Abstract
Background. Human immunodeficiency virus (HIV) infection and associated immune activation predict the risk of cardiovascular disease in resource-rich areas. Less is known about these relationships in sub-Saharan Africa.
Methods. Beginning in 2005, we enrolled subjects in southwestern Uganda into a cohort at the time of antiretroviral therapy (ART) initiation. Multiple immune activation measures were assessed before and 6 months after ART initiation. Beginning in 2013, participants aged >40 years underwent metabolic profiling, including measurement of hemoglobin A1c and lipid levels and carotid ultrasonography. We fit regression models to identify traditional and HIV-specific correlates of common carotid intima media thickness (CCIMT).
Results. A total of 105 participants completed carotid ultrasonography, with a median completion time of 7 years following ART initiation. Age, low-density lipoprotein cholesterol level, and pre-ART HIV load were correlated with CCIMT. No association was found between CCIMT and any pre-ART biomarkers of immune activation. However, in multivariable models adjusted for cardiovascular disease risk factors, lower absolute levels of soluble CD14 and interleukin 6 and greater declines in the CD14 level and kynurenine-tryptophan ratio after 6 months of ART predicted a lower CCIMT years later (P < .01).
Conclusions. Persistent immune activation despite ART-mediated viral suppression predicts the future atherosclerotic burden among HIV-infected Ugandans. Future work should focus on clinical correlates of these relationships, to elucidate the long-term health priorities for HIV-infected people in the region.
Keywords: HIV/AIDS, Uganda, aging, inflammation, atherosclerosis, carotid intima media thickness, antiretroviral therapy
The era of antiretroviral therapy (ART) has brought remarkable improvement in life expectancy for human immunodeficiency virus (HIV)–infected individuals [1, 2], but it has also introduced new challenges. Soon after it became evident that ART prolonged survival, data from the United States and Europe began demonstrating increasing incidence of cardiovascular disease [3]. Large cohort studies have corroborated that the risk of both myocardial infarction and cerebrovascular disease (CVD) is 40%–70% greater among HIV-infected individuals than among age- and sex-matched HIV-uninfected controls [4–7].
Whereas some ART medications have been implicated in CVD risk [3], the elevated risk in this population appears to be mediated to a greater extent by HIV infection itself. Studies have demonstrated an increased risk of CVD events among those discontinuing ART [8] and in those with detectable viral loads [7]. It has been hypothesized that the increased attributable risk among HIV-infected individuals is due to increased immune activation and chronic inflammation, which remain abnormally high among those infected with HIV even after viral suppression [9, 10], and are associated with preclinical and clinical atherosclerosis [11–16].
To date, there are few data describing these same correlations between HIV infection, immune activation, and CVD risk in sub-Saharan Africa, where >70% of the world's population of HIV-infected individuals reside [17]. We measured a well-validated surrogate marker of CVD risk [18–21], common carotid intima media thickness (CCIMT), a median of 7 years after ART initiation among participants enrolled in a prospective, longitudinal cohort of HIV-infected individuals. We sought to assess relationships between ART, immune activation, and risk of atherosclerotic disease among HIV-infected individuals in Uganda.
METHODS
Study Participants and Procedures
Beginning in 2005, participants were enrolled in the Ugandan AIDS Rural Treatment Outcomes study. Full cohort details have been described previously [22, 23]. Briefly, participants provided blood samples just prior to ART initiation (baseline) and then every 3–4 months thereafter for determination of the CD4+ T-cell count and viral load. At baseline, for all participants, and 6 months after ART initiation, for those who achieved viral suppression by that time (n = 90), we assessed cryopreserved plasma samples for levels of soluble CD14 (sCD14; R&D Systems), soluble CD163 (sCD163; Trillium Diagnostics), interleukin 6 (IL-6; Human IL-6 Ultra-Sensitive Kit, Meso Scale Diagnostics), D-dimer (Diagnostico Stago), lipopolysaccharide (LPS; Limulus Amebocyte Assay, Cambrex) and the ratio of kynurenine to tryptophan (KT) [24]. The percentage of CD38+DR+ T cells among CD8+ T-cells was assessed in fresh whole-blood specimens processed the same day, as described previously [22, 24]. Approximately 95% of samples were stored in acid citrate dextrose (ACD), with the remainder stored in ethylenediaminetetraacetic acid. To account for differences in diluents, an adjustment factor of 1.276 was multiplied to the results of plasma markers tested from ACD tubes. Depending on the year of enrollment, HIV type 1 (HIV-1) RNA load testing was performed with the Roche Amplicor HIV Monitor assay or the Roche Cobas Taqman HIV-1 test, version 1.0.
In December 2013, we began a substudy of cardiovascular disease risk assessment, titled the Ugandan Noncommunicable Diseases and Aging Cohort (clinical trials registration NCT02445079). Eligibility criteria for the substudy are age >40 years and a minimum of 3 years of ART use. Additional substudy procedures included completion of a questionnaire to assess smoking history, measurement of anthropomorphic characteristics, testing of blood specimens for the serum lipid profile and hemoglobin A1c level, measurement of blood pressure, and performance of carotid ultrasonography for CCIMT estimation. Serum chemistry tests were performed with the Abbott Architect Clinical Chemistry Analyzer (Abbott Diagnostics, Abbot Park, Illinois). Hemoglobin A1c levels were measured with the Bayer A1c Now+ point of care assay (Bayer, Pittsburgh, Pennsylvania).
