1995; 33:99–104. [PubMed] [Google Scholar]
- 15.Boneschansker L, Yan J, Wong E, Briscoe DM, Irimia D. Microfluidic platform for the quantitative analysis of leukocyte migration signatures. Nat Commun 2014; 5:4787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Latgé J-P, Steinbach WJ. Aspergillus fumigatus and aspergillosis. 2009; xviii, 568 p., Washington DC, ASM Press. [Google Scholar]
- 17.Meletiadis J, Meis JF, Mouton JW, Verweij PE. Analysis of growth characteristics of filamentous fungi in different nutrient media. J Clin Microbiol 2001; 39:478–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heit B, Robbins SM, Downey CM et al. . PTEN functions to ‘prioritize’ chemotactic cues and prevent ‘distraction’ in migrating neutrophils. Nat Immunol 2008; 9:743–52. [DOI] [PubMed] [Google Scholar]
- 19.Heit B, Liu L, Colarusso P, Puri KD, Kubes P. PI3K accelerates, but is not required for, neutrophil chemotaxis to fMLP. J Cell Sci 2008; 121:205–14. [DOI] [PubMed] [Google Scholar]
- 20.Cho H, Hamza B, Wong EA, Irimia D. On-demand, competing gradient arrays for neutrophil chemotaxis. Lab Chip 2014; 14:972–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Waldorf AR, Diamond RD. Neutrophil chemotactic responses induced by fresh and swollen Rhizopus oryzae spores and Aspergillus fumigatus conidia. Infect Immun 1985; 48:458–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Braedel S, Radsak M, Einsele H et al. . Aspergillus fumigatus antigens activate innate immune cells via toll-like receptors 2 and 4. Br J Haematol 2004; 125:392–9. [DOI] [PubMed] [Google Scholar]
- 23.Diamond RD, Clark RA. Damage to Aspergillus fumigatus and Rhizopus oryzae hyphae by oxidative and nonoxidative microbicidal products of human neutrophils in vitro. Infect Immun 1982; 38:487–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prufer S, Weber M, Stein P et al. . Oxidative burst and neutrophil elastase contribute to clearance of Aspergillus fumigatus pneumonia in mice. Immunobiology 2014; 219:87–96. [DOI] [PubMed] [Google Scholar]
- 25. A controlled trial of interferon gamma to prevent infection in chronic granulomatous disease. The International Chronic Granulomatous Disease Cooperative Study Group. N Engl J Med 1991; 324:509–16. [DOI] [PubMed] [Google Scholar]
- 26.Marciano BE, Spalding C, Fitzgerald A et al. . Common severe infections in chronic granulomatous disease. Clin Infect Dis 2015; 60:1176–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Levitz SM, Diamond RD. Mechanisms of resistance of Aspergillus fumigatus Conidia to killing by neutrophils in vitro. J Infect Dis 1985; 152:33–42. [DOI] [PubMed] [Google Scholar]
- 28.Winn RM, Gil-Lamaignere C, Roilides E et al. . Selective effects of interleukin (IL)-15 on antifungal activity and IL-8 release by polymorphonuclear leukocytes in response to hyphae of Aspergillus species. J Infect Dis 2003; 188:585–90. [DOI] [PubMed] [Google Scholar]
- 29.Sato T, Iwabuchi K, Nagaoka I et al. . Induction of human neutrophil chemotaxis by Candida albicans-derived beta-1,6-long glycoside side-chain-branched beta-glucan. J Leukoc Biol 2006; 80:204–11. [DOI] [PubMed] [Google Scholar]
- 30.Rex JH, Bennett JE, Gallin JI, Malech HL, Melnick DA. Normal and deficient neutrophils can cooperate to damage Aspergillus fumigatus hyphae. J Infect Dis 1990; 162:523–8. [DOI] [PubMed] [Google Scholar]
- 31.Inoue K, Takano H, Oda T et al. . Candida soluble cell wall beta-D-glucan induces lung inflammation in mice. Int J Immunopathol Pharmacol 2007; 20:499–508. [DOI] [PubMed] [Google Scholar]
- 32.Robertson AL, Holmes GR, Bojarczuk AN et al. . A zebrafish compound screen reveals modulation of neutrophil reverse migration as an anti-inflammatory mechanism. Sci Transl Med 2014; 6:225ra29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Woodfin A, Voisin MB, Beyrau M et al. . The junctional adhesion molecule JAM-C regulates polarized transendothelial migration of neutrophils in vivo. Nat Immunol 2011; 12:761–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grogan JB, Smith GV. Neutrophil function in clinical kidney allograft recipients. Surgery 1975; 78:316–21. [PubMed] [Google Scholar]
- 35.Clark RA, Gallin JI, Fauci AS. Effects of in vivo prednisone on in vitro eosinophil and neutrophil adherence and chemotaxis. Blood 1979; 53:633–41. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.