CCIMT Measurement
CCIMT measurements were performed using a standardized protocol [25] by a single operator who completed the CCIMT training course at the University of Wisconsin [26]. All ultrasonography was performed with a Sonosite M-Turbo (Sonosite, Bothell, Washington). We collected images from the anterior, lateral, and posterior position on both the left and right carotid artery for a total of 6 images per participant. All study images were scored by a board-certified cardiologist. Images with a score of 3 were discarded from the analysis. Far-wall CCIMT was measured in 1-cm segments directly proximal to the carotid bulb, using semiautomated border-detection software (SonoCalc, version 5.0; Sonosite), as supported by published recommendations of CCIMT interpretation [25]. The mean value of all adequate images was summarized as the mean CCIMT estimate for each participant. Study staff were blinded to participant information during image quality assurance and CCIMT measurement.
Ultrasonography Quality Assurance
The ultrasonography technologist completed paired, prestudy ultrasonography on 2 separate days for 10 volunteers. The mean CCIMTs on day 1 and day 2 were both 0.538 mm (t = 0.0012 and P = .999, by the paired t test), with an absolute value mean difference (±standard deviation [SD]) between days of 0.017 ± 0.018 mm. The coefficient of regression (β) between the 2 days was 1.001 (P < .001 and R2 = 0.87), and the coefficient of variation was 4.9%. The mean image quality score (±SD) for images collected during the study, as graded by the study cardiologist (L. H.), was 1.40 ± 0.71, with 1 indicating a high-quality image and 3 indicating a poor-quality image. This mean score falls well within recommended thresholds for adequate image quality [26]. Approximately three quarters of images (453 of 616 [74%]) were scored as high quality, 13% (78 of 616) were scored as average quality, and 14% (85 of 616) were scored as unsuitable for interpretation and discarded from the analysis. After discarding poor-quality images, most participants (60 of 105 [57%]) contributed all 6 anatomical images to the calculation of CCIMT, and nearly all participants (100 of 105 [95%]) contributed at least 4 images, a proportion higher than reported in other similar published studies [27].
Statistical Analyses
We used standardized statistical summarization techniques to describe cohort characteristics. To confirm assumptions about linear relationships, we graphically depicted scatterplots between mean CCIMT and relevant predictors of interest, including standard CVD risk factors and markers of immune activation. We then fit linear univariable regression models for each predictor of interest, including age, sex, smoking status (ever vs never), hemoglobin A1c level on the date of CCIMT, high blood pressure (defined as a systolic pressure of >140 mm Hg or a diastolic pressure of >90 mm Hg on the date of CCIMT), low-density lipoprotein (LDL) cholesterol level on the date of CCIMT, high-density lipoprotein (HDL) cholesterol level on the date of CCIMT, CD4+ T-cell count on the date of CCIMT, CD4+ T-cell count nadir, pre-ART viral load, current viral load (dichotomized as less than or greater than 400 copies/mL), and total ART duration through the date of CCIMT. To determine covariates for inclusion in final models, we performed backward stepwise multivariable logistic regression, retaining all variables with a level of significance of ≤0.25.
We added each marker of immune activation to the multivariable model individually, using the pre-ART absolute level, the 6-month post-ART absolute level, and the difference between 6 months and the baseline value (meaning that a positive value represents an increase in the level of the inflammatory marker from baseline to 6 months). All markers of immune activation were log transformed and divided by the cohort interquartile range (IQR), such that each unit of increase in the coefficient represented a change in the IQR of the marker. Because any association between markers of immune activation and atherosclerosis risk might be mediated by HIV viremia, we also fit additional models excluding pre-ART viral load. Last, we assessed for interaction in associations between immune activation and atherosclerosis, by sex. All statistical analyses were conducted with Stata, version 13 (Statacorp, College Station, Texas).
Ethical Considerations
Study procedures were reviewed and approved by the institutional review committees of the Mbarara University of Science and Technology, the Ugandan National Council of Science and Technology, and Partners Healthcare. All participants gave written informed consent.
RESULTS
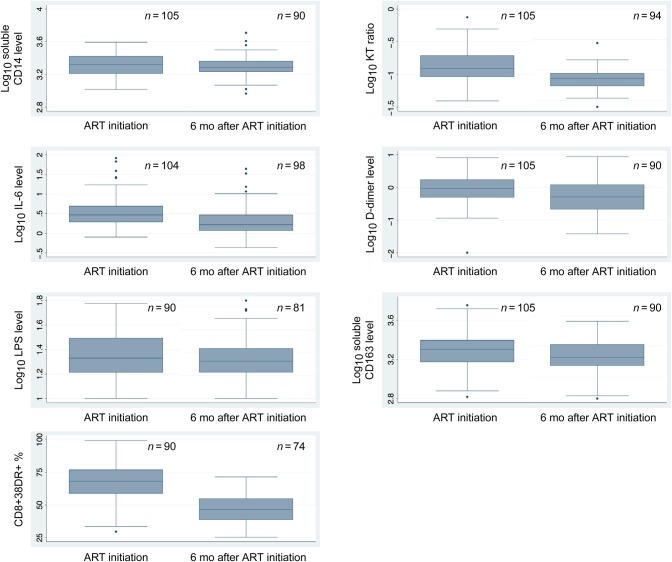
One hundred and five participants completed the CVD substudy. A total of 51% of participants were female, with a median age of 49 years (IQR, 45–51 years; Table 1). Twenty-eight participants (27%) were overweight or obese (body mass index, >25), and 39 (37%) were former or current smokers. The median LDL cholesterol level was 74 mg/dL (IQR, 56–93 mg/dL), and the median HDL cholesterol level was 44 mg/dL (IQR, 37–53 mg/dL). At the time of CCIMT, participants had a median of 7.0 years of ART exposure (IQR, 6.4–7.5 years) and a median CD4+ T-cell count of 430 cells/mm3 (IQR, 334–546 cells/mm3), and 85 (81%) had a viral load of <400 copies/mL. The median nadir CD4+ T-cell count was 122 cells/mm3 (IQR, 80–175 cells/mm3). Distributions of each inflammatory marker at baseline and for those with an undetectable viral load at 6 months are displayed in Figure 1. Levels of most biomarkers decreased substantially (P < .001) between the pre-ART and 6-month measurements, with the exception of sCD14 (3.32 vs 3.29; P = .04) and LPS (1.33 vs 1.34; P = .90).
Table 1.
Cohort Characteristics
| Characteristic | Summary Statistic (n = 105) |
|---|---|
| At time of CCIMT measurement | |
| Female sex | 54 (51) |
| Age, y | 49 (45–51) |
| Body mass index | |
| <18 | 6 (6) |
| 18–25 | 71 (68) |
| 25–30 | 22 (21) |
| >30 | 6 (6) |
| Smoking history | |
| Never | 66 (63) |
| Former | 35 (33) |
| Current | 4 (4) |
| Hb A1c level, % of total Hb | 5.3 (5.0–5.7) |
| High blood pressure | 12 (11) |
| LDL cholesterol level, mg/dL | 74 (56–93) |
| HDL cholesterol level, mg/dL | 44 (37–53) |
| Creatinine level, mg/dL | 0.77 (0.72–0.84) |
| ART duration, y | 7.0 (6.4–7.5) |
| ART regimen | |
| AZT/3TC/NVP | 68 (65) |
| AZT/3TC/EFV | 21 (20) |
| TDF/3TC/LPV/r | 9 (9) |
| Other | 7 (7) |
| Historical HIV-specific indicators | |
| CD4+ T-cell count, cells/mm3 | |
| Nadir | 122 (80–175) |
| 6 mo after ART initiation | 218 (152–301) |
| At time of CCIMT measurement | 430 (334–546) |
| Viral load, log10 copies/mL | |
| Baseline | 5.1 (4.8–5.6) |
| <400 6 mo after ART initiation | 96 (91) |
Data are no. (%) of subjects or median value (interquartile range). Blood test results were collected on the day of carotid ultrasonography, unless otherwise indicated.
Abbreviations: 3TC, lamivudine; ART, antiretroviral therapy; AZT, zidovudine; CCIMT, common carotid intima media thickness; Hb, hemoglobin; HDL, high-density lipoprotein; HIV, human immunodeficiency virus; LDL, low-density lipoprotein; LPV/r, lopinavir/ritonavir; NVP, nevirapine; TDF, tenofovir.
Figure 1.
Distribution of markers of immune activation before and 6 months after initiation of antiretroviral therapy (ART) among a cohort of people in southwestern Uganda infected with human immunodeficiency virus. Abbreviations: IL-6, interleukin 6; KT, kynurenine-tryptophan; LPS, lipopolysaccharide; sCD14, soluble CD14; sCD163, soluble CD163.
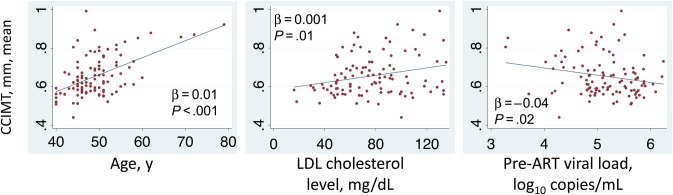
The mean CCIMT (±SD) in the cohort was 0.656 ± 0.10 mm (range, 0.513–1.153 mm). In univariable models, greater mean CCIMT was associated with older age (β = 0.088 for each 10-year increase; P < .001), higher hemoglobin A1c level (0.018; P = .02), higher LDL (0.048 for each 50-mg/dL increase; P = .01), lower baseline viral load (−0.041 for each log10 copies/mL increase; P = .02), and higher nadir CD4+ T-cell count (0.014 for each 50-cell/mm3 increase; P = .02; Table 2). In multivariable models, only age (β = 0.081; P < .001), LDL cholesterol level (0.038; P = .01), and baseline viral load (−0.038; P = .01) were independently associated with mean CCIMT (Table 2 and Figure 2).
Table 2.
Correlates of Common Carotid Intima Media Thickness
| Correlate | Univariable Model |
Multivariable Modela |
||
|---|---|---|---|---|
| β coefficient (95% CI) | P Value | β coefficient (95% CI) | P Value | |
| Female sex | 0.028 (−.012 to .068) | .17 | ||
| Age (each 10-y increase) | 0.088 (.060–.116) | <.001 | 0.081 (.054–.108) | <.001 |
| Body mass index | ||||
| <18 | 0.017 (−.072 to .106) | .70 | ||
| 18–25 | Reference | |||
| 25–30 | 0.010 (−.041 to .061) | .71 | ||
| >30 | 0.010 (−.079 to .099) | .82 | ||
| Ever smoker | −0.022 (−.064 to .019) | .29 | ||
| Hemoglobin A1c level | 0.018 (.003–.341) | .02 | ||
| High blood pressure | 0.010 (−.053 to .074) | .75 | ||
| LDL cholesterol level (each 50-mg/dL increase) | 0.048 (.012–.083) | .01 | 0.038 (.008–.068) | .01 |
| HDL cholesterol level (each 50-mg/dL increase) | 0.006 (−.077 to .090) | .88 | ||
| Creatinine level | −0.037 (−.179 to .104) | .60 | ||
| Nadir CD4+ T-cell count (each 50-cell increase) | 0.014 (.002–.026) | .02 | ||
| Baseline viral load (each log10 copies/mL increase) | −0.041 (−.074 to −.008) | .02 | −0.038 (−.066 to .010) | .01 |
| Current viral load undetectable | 0.010 (−.042 to .061) | .71 | ||
| Duration of ART (each additional year) | −0.006 (−.026 to .014) | .54 | ||
| ART regimen (no. [%]) | ||||
| AZT/3TC/NVP | Reference | |||
| AZT/3TC/EFV | 0.035 (−.017 to .086) | .19 | ||
| TDF/3TC/LPV/r | 0.023 (−.050 to .097) | .53 | ||
| Other | 0.038 (−.044 to .120) | .36 | ||
Abbreviations: 3TC, lamivudine; ART, antiretroviral therapy; AZT, zidovudine; CI, confidence interval; HDL, high-density lipoprotein; LDL, low-density lipoprotein; LPV/r, lopinavir/ritonavir; NVP, nevirapine; TDF, tenofovir.
a Backward stepwise regression with a P value threshold of .25 was used to select covariates for the multivariable model.
Figure 2.
Scatterplots demonstrating relationships between common carotid intima media thickness (CCIMT) and age, low-density lipoprotein (LDL) cholesterol level, and viral load before antiretroviral therapy (ART) initiation among a cohort of people in southwestern Uganda receiving therapy for a median of 7 years. Fit lines, β statistics, and P values were generated by bivariate linear regression models.
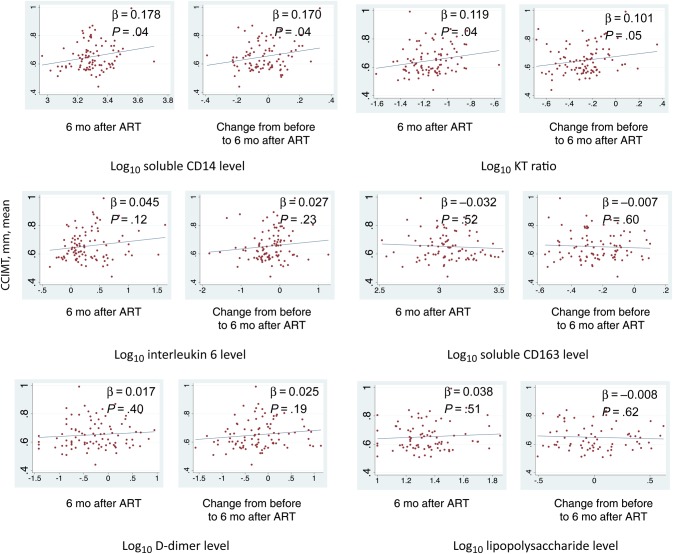
None of the pre-ART markers of immune activation (sCD14, sCD163, IL-6, d-dimer, and LPS levels; KT ratio; and proportion CD38+DR+ T cells among CD8+ T-cells) were associated with future mean CCIMT either in univariable models or after adjustment for age, LDL cholesterol level, and baseline viral load (Table 3). In contrast, in models restricted to those with an undetectable viral load at 6 months, higher absolute values of sCD14 (0.022 mm per IQR increase; P = .04) and KT ratio (0.024 mm per IQR increase; P = .04) were associated with a higher mean CCIMT (Figure 2). Although not statistically significant, a similar relationship was seen with IL-6 levels at 6 months after ART initiation (0.018 mm per IQR increase; P = .120). These relationships remained (or became) significant after adjustment of models for age, LDL cholesterol level, and baseline viral load for sCD14 level (0.026 mm per IQR increase; P = .006) and IL-6 level (0.020 mm per IQR increase; P = .04) but not for KT ratio (0.013 mm per IQR increase; P = .25; Table 3). Similarly, after adjustment for age, LDL cholesterol level, and baseline viral load, greater decreases in sCD14 level (0.041 mm per IQR decrease; P = .003) and KT ratio (0.032 mm per IQR decrease; P = .005) between baseline and 6 months after ART-mediated viral suppression were associated with lower CCIMT years later (Table 3 and Figure 3). Decreases in IL-6 and d-dimer levels during the first 6 months of ART had similar but nonsignificant associations with future mean CCIMT (Table 3). We found no association between changes in sCD163 level, plasma LPS level, or percentage of CD38+HLA-DR+ T cells among CD8+ T cells during the first 6 months of ART and future CCIMT (Table 3). Removal of the pre-ART viral load from models did not alter any of the above associations. Last, we found that the association of sCD14 appeared to be independent of other inflammatory markers. For example, in a multivariable model including changes between the pre-ART value and the 6-month value for both the sCD14 level and KT ratio, we found only minimal confounding effects in either marker, with regression coefficients decreasing by approximately 30% in each and unchanged P values (P = .03 vs .04 for the sCD14 level, and P = .04 vs .06 for the KT ratio). In a model with 6-month levels of both sCD14 and IL-6, the sCD14 coefficient was largely unchanged (β changed from 0.026 to 0.022 mm per IQR), and the association remained significant (P = .03), whereas the IL-6 coefficient decreased substantially and became nonsignificant (P = .38). We found no evidence of interaction between markers of immune activation and atherosclerosis, by sex.
Table 3.
Relationships Between Carotid Intima Media Thickness a Median of 7 Years After Antiretroviral Therapy (ART) Initiation and Levels of Soluble Biomarkers of Immune Activation at the Time of ART Initiation, Levels 6 Months After ART Initiation, and Changes in Levels Over the First 6 Months of Therapy
| Marker | Level Before ART Initiation, Per IQR Increasea |
Level 6 Mo After ART Initiation, Per IQR Increasea |
Difference in Levels Between 6 Mo After ART and Before ARTa |
|||
|---|---|---|---|---|---|---|
| β coefficient (95% CI) | P Value | β coefficient (95% CI) | P Value | β coefficient (95% CI) | P Value | |
| Soluble CD14 | −0.005 (−.032 to .020) | .68 | 0.026 (.008–.044) | .006 | 0.041 (−.067 to .014) | .003 |
| KT ratio | −0.017 (−.040 to .006) | .15 | 0.013 (−.009 to .035) | .25 | 0.032 (−.055 to −.010) | .005 |
| IL-6 | −0.002 (−.019 to .016) | .85 | 0.020 (.001–.039) | .04 | 0.015 (−.032 to .002) | .08 |
| D-dimer | −0.000 (−.022 to .021) | .98 | 0.016 (−.010 to .041) | .24 | 0.019 (−.042 to .005) | .11 |
| Lipopolysaccharide | 0.006 (−.015 to .028) | .58 | −0.002 (.024–.020) | .87 | −0.012 (−.016 to .040) | .40 |
| Soluble CD163 | 0.016 (−.035 to .003) | .09 | −0.016 (−.040 to .008) | .18 | −0.004 (−.021 to .029) | .78 |
| Percentage of CD38+DR+ T-cell lymphocytes | −0.006 (−.031 to .018) | .61 | −0.014 (−.042 to .015) | .35 | 0.002 (−.028 to .024) | .86 |
All models were adjusted for age, low-density lipoprotein cholesterol level, and baseline viral load.
Abbreviations: CI, confidence interval; IL-6, interleukin 6; IQR, interquartile range; KT, kynurenine-tryptophan.
a Inflammatory markers are log-transformed and divided by the IQR of the cohort, such that the β coefficient signifies the estimated change in mean common carotid intima media thickness for each IQR in the marker of inflammation. In the models with a change in inflammatory markers, the variable is calculated as 6 months – baseline, such that a larger number represents less of a decrease over that period.
Figure 3.
Unadjusted scatter plots demonstrating relationships between common carotid intima media thickness (CCIMT) and markers of immune activation at 6 months after antiretroviral therapy (ART) initiation (left) and changes from pre-ART to 6 months after initiation of ART (right) among a cohort of people in southwestern Uganda receiving therapy for a median of 7 years. Inflammatory markers are log transformed. Fit lines, β-statistics, and P values were generated by bivariate linear regression models. Abbreviation: KT, kynurenine-tryptophan.
DISCUSSION
To our knowledge, we present the first study describing prospective associations between markers of immune activation and future risk of atherosclerotic burden in HIV-infected individuals in sub-Saharan Africa. Our data demonstrate that persistent immune activation, despite 6 months of ART-mediated viral suppression, is associated with an increased burden of carotid atherosclerosis a median of 7 years later in a population of older-aged people receiving stable ART in southwestern Uganda. Further data are needed to corroborate these findings and to assess the population health relevance of these associations. If our results are confirmed, they reinforce the important need to consider expanding the breadth of HIV care programs in the region to include CVD screening, prevention, and treatment [28–30].
The association we found between sCD14, a macrophage-associated marker of immune activation, and subclinical atherosclerosis among HIV-infected individuals in Uganda builds upon prior work from the United States and Europe [31, 32]. This specific marker is of particular relevance to this population because it appears to remain elevated in HIV-infected individuals, compared with HIV-uninfected controls, despite receipt of long-term suppressive ART [33] and because of its association with both cardiovascular disease risk and all-cause mortality in the general population [34]. A subanalysis of HIV-infected persons within the SATURN randomized controlled trial, which evaluated the effect of rosuvastatin on atherosclerotic disease among people with elevated C-reactive protein (CRP) levels, noted independent cross-sectional associations between sCD14 and coronary artery calcification [35]. More recently, a large analysis among participants in the Multicenter AIDS Cohort Study also noted significant cross-sectional correlations between sCD14 levels and noncalcified coronary artery plaque, coronary stenosis >50%, and total plaque score [13]. Other studies that have assessed inflammatory markers and progression of carotid atherosclerosis have found no association between sCD14 or high-sensitivity C-reactive protein levels and CCIMT at baseline, but strong associations between sCD14 levels and future CCIMT progression were observed [36, 37]. An important and unique feature of our study is that we were able to assess relationships among a population before and after ART-mediated viral suppression and subsequent atherosclerosis years later. We found that those with the highest levels of sCD14 after 6 months of ART had the greatest future CCIMT. One potential explanation for this association is that persistent monocyte activation despite suppression of HIV viremia could be a key driver of cardiovascular disease risk in this setting. Alternatively, other potential drivers of immune activation that are not directly affected by ART (eg, helminthic infection and subclinical malaria) could also be responsible. Future studies involving HIV-uninfected persons will help to elucidate these relationships.
Our study is the first report, to our knowledge, to correlate the KT ratio with the atherosclerotic disease risk among an HIV-infected population. The KT ratio largely reflects the activity of host indoleamine 2,3-dixoygenase-1 (IDO), an inducible enzyme in macrophages and dendritic cells that catabolizes tryptophan to kynurenine. IDO is activated in response to type I and II interferons and microbial products [38, 39]. We have previously shown that the KT ratio is elevated in HIV-infected Ugandans initiating ART, that levels decrease after ART, and that levels before initiation of and during ART independently predict all-cause mortality in this setting [24]. Whereas IDO activity has been associated with both cardiovascular disease risk factors [40] and coronary artery disease [41] in uninfected populations, as well as with CCIMT among patients undergoing dialysis [42], less is known about the KT ratio and cardiovascular disease risk among HIV-infected populations. Our finding that the failure to decrease KT ratios despite 6 months of suppressive ART is associated with future CCIMT, independent both of traditional cardiovascular risk factors and sCD14, suggests that this pathway might also play a similarly unique role in atherogenesis in HIV-infected persons and should prompt further investigation of these relationships.
We also detected associations between persistent elevations in IL-6 levels despite 6 months of ART-mediated viral suppression and carotid atherosclerosis. Data from the SMART study demonstrated that IL-6 is an independent correlate of both CVD outcomes and all-cause mortality in HIV-infected persons [12, 43]. Notably, as in our study, that study detected associations between IL-6 levels and clinical outcomes even among those receiving suppressive ART. Similarly, a cross-sectional study of people with HIV infection who were receiving suppressive ART found nonsignificant associations between plasma IL-6 levels and abnormal carotid atherosclerosis (P = .08), defined as an internal carotid intima media thickness of >1.0 mm [44].
We did not find relationships between carotid atherosclerosis and baseline or early post-ART changes in sCD163 levels, another marker of monocyte activation and, like sCD14 levels, a marker of HIV-related immune activation [45]. This finding is in contrast to prior work noting relationships between sCD163 and noncalcified coronary artery plaque, coronary artery stenosis, and arterial wall inflammation [11, 13, 46]. Ours is the first study, to our knowledge, to assess this interaction in sub-Saharan Africa. Interestingly, while associations between sCD163 levels and CCIMT have been noted in the general population [47], the only other study, to our knowledge, to assess correlations between sCD163 levels and carotid atherosclerosis among an HIV-infected population also failed to find a relationship between the two [48].
An unexpected finding of our study was that higher pre-ART viral load and lower nadir CD4+ T-cell count predicted decreased future CCIMT. This finding is similar to that from a prior study in the United States, which found that higher nadir CD4+ T-cell counts were associated with an increased risk of CCIMT progression during ART [49], but it differs from those from larger cohort studies in the United States that have noted a decreasing risk of stroke and myocardial infarction with increasing nadir CD4+ T-cell counts [6, 50]. Because our study evaluated participants who remained alive and in care a median of 7 years after ART initiation, we could be observing a survivorship bias. Alternatively, there could be a lagged relationship between nadir CD4+ T-cell count and atherosclerosis, such that the risk begins once a relative return to health is achieved. Future work will be necessary to help elucidate the precise relationships between HIV viremia, immune activation, and atherosclerotic disease burden in this setting.
Our study should be considered while keeping several limitations in mind. Most importantly, only study participants who remained in the parent study from enrollment through initiation of the cardiovascular disease procedures in late 2013 were eligible to be included in the substudy. Notably only 80 of 762 participants (10%) in the parent cohort died, withdrew, or were lost to follow-up from the parent study in the interim. Of these, only 49 (6%) would have been >40 years of age in 2014 and eligible for this study, including 35 (5%) who died in the interim. Furthermore, our 6-month measures of immune activation were restricted to those with viral suppression at that time. As such, our study should be taken to represent associations between baseline inflammatory markers and atherosclerotic progression among those who suppress HIV during the first 6 months of ART and survive in the 5–10-year period after ART initiation. As with all nonrandomized observational studies, our study could also by limited by unmeasured or residual confounding from the associations between immune activation and atherosclerosis. Last, we currently lack similar data from an HIV-uninfected control group sampled from the same environment, so we are not able to address whether the associations between inflammatory markers and atherosclerosis observed in this study are unique to HIV infection. We plan to address this issue in the future as part of an ongoing extension of this study.
In conclusion, we found that persistent immune activation following ART initiation is strongly and independently associated with the subsequent extent of carotid atherosclerosis among ART-treated, HIV-infected individuals in Uganda. These data are among the first to demonstrate this association in the sub-Saharan African region and signal an important need to further explore the long-term health priorities for the large and aging population of HIV-infected individuals in the region. Future work should focus on relationships between HIV infection, immune activation, and clinical outcomes and, if significant relationships are confirmed, should evaluate preventive and interventional strategies to reduce the CVD risk in this population.
Notes
Disclaimer. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Financial support. This work was supported by the National Institutes of Health (grants R21HL124712, K23MH099916, R56AI100765, R21AI078774, R01MH054907, P01AI027763 and P01AI076174), the Harvard Center for AIDS Research, the Doris Duke Charitable Foundation, and the Sullivan Family Foundation.
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Antiretroviral Therapy Cohort Collaboration. Life expectancy of individuals on combination antiretroviral therapy in high-income countries: a collaborative analysis of 14 cohort studies. Lancet 2008; 372:293–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lima VD, Hogg RS, Harrigan PR et al. Continued improvement in survival among HIV-infected individuals with newer forms of highly active antiretroviral therapy. AIDS 2007; 21:685–92. [DOI] [PubMed] [Google Scholar]
- 3.Friis-Moller N, Reiss P, Sabin CA et al. Class of antiretroviral drugs and the risk of myocardial infarction. N Engl J Med 2007; 356:1723–35. [DOI] [PubMed] [Google Scholar]
- 4.Chow FC, Regan S, Feske S, Meigs JB, Grinspoon SK, Triant VA. Comparison of ischemic stroke incidence in HIV-infected and non-HIV-infected patients in a US health care system. J Acquir Immune Defic Syndr 2012; 60:351–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab 2007; 92:2506–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marcus JL, Leyden WA, Chao CR et al. HIV infection and incidence of ischemic stroke. AIDS 2014; 28:1911–9. [DOI] [PubMed] [Google Scholar]
- 7.Freiberg MS, Chang CC, Kuller LH et al. HIV infection and the risk of acute myocardial infarction. JAMA Intern Med 2013; 173:614–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.El-Sadr WM, Lundgren JD, Neaton JD et al. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med 2006; 355:2283–96. [DOI] [PubMed] [Google Scholar]
- 9.Brenchley JM, Price DA, Schacker TW et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med 2006; 12:1365–71. [DOI] [PubMed] [Google Scholar]
- 10.Hunt PW, Brenchley J, Sinclair E et al. Relationship between T cell activation and CD4+ T cell count in HIV-seropositive individuals with undetectable plasma HIV RNA levels in the absence of therapy. J Infect Dis 2008; 197:126–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Subramanian S, Tawakol A, Burdo TH et al. Arterial inflammation in patients with HIV. JAMA 2012; 308:379–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duprez DA, Neuhaus J, Kuller LH et al. Inflammation, coagulation and cardiovascular disease in HIV-infected individuals. PLoS One 2012; 7:e44454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McKibben RA, Margolick JB, Grinspoon S et al. Elevated levels of monocyte activation markers are associated with subclinical atherosclerosis in men with and those without HIV infection. J Infect Dis 2015; 211:1219–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ross AC, Rizk N, O'Riordan MA et al. Relationship between inflammatory markers, endothelial activation markers, and carotid intima-media thickness in HIV-infected patients receiving antiretroviral therapy. Clin Infect Dis 2009; 49:1119–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Triant VA, Grinspoon SK. Immune dysregulation and vascular risk in HIV-infected patients: implications for clinical care. J Infect Dis 2011; 203:439–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Triant VA, Meigs JB, Grinspoon SK. Association of C-reactive protein and HIV infection with acute myocardial infarction. J Acquir Immune Defic Syndr 2009; 51:268–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. UNAIDS Report on the Global AIDS Epidemic. UNAIDS. 2013. http://www.unaids.org/sites/default/files/en/media/unaids/contentassets/documents/epidemiology/2013/gr2013/UNAIDS_Global_Report_2013_en.pdf Accessed 1 March 2015.
- 18.Chambless LE, Heiss G, Folsom AR et al. Association of coronary heart disease incidence with carotid arterial wall thickness and major risk factors: the Atherosclerosis Risk in Communities (ARIC) Study, 1987–1993. Am J Epidemiol 1997; 146:483–94. [DOI] [PubMed] [Google Scholar]
- 19.Hodis HN, Mack WJ, LaBree L et al. The role of carotid arterial intima-media thickness in predicting clinical coronary events. Ann Intern Med 1998; 128:262–9. [DOI] [PubMed] [Google Scholar]
- 20.Lorenz MW, Markus HS, Bots ML, Rosvall M, Sitzer M. Prediction of clinical cardiovascular events with carotid intima-media thickness: a systematic review and meta-analysis. Circulation 2007; 115:459–67. [DOI] [PubMed] [Google Scholar]
- 21.O'Leary DH, Polak JF, Kronmal RA, Manolio TA, Burke GL, Wolfson SK Jr. Carotid-artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. Cardiovascular Health Study Collaborative Research Group. N Engl J Med 1999; 340:14–22. [DOI] [PubMed] [Google Scholar]
- 22.Hunt PW, Cao HL, Muzoora C et al. Impact of CD8+ T-cell activation on CD4+ T-cell recovery and mortality in HIV-infected Ugandans initiating antiretroviral therapy. AIDS 2011; 25:2123–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Siedner MJ, Lankowski A, Tsai AC et al. GPS-measured distance to clinic, but not self-reported transportation factors, are associated with missed HIV clinic visits in rural Uganda. AIDS 2013; 27:1503–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Byakwaga H, Boum Y II, Huang Y et al. The kynurenine pathway of tryptophan catabolism, CD4+ T-cell recovery, and mortality among HIV-infected Ugandans initiating antiretroviral therapy. J Infect Dis 2014; 210:383–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stein JH, Korcarz CE, Hurst RT et al. Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: a consensus statement from the American Society of Echocardiography Carotid Intima-Media Thickness Task Force. Endorsed by the Society for Vascular Medicine. J Am Soc Echocardiogr 2008; 21:93–111; quiz 89–90. [DOI] [PubMed] [Google Scholar]
- 26.Korcarz CE, Hirsch AT, Bruce C et al. Carotid intima-media thickness testing by non-sonographer clinicians: the office practice assessment of carotid atherosclerosis study. J Am Soc Echocardiogr 2008; 21:117–22. [DOI] [PubMed] [Google Scholar]
- 27.Touboul PJ, Hernandez-Hernandez R, Kucukoglu S et al. Carotid artery intima media thickness, plaque and Framingham cardiovascular score in Asia, Africa/Middle East and Latin America: the PARC-AALA study. Int J Cardiovasc Imaging 2007; 23:557–67. [DOI] [PubMed] [Google Scholar]
- 28.Bendavid E, Ford N, Mills EJ. HIV and Africa's elderly: the problems and possibilities. AIDS 2012; 26(suppl 1):S85–91. [DOI] [PubMed] [Google Scholar]
- 29.Mills EJ, Barnighausen T, Negin J. HIV and aging--preparing for the challenges ahead. N Engl J Med 2012; 366:1270–3. [DOI] [PubMed] [Google Scholar]
- 30.Negin J, Barnighausen T, Lundgren JD, Mills EJ. Aging with HIV in Africa: the challenges of living longer. AIDS 2012; 26(suppl 1):S1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hsue PY, Deeks SG, Hunt PW. Immunologic basis of cardiovascular disease in HIV-infected adults. J Infect Dis 2012; 205(suppl 3):S375–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sandler NG, Wand H, Roque A et al. Plasma levels of soluble CD14 independently predict mortality in HIV infection. J Infect Dis 2011; 203:780–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mendez-Lagares G, Romero-Sanchez MC, Ruiz-Mateos E et al. Long-term suppressive combined antiretroviral treatment does not normalize the serum level of soluble CD14. J Infect Dis 2013; 207:1221–5. [DOI] [PubMed] [Google Scholar]
- 34.Reiner AP, Lange EM, Jenny NS et al. Soluble CD14: genomewide association analysis and relationship to cardiovascular risk and mortality in older adults. Arterioscler Thromb Vasc Biol 2013; 33:158–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Longenecker CT, Jiang Y, Orringer CE et al. Soluble CD14 is independently associated with coronary calcification and extent of subclinical vascular disease in treated HIV infection. AIDS 2014; 28:969–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hsue PY, Scherzer R, Hunt PW et al. Carotid intima-media thickness progression in HIV-infected adults occurs preferentially at the carotid bifurcation and is predicted by inflammation. J Am Heart Assoc 2012; 1:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kelesidis T, Kendall MA, Yang OO, Hodis HN, Currier JS. Biomarkers of microbial translocation and macrophage activation: association with progression of subclinical atherosclerosis in HIV-1 infection. J Infect Dis 2012; 206:1558–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Favre D, Mold J, Hunt PW et al. Tryptophan catabolism by indoleamine 2,3-dioxygenase 1 alters the balance of TH17 to regulatory T cells in HIV disease. Sci Transl Med 2010; 2:32ra6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vujkovic-Cvijin I, Dunham RM, Iwai S et al. Dysbiosis of the gut microbiota is associated with HIV disease progression and tryptophan catabolism. Sci Transl Med 2013; 5:193ra91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Niinisalo P, Raitala A, Pertovaara M et al. Indoleamine 2,3-dioxygenase activity associates with cardiovascular risk factors: the Health 2000 study. Scand J Clin Lab Invest 2008; 68:767–70. [DOI] [PubMed] [Google Scholar]
- 41.Wirleitner B, Rudzite V, Neurauter G et al. Immune activation and degradation of tryptophan in coronary heart disease. Eur J Clin Invest 2003; 33:550–4. [DOI] [PubMed] [Google Scholar]
- 42.Kato A, Suzuki Y, Suda T et al. Relationship between an increased serum kynurenine/tryptophan ratio and atherosclerotic parameters in hemodialysis patients. Hemodial Int 2010; 14:418–24. [DOI] [PubMed] [Google Scholar]
- 43.Kuller LH, Tracy R, Belloso W et al. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med 2008; 5:e203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Merlini E, Luzi K, Suardi E et al. T-cell phenotypes, apoptosis and inflammation in HIV+ patients on virologically effective cART with early atherosclerosis. PLoS One 2012; 7:e46073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Burdo TH, Lentz MR, Autissier P et al. Soluble CD163 made by monocyte/macrophages is a novel marker of HIV activity in early and chronic infection prior to and after anti-retroviral therapy. J Infect Dis 2011; 204:154–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Burdo TH, Lo J, Abbara S et al. Soluble CD163, a novel marker of activated macrophages, is elevated and associated with noncalcfied coronary plaque in HIV-infected patients. J Infect Dis 2011; 204:1227–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moreno JA, Munoz-Garcia B, Martin-Ventura JL et al. The CD163-expressing macrophages recognize and internalize TWEAK: potential consequences in atherosclerosis. Atherosclerosis 2009; 207:103–10. [DOI] [PubMed] [Google Scholar]
- 48.Longenecker CT, Funderburg NT, Jiang Y et al. Markers of inflammation and CD8 T-cell activation, but not monocyte activation, are associated with subclinical carotid artery disease in HIV-infected individuals. HIV Med 2013; 14:385–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Volpe GE, Tang AM, Polak JF, Mangili A, Skinner SC, Wanke CA. Progression of carotid intima-media thickness and coronary artery calcium over 6 years in an HIV-infected cohort. J Acquir Immune Defic Syndr 2013; 64:51–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Klein DB, Leyden WA, Xu L et al. Declining relative risk for myocardial infarction among HIV-positive compared with HIV-negative individuals with access to care. Clin Infect Dis 2015; 60:1278–80. [DOI] [PubMed] [Google Scholar